Abstract
Human erythrocytes are continuously exposed to glucose, which reacts with the amino terminus of the β-chain of hemoglobin (Hb) to form glycated Hb, HbA1c, levels of which increase with the age of the circulating cell. In contrast to extensive insights into glycation of hemoglobin, little is known about glycation of erythrocyte membrane proteins. In the present study, we explored the conditions under which glucose and ribose can glycate spectrin, both on the intact membrane and in solution and the functional consequences of spectrin glycation. Although purified spectrin could be readily glycated, membrane-associated spectrin could be glycated only after ATP depletion and consequent translocation of phosphatidylserine (PS) from the inner to the outer lipid monolayer. Glycation of membrane-associated spectrin led to a marked decrease in membrane deformability. We further observed that only PS-binding spectrin repeats are glycated. We infer that the absence of glycation in situ is the consequence of the interaction of the target lysine and arginine residues with PS and thus is inaccessible for glycation. The reduced membrane deformability after glycation in the absence of ATP is likely the result of the inability of the glycated spectrin repeats to undergo the obligatory unfolding as a consequence of interhelix cross-links. We thus postulate that through the use of an ATP-driven phospholipid translocase (flippase), erythrocytes have evolved a protective mechanism against spectrin glycation and thus maintain their optimal membrane function during their long circulatory life span.
Keywords: ATP, Erythrocyte, Glucose, Membrane Lipids, Membrane Proteins, Glycation, Pentosidine, Phosphatidylserine, Ribose, Spectrin
Introduction
Proteins undergo nonenzymatic glycation when exposed to monosaccharides. The reaction proceeds in three steps: reversible formation of a Schiff-base adduct between the aldehyde or ketone moieties of the sugar and protein amino groups, followed by almost irreversible transformation to Amadori products, terminating in completely irreversible advanced glycation end products constituting a heterogeneous class of structures (1). Among them is pentosidine which is characterized by a pentose-derived fluorescent cross-link formed between lysine and arginine residues (2). Pentosidine can be generated not only by a pentose (such as ribose) but also by hexoses (such as glucose) (3). Because pentosidine formation is a very slow process, the most likely candidates for such modification in vivo are proteins that turn over very slowly.
Human erythrocytes are continuously exposed to glucose in plasma during their circulatory life span of 120 days. Passive transport through insulin-independent glucose transporter, GLUT1, ensures that the glucose concentration in the cytosol is close to that in the plasma, normally about 5 mm. Because there is no protein synthesis or degradation in the enucleate erythrocyte, a large fraction of membrane cytoskeletal proteins, including spectrin, have long life spans and are therefore amenable to glycation. Intracellular glucose reacts with the amino terminus of the β-chain of hemoglobin (Hb)2 to form HbA1c, levels of which increase with the age of the circulating cell. HbA1c levels are higher in individuals with diabetes due to higher blood glucose levels and are thus routinely used to monitor these patients (4). Ribose also forms Amadori products but at a much faster rate than with glucose (3). It should be noted, however, that in contrast to glucose, plasma ribose levels are 2–3 orders of magnitude lower (2). In contrast to extensive insights into glycation of Hb, little is known about glycation of erythrocyte membrane proteins.
The ability to undergo extensive membrane deformation is critical for the erythrocyte to pass through narrow capillaries and perform its function of oxygen delivery to the tissues. This property of the membrane is primarily governed by the spectrin-based membrane skeleton, situated beneath the lipid bilayer (5). Recent studies have shown that structural unfolding of certain of the constituent triple-helical repeats of α- and β-spectrin accompanies membrane deformation (6). Hence, if spectrin repeats are glycated during the erythrocyte life span, glycation-induced cross-links in these repeats could impede this response to deformation and render the cell more rigid.
In the present study we have explored the conditions under which glucose and ribose can generate pentosidine formation in spectrin, both on intact membrane and in solution. Although purified spectrin in solution could be readily glycated, membrane-associated spectrin could be glycated only after translocation of phosphatidylserine (PS) from the inner to the outer lipid monolayer as a result of ATP depletion. Glycation of membrane-associated spectrin led to a marked decrease in membrane deformability. We further observed that only a subset of spectrin repeats (11 of 37) are glycated and that every one of the glycated repeats is a PS-binding repeat. We infer that the absence of glycation in situ is the result of the interaction of the target lysine and arginine residues in these repeats with PS, in the inner monolayer of the erythrocyte membrane, thereby preventing them from being glycated. Thus, maintenance of the activity of the ATP-driven phospholipid translocase responsible for localizing PS to the inner monolayer is critical for presenting glycation of spectrin in intact erythrocytes. We thus postulate that erythrocytes have evolved this ATP-dependent protective mechanism against spectrin glycation by localizing PS to the inner monolayer and thereby enabling the membrane to maintain its remarkable elasticity through its long life span.
EXPERIMENTAL PROCEDURES
Materials
d-Ribose (catalog no. R7500) was purchased from Sigma. Anti-pentosidine antibody (clone PEN-12) was purchased from Trans Genic Inc. N-Ethylmaleimide (catalog no. 054-02063) was purchased from Wako Pure Chemical Industries. PS (AVT 840034C), phosphatidylcholine (PC) (AVT 850457C) and phosphatidylethanolamine (PE) (AVT 850757C) were purchased from Avanti Polar Lipids. Pyridyldithioethanolamine (PDA) was synthesized as described previously (7, 8).
Methods
Glycation of Erythrocyte Ghosts and Purified Spectrin
After obtaining informed consent, 200 ml of venous blood was collected from healthy volunteer donors. The leukocytes were removed using IMUGARD III-RC filter (Terumo Inc., Tokyo, Japan), and erythrocytes were washed three times with ice-cold phosphate buffered saline (PBS; 10 mm sodium phosphate, 150 mm NaCl, pH 7.4). Intact erythrocytes were lysed and washed three times in 35 volumes of the hypotonic buffer (5P7.4; 5 mm sodium phosphate, pH 7.4) at 4 °C. The resulting white ghost membranes were gently stirred at 0 °C for 5 min in 0.35 m phosphate buffer (buffer A, pH 7.4) containing 0.35 m ribose and subsequently incubated at 37 °C for 0–6 h.
Spectrin dimer was purified from erythrocytes as we described previously (9) and incubated at 37 °C with buffer A containing 0.35 m glucose for 0–3 days or 0.35 m ribose for 0–5 h. To prevent further glycation, each solution was applied onto a gel filtration column (Econo-Pac 10 DG; Bio-Rad) to remove unreacted sugars. Spectrin dimers were collected from the eluate, monitored by absorbance at 280 nm (A280). Membrane proteins and purified spectrin were analyzed by SDS-PAGE on either 5% or 9% acrylamide gels. The proteins were either stained with Coomassie Brilliant Blue R-250 (CBB), or transferred to PVDF membranes (Immobilon P; Millipore). The PVDF membranes with transferred proteins were then incubated with anti-pentosidine antibody (PEN-12) and washed extensively, followed by incubation with secondary horseradish peroxidase-conjugated anti-mouse antibody. Immunoreactive proteins were visualized with chemiluminescence detection reagent ECL (Amersham Biosciences).
Glycation of Erythrocytes
The erythrocytes were suspended at 20% hematocrit in PBS containing 0–0.35 m ribose followed by incubation at 37 °C for 48 h. Glycated erythrocytes were lysed and washed three times in 35 volumes of 5P7.4 at 4 °C and subsequently incubated with PBS at 37 °C for 40 min to allow membrane resealing for analysis of pentosidine formation as well as measurement of membrane deformability.
Measurement of Membrane Deformability
Resealed ghosts were resuspended in 35% dextran solution and examined by ektacytometry as described previously (10). Briefly, resealed ghosts were subjected to increasing levels of applied shear stress (0–150 dynes/cm2) and changes in deformability index was recorded as a function of applied shear stress.
Identification of Pentosidine Formation Site of Spectrin
Erythrocytes suspended at a hematocrit of 20% in PBS containing 0.35 m ribose were incubated at 37 °C for 48 h. Ghosts were then prepared by lysis with 5P7.4 as described and washed once with 0.1 mm EDTA. After adjustment of pH to 8.5, the ghost suspension was incubated at 37 °C for 30 min to extract spectrin dimers, which were separated from membrane vesicles by centrifugation at 40,000 × g at 4 °C for 72 min. The isolated spectrin dimer was treated with reducing and alkylating agents and was subsequently digested with lysyl-endopeptidase at an enzyme to substrate ratio of 1:650 at 37 °C for 0–60 min. Partially digested peptides were separated by one-dimensional NuPAGE Novex BisTris Mini Gel (12%) in MES as running buffer and transferred onto two PVDF membranes (Immobilon-PSQ; Millipore), one for staining with CBB and the other for immunoblotting with the pentosidine antibody. The CBB bands corresponding to immunoreactive bands were sequenced for amino-terminal amino acids by Shimadzu Technoresearch.
Preparation and Glycation of α- and β-Spectrin Repeats
cDNA of β-spectrin repeat 1 + 2 was chemically synthesized by Operon Biotechnologies, K. K. Japan based on the previously reported sequence (11–13). Repeat 1 was prepared by insertion of the stop codon between repeats 1 and 2. cDNAs of all other repeats of α- and β-spectrin (13, 14) were kind gifts from Xuili An (Red Cell Physiology Laboratory, New York Blood Center). These DNA fragments were subcloned into pGEX-4T-2 vector using BamHI and EcoRI as upstream and downstream cloning sites. The expression of recombinant proteins was induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside at 16 °C for 18 h. The GST fusion polypeptides were purified using a glutathione-Sepharose 4B affinity column, and GST was cleaved with thrombin. All spectrin repeat polypeptides were dialyzed against buffer A and incubated in the same buffer containing 0.35 m ribose at 37 °C for 4 h.
Single mutations introduced into repeat 1 + 2 of β-spectrin consisted of replacing Lys461, Lys462, or Lys463 by Gln, and either Arg520 or Arg521 by Leu, as described previously (15). Expressed GST peptides carrying the mutations were dialyzed against buffer A and incubated in same buffer containing 0.35 m ribose at 37 °C for 4 h. The glycated peptides were analyzed by SDS-PAGE on 15% acrylamide gels and by immunoblotting with anti-pentosidine antibody.
Modeling of β-Spectrin
Modeling of erythrocyte spectrin repeats was performed using Swiss Model software based on the structure of brain βII-spectrin (16).
Binding of Repeat 1 + 2 of β-Spectrin to PS-Liposomes
PS-liposomes were prepared by sonication. Peptide-liposome interaction was measured by a pelleting assay (14). Briefly, GST fusion peptide of repeat 1 + 2 of β-spectrin was incubated with 0.5 m ribose in 50 mm phosphate buffer, pH 7.4, at 37 °C for 20 h and then dialyzed against the same buffer without ribose. Glycated and unglycated peptides were incubated with PS-liposomes at room temperature for 60 min. The liposomes were pelleted by centrifugation at 230,000 × g for 30 min at 4 °C. They were subsequently washed with 50 mm phosphate buffer, pH 7.4, and resuspended in the same volume as the original supernatant. Equal volumes of supernatant and pellet suspension were analyzed by SDS-PAGE and stained with CBB.
Glycation of Purified Spectrin Dimer in the Presence of PS-Liposomes
Spectrin dimer purified from native erythrocyte ghosts were glycated in the presence of PS-, PC-, or PE-liposomes (molar ratio of lipid to spectrin dimer of 7:1) at 37 °C for 8 h. The glycated spectrin was analyzed by SDS-PAGE and by immunoblotting with anti-pentosidine antibody.
Preparation of Ghosts in the Presence or Absence of ATP
Ghosts were prepared in the presence or absence of ATP as we reported previously (8). Briefly, erythrocytes were lysed and washed three times in 35 volumes of 5 mm Tris-HCl, pH,7.4, containing 5 mm KCl with or without 0.6 mm MgATP, 1.0 mm MgCl2 at 4 °C. To restore isotonicity, a small volume of a concentrated solution of KCl, MgCl2, and DTT was added to the membrane suspension, to give final concentrations of 150, 1.6, and 1 mm, respectively. The ghost suspension was washed once in 0.35 m phosphate buffer, pH 7.4, with or without 0.5 m ribose followed by incubation in the same buffer at 37 °C for 30 or 60 min. Ghosts were then washed three times in 5P7.4 followed by an analysis of pentosidine formation of spectrin dimer by immunoblotting. For some experiments, flippase inhibitors, 10 mm PDA and 2 mm N-ethylmaleimide, were incorporated into ATP ghosts.
Measurement of ATP Concentration in Erythrocytes
Erythrocytes were treated with 10 volumes of 0.6 n perchloric acid followed by centrifugation at 1,500 × g at 4 °C for 10 min. The supernatant was collected and neutralized with triethanolamine solution containing potassium carbonate. ATP contents of supernatants were measured with the bioluminescence kit (KIKKOman).
RESULTS
Glycation of Spectrin
Western blot analysis with an anti-pentosidine antibody of membrane proteins of erythrocyte ghosts prepared in the absence of ATP and incubated with 0.35 m ribose at 37 °C revealed that glycation occurred predominantly on spectrin and that the β-chain was more strongly glycated than the α-chain (Fig. 1A). Vestigial glycation was also observed in some other proteins. Incubation of isolated spectrin dimers with either glucose or ribose also caused pentosidine formation, confirming that the reaction is nonenzymatic (Fig. 1B). Although glycation of spectrin dimers in solution by ribose was evident within a few hours, more than 24 h were required for documenting noticeable amount of modification by glucose. This confirms that the reaction with the pentose proceeds more rapidly than with the hexose.
FIGURE 1.
Glycation of erythrocyte ghosts membrane and purified spectrin. Erythrocyte ghosts membranes were prepared by hypotonic lysis and incubated with ribose at 37 °C for up to 6 h. A, glycated ghosts membrane proteins were separated on 9% SDS-polyacrylamide gel and stained with CBB (left panel); a Western blot of a replicate gel probed with the anti-pentosidine antibody is shown (right panel). B, purified spectrin dimers prepared from unglycated erythrocyte membranes were incubated with glucose (upper panel) or ribose (lower panel) for the indicated periods of days or hours, respectively.
Decrease in Membrane Deformability after Spectrin Glycation
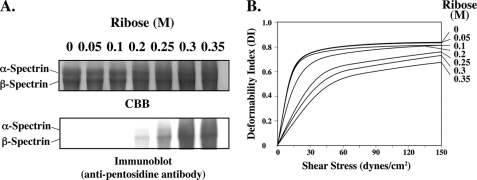
Glycation of spectrin in intact cells increased after incubation for 48 h at 37 °C with increasing concentrations of ribose of up to 0.35 m (Fig. 2A). Pentosidine formation of spectrin could be readily detected at a ribose concentration of 0.2 m. It is again evident that β-spectrin reacts more abundantly than α-spectrin (Fig. 2A). Deformability of membranes prepared from glycated erythrocytes as measured by ektacytometry showed a progressive decrease with increasing ribose concentration during incubation, as reflected by the decreased extent of increase in deformability index with increasing values of applied shear stress (Fig. 2B). Although there was no perceptible change in membrane deformability after incubation with 0.05 or 0.1 m ribose, it decreased by factors of 1.6, 3.2, 3.8, and 4.9 after incubation with 0.2, 0.25, 0.3, and 0.35 m ribose, respectively, as reflected by the extent of excess shear stress required to reach equivalent value of deformability index (10). The extent of decrease in membrane deformability was related to amount of spectrin pentosidine formation. We note that pentosidine could be detected in spectrin even after 24 h of incubation with ribose and increased with time (data not shown). A parallel incubation time-dependent decrease in membrane deformability was also documented (data not shown).
FIGURE 2.
Effects of ribose on spectrin glycation in erythrocytes and on membrane deformability. The membranes from erythrocytes incubated with the indicated concentrations of ribose at 37 °C for 48 h were prepared by hypotonic lysis and resealed in PBS. A, membrane proteins separated on 5% SDS-acrylamide gel and stained with CBB (upper panel) and blotted onto PVDF and probed with anti-pentosidine antibody (lower panel) are shown. B, membrane deformability of the ghosts was analyzed by ektacytometry.
Identification of Pentosidine Formation Site of Spectrin
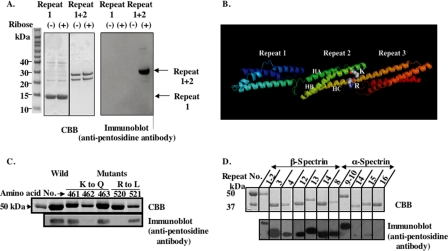
To identify the specific pentosidine formation sites in spectrin, spectrin dimers were purified from erythrocytes incubated with 0.35 m ribose for 48 h and subjected to lysyl-endopeptidase digestion for 60 min. Western blot analysis revealed several bands that reacted with anti-pentosidine antibody (supplemental Fig. 1). Sequence analysis of the pentosidine-containing component of the lowest molecular mass revealed that it consisted of repeats 1 and 2 of β-spectrin (data not shown). To confirm this finding, we examined the ability of ribose to glycate recombinant peptides representing either repeat 1 or repeats 1 + 2 of β-spectrin. Pentosidine formation was detected only in the latter (Fig. 3A), implying that repeat 2 is glycated. Modeling of erythroid βI-spectrin repeats 1, 2, and 3, based on the crystal structure of nonerythroid βII-spectrin repeat, identified Lys462 and Arg520 of repeat 2 in close apposition and therefore a likely target for pentosidine formation (Fig. 3B). To validate this hypothesis, we generated several recombinant repeat 1 + 2 polypeptides with single amino acid substitutions, replacing Lys461, Lys462 or Lys463, by Gln, and Arg520 or Arg521 by Leu. Pentosidine formation was significantly reduced only in recombinant polypeptides in which Lys462 and Arg520 were changed to Gln and Leu, respectively, indicating that these residues are essential for cross-link formation (Fig. 3C).
FIGURE 3.
Identification of pentosidine formation site of spectrin. Recombinant peptides of PS-binding repeats of α- and β-spectrin were prepared. A, repeat 1 and repeats 1 + 2 without GST were glycated and were separated on a one-dimensional 12% NuPAGE Novex BisTris Mini Gel in MES running buffer and blotted onto two PVDF membranes; one was stained with CBB (left panel) and the other probed with anti-pentosidine antibody (right panel). B, predicted ribbon model of repeats 1–3 of human erythroid β-spectrin based on the defined structure of brain βII-spectrin derived using software of Swiss Model are shown. HA, HB, and HC represent the helices of the triple-helical repeat 2. C, recombinant peptides of repeats 1 + 2 with indicated single-amino acid substitutions were glycated with ribose. They were separated by SDS-PAGE as indicated above. Gels were stained with CBB (upper panel), blotted onto PVDF, and probed with anti-pentosidine antibody (lower panel). D, various PS-binding and non-PS-binding repeats of α- and β-spectrin were glycated with ribose. They were separated by SDS-PAGE as above. Gels were stained with CBB (upper panel), blotted onto PVDF, and probed with anti-pentosidine antibody (lower panel).
Pentosidine Formation of PS-binding Repeats of Spectrin
Having documented that a previously identified PS-binding repeat of spectrin, repeat 2 of β-spectrin (13), can be glycated, we explored whether all other PS-binding repeats in α- and β-spectrin can also be glycated. As shown in Fig. 3D, all previously documented PS-binding repeats (repeats 3, 4, 12, 13, and 14 of β-spectrin and 8, 9, and 10 of α-spectrin) were indeed glycated by ribose. Importantly, non-PS-binding repeats such as repeats 14, 15, and 16 of α-spectrin could not be glycated (Fig. 3D).
Glycation Inhibits Spectrin-PS Interaction
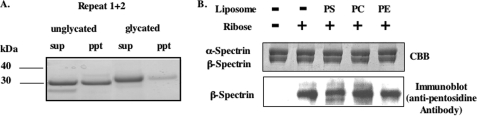
We investigated whether glycation of spectrin can inhibit the spectrin-PS interaction and conversely whether binding of spectrin to PS can suppress glycation. Unglycated or glycated recombinant polypeptides of repeats 1 + 2 of β-spectrin were incubated with PS-containing liposomes, and binding of these peptides was determined by the pelleting assay (Fig. 4A). The binding of the glycated fragment to the liposomes was markedly diminished relative to that of the unglycated polypeptide. Thus, glycation indeed suppresses the interaction with PS.
FIGURE 4.
Effect of glycation on binding of spectrin to PS-liposomes and extent of glycation of spectrin in the presence of PS-liposomes. A, repeats 1 + 2 were glycated with ribose. Glycated or unglycated repeat 1 + 2 polypeptides were incubated with PS-liposomes at room temperature for 60 min. The liposomes were then collected by centrifugation. The supernatants (sup) and liposome pellets (ppt) were analyzed by SDS-PAGE and stained with CBB. B, purified spectrin was incubated with ribose in the presence of PS-, PC-, and PE-liposomes (molar ratio of 7 to spectrin dimer) at 37 °C for 8 h. Pentosidine formation of spectrin molecules was analyzed by SDS-PAGE. Gels were stained with CBB (upper panel), blotted onto PVDF, and probed with anti-pentosidine antibody (lower panel).
We next examined whether PS-bound spectrin can be glycated in vitro. Purified spectrin dimer was incubated with ribose in the presence of PS-, PC- or PE-liposomes. Glycation of spectrin was reduced by more than 50% in the presence of PS-liposomes (Fig. 4B). In contrast, spectrin glycation was only marginally decreased to 85 and 87% of control in the presence PC- and PE-liposomes, respectively (Fig. 4B). These results imply that PS binding specifically suppresses spectrin glycation.
Binding of Spectrin to PS Protects against Glycation on the Membrane
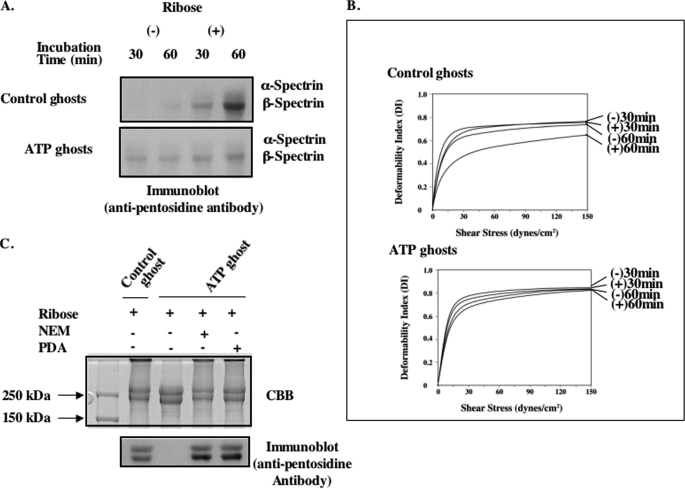
To determine whether the spectrin-PS interaction can inhibit glycation of spectrin in intact membranes, two types of ghosts were prepared: when cells are lysed in the presence of ATP, spectrin remains bound to PS, whereas in ghosts prepared in the absence of ATP, spectrin is no longer bound to PS due to translocation of PS to the outer leaflet of the membrane (8). Following incubation with ribose for 60 min, pentosidine formation of β-spectrin could be readily detected in ghosts prepared without ATP, whereas the extent of glycation is markedly decreased in ghosts prepared in the presence of ATP (Fig. 5A). Correspondingly, the glycation-dependent decrease in deformability was much less in ghosts prepared in the presence of ATP rather than in its absence (Fig. 5B). These results indicate that interaction of spectrin with PS in erythrocyte membrane protects it against glycation and the resultant decrease in membrane deformability.
FIGURE 5.
Glycation of ATP ghosts. The ghosts were prepared by lysis of cells in the presence or absence of ATP and resealed as described under “Methods.” These ghosts were glycated with ribose at 37 °C for up to 60 min. A, membrane proteins were separated on 5% SDS-PAGE followed by immunoblotting with anti-pentosidine antibody. B, membrane deformability of these ghosts was measured by the ektacytometry. C, flippase inhibitor, PDA or N-ethylmaleimide (NEM), was incorporated into ATP ghosts. Pentosidine formation of spectrin molecules was analyzed by SDS-PAGE. Gels were stained with CBB, blotted onto PVDF, and probed with anti-pentosidine antibody.
To ascertain whether the observed effect of ATP is mediated by ATP-dependent flippase that maintains PS in the inner leaflet, we incorporated flippase inhibitors, PDA and N-ethylmaleimide, into ATP ghosts. In the presence of these inhibitors, spectrin was extensively glycated, implying that ATP prevents spectrin glycation by its activation of flippase (Fig. 5C).
Importance of ATP in Preventing Pentosidine Formation of Spectrin in Intact Erythrocytes
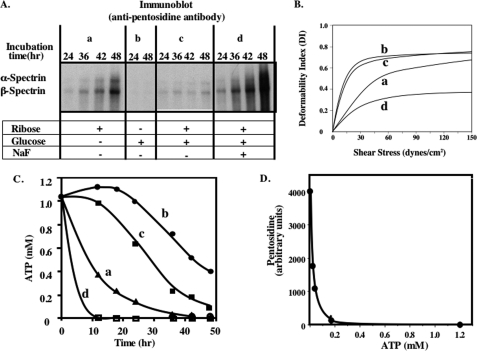
To establish whether the observations on ghosts are equally applicable to intact erythrocytes, we measured spectrin glycation in erythrocytes incubated with glucose or ribose or both together for up to 48 h (Fig. 6A). In contrast to the extensive glycation of spectrin observed after incubation with ribose, no such glycation could be detected after incubation of the cells with glucose. Furthermore, ribose-induced glycation of spectrin was markedly inhibited by the presence of glucose in the incubation medium. Because glucose but not ribose is used by cells to generate ATP by glycolysis, we inferred that the inability to maintain an adequate cytosolic ATP concentration may account for this striking difference. To test this hypothesis directly, NaF was added, together with glucose and ribose, to inhibit glycolysis and thus ATP synthesis. Addition of NaF to the incubation medium containing both ribose and glucose, indeed restored the ability of ribose to induce extensive spectrin glycation. Importantly, as with ghosts, the extent of decreased membrane deformability of the intact red cells was directly related to the extent of spectrin glycation (Fig. 6B). These findings suggest that maintenance of cellular ATP levels protects against spectrin glycation.
FIGURE 6.
Effects of ATP on pentosidine formation and membrane deformability. Erythrocytes were incubated for up to 48 h with ribose alone (a), glucose alone (b), a mixture of ribose and glucose (c), or the same mixture with NaF (d). Ghosts were prepared from these erythrocytes by hypotonic lysis and resealed in isotonic buffer. A, ghost proteins were separated on 5% SDS-PAGE and blotted onto PVDF membranes and probed with anti-pentosidine antibody. B, deformability of these ghosts was measured in the ektacytometer. C, measured ATP concentrations were plotted against incubation time. D, pentosidine formation was plotted against intracellular ATP concentrations.
To validate this conclusion, intracellular ATP concentrations of erythrocytes were measured under the various incubation conditions (Fig. 6C). ATP concentration was maintained after incubation with glucose alone for at least 20 h and then started to decline gradually to 0.4 mm after 48 h (due to consumption of ATP and glucose). In marked contrast, after incubation with ribose alone it decreased rapidly to 0.2 mm or less after 24 h because ribose could not be glycolysed to produce ATP. The ATP profile with a mixture of glucose and ribose showed an intermediate pattern of decline in ATP levels, whereas the highest rate of ATP consumption occurred after the addition of NaF to the incubation medium (Fig. 6C). These results in conjunction with the spectrin glycation data imply that pentosidine formation is inhibited as long as cytosolic ATP concentrations is 0.2 mm or higher (Fig. 6D).
DISCUSSION
In the present study we have shown that isolated spectrin in solution, as well as spectrin in erythrocyte ghosts and intact cells, can be glycated by both glucose and ribose to form pentosidine under appropriate incubation conditions. This posttranslational modification was nonenzymatic, irreversible, and time-dependent. Ribose induced spectrin glycation at a much faster rate than glucose because ribosylated lysine residue in the Amadori product formed by ribose (pentose) readily reacts with the guanide group of arginine residues to form a cross-link. In contrast, glucated lysine formed by glucose (hexose) must be decarboxylated before it can form a cross-link with arginine, and therefore pentosidine formation with glucose is much slower.
Among major erythrocyte membrane proteins, it is α- and β-spectrins that are predominantly glycated in the intact cell, with more abundant modification of β-spectrin than α-spectrin. Spectrin, a long filamentous molecule, is composed of a string of triple-helical repeats, 22 in α-spectrin and 17 in β-spectrin (17). A number of these repeats, including repeat 2 in β-spectrin, contain lysine clusters with proximal arginines, which are potential substrates for nonenzymatic glycation. We documented that Lys462 in the helix B (Fig. 3B) and Arg520 in the helix C (Fig. 3B) of repeat 2 are involved in glycation. Based on homology modeling, these two residues are only 5.8 Å apart and could potentially form a cross-link. Studies with hemoglobin (18, 19) and human serum albumin (20) have shown that among the various lysines in these proteins, clusters such as KKK as well as KH are preferential substrates for glycation. Studies with RNase have shown that the reactive lysine residues in this enzyme are located in a fairly basic region of the protein (21). In addition to one KKK cluster in repeat 2 of β-spectrin, we also noted the presence of KK clusters in repeats 4 and 12 and KH in repeats 3 and 4 of β-spectrin. In addition, α-spectrin has KK clusters in repeats 8, 9, 10, 14, 15, and 16. Indeed, we were able to document that β-spectrin repeats 3, 4, 12, 13, and 14 and α-spectrin repeats 8, 9, and 10 can all be glycated. Interestingly, although α-spectrin repeats 14, 15, and 16 contain KK, they were not glycated, indicating that they may not have Arg at an appropriate distance.
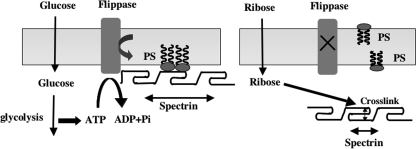
Previous studies have established that spectrin interacts directly with aminophospholipids of the inner membrane leaflet (23–26) and that the spectrin-lipid interactions can play an important part in stabilizing the red cell membrane (8). A major finding of the present study is that every spectrin repeat that has previously been identified to bind PS (13) can be glycated, whereas the non-PS-binding repeats are not glycated. Furthermore, we noted that glycation of repeat 2 suppresses its ability to bind PS and that spectrin in intact membranes can only be glycated when its interaction with PS is lost as a consequence of loss of phospholipid asymmetry through inactivation of the ATP-dependent flippase. Further support for this thesis is provided by our finding that spectrin that is not glycated in ATP ghosts is readily glycated after inhibition of the flippase. These findings strongly suggest that metabolically regulated confinement of PS to the inner lipid monolayer in intact erythrocytes protects against spectrin glycation by masking the glycation sites (Fig. 7).
FIGURE 7.
Working model for glycation of spectrin induced by either glucose or ribose in human erythrocytes. In normal erythrocytes, glycolysis-derived ATP driving the ATP-dependent flippase maintains PS in the inner monolayer, enabling the PS-binding spectrin repeats to be anchored in the bilayer (left panel). In the absence of ATP, PS is translocated to the outer monolayer, enabling the dissociation of PS-binding repeats from the membrane and thereby making the lysine and arginine residues accessible for glycation.
Spectrin contributes to erythrocyte elasticity by local folding and unfolding of certain of its structural repeats. This has been shown by atomic force microscopy to occur at forces in the range of 25–50 piconewtons (6). More recently, such unfolding events have been demonstrated in some spectrin repeats in both chains when the intact membrane is deformed (22). Our finding that glycation of spectrin reduces membrane deformability of erythrocytes could be accounted for by the inability of the large number of glycated repeats to undergo the obligatory unfolding as a consequence of interhelix cross-links in the normally structurally labile repeats.
We thus infer that the erythrocyte has evolved an ATP-dependent protective mechanism against spectrin glycation in the face of constant exposure to glucose through out its long life span of 120 days, allowing it to maintain its ability to undergo the extensive deformations demanded by its function of oxygen delivery.
Supplementary Material
Acknowledgments
We thank Xuili An of the Red Cell Physiology Laboratory, New York Blood Center, and Wataru Nunomura of the Department of Biochemistry, Tokyo Women's Medical University, for valuable discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants HL 31579 DK26263 and DK32094. This work was also supported by Grant-in-aid for Scientific Research 19590289 from the Ministry of Education Culture, Sports, Science, and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig 1.
- Hb
- hemoglobin
- HbA1c
- glycated Hb
- BisTris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
- CBB
- Coomassie Brilliant Blue R-250
- PC
- phosphatidylcholine
- PDA
- pyridyldithioethanolamine
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine.
REFERENCES
- 1.Bierhaus A., Hofmann M. A., Ziegler R., Nawroth P. P. (1998) Cardiovasc. Res. 37, 586–600 [DOI] [PubMed] [Google Scholar]
- 2.Sell D. R., Monnier V. M. (1989) J. Biol. Chem. 264, 21597–21602 [PubMed] [Google Scholar]
- 3.Grandhee S. K., Monnier V. M. (1991) J. Biol. Chem. 266, 11649–11653 [PubMed] [Google Scholar]
- 4.Bunn H. F., Gabbay K. H., Gallop P. M. (1978) Science 200, 21–27 [DOI] [PubMed] [Google Scholar]
- 5.Mohandas N., Gallagher P. G. (2008) Blood 112, 3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson C. P., Tang H. Y., Carag C., Speicher D. W., Discher D. E. (2007) Science 317, 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor J., Schroit A. J. (1988) Biochemistry 27, 848–851 [DOI] [PubMed] [Google Scholar]
- 8.Manno S., Takakuwa Y., Mohandas N. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1943–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manno S., Takakuwa Y., Nagao K., Mohandas N. (1995) J. Biol. Chem. 270, 5659–5665 [DOI] [PubMed] [Google Scholar]
- 10.Chasis J. A., Mohandas N., Shohet S. B. (1985) J. Clin. Invest. 75, 1919–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begg G. E., Harper S. L., Morris M. B., Speicher D. W. (2000) J. Biol. Chem. 275, 3279–3287 [DOI] [PubMed] [Google Scholar]
- 12.Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. (1990) J. Biol. Chem. 265, 11827–11832 [PubMed] [Google Scholar]
- 13.An X., Guo X., Sum H., Morrow J., Gratzer W., Mohandas N. (2004) Biochemistry 43, 310–315 [DOI] [PubMed] [Google Scholar]
- 14.An X. L., Takakuwa Y., Manno S., Han B. G., Gascard P., Mohandas N. (2001) J. Biol. Chem. 276, 35778–35785 [DOI] [PubMed] [Google Scholar]
- 15.Nunomura W., Takakuwa Y., Parra M., Conboy J., Mohandas N. (2000) J. Biol. Chem. 275, 24540–24546 [DOI] [PubMed] [Google Scholar]
- 16.Davis L., Abdi K., Machius M., Brautigam C., Tomchick D. R., Bennett V., Michaely P. (2009) J. Biol. Chem. 284, 6982–6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Speicher D. W., Marchesi V. T. (1984) Nature 311, 177–180 [DOI] [PubMed] [Google Scholar]
- 18.Shapiro R., McManus M. J., Zalut C., Bunn H. F. (1980) J. Biol. Chem. 255, 3120–3127 [PubMed] [Google Scholar]
- 19.Garlick R. L., Mazer J. S., Higgins P. J., Bunn H. F. (1983) J. Clin. Invest. 71, 1062–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iberg N., Flückiger R. (1986) J. Biol. Chem. 261, 13542–13545 [PubMed] [Google Scholar]
- 21.Watkins N. G., Neglia-Fisher C. I., Dyer D. G., Thorpe S. R., Baynes J. W. (1987) J. Biol. Chem. 262, 7207–7212 [PubMed] [Google Scholar]
- 22.Law R., Carl P., Harper S., Dalhaimer P., Speicher D. W., Discher D. E. (2003) Biophys. J. 84, 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maksymiw R., Sui S. F., Gaub H., Sackmann E. (1987) Biochemistry 26, 2983–2990 [DOI] [PubMed] [Google Scholar]
- 24.Cohen A. M., Liu S. C., Derick L. H., Palek J. (1986) Blood 68, 920–926 [PubMed] [Google Scholar]
- 25.MacDonald R. I. (1993) Biochemistry 32, 6957–6964 [DOI] [PubMed] [Google Scholar]
- 26.Michalak K., Bobrowska M., Sikorski A. F. (1993) Gen. Physiol. Biophys. 12, 163–170 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.