Abstract
We previously demonstrated that Saccharomyces cerevisiae vnx1Δ mutant strains displayed an almost total loss of Na+ and K+/H+ antiporter activity in a vacuole-enriched fraction. However, using different in vitro transport conditions, we were able to reveal additional K+/H+ antiporter activity. By disrupting genes encoding transporters potentially involved in the vnx1 mutant strain, we determined that Vcx1p is responsible for this activity. This result was further confirmed by complementation of the vnx1Δvcx1Δ nhx1Δ triple mutant with Vcx1p and its inactivated mutant Vcx1p-H303A. Like the Ca2+/H+ antiporter activity catalyzed by Vcx1p, the K+/H+ antiporter activity was strongly inhibited by Cd2+ and to a lesser extend by Zn2+. Unlike as previously observed for NHX1 or VNX1, VCX1 overexpression only marginally improved the growth of yeast strain AXT3 in the presence of high concentrations of K+ and had no effect on hygromycin sensitivity. Subcellular localization showed that Vcx1p and Vnx1p are targeted to the vacuolar membrane, whereas Nhx1p is targeted to prevacuoles. The relative importance of Nhx1p, Vnx1p, and Vcx1p in the vacuolar accumulation of monovalent cations will be discussed.
Keywords: Exchangers, Membrane Isolation, Mutant, Potassium Transport, Yeast, Ion Homeostasis, Vacuolar Membrane
Introduction
In prokaryotes as well as in eukaryotes, the tight control of alkali metal concentrations is necessary for the maintenance of important biological processes, such as pH regulation, enzyme activity, osmoregulation, and signaling (1–3). Cation/proton antiporters play a major role in the control of cytosolic ion concentrations (4–7). In Escherichia coli, NhaA contributes to the maintenance of a low cytosolic Na+ concentration and also enables growth in alkaline conditions (8). Based on recent structure determination and experimental data, a detailed model of Na+ extrusion has been proposed for NhaA (9, 10). The E. coli genome encodes two other functional Na+/H+ homologs, NhaB and ChaA. NhaB unlike NhaA transports Na+ out of the cell in a pH-independent manner (11). Interestingly, ChaA, a Ca2+/H+ antiporter, also catalyzes direct Na+/H+ (12) and K+/H+ antiport (13), conferring resistance to the presence of high concentrations of salts (Ca2+, Na+, or K+) in the medium.
Comparatively, in Saccharomyces cerevisiae four Na+(K+)/H+ exchangers have been described so far. Three belong to the monovalent cation proton antiporter superfamily (Nha1p, Nhx1p, and Kha1p) (5, 14, 15), and the fourth and last one to be characterized, Vnx1p, is a member of the type II calcium exchanger family (CAX)4 of the calcium cation antiporter (CaCA) superfamily (16). Despite its homology with Vcx1p, a well characterized vacuolar Ca2+/H+ antiporter (17–20), Vnx1p is unable to transport Ca2+ into the vacuole. We demonstrated its direct implication in the ΔpH-dependent monovalent cation transport across the yeast vacuolar membrane (16). Indeed, a vacuole-enriched fraction of vnx1Δ cells was totally devoid of exchange activity in the presence of NaCl or KCl, whereas vacuoles from nhx1Δ cells behaved like wild type cells as reported previously (21, 22).
Recently, transport of monovalent cations has been reported for three other members of the CaCA superfamily. It was shown that CAX1 from the chlorophyllian unicellular alga Chlamydomonas reinhardtii mediates vacuolar proton gradient-dependent Ca2+ and Na+ exchange activity when expressed in yeast (23). Furthermore, co-expression of AtCAX1 and AtCAX3 in yeast cells leads to the formation of “hetero-CAX” complexes able to transport Li+ without affecting Ca2+ transport rate (24). Finally, expression of AtCCX3 (formerly AtCAX9) in yeast cells induced a high capacity uptake of radioactive Rb+ in the vacuolar membrane-enriched fraction, which could be inhibited by an excess of cold K+, Na+, and Mn2+ but not Ca2+ (25). AtCAX9 as well as four other CAX transporters from Arabidopsis (AtCAX7–11) have been reassigned to the cation calcium exchanger family together with a mammalian K+-dependent Na+/Ca2+ antiporter (NCKX6) (26, 27), based on sequence homology and the presence of a characteristic α-repeat.
These observations would indicate that yeast CaCA evolved differently from other organisms attributing monovalent or divalent cation transport to two distinct members of the same family. However, we were able to detect a “residual” K+/H+ exchange activity in the vacuolar fraction extracted from vnx1Δ or vnx1Δnhx1Δ cells by changing the sodium and the potassium chloride, used in the transport assay, for their corresponding sulfate salts. Disruption of potential candidate genes in vnx1Δ background strains permitted us to assign this residual activity to Vcx1p. Overexpression of VCX1, or its inactive mutant form VCX1-H303A, in the yeast triple mutant vnx1Δvcx1Δnhx1Δ confirmed this newly discovered transport activity of Vcx1p.
EXPERIMENTAL PROCEDURES
Yeast Strains and Media
The vcx1Δ yeast strains (Table 1) were generated in the W303-1A background by replacing the YDL128w ORF with the kanMX6, natNT2, or hphNT1 gene by PCR-based gene deletion method (28, 29) using the following primers: Vcx1-F1 (5′-CGCATATCATTCATCGGCTGCTGATAGCAAATAAAACAGCATAGGCCACTAGTGGATCTG-3′) and Vcx1-R1 (5′-TTACGAAGAAGATAAAATATAAAAAAAAAGAGAATGGTGCAGCTGAAGCTTCGTACGC-3′). The deletion constructs contain 40 bp of homology with the beginning and end of the YDL128w ORF. Yeast strains were transformed with the deletion cassette using the standard lithium acetate method (30). Genomic DNA from antibiotic-resistant strains was isolated as described previously (31). Insertion of the disruption cassette into the correct locus was verified by PCR.
TABLE 1.
List of strains used in this study
BY double mutant strains were obtained by crossing the strain BY α vcx1Δ with strain BY from the other disruption mutants listed in Table 1 to generate diploid strains. Diploids were allowed to sporulate and were then subjected to tetrad dissection to generate haploid strains, according to Guthrie and Fink (32). All yeast strains used in this study are listed in Table 1. Yeast cells were grown in YPD (1% yeast extract, 2% peptone, 2% glucose), YPG (1% yeast extract, 2% peptone, 2% galactose), or SD media (0.67% yeast nitrogen base 2% glucose) with appropriate amino acid supplements as indicated.
Isolation of Intact Vacuoles from Yeast
Intact vacuoles were isolated as described by Ohsumi and Anraku (33). The intact vacuoles were collected on top of the gradient and resuspended in 5 mm Tris/Mes (pH 6.9). Protein concentrations were determined by the Bio-Rad DC protein assay according to the manufacturer's protocol.
Transport Assays
The fluorescence quenching of acridine orange was used to monitor the establishment and dissipation of vacuolar inside acidic pH gradients (supplemental Fig. 1S) (34, 35). Intact vacuoles (25 μg of protein) were used for each assay. Vacuoles were added to a buffer containing 50 mm tetramethylammonium chloride, 5 μm acridine orange, 5 mm Tris/Mes (pH 7.5), and 3.125 mm MgSO4. The vacuolar ATPase was activated by the addition of 2.5 mm Tris-ATP, and time-dependent acridine orange fluorescence changes were monitored using a QuantaMasterTM QM-4 fluorescent spectrophotometer (PTI® Photon Technology International, Lawrenceville, NJ) with excitation and emission wavelengths of 495 and 540 nm, respectively. When a minimum fluorescence point was reached, the vacuolar ATPase activity was slowed down by addition of bafilomycin A to establish a steady-state pH gradient. Chloride salts or sulfate salts were added as indicated. All curves were normalized to 100% quenching.
Plasmid Construction
The yeast expression vector, pRS306 GAL1 ccdB V5 His6, used in this study is a modification of the pRS306 (36). The primers GAL1-For (5′-ATGATCCACTAGTACGGATT-3′) and CYC1-Rev (5′-TTGGCCGATTCATTAATGCA-3′) were used to amplify, from the commercial vector pYES-DEST52 (Invitrogen), a DNA fragment starting from the GAL1 promoter to the CYC1 terminator that includes the Gateway® recombination cassette and the V5 and the His6 epitopes. This PCR product was then inserted in the SmaI restriction site of pRS306. The Vcx1 coding sequence was cloned by PCR using the Phusion® hot start high fidelity DNA polymerase (Finnzymes, Espoo, Finland). The PCR was performed on genomic DNA with primers Vcx1-For (5′-CACCATGGATGCAACTACCCCAC-3′) in combination with Vcx1-Rev (5′-TCATAAACTATTTCCAATAGAGTC-3′) for the full ORF or Vcx1-Tag-Rev (5′-TAAACTATTTCCAATAGAGTC-3′) for the Vcx1-TAG fusion construct. Each resulting PCR product was introduced into pENTR SD/D TOPO and then in pDEST vectors as described by the manufacturer (Invitrogen). All pAG destination vectors (37) used in this study are available via Addgene.
Site-directed Mutagenesis
The single point mutant Vcx1p-H303A was generated using the QuikChange® II site-directed mutagenesis kit (Stratagene) according to modifications described by Wang and Malcom (38). The complementary primers used to create VCX1-H303A were as follows: VCX1-H(303)A-For (5′-GGGTAATGCCGCAGAGGCTGTCACTTCAGTCTTGG-3′) and VCX1-H(303)A-Rev (5′-CCAAGACTGAAGTGACAGCCTCTGCGGCATTACCC-3′). Underlined letters indicate the changes in nucleotide sequence, and boldface letters indicate the introduced silent restriction site BglI.
Growth Tests
Yeasts were grown in YPD medium to saturation and washed twice in sterile water. All cell suspensions were adjusted to an optical density of 0.2, 0.02, and 0.002 in sterile water. 5 μl of each dilution were spotted onto YPD, or YPG, media supplemented with salts or hygromycin B. Growth was assessed at 30 °C after 2–5 days.
Fluorescence Microscopy
A Nikon Eclipse 50i fluorescent microscope was used to visualize the transformed cells. GFP- and eYFP-fused proteins were visualized by using B-2A filters (excitation filter, 450–490 nm; Dichromatic Mirror cut-on wavelength, 500 nm; Barrier filter wavelength, 515-nm long pass filter). All images were taken at ×100 magnification, with a Nikon DS-Fi1 CCD camera.
RESULTS
Detection of an Additional Vacuolar Cation/H+ Exchange Component
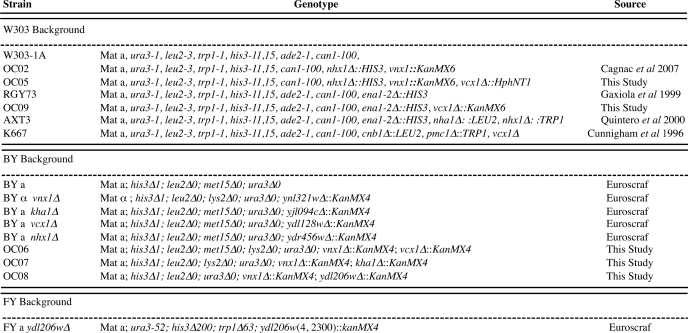
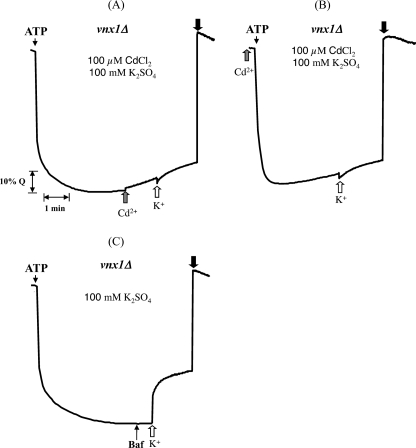
We previously showed that Vnx1p accounts for the monovalent cation/H+ antiport activity from isolated yeast vacuoles as vnx1 mutants displayed a total loss of Na+(K+)/H+ antiporter activity (16). By replacing chloride salts (Fig. 1A) with sulfate salts (Fig. 1B), or gluconate salts, we were able to reveal another type of K+/H+ antiporter activity in the vacuolar fraction extracted from vnx1Δnhx1Δ cells (Fig. 1B) as well as from vnx1Δ single mutant cells (data not shown). A similar background activity, using the OC02 strain (vnx1Δnhx1Δ), can be seen in the study of Hernandez et al. (39). Compared with the activity that is dependent on the presence of Vnx1p, the exchange activity was much lower. Also in the wild type strain W303-1A, the relative magnitude of K+/H+ antiport is dependent on the presence of chloride or sulfate salts. In the presence of chloride salts, antiport activity is higher with Na+ as compared with K+, although in the presence of sulfate salts this is inverted (Fig. 1C). Together, these data suggest the presence of one or several other vacuolar membrane-bound transporter(s), distinct from Nhx1p and Vnx1p, and are able to drive the transport of K+ in a pH-dependent manner. However, it is not clear why the detection of this activity is dependent on the counter anion used during the transport assay. It is possible that the relatively permeant Cl− and H+ ions induce passive leaks that mask directly coupled K+/H+ antiport. In this sense, addition of the more permeant monovalent Cl− ions compared with SO42− ions would lead to a dissipation of the inside positive membrane potential created by the H+-ATPase, which would counteract H+ efflux from the vesicles, and thus mask antiporter activity.
FIGURE 1.
Salt-dependent cation/H+ transport across the vacuolar membrane. Proton movements were monitored by following the fluorescence quenching of acridine orange as described under “Experimental Procedures” and supplemental Fig. 1S. At the indicated times, vacuole acidification was initiated by the addition of ATP. After a steady-state acidic inside pH gradient was attained, the activity of the H+-ATPase was partially inhibited by the addition of bafilomycin (Baf). A and B, cation/proton antiport using isolated vacuoles of the OC02 yeast strain (nhx1Δ, vnx1Δ) was monitored as the recovery of fluorescence quench upon addition of 100 mm of Na+ or K+ (white arrows). At the indicated time, 20 mm of (NH4)2SO4 was added to collapse the ΔpH across the vacuolar membrane to recover 100% of the fluorescence (black arrows). Traces are representative of at least three independent experiments. C, effect of the counter anion on the transport rate has been measured in isolated vacuoles from the wild type strain W303. The initial rates of cation-dependent H+ movement were assayed by measuring the initial rates of fluorescence quench recovery after addition of 100 mm chloride salts or 50 mm sulfate salts as indicated in the figure. Data represent the means ±S.D. of six measurements from three independent preparations. Bars with different letters indicate values that are significantly different when compared with the other three conditions tested as determined by a two-tailed t test (p < 0.009) (GraphPad software).
Identification of Vcx1 as K+/H+ Antiporter
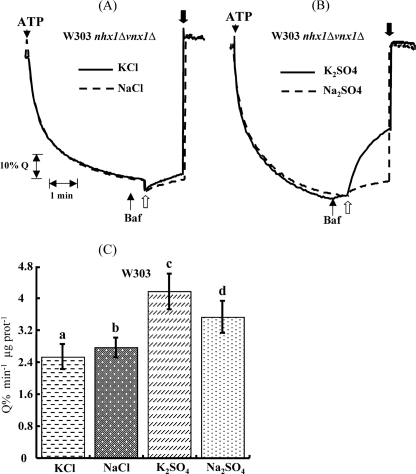
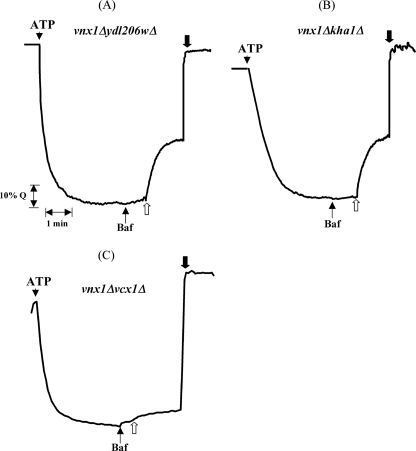
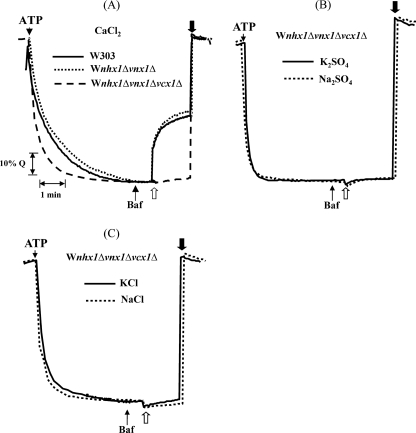
Based on the same strategy used to identify Vnx1p, where we screened single disruption mutants, we screened double disruption mutants to identify the gene(s) coding for the transporter responsible for the residual K+/H+ activity in the vacuolar membrane preparation of the VNX1 disruption mutant. We tested three candidate genes, KHA1, a monovalent/cation antiporter of the CPA2 family localized in Golgi membranes (15), and Vcx1 and YDL206w, two of the three remaining CaCA family proteins besides Vnx1. Each disruption mutant of candidate genes was crossed with the BY Mat α vnx1Δ strain. The resulting diploids were then sporulated and micro-dissected to collect the spores. The progeny of G418-resistant spores presenting a 2:2 segregation was then tested for mating type assignment. To be consistent with the previous transport experiments, only mating type a strains were conserved. Freshly extracted vacuoles were isolated from the above mentioned double mutant strains and tested for their ability to mediate vacuolar alkalinization upon addition of 50 mm K2SO4. Surprisingly, the K+/H+ antiport activity was abolished only in the strain harboring the vcx1Δ disruption (Fig. 2). Vcx1p has been characterized before as a low affinity Ca2+/H+ antiporter (40–42), but to our knowledge nothing has been mentioned about the role of Vcx1p in K+ transport against protons at the vacuolar membrane. Therefore, to ensure that the transport activity was dependent on Vcx1 expression, this gene was disrupted in the OC02 W303-1A background strain to generate a triple mutant OC05 (Table 1), using a PCR-based method (28, 29). The disruption was confirmed, first by PCR on genomic DNA to ensure the correct integration of the selection marker hphNT1 and then by testing the Ca2+/H+ exchange across the vacuolar membrane of isolated vacuoles compared with controls, W303 and OC02, respectively. Disruption of VCX1 completely abolished the exchange of Ca2+ in the enriched vacuolar fraction confirming what had been described before (Fig. 3A) (16, 18), and it also abolished the residual K+/H+ exchange activity observed in the presence of sulfate salts, confirming the results obtained using the BY strains (Fig. 3B). No transport is observed using NaCl and KCl in isolated vacuoles from nhx1Δvnx1Δvcx1Δ mutant strains (Fig. 3C). Overexpression of VCX1 under the control of the GAL1 promoter in this yeast strain restores all exchange activities in isolated vacuoles only when grown in the presence of galactose as a carbon source (Fig. 4A). Overexpression of VCX1 not only restores transport, but Ca2+ and K+ transport rates are even higher when compared with the OC2 strain (nhx1Δvnx1Δ) (Fig. 4A). This clearly indicates that monovalent and divalent transport activities are catalyzed by Vcx1p.
FIGURE 2.
Identification of Vcx1p as a potential K+/H+ antiporter by a reverse genetic approach. Exchange of potassium against protons across the vacuolar membrane of BY double mutant strains was monitored using K2SO4 as indicated. At the indicated times bafilomycin (Baf), 100 mm K+ equivalent (white arrows), and 20 mm of (NH4)2SO4 were added (black arrows). Traces are representative of three measurements of the same vacuolar preparation.
FIGURE 3.
Effect of VCX1 disruption on vacuolar transport of potassium and calcium. At the indicated time (white arrow), 12.5 μm CaCl2 was added to visualize the recovery of fluorescence in the presence or absence of Vcx1p (A). At the indicated time (white arrow) 50 mm Na2SO4 and K2SO4 (B) or 100 mm of NaCl and KCl (C) was added to collapse the pH gradient across the vacuolar membrane of W303 nhx1Δvnx1Δvcx1Δ resulting in a cation-dependent H+ movement and alkalinization of the vesicular lumen. Traces are representative of at least three independent experiments. Baf, bafilomycin.
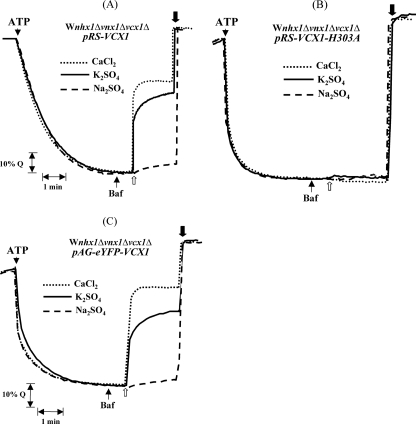
FIGURE 4.
Transport activity measured in isolated vacuoles of the W303 triple mutant strain complemented with VCX1 genes and derivatives. The yeast strain W303 nhx1Δvnx1Δvcx1Δ was transformed with the yeast integrative vectors pRS306 Gal1 or pAG306 eYFP into which VCX1 or VCX1-H303A had been previously introduced by recombination as described under “Experimental Procedures.” The correct genomic integration of plasmids was verified by PCR. Acridine orange fluorescence recovery mediated by Vcx1p (A), Vcx1p-H303A (B), and eYFP::Vcx1p (C), by the addition of 50 mm Na2SO4 (- - -), 50 mm K2SO4 ( ), or 12.5 μm CaCl2 (···), was monitored after the establishment of a pH gradient and partial inhibition of the H+-ATPase by bafilomycin A (Baf). Traces are representative of three experiments using independent membrane preparations from independent clones.
), or 12.5 μm CaCl2 (···), was monitored after the establishment of a pH gradient and partial inhibition of the H+-ATPase by bafilomycin A (Baf). Traces are representative of three experiments using independent membrane preparations from independent clones.
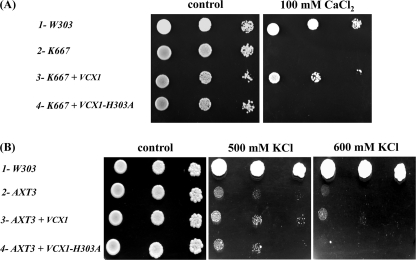
To further test the involvement of Vcx1p in K+/H+ antiport activity, we constructed an inactivated mutant enzyme. Several amino acid residues were identified as crucial for calcium transport in CAXs from plants (43, 44). These residues are highly conserved among the different species. Expression in yeast of a mutated AtCAX1 or OsCAX1a, in which histidine at position 338 or 330, respectively, has been replaced by an alanine did not rescue yeast Ca2+ sensitivity or vacuolar transport activity. Using PCR-based site-directed mutagenesis, we changed the corresponding conserved His303 in Vcx1p for Ala (supplemental Fig. 2S). Overexpression of this mutant enzyme did not restore Ca2+ and K+ cation transport in the nhx1Δvnx1Δvcx1Δ yeast strain, indicating that this substitution found in the C-2-specific domain is essential for general functioning (Fig. 4B). Similarly to what has been described previously, expression of wild type VCX1 in the K667 calcium-sensitive strain did confer calcium tolerance, whereas VCX1-H303A did not (Fig. 5A). Overexpression of VCX1 only slightly increased the growth of the highly K+-sensitive AXT3 strain at high external K+ concentrations, although overexpression of the mutant Vcx1-H303A mutant had no effect (Fig. 5B). Disruption of VCX1 in a less K+-sensitive yeast (ena1Δ-ena4Δ) did not result in any growth delay (data not shown) indicating only a minor contribution of Vcx1p to high K+ tolerance. Neither the disruption of VCX1 nor its overexpression in a nhx1Δ strain resulted in an increase of sensitivity or resistance toward hygromycin (data not shown).
FIGURE 5.
Growth of various mutant strains expressing VCX1 and VCX1-H303A in the presence of toxic concentrations of cations. Both VCX1 and VCX1-H303A were introduced into the yeast shuttle vector pYES-DEST52 using Gateway recombination as described under “Experimental Procedures.” Each construct was introduced into K667 (cnb1Δ pmc1Δ vcx1Δ) and AXT3 (ena1-2Δ nha1Δ nhx1Δ) mutant strains. Each strain was grown, and serial dilutions were made as described under “Experimental Procedures.” Five microliters of each dilution were spotted onto YPG medium with addition of a high concentration of CaCl2 (A) or KCl (B) as indicated. Plates were incubated at 30 °C for 2–5 days.
Does the Point Mutation in Vcx1p Change Its Subcellular Localization?
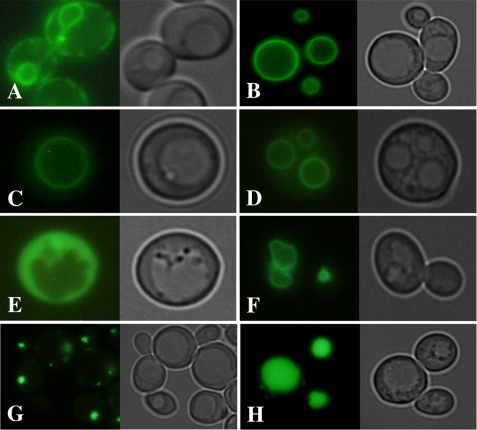
Failure of the mutant enzyme to confer resistance to high K+ concentrations or to catalyze K+/H+ antiport in vacuolar fractions could also be due to a mislocalization of the enzyme. In yeast, a single point mutation can lead to a retention of the mutant protein in the ER (45), where it will be degraded (46). In addition to the potential mislocalization related to the mutation, accumulation of membrane proteins in the ER due to overexpression using high copy shuttle vectors or to the fusion of GFP to the C terminus of the protein has been reported (16, 47, 48). According to the Yeast GFP Fusion Localization Database, the chromosomal C-terminal Vcx1p::GFP-tagged fusion protein was predicted to be localized in the ER (Fig. 6A) (48). Therefore, we checked the correct localization of Vcx1p and Vcx1p-H303A by fusing it to a fluorescent protein. Fusion of eYFP to the N terminus of Vnx1 (eYFP::Vnx1p) or Vcx1 (eYFP::Vcx1p) gave us a vacuolar membrane-bound localization without any residual signal trapped in the ER (Fig. 6, B and C). Yeast cells (OC05) expressing the chimeric construct eYFP::VCX1-H303A (Fig. 6D) displayed a vacuolar membrane localization identical to controls. The exact same pattern of localization was observed in all the yeast strains used in this study (data not shown). The addition of the GFP at the N terminus of Vcx1p did not affect its ability to mediate cation accumulation in the vacuolar lumen (Fig. 4C). Typical localizations in the cytosol, the vacuolar membrane, the PVC, and vacuolar lumen were observed by expression of eYFP alone, Vph1p-GFP, Nhx1p-GFP, and CPY-GFP respectively (Fig. 6, E–H). Altogether, these data confirm that the absence of Ca2+ and K+ tolerance observed in yeast cells expressing the mutated version of Vcx1p was due to inactivation and not mislocalization or degradation.
FIGURE 6.
Vacuolar localization of Vcx1p and Vcx1p-H303A mutant. The localization was performed using the triple mutant strain OC05 (nhx1Δvnx1Δvcx1Δ). Cells were transformed with yeast expression vectors of the pAG series (see “Experimental Procedures”). A, vacuolar Ca2+/H+ exchanger Vcx1p fused to GFP at the C terminus displayed a typical signal of ER localization as observed for the vacuolar Na+/H+ exchanger Vnx1p in the same conditions (16). When Vnx1p was fused to eYFP at the N terminus (B), the fluorescent signal was observed at the vacuolar membrane. An identical localization for Vcx1p (C) and its inactive mutant form Vcx1p-H303A (D) was observed when they were fused to eYFP at their N termini. The same results were obtained using the yeast strain K667 and AXT3 (data not shown). Fluorescence was observed in the cytosol of cells expressing a free form of eYFP (E). A positive control of vacuolar membrane localization was obtained using the vacuolar ATPase Vph1p subunit fused to GFP at the C terminus (F). Nhx1p::eGFP chimeric protein was used to visualize PVC localization (G), although the vacuolar lumen was stained by accumulation of GFP specifically targeted to the vacuolar lumen by addition of the signal peptide of carboxypeptidase Y at the N terminus (H). Images were acquired as described under “Experimental Procedures” using an excitation 450–490-nm bandpass filter and an emission 515-nm cuton long pass filter.
Inhibition of Vcx1p K+/H+ Exchange Activity by Cd2+ and Zn2+
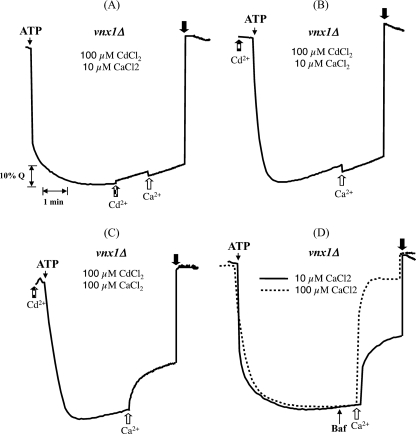
Vcx1p is a Ca2+/H+ antiporter whose activity is strongly inhibited by Cd2+ and to a lesser extent by Zn2+ (19). Therefore, the effect of these ions on K+/H+ exchange mediated by Vcx1p was tested. Use of 200 μm Zn2+ or Cd2+ induced considerable leakage of H+ similar to what can be observed when bafilomycin A, a specific inhibitor of the V-ATPase, is added in excess (supplemental Fig. 2S) (16). These results are supported by previously published data showing a strong noncompetitive inhibition of the V-ATPase by Zn2+ (49). Therefore, we used a lower concentration of inhibitors (100 μm) to allow for the measurement of transport activity related to the addition of CaCl2 or K2SO4. We also ensured that the integrity of the vacuoles was not affected by the high concentration of toxic heavy metal (supplemental Fig. 2S). For more convenience, the inhibition experiments were conducted with vacuoles isolated from the vnx1Δ mutant strain (BY background). The alkalinization of vacuoles isolated from cells expressing Vcx1 induced by the addition of 10 μm Ca2+ was completely inhibited in the presence of 100 μm Cd2+ (Fig. 7, A and B) and strongly inhibited when equal amounts of both cations were used (Fig. 7C) as compared with control conditions (Fig. 7D). Interestingly, the K+/H+ exchange reaction induced by the addition of 100 mm K+ was completely inhibited in the presence of Cd2+, despite a 1000 times higher K+ concentration as compared with Cd2+ (Fig. 8). A total inhibition of the exchange reaction induced by 10 μm Ca2+ transport was also observed in the presence of Zn2+ (supplemental Fig. 4S). Ohsumi and Anraku (41) have reported a similar inhibition of Zn2+ on Ca2+ transport in vacuolar membrane vesicles. However, when compared with Cd2+, the effect of Zn2+ is less pronounced when used in a 1:1 ratio to Ca2+, and the K+-induced K+/H+ exchange reaction was only partially inhibited in the presence of Zn2+ (supplemental Fig. 4S).
FIGURE 7.
Inhibition of Ca2+/H+ exchange activity in presence of Cd2+. Vacuoles isolated from the yeast strain BY vnx1Δ were used to visualize the effect of 100 μm CdCl2 on the Ca2+/H+ exchange activity mediated by Vcx1p. CdCl2 was added to the reaction mixture 1 min before the addition of Ca2+ (A) or before the initiation of fluorescence quenching by the addition of ATP (B) as indicated by the gray arrow. The fluorescence recovery was followed after addition of 10 μm (A and B) or 100 μm CaCl2 (C) as indicated by the white arrow. The same experiments were performed in the absence of CdCl2 (D). Traces are representative of two experiments using independent membrane preparations. Baf, bafilomycin A.
FIGURE 8.
Inhibition of K+/H+ exchange activity in presence of Cd2+. Inhibition of the K+/H+ exchange reaction by Cd2+ was assayed as described in Fig. 7. The fluorescence recovery was followed after the addition of 50 mm K2SO4 (A and B) as indicated by the white arrow. The same experiments were performed in the absence of CdCl2 (C). Traces are representative of two experiments using independent membrane preparations. Baf, bafilomycin A.
DISCUSSION
Although the influx and the efflux of K+ across the plasma membrane have been well documented in the last few decades (for review see Refs. 2, 52), little is known about the mechanisms controlling the vacuolar pool of K+.
Until recently, monovalent cation/H+ exchange was ascribed to transporters of the cation proton antiporter superfamily. The E. coli ChaA antiporter was the only example of a CaCA family member able to promote movement of Ca2+, Na+, and K+ (12, 13). Despite this example, the sequence comparison and homology analysis, as well as experimental data, attributed distinct ion selectivity to each superfamily. However, within the last 3 years several other members of the CaCA superfamily have been shown to be involved in the vacuolar compartmentalization of alkali ions (16, 23, 53). To our knowledge, Vnx1p was the first eukaryotic CaCA antiporter homolog to be characterized as a monovalent/H+ exchanger (16). In this previous work, Vnx1p was described as the only antiporter responsible for the accumulation of alkali cations against protons in the vacuolar lumen. However, by using sulfate instead of chloride salts, it is now shown that the monovalent cation transport in vacuoles is a composite of several activities (Fig. 1C). A reverse genetic approach allowed us to identify a double mutant vnx1Δvcx1Δ strain apparently devoid of all vacuolar K+/H+ antiporter activity, indicating that Vcx1p was catalyzing K+/H+ exchange. The role of Vcx1p was further confirmed by overexpression of VCX1 and VCX1-H303A, an inactivated version, in various yeast strains. Unlike the wild type gene, the His303 substituted Vcx1 mutant did not complement the Ca2+- or K+-sensitive strain K667 or AXT3, respectively. In accordance with these complementation data, transport assays performed with isolated vacuoles of the mutant strains OC5 overexpressing both versions of the gene (Fig. 4) showed that the wild type protein displayed Ca2+/H+ and K+/H+ antiporter activity, although the mutant protein was inactive. Moreover, both Ca2+ and K+ transport activities mediated by Vcx1p are strongly affected by the presence of Cd2+ and to a lesser extent by Zn2+.
The fact that the additional K+/H+ antiport can only be observed using sulfate salts can have several causes. First, it is possible that Cl− or SO42− specifically modify antiporter activity. Second, specific anion transport systems in the vacuolar membrane, for instance a Cl−:H+ symporter, could be responsible for anion-coupled H+ influx, thus masking H+/K+ antiporter activity. This would point to the physiological significance of the differences observed. However, using several purified plant antiporters reconstituted in liposomes, we generally observe higher antiporter activities using sulfate salts as compared with chloride salts.5 The observed effects are thus more likely related to nonspecific electrical effects of the anion. Cl− ions are much more permeant than SO42− or gluconate ions. In that sense, addition of relatively permeable Cl− ions to the outside of the vesicles would shift membrane potential to a more negative inside value as compared with the situation using impermeant anions, counteracting net H+ efflux from the vesicles. Especially when antiporter activity is relatively low, a considerable fraction of the protons that are transported out of the vesicle in the exchange reaction would leak back into the vesicle together with Cl− anions. Therefore, because of the leakiness of the system, real antiporter activity can only be adequately measured using relatively impermeant anions like sulfate or gluconate.
Role of Vcx1p, Vnx1p, and Nhx1p in Vacuolar Ion Compartmentalization
The vacuole is the main calcium store and sink of yeast cells (54). Vcx1p along with Pmc1p (a high affinity Ca2+ ATPase) were shown to play a key role in the vacuolar translocation of Ca2+ after a cytosolic spike induced by various stimuli (55–57). In addition to regulating calcium homeostasis, in this paper we show that Vcx1p along with Vnx1p play a role in the sequestration of cytosolic K+ into the vacuole. Like Ca2+, K+ is mainly stored in the vacuoles. The ratio [K+]cyto/[K+]vac is close to 1:8 during the exponential growth phase but would appear to be inferior during the stationary phase (58). A third antiporter that has been proposed to be involved in vacuolar K+ and Na+ sequestration is Nhx1p. However, although Vnx1p and Vcx1p are clearly shown to be targeted to the vacuolar membrane, the Nhx1 protein resides in the prevacuolar compartment (44, 56, 58–61). Accordingly, we could not detect an effect of NHX1 disruption on vacuolar Na+/H+ or K+/H+ exchange activity (16). Despite this, the biochemical characterization of several vacuolar antiporters from plants and green alga has been carried out using a yeast strain disrupted on NHX1 only. According to these studies, the enriched vacuolar fraction used for transport assays did not have any significant Na+/H+ or K+/H+ exchange activity despite the presence of Vnx1p and Vcx1p (23, 25, 53), although cells expressing NHX1 did have antiporter activity (23). In these studies yeast vacuolar membrane vesicles were prepared using a two-step sucrose gradient as previously described by Nakanishi et al. (59). These results are in contradiction with data obtained with vacuoles isolated by the Ficoll (polysucrose) floating method (16, 21, 22, 39). The most plausible explanation for detection of Nhx1p activity in vacuolar membranes isolated by the two-step density gradient centrifugation is that these membranes are contaminated with many other endosomal membranes, although the Ficoll floating method could give rise to a purer vacuolar preparation. Recently, Wiederhold et al. (60) published an S. cerevisiae vacuolar membrane proteome using vacuoles purified with a similar Ficoll floating method. A subtractive proteomic approach (LOPIT) with iTRAQ labeling was used to distinguish vacuolar proteins from contaminants and allowed the identification of 148 proteins that were significantly enriched. In this cluster of proteins, Vcx1p and Vnx1p were annotated as vacuolar along with a list of 46 other transporter proteins from which Nhx1p is absent. However, it is not clear why no Vnx1p activity could be detected in vesicles prepared with the sucrose gradient method. This could be related to the obtainment of less sealed vacuoles, leading to considerable proton leaks masking the activity.
Nevertheless, the role of Nhx1p in cation sequestration in the late endosomes and the vacuole has been clearly demonstrated in vivo (5, 61). Observations of Ste3-tagged protein and FM4-64 movements in wild type and nhx1Δ cells strongly indicated that the dominant role of Nhx1p is the control of the vesicular trafficking from late endosome to the vacuole by regulating cellular pH (47, 61–63).
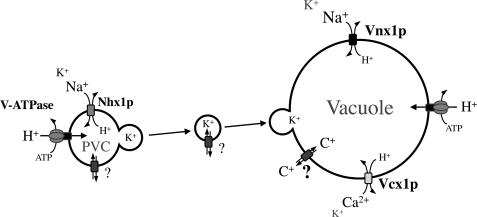
Altogether, these data indicate that in yeast Vnx1p, Vcx1p, and Nhx1p are involved in the sequestration of cations in the vacuole. Unlike Vnx1p and Vcx1p, Nhx1p is not involved in a direct transport of cations from the cytosol to the vacuole through the vacuolar membrane. However, it could be responsible for vacuolar cation accumulation by fusion of cation-loaded vesicles trafficked from the PVC to the vacuole or by controlling the trafficking to the vacuole of transporters involved in alkali cation homeostasis (Fig. 9).
FIGURE 9.
Model for cation accumulation in the vacuole. Vacuolar membrane-bound antiporters Vcx1p and Vnx1p use the proton gradient generated by the V-ATPase to energize the transport of cations into the lumen of the main vacuole. The contribution of the pre-vacuolar alkali cation/H+ antiporter, Nhx1p, in the accumulation of K+ and Na+ remains to be determined. Nhx1p could play a role in the loading of the PVC from which vesicles containing ions are sent to the main vacuoles and therefore contribute to the accumulation of ions in the vacuolar lumen. Knowing the importance of Nhx1p in the control of the vesicular trafficking, this antiporter could play a role in the targeting of uncharacterized cation transporters (?) to the vacuolar membrane and indirectly affect ion homeostasis of the vacuole.
Sequence Analysis of Yeast CAX Antiporters
The Genolevure website (50) reveals that most yeast species contain two types of Na+/Ca2+ exchangers identified by the Pfam PF01699 conserved domain as follows: the Vcx1p-like (type I CAX) and the Vnx1p-like transporters (type II CAX) (supplemental Fig. 5S). One exception is the yeast Yarrowia lipolytica, which also has a third antiporter, quite different from the two others although it contains the PF01699 conserved domain. Interestingly, in the YETI data base, Y. lipolytica and Candida glabrata appear to contain two Vcx1-like proteins but no Vnx1p. However, a new amino acid sequence alignment revealed that for each species one of the Vcx1p is classified together with the other Vnx1 proteins (YALI0D24233g and CAGL0M04147g). This result shows that Vcx1 and Vnx1 proteins are both ubiquitous and that Vnx1 proteins are not homoplasic as suggested by De Hertogh et al. (51).
Two Amino Acids Might Determine Alkali Cation Selectivity
Two highly conserved sequence repeats from CAX proteins, named c1 and c2, which were first identified in plants, were shown to be involved in cation translocation (43). The newly discovered K+/H+ exchange activity of Vcx1p, along with data on CrCAX1 and Vnx1p transport specificity, could be related to the potential role of two amino acids of these conserved regions in ion selectivity (supplemental Fig. 2S). In the c-1 region, a serine residue conserved in CAX1 from C. reinhardtii and Vnx1p of S. cerevisiae, both known to transport Na+ (16, 23), is absent from Vcx1-like protein sequences. Moreover, the heterodimer formed between CAX1 and CAX3 from Arabidopsis thaliana, bearing this conserved serine, appears to promote the transport of Li+ and/or Na+ (53). Consequently, this serine residue could be important for Na+ transport. Similarly, a conserved aspartate residue in the c-2 region of Vcx1-like proteins from yeast is substituted by an asparagine at the corresponding position in CrCAX1 and CrCAX2. This repartition suggests that an aspartate or an asparagine at this position could determine the ability of the protein to transport K+ or Na+. The alignment of the c-1 and c-2 regions also reveals that one AtCAX subgroup (AtCAX1, AtCAX3, and AtCAX4) is closer to CrCAX1, and the other subgroup (AtCAX2, AtCAX5, and AtCAX6) has more similarities to Vcx1p (supplemental Fig. 2S) and could reflect their potential ability to transport Na+ or K+, respectively. The fact that these two residues are in general conserved in species belonging to different kingdoms such as plants and yeast but not in all CAXs members of A. thaliana reinforces the idea that they could be functionally important. Other residues have also been suggested to play a role in cation selectivity such as Met170 and Cys350 in CrCAX1 (supplemental Fig. 2S) (23). A site-directed mutagenesis approach could easily confirm whether the exchange of one or two amino acids is sufficient to change the ion selectivity of the transporter.
Supplementary Material
Acknowledgments
We thank Eduardo Blumwald and Kyle W. Cunningham for the kind gift of some of the yeast strains used in this study, Concepcion Azcon-Aguilar for access to her microscope, and Maria Isabel Gaspar Vidal for technical help. We also thank Mario Fon for the critical review of this manuscript.
This work was supported in part by Grants BIO2008-01691 from the Spanish Plan Nacional de Biotecnología-FEDER and AGR 436 from the Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía (to M.-P. R.-R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1S–5S.
K. Venema, unpublished results.
- CAX
- calcium exchanger
- CaCA
- calcium cation antiporter
- ER
- endoplasmic reticulum
- eYFP
- enhanced YFP.
REFERENCES
- 1.Epstein W. (2003) Prog. Nucleic Acid Res. Mol. Biol. 75, 293–320 [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Navarro A. (2000) Biochim. Biophys. Acta 1469, 1–30 [DOI] [PubMed] [Google Scholar]
- 3.Maathuis F. J. (2009) Curr. Opin. Plant. Biol. 12, 250–258 [DOI] [PubMed] [Google Scholar]
- 4.Prior C., Potier S., Souciet J. L., Sychrova H. (1996) FEBS Lett. 387, 89–93 [DOI] [PubMed] [Google Scholar]
- 5.Nass R., Cunningham K. W., Rao R. (1997) J. Biol. Chem. 272, 26145–26152 [DOI] [PubMed] [Google Scholar]
- 6.Pinner E., Kotler Y., Padan E., Schuldiner S. (1993) J. Biol. Chem. 268, 1729–1734 [PubMed] [Google Scholar]
- 7.Leidi E. O., Barragan V., Rubio L., El-Hamdaoui A., Ruiz M. T., Cubero B., Fernandez J. A., Bressan R. A., Hasegawa P. M., Quintero F. J., Pardo J. M. (2010) Plant J. 61, 495–506 [DOI] [PubMed] [Google Scholar]
- 8.Padan E., Bibi E., Ito M., Krulwich T. A. (2005) Biochim. Biophys. Acta 1717, 67–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arkin I. T., Xu H., Jensen M. Ø., Arbely E., Bennett E. R., Bowers K. J., Chow E., Dror R. O., Eastwood M. P., Flitman-Tene R., Gregersen B. A., Klepeis J. L., Kolossváry I., Shan Y., Shaw D. E. (2007) Science 317, 799–803 [DOI] [PubMed] [Google Scholar]
- 10.Hunte C., Screpanti E., Venturi M., Rimon A., Padan E., Michel H. (2005) Nature 435, 1197–1202 [DOI] [PubMed] [Google Scholar]
- 11.Pinner E., Padan E., Schuldiner S. (1992) J. Biol. Chem. 267, 11064–11068 [PubMed] [Google Scholar]
- 12.Ivey D. M., Guffanti A. A., Zemsky J., Pinner E., Karpel R., Padan E., Schuldiner S., Krulwich T. A. (1993) J. Biol. Chem. 268, 11296–11303 [PubMed] [Google Scholar]
- 13.Radchenko M. V., Tanaka K., Waditee R., Oshimi S., Matsuzaki Y., Fukuhara M., Kobayashi H., Takabe T., Nakamura T. (2006) J. Biol. Chem. 281, 19822–19829 [DOI] [PubMed] [Google Scholar]
- 14.Banuelos M. A., Sychrova H., Bleykasten-Grosshans C., Souciet J. L., Potier S. (1998) Microbiol-Uk. 144, 2749–2758 [DOI] [PubMed] [Google Scholar]
- 15.Maresova L., Sychrova H. (2005) Mol. Microbiol. 55, 588–600 [DOI] [PubMed] [Google Scholar]
- 16.Cagnac O., Leterrier M., Yeager M., Blumwald E. (2007) J. Biol. Chem. 282, 24284–24293 [DOI] [PubMed] [Google Scholar]
- 17.Cunningham K. W., Fink G. R. (1996) Mol. Cell. Biol. 16, 2226–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Pozo L., Osaba L., Corchero J., Jimenez A. (1999) Yeast 15, 371–375 [DOI] [PubMed] [Google Scholar]
- 19.Pittman J. K., Cheng N. H., Shigaki T., Kunta M., Hirschi K. D. (2004) Mol. Microbiol. 54, 1104–1116 [DOI] [PubMed] [Google Scholar]
- 20.Pozos T. C., Sekler I., Cyert M. S. (1996) Mol. Cell. Biol. 16, 3730–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirata T., Wada Y., Futai M. (2002) J. Biochem. 131, 261–265 [DOI] [PubMed] [Google Scholar]
- 22.Martínez-Muñoz G. A., Peña A. (2005) Yeast 22, 689–704 [DOI] [PubMed] [Google Scholar]
- 23.Pittman J. K., Edmond C., Sunderland P. A., Bray C. M. (2009) J. Biol. Chem. 284, 525–533 [DOI] [PubMed] [Google Scholar]
- 24.Zhao J., Connorton J. M., Guo Y., Li X., Shigaki T., Hirschi K. D., Pittman J. K. (2009) J. Biol. Chem. 284, 34075–34083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris J., Tian H., Park S., Sreevidya C. S., Ward J. M., Hirschi K. D. (2008) Plant Physiol. 148, 1474–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X., Lytton J. (2004) Mol. Biol. Evol. 21, 1692–1703 [DOI] [PubMed] [Google Scholar]
- 27.Shigaki T., Rees I., Nakhleh L., Hirschi K. D. (2006) J. Mol. Evol. 63, 815–825 [DOI] [PubMed] [Google Scholar]
- 28.Longtine M. S., McKenzie A., 3rd, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998) Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- 29.Janke C., Magiera M. M., Rathfelder N., Taxis C., Reber S., Maekawa H., Moreno-Borchart A., Doenges G., Schwob E., Schiebel E., Knop M. (2004) Yeast 21, 947–962 [DOI] [PubMed] [Google Scholar]
- 30.Gietz R. D., Schiestl R. H., Willems A. R., Woods R. A. (1995) Yeast 11, 355–360 [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J., Russell D. W. (2001) Molecular Cloning: a Laboratory Manual, pp. 6.31–6.32, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 32.Sherman F. (2002) Methods Enzymol. 350, 3–41 [DOI] [PubMed] [Google Scholar]
- 33.Ohsumi Y., Anraku Y. (1981) J. Biol. Chem. 256, 2079–2082 [PubMed] [Google Scholar]
- 34.Blumwald E., Rea P. A., Poole R. J. (1987) Method Enzymol. 148, 115–123 [Google Scholar]
- 35.Yamaguchi T., Apse M. P., Shi H., Blumwald E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12510–12515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikorski R. S., Hieter P. (1989) Genetics 122, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberti S., Gitler A. D., Lindquist S. (2007) Yeast 24, 913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W., Malcolm B. A. (2002) Methods Mol. Biol. 182, 37–43 [DOI] [PubMed] [Google Scholar]
- 39.Hernández A., Jiang X., Cubero B., Nieto P. M., Bressan R. A., Hasegawa P. M., Pardo J. M. (2009) J. Biol. Chem. 284, 14276–14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn T., Gable K., Beeler T. (1994) J. Biol. Chem. 269, 7273–7278 [PubMed] [Google Scholar]
- 41.Ohsumi Y., Anraku Y. (1983) J. Biol. Chem. 258, 5614–5617 [PubMed] [Google Scholar]
- 42.Okorokov L. A., Kulakovskaya T. V., Lichko L. P., Polorotova E. V. (1985) FEBS Lett. 192, 303–306 [DOI] [PubMed] [Google Scholar]
- 43.Kamiya T., Maeshima M. (2004) J. Biol. Chem. 279, 812–819 [DOI] [PubMed] [Google Scholar]
- 44.Shigaki T., Barkla B. J., Miranda-Vergara M. C., Zhao J., Pantoja O., Hirschi K. D. (2005) J. Biol. Chem. 280, 30136–30142 [DOI] [PubMed] [Google Scholar]
- 45.DeWitt N. D., dos Santos C. F., Allen K. E., Slayman C. W. (1998) J. Biol. Chem. 273, 21744–21751 [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Chang A. (1999) EMBO J. 18, 5972–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowers K., Levi B. P., Patel F. I., Stevens T. H. (2000) Mol. Biol. Cell 11, 4277–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'Shea E. K. (2003) Nature 425, 686–691 [DOI] [PubMed] [Google Scholar]
- 49.Kakinuma Y., Ohsumi Y., Anraku Y. (1981) J. Biol. Chem. 256, 10859–10863 [PubMed] [Google Scholar]
- 50.Sherman D. J., Martin T., Nikolski M., Cayla C., Souciet J. L., Durrens P. (2009) Nucleic Acids Res. 37, D550–D554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Hertogh B., Hancy F., Goffeau A., Baret P. V. (2006) Genetics 172, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ariño J., Ramos J., Sychrová H. (2010) Microbiol Mol. Biol. Rev. 74, 95–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao J., Shigaki T., Mei H., Guo Y. Q., Cheng N. H., Hirschi K. D. (2009) J. Biol. Chem. 284, 4605–4615 [DOI] [PubMed] [Google Scholar]
- 54.Halachmi D., Eilam Y. (1996) FEBS Lett. 392, 194–200 [DOI] [PubMed] [Google Scholar]
- 55.Miseta A., Kellermayer R., Aiello D. P., Fu L., Bedwell D. M. (1999) FEBS Lett. 451, 132–136 [DOI] [PubMed] [Google Scholar]
- 56.Kellermayer R., Szigeti R., Kellermayer M., Miseta A. (2004) FEBS Lett. 571, 55–60 [DOI] [PubMed] [Google Scholar]
- 57.Denis V., Cyert M. S. (2002) J. Cell. Biol. 156, 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okorokov L. A., Lichko L. P., Kulaev I. S. (1980) J. Bacteriol. 144, 661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakanishi Y., Saijo T., Wada Y., Maeshima M. (2001) J. Biol. Chem. 276, 7654–7660 [DOI] [PubMed] [Google Scholar]
- 60.Wiederhold E., Gandhi T., Permentier H. P., Breitling R., Poolman B., Slotboom D. J. (2009) Mol. Cell. Proteomics. 8, 380–392 [DOI] [PubMed] [Google Scholar]
- 61.Brett C. L., Tukaye D. N., Mukherjee S., Rao R. (2005) Mol. Biol. Cell 16, 1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali R., Brett C. L., Mukherjee S., Rao R. (2004) J. Biol. Chem. 279, 4498–4506 [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee S., Kallay L., Brett C. L., Rao R. (2006) Biochem. J. 398, 97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 Profesor Albareda No. 1, E-18008 Granada, Spain. Fax: 34-958-129-600. E-mail:
Profesor Albareda No. 1, E-18008 Granada, Spain. Fax: 34-958-129-600. E-mail: