Abstract
G-protein signaling modulators (GPSM) play diverse functional roles through their interaction with G-protein subunits. AGS3 (GPSM1) contains four G-protein regulatory motifs (GPR) that directly bind Gαi free of Gβγ providing an unusual scaffold for the “G-switch” and signaling complexes, but the mechanism by which signals track into this scaffold are not well understood. We report the regulation of the AGS3·Gαi signaling module by a cell surface, seven-transmembrane receptor. AGS3 and Gαi1 tagged with Renilla luciferase or yellow fluorescent protein expressed in mammalian cells exhibited saturable, specific bioluminescence resonance energy transfer indicating complex formation in the cell. Activation of α2-adrenergic receptors or μ-opioid receptors reduced AGS3-RLuc·Gαi1-YFP energy transfer by over 30%. The agonist-mediated effects were inhibited by pertussis toxin and co-expression of RGS4, but were not altered by Gβγ sequestration with the carboxyl terminus of GRK2. Gαi-dependent and agonist-sensitive bioluminescence resonance energy transfer was also observed between AGS3 and cell-surface receptors typically coupled to Gαi and/or Gαo indicating that AGS3 is part of a larger signaling complex. Upon receptor activation, AGS3 reversibly dissociates from this complex at the cell cortex. Receptor coupling to both Gαβγ and GPR-Gαi offer additional flexibility for systems to respond and adapt to challenges and orchestrate complex behaviors.
Keywords: Drug Action, G-protein-coupled Receptors (GPCR), G-proteins, 7-Helix Receptor, Heterotrimeric G Proteins, Activator of G-protein Signaling, Receptor Signaling Complex
Introduction
Cell-surface receptors coupled to heterotrimeric G-proteins (Gαβγ) serve as a major mode of cellular communication via their specific interaction with a variety of hormones and neurotransmitters. Gαβγ functions as a transducer in this process by sensing receptor activation with subsequent regulation of various effectors by both Gα and Gβγ. Central to this process is the exchange of GTP for GDP bound to Gα, dissociation or rearrangement of Gα and Gβγ with subsequent effector engagement, hydrolysis of GTP bound to Gα, and regeneration of the heterotrimer Gαβγ. Beyond the core triad of receptor, G-protein, and effector, there are multiple accessory proteins that provide alternative modes of signal input to G-protein signaling systems, segregate a signaling complex to microdomains of the cell and/or regulate the basal activity, efficiency, and specificity of signal transfer. Accessory proteins may directly regulate guanine nucleotide exchange and/or hydrolysis by the G-protein transducers or serve as alternative binding partners for Gα and Gβγ independent of the typical Gαβγ heterotrimer (1–2). Such entities provide attractive mechanisms for tissues to respond and adapt to physiological and pathological challenges and present potential therapeutic targets.
One such group of accessory proteins were discovered as receptor-independent activators of G-protein signaling or AGS proteins in a yeast-based functional screen of mammalian cDNAs (2–6). The discovery of AGS proteins and related entities in parallel with a variety of studies that revealed unexpected functional roles for G-protein subunits in asymmetric cell division in Caenorhabditis elegans and Drosophila melanogaster led to the postulate that Gα and Gβγ regulate intracellular events distinct from their role as transducers for cell-surface seven-transmembrane span receptors (2, 3, 7–13). There are essentially three general groups of AGS proteins. Group I AGS proteins refer to non-receptor entities that act as guanine nucleotide exchange factors for Gαi, Gαo, or brain Gαβγ. Group II AGS proteins behave as guanine nucleotide dissociation inhibitors by stabilizing the GDP-bound conformation of Gαi (7, 14–18). Group III AGS proteins generally interact with Gβγ (5, 19–20). Other proteins have similar properties, but were not isolated in the initial functional screen used to identify AGS proteins and are known by different names (1, 21–23).
The Group II AGS proteins (AGS3 (GPSM1), LGN (GPSM2, AGS5), AGS4 (GPSM3), RGS12 (AGS6), RGS14, PCP2/L7) serve as alternative binding partners for Gα-GDP (Gαi, Gαt, Gαo) independent of the classical heterotrimer Gαβγ and this interaction is mediated by a stretch of 20–25 amino acids termed a G-protein regulatory (GPR)3 or GoLoco motif (2, 24). Multiple GPR motifs may exist within a single protein thus presenting a scaffold for assembly of Gαi molecules free of Gβγ. However, the mechanisms controlling the interactions between GPR-containing proteins and their G-protein partners remain poorly understood. Such signals may involve coordinated positioning of GPR proteins within the cell, receptor-activated signaling cascades, regulatory proteins binding to non-GPR domains of the proteins, and/or non-receptor guanine nucleotide exchange factors acting on a GPR·Gαi complex (2, 3, 25–32).
The GPR·Gαi signaling cassette is utilized across a wide range of organisms including C. elegans (Nematoda), D. melanogaster (Arthropoda), and mammalian systems (Rodentia, Mus, rattus; primates, Homo) where it is implicated in the process of asymmetric cell division and cell polarity in embryonic and adult stem cells from multiple tissues through regulation of spindle pulling forces and spindle orientation (9, 10, 26, 33–37). In C. elegans, GPR proteins actually act in concert with a regulator of G-protein signaling (RGS) protein, which accelerates the GTPase activity of Gα, to differentially regulate the movement of the two spindle poles driving asymmetry in the early embryo (38). Further studies in mammalian systems with AGS3 in vitro and in vivo indicate additional functional roles for the protein in various physiological and pathological processes including neuronal plasticity and addiction, autophagy, membrane protein trafficking, polycystic kidney disease, cardiovascular regulation, and metabolism (2, 39–45). The role of the GPR·Gαi signaling cassette in asymmetric cell division appears independent of the classical receptor-heterotrimeric G-protein-effector concept (9, 34, 46–47). In other functional roles, the GPR·Gαi signaling module may regulate signaling events initiated by a cell-surface seven-transmembrane receptor (G-protein-coupled receptor), although the mechanism of this modulation is not well understood and such modulation is not operative in all of the various systems studied (32, 33, 39, 40, 48–51).
As GPR proteins often have multiple GPR motifs and other defined protein interaction domains, they may play key roles within the regulated assembly or disassembly of larger signaling complexes. Of particular note, both AGS3 and LGN (AGS5) possess seven tetratricopeptide repeats, a linker region, and four GPR motifs. The four GPR motifs are separated by 14–35 amino acids essentially allowing the focused assembly of Gαi·GDP proteins for signal processing. Such complexes provide a very attractive system for signal integration via cell-surface receptors and perhaps relate to the multimeric G-protein complexes suggested by work in the laboratory of Dr. Rodbell (52). In this study, we report the regulation of the AGS3·Gαi signaling cassette by a cell-surface seven-transmembrane span receptor and its existence as part of a signaling complex at the cell cortex.
EXPERIMENTAL PROCEDURES
Materials
UK-14304, rauwolscine HCl, clonidine, guanabenz, (−)-epinephrine, [d-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO), isoproterenol, pertussis toxin, forskolin, PMSF (phenylmethylsulfonyl fluoride), EDTA, and EGTA were purchased from Sigma. Ionomycin and Gαi1/2 antibody were obtained from Calbiochem (La Jolla, CA). Anti-GFP antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). AGS3-antisera generated by immunization of rabbits with a GST-AGS3 fusion protein encoding the GPR domain (Ala461–Ser650) of AGS3 was kindly provided by Dr. Ma (University of California, Santa Barbara). Protease inhibitor mixture tablets (Complete Mini) were obtained from Roche Applied Science. Polyethylenimine (PEI, linear, Mr 25,000) was obtained from Polysciences, Inc. (Warrington, PA). Lipofectamine 2000 and all cell culture materials were obtained from Invitrogen. Bio-Rad DC protein assay kit was obtained from Bio-Rad. Benzyl-coelenterazine was obtained from NanoLight Technology (Pinetop, AZ). 96-Well gray optiplates were obtained from PerkinElmer Life Sciences. Gαi3, Gαi3-G202T, and Gαi3-Q204L cDNAs were obtained from cDNA Resource Center (University of Missouri, Rolla, MO). GRK2 antibody (ab50633) was obtained from Abcam Inc. (Cambridge, MA). G-protein β-subunit antiserum was a kind gift from Dr. John Hildebrandt (Medical University of South Carolina, Charleston, SC). RGS4 antiserum was a kind gift from Dr. Ron Taussig and the Alliance for Cell Signaling (University of Texas, Southwestern Medical Center, Dallas, TX). Dexmedetomidine was a kind gift from Orion Pharma (Turku, Finland). pcDNA3.1::Gαi1-YFP was generated by Dr. Scott Gibson (53) and obtained from Dr. Gregory Tall (University of Rochester School of Medicine and Dentistry, Rochester, NY). pVenus-N1 was obtained from Dr. Atsushi Miyawaki (Laboratory for Cell Function and Dynamics, Advanced Technology Development Center, Wako City, Saitama, Japan) (54). Venus is a variant of yellow fluorescent protein with mutations that optimize maturation and stability. FLAG-tagged and YFP-tagged μ-opioid receptor (μ-OR) cDNA constructs were kindly provided by Dr. Lakshmi A. Devi (Mount Sinai Medical Center, New York) (55). pcDNA3::GRK2-CT, which encodes amino acids Tyr466–Leu689 in the carboxyl terminus of GRK2, was kindly provided by Dr. Jeffrey Benovic (Thomas Jefferson University, Philadelphia, PA). pcDNA3::RGS4-C2S was kindly provided by Dr. Richard Neubig (University of Michigan, Ann Arbor, MI). The C2S mutation in RGS4 inhibits protein degradation by the proteosome (56). GPR mutants, Gαi1-G202T-YFP, Gαi1-Q204L-YFP, Gαi1-G184S-YFP, and RGS4-C2S-N128A were generated by site-directed mutagenesis using the QuikChange Site-directed Mutagenesis (Stratagene, La Jolla, CA). Rat AGS3, AGS3-Q/A, AGS3-SHORT, and AGS3-MYR-SHORT cDNAs were cloned into pNPY-Venus-N1 (54), pRLuc-C1 and pRLuc-N3 (57). All other reagents and materials were obtained as described elsewhere (50, 58, 59).
Cell Culture and Transfection
The human epithelial cell line HEK-293 was maintained in Dulbecco's minimal essential medium (high glucose, without phenol red) supplemented with 5% fetal bovine serum, 2 mm glutamine, 100 units/ml of penicillin, and 100 mg/ml of streptomycin. Cells were grown in a humidified incubator in the presence of 5% CO2 at 37 °C. For transfection, HEK-293 cells were seeded at a density of 8 × 105 cells/well on 6-well plates and cultured overnight at 37 °C. Cells were then transiently transfected using PEI (1 mg/ml in distilled H2O) at a DNA:PEI ratio of 1:4. Plasmid DNA and PEI were diluted, each in separate tubes, with 100 μl of serum-free medium. The PEI and DNA solutions were mixed, vortexed at maximum speed for 3–5 s, and incubated at room temperature for 15 min prior to addition to the cells. Cells were then incubated for 48 h and harvested for experiments. Extracts were processed for gel electrophoresis and immunoblotting as previously described (25).
Bioluminescence Resonance Energy Transfer (BRET)
AGS3 was tagged with Venus or RLuc at the carboxyl terminus and YFP or RLuc was inserted into the loop connecting helices αB and αC (residue 122) of Gαil (57, 59, 60). AGS3 tagged at the carboxyl terminus behaves similarly to untagged protein in terms of its co-immunoprecipitation with Gαi and its subcellular distribution (25, 58, 61, 62). Insertion of YFP or RLuc at residue 122 in Gαi or placement of Venus at the carboxyl terminus of α2A-AR does not alter the apparent functionality of the proteins (53, 57, 59, 60). A series of preliminary experiments were conducted to optimize the BRET system for AGS3-Gαi1 interactions and to provide robust internal controls to ensure the specificity of observed signals. All studies involved saturation BRET analysis, different donor/acceptor ratios, full spectrum scanning, and/or time course analysis. Forty-eight hours after transfection, the culture medium was removed and cells were washed once with phosphate-buffered saline and harvested with Tyrode's solution (140 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 0.37 mm NaH2PO4, 24 mm NaHCO3, 10 mm HEPES, pH 7.4, and 0.1% glucose (w/v)). Cells (1 × 105) were distributed in triplicate into gray 96-well optiplates. All fluorescence and luminescence signals were measured with the TriStar LB 941 plate reader (Berthold Technologies, Oak Ridge, TN). In individual BRET experiments, total fluorescence (excitation, 485 nm; emission, 535 nm) was first measured as an internal control for acceptor expression. Subsequently, the luciferase substrate coelenterazine H (5 μm final concentration) was added and luminescence was measured (donor, 480 ± 20; acceptor, 530 ± 20 nm). BRET signals were determined by calculating the 530 ± 20/480 ± 20 ratio of light intensity 2 min following addition of coelentrazine. Net BRET values were determined by subtracting the background signal detected from expression of the RLuc-tagged construct alone. Spectral measurement was performed with the protocol described above using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA).
AGS3 and Gαi Association with Cell Membrane
Cells were split into a 6-well plate and transfected with pRLuc::AGS3, pcDNA3::Gαi1-YFP, and/or pcDNA3::α2A/D-AR. 48 h later, cells were suspended in BRET buffer and incubated with the α2-AR agonist UK-14304 (10 μm) or vehicle for 5 min. Cells were then pelleted (200 × g, 5 min) and lysed in 1 ml of hypotonic lysis buffer (5 mm EDTA, 5 mm EGTA, 5 mm Tris-HCl, pH 7.4, and protease inhibitor mixture) per 6-well dish with a 26-guage syringe followed by centrifugation at 10,000 × g for 10 min to obtain crude membrane and cytosol fractions. UK-14304 or vehicle were maintained throughout cell lysis and centrifugation. Membranes were resuspended in 150 μl of membrane buffer (50 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, and protease inhibitor mixture). Membrane (50 μg of protein) and cytosol (50 μg of protein) samples from each group were diluted into 100 μl of BRET buffer. Total fluorescence (excitation, 485 nm; emission, 535 nm) was measured to determine the relative distribution of Gαi1-YFP. Luciferase substrate coelenterazine H (5 μm final concentration) was then added and luminescence was measured at 480 ± 20 nm to determine the relative distribution of AGS3-RLuc.
Data Analysis
BRET saturation curves were fitted using a nonlinear regression equation assuming a single binding site. Data were analyzed by analysis of variance and significant differences between groups determined by the Tukey post test using GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA). Data are expressed as mean ± S.E. unless indicated otherwise.
RESULTS
Interaction of AGS3 and Gαi in HEK-293 Cells
We developed an assay system with BRET to study the interaction between AGS3 and Gαi and its regulation in the cell. Co-expression of AGS3-RLuc and Gαi1-YFP in HEK-293 cells generated robust BRET as defined by the strong additional emission peak at 535 nm (Fig. 1A, left panel). The emission peak at 535 nm was not observed with the AGS3-Q/A variant, which contains a single amino acid substitution in each GPR motif (GPR1-Q488A, GPR2-Q541A, GPR3-Q589A, and GPR4-Q623A) that renders them incapable of binding Gα (Fig. 1A, left panel). Net BRET values were determined with a fixed amount of donor and increasing concentrations of the acceptor (Fig. 1A, right panel).4 When the BRET was measured using the reverse configuration for the energy donor and acceptor (AGS3-YFP/Gαi1-RLuc), a significantly lower BRET was observed (Fig. 1B). Both the AGS3-RLuc·Gαi1-YFP BRET and the AGS3-YFP·Gαi1-RLuc BRET were specific as BRET was not observed with the AGS3-Q/A variant that does not bind Gα (Fig. 1, A, right panel, and B).
FIGURE 1.
Interaction between AGS3 and Gαi as determined by BRET. A, interaction between AGS3-RLuc and Gαi1-YFP. Left panel, emission spectra for luminescence in HEK-293 cells transfected with pRLuc::AGS3 (10 ng) or pRLuc::AGS3-Q/A (10 ng) and pcDNA3::Gαi1-YFP (750 ng). Data presented are representative of 5–10 experiments. Right panel, HEK-293 cells were transfected with a fixed amount of pRLuc::AGS3 (10 ng) (n = 9) or pRLuc::AGS3-Q/A (n = 4) and increasing amounts of pcDNA3::Gαi1-YFP (0–750 ng) and processed for BRET measurements as described under “Experimental Procedures.” B, interaction of AGS3-Venus and Gαi1-RLuc. HEK-293 cells were transfected with pRLuc::Gαi1 (50 ng) and increasing amounts of pVenusN1::AGS3 (n = 3) or pVenusN1::AGS3-Q/A (0–500 ng) (n = 3) and processed for BRET measurements as described under “Experimental Procedures.” BRET saturation curves were fitted using a non-linear regression equation assuming a single binding site. Results are expressed as the mean ± S.E. of three independent experiments.
The AGS3-RLuc·Gαi1-YFP BRET was inhibited by co-expression of Gβγ (Fig. 2A), which competes with GPR domains for interaction with Gαi (63). The AGS3-RLuc·Gαil-YFP BRET was also markedly reduced by switch II mutations in Gαi1 (G202T or Q204L) further indicating the specificity of the interaction and the potential role of the guanine nucleotide binding and hydrolysis cycle in regulating the AGS3-Gαi interaction (Fig. 2B and supplemental Fig. S2). We next asked if the AGS3-RLuc·Gαil-YFP BRET involved Gαi positioned on any specific GPR motif within AGS3 by measuring BRET following disruption of individual GPR motifs with the Q/A substitution (14, 50). The magnitude of the AGS3-RLuc·Gαil-YFP BRET was generally related to the number of GPR motifs available for binding Gαi (Fig. 2C) indicating that AGS3 binds multiple Gαi (63, 64). However, the relative contributions of the individual GPR motifs to the overall AGS3-RLuc·Gαil-YFP BRET were not the same and disruption of the GPR motifs in various combinations differentially influenced the net BRET (see “Discussion”). The magnitude of AGS3-RLuc·Gαil-YFP BRET for constructs containing one functional GPR motif was similar to that observed for AGS3-Venus and Gαil-YFP (Figs. 1B and 2C).
FIGURE 2.
Interaction between AGS3-RLuc and Gαi1-YFP. A, HEK-293 cells were transfected with a fixed amount of pRLuc::AGS3 (10 ng) and increasing concentrations of pcDNA3::Gαi1-YFP (0–750 ng) in the absence and presence of pcDNA3::Gβ1 (500 ng) and pcDNA3::Gγ2 (500 ng) (n = 9). Inset, Gβ immunoblot. Each lane contains 50 μg of lysate protein. The fluorescent signal increased by 5–7-fold as the amount of pcDNA3::Gαi1-YFP increased from 15 to 750 ng. BRET saturation curves were fitted using a non-linear regression equation assuming a single binding site. B, net BRET signal generated from HEK-293 cells transfected with pRLuc::AGS3 and pcDNA3 containing Gαi1-YFP, Gαi1-G202T-YFP, or Gαi1-Q204L-YFP (750 ng) (n = 4). C, net BRET generated from HEK-293 cells expressing Gαi1-YFP (pcDNA3::Gαi1-YFP, 750 ng) and wild type AGS3-RLuc (WT, 10 ng) or AGS3-RLuc (10 ng) with single residue mutations in one to four of the GPR motifs (GPR1-Q488A, GPR2-Q541A, GPR3-Q589A, GPR4-Q623A) that disrupt Gαi binding to the individual GPR motif (n = 5–9). Expression of Gαi1-YFP was monitored in all experiments by fluorescence measurements (excitation, 485 nm; emission, 535 nm) with no significant difference in Gαi1-YFP expression among the different experimental groups. The net BRET signal observed with GPR1-Q/A was not significantly different from WT and GPR1–4-Q/A was not significantly different from any of the constructs containing three GPR-Q/A substitutions. A-C, *, p < 0.05 compared with GPR2-Q/A; **, p < 0.05 compared with GPR1,2-Q/A and GPR4-Q/A; #, p < 0.05 compared with GPR1,3-Q/A; ##, p < 0.05 compared with GPR2,4-Q/A; ***, p < 0.05 compared with GPR3,4-Q/A. WT, wild type.
Regulation of the Interaction of AGS3 and Gαi in HEK-293 Cells
We next asked if the interaction of a GPR protein with Gαi was regulated by a seven-transmembrane span receptor typically coupled to Gαiβγ or Gαoβγ. Activation of the α2A/D-adrenergic receptor (α2A/D-AR) by the agonist UK-14304 reduced AGS3-RLuc·Gαil-YFP BRET by 30–40% (Fig. 3 and supplemental Fig. S3). The agonist-mediated reduction in BRET was concentration dependent (Fig. 3C) (IC50 = 10 ± 0.1 nm) and a similar reduction was observed with the α2-AR agonists clonidine, guanabenz, dexmedetomidine, and epinephrine (Fig. 3D). The α2-AR antagonist rauwolscine did not alter AGS3-RLuc·Gαil-YFP BRET (Fig. 3D), but it inhibited the effect of UK-14304 (Fig. 3A). The agonist-induced reduction in AGS3-RLuc·Gαil-YFP BRET was also blocked by cell incubation with pertussis toxin (Fig. 3A), which ADP-ribosylates a cysteine residue four amino acids from the carboxyl terminus of Gαi1 eliminating effective coupling of the receptor and G-proteins of the Gi, Go, or Gt class. Notably, such toxin-mediated modification of the protein did not alter the interaction between AGS3-RLuc and Gαil-YFP as there was no change in the basal AGS3-RLuc·Gαil-YFP net BRET signal.
FIGURE 3.
Regulation of AGS3 and Gαi interaction by the α2A/D-AR. A, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng) and pcDNA3::Gαi1-YFP (750 ng) in the presence or absence of pcDNA3::α2A/D-AR (1 μg). Control (n = 8) and α2A/D-AR-transfected cells were treated with vehicle or UK-14304 (10 μm) (n = 22) with and without rauwolscine (100 μm, n = 6) for 13 min. Similar experiments were conducted with cells treated with 100 ng/ml of pertussis toxin (n = 6) for 16 h. Cells were then processed for BRET measurements as described under “Experimental Procedures.” *, p < 0.05 compared with basal; **, p < 0.05 compared with UK-14304 treatment. B, HEK-293 cells were transfected with a fixed amount of pRLuc::AGS3 (10 ng) and increasing concentrations of pcDNA3::Gαi1-YFP (0–750 ng) in the presence of pcDNA3::α2A/D-AR (1 μg) (n = 8). Cells were treated with vehicle or UK-14304 (10 μm) for 13 min and BRET measurements were performed. C and D, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng) and pcDNA3::Gαi1-YFP (750 ng) in the presence of pcDNA3::α2A/D-AR (1 μg). Cells were incubated with different concentrations of UK-14304 (n = 5) (C), with different α2-AR agonists (10 μm) or the α2-AR antagonist rauwolscine (100 μm) for 13 min (D), and BRET measurements were performed. D, *, p < 0.05 compared with vehicle (n = 5). A–D, results are expressed as the mean ± S.E. of 4 to 20 experiments.
The AGS3-RLuc·Gαil-YFP BRET was also regulated by activation of the μ-opioid receptor (μ-OR) and the α2B-AR, both of which couple to Gi/Go, but it was not altered by activation of the Gq-coupled M3-muscarinic receptor (M3-MR) (Fig. 4A). The inhibition of AGS3-RLuc·Gαil-YFP BRET by activation of the μ-OR with DAMGO was also dependent upon the concentration of agonist (Fig. 4B). Neither elevation of intracellular calcium with ionomycin nor activation of the adenylyl cyclase pathway by forskolin altered AGS3-RLuc·Gαi1-YFP BRET (Fig. 4C).
FIGURE 4.
Regulation of AGS3 and Gαi interaction by G-protein-coupled receptors and second messengers. A, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng) and pcDNA3::Gαi1-YFP (750 ng) in the presence of pcDNA3 (1 μg) (Control), pcDNA3::α2B-AR (1 μg) (n = 3), pcDNA3::μ-opioid receptor (1 μg) (n = 4), or pcDNA3::M3-MR (1.5 μg) (n = 3). Cells were incubated with agonist for 13 min prior to addition of coelenterazine H and processed for BRET measurements as described under “Experimental Procedures.” *, p < 0.05 compared with receptor-transfected cells without agonist treatment (Basal). B, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng), pcDNA3::Gαi-YFP (750 ng), and pcDNA3::μ-opioid receptor (1 μg) (n = 4). Cells were incubated with increasing concentrations of DAMGO for 13 min prior to addition of coelenterazine H, and the cells were processed for BRET measurements as described under “Experimental Procedures.” C, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng) and pcDNA3::Gαi1-YFP (750 ng) and treated with forskolin (10 μm) or ionomycin (1 μm) for 5 min (n = 4) to increase intracellular cAMP and calcium, respectively. Cells were then processed for BRET measurements as described under “Experimental Procedures.” Results are expressed as the mean ± S.E. of four independent experiments. D, HEK-293 cells were transfected with pRLuc::AGS3-SH (1 ng) or pRLuc::AGS3-MYR-SH (2 ng), pcDNA3::Gαi1-YFP (750 ng), and pcDNA3:: α2A/D-AR (1 μg). Cells were treated with vehicle or UK-14304 (10 μm) (n = 7) for 13 min and processed for BRET measurements. Results are expressed as the mean ± S.E. *, p < 0.05 compared with its vehicle treated.
AGS3 appears to oscillate between a membrane-bound and cytosolic distribution that is regulated by binding partners that interact with the TPR and/or GPR domains (25, 61, 62). We thus explored the role of the TPR domain and membrane localization in the interaction between AGS3-Gαil and its regulation by receptor activation. The agonist-induced reduction in AGS3-RLuc·Gαil-YFP BRET was independent of the TPR domain of AGS3 as it was also observed with AGS3-SHORT (Fig. 4D and supplemental Fig. S4), an AGS3 variant that lacks the TPR domain, but contains three GPR motifs (61). Insertion of a consensus myristoylation sequence at the amino terminus of AGS3-SHORT also stabilizes the protein at the cell cortex (50) and this was associated with an increase in basal AGS3-RLuc·Gαil-YFP BRET that was similarly regulated by activation of the α2A/D-AR (Fig. 4D and supplemental Fig. S4).
The population of AGS3-Gαil regulated by a seven-transmembrane span receptor likely resides at the cell cortex, but it is not clear if the receptor is actually coupling directly to an AGS3·Gαil complex or whether receptor coupling to endogenous Gαβγ leads to altered AGS3-Gαil interaction by direct action of Gα or Gβγ on the AGS3·Gαi1 complex or through specific signaling pathways. To begin to address these questions, we first asked if the receptor-mediated regulation of AGS3-RLuc·Gαil-YFP BRET was reversible.
The agonist-induced reduction in AGS3-RLuc·Gαil-YFP BRET was observed as early as 1–2 min following agonist exposure (Fig. 5A). The magnitude of net BRET reduction by agonist at 2 min was similar to that observed with 15 min of agonist incubation (Fig. 5A), and the effect of agonist was reversible. Following measurement of AGS3-RLuc·Gαil-YFP BRET after 2 min of agonist incubation (1 μm UK-14304), 10 μm rauwolscine was added to the same sample well containing agonist, and BRET measurements were repeated after 1 min incubation with rauwolscine. Addition of the receptor antagonist reversed the agonist-mediated inhibition of AGS3-RLuc·Gαil-YFP BRET (Fig. 5B).
FIGURE 5.
Influence of agonist incubation time on the regulation of AGS3-RLuc·Gαi1-YFP BRET and reversal of the agonist-induced reduction in AGS3-RLuc·Gαi1-YFP BRET. HEK-293 cells were processed for BRET assays as described in the legend to Fig. 3. In A, agonist and antagonist were added at the same time. B, reversal of agonist-mediated effects on AGS3-RLuc·Gαi1-YFP BRET by rauwolscine. BRET measurements were obtained twice from three sets of tubes. One set of tubes contained UK-14304 (1 μm). In a second set of tubes, UK-14304 (1 μm) and rauwolscine (10 μm) were added at the same time. In a third set of tubes, samples were first incubated with UK-14304 (1 μm) for 2 min and the first BRET measurement was performed and then rauwolscine (10 μm final) was added and the incubation continued for 1 min before the second BRET measurement. *, p < 0.05 compared with UK-14304 (n = 4). **, p < 0.05 compared with the initial BRET measurement for UK-14304 alone after a 2-min incubation with agonist (n = 4).
We next asked if the receptor-mediated regulation of AGS3-RLuc·Gαil-YFP BRET involved Gβγ or whether it was dependent upon nucleotide exchange and hydrolysis by Gαi. Neither basal nor receptor-mediated regulation of AGS3-RLuc·Gαil-YFP BRET was altered by co-expression of the carboxyl terminus of GRK2, which binds Gβγ and interferes with Gβγ interaction with effectors (Fig. 6, A–C, and supplemental Fig. S5). These data suggest that Gβγ itself is not directly involved in the receptor-mediated effects on the AGS3-Gαi interaction.
FIGURE 6.
Influence of GRK2-CT and RGS4 on basal and receptor-mediated regulation of AGS3-RLuc·Gαi1-YFP BRET. A, HEK-293 cells were transfected with a fixed amount of pRLuc::AGS3 (10 ng) and increasing concentrations of pcDNA3::Gαi1-YFP (0–750 ng) in the presence and absence of pcDNA3::RGS4-C2S (500 ng) or pcDNA3::GRK2-CT (500 ng) (n = 5). Cells were processed for BRET measurements as described under “Experimental Procedures.” B, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng), pcDNA3::α2A/D-AR (750 ng), and increasing concentrations of pcDNA3::Gαi1-YFP (0–750 ng) in the presence and absence of pcDNA3::RGS4-C2S (500 ng) or pcDNA3::GRK2-CT (500 ng) (n = 5). Cells were treated with vehicle or UK-14304 (10 μm) (n = 5) for 13 min and processed for BRET measurements. *, p < 0.05 compared with control. C, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng), pcDNA3::α2A/D-AR (750 ng), and pcDNA3::Gαi1-YFP (750 ng) or pcDNA3::Gαi1-YFP-G184S (750 ng) in the presence and absence of pcDNA3::GRK2-CT (500 ng), pcDNA3::RGS4-C2S (500 ng), or pcDNA3::RGS4-C2S-N128A (500 ng). Cells were treated with vehicle or UK-14304 (10 μm) for 13 min and processed for BRET measurements. Results are expressed as percent inhibition of net BRET by agonist. Results are expressed as the mean ± S.E. of 3–5 independent experiments. *, p < 0.05 compared with control. #, p < 0.05 compared with the pcDNA3::RGS4-C2S transfected group. Right panel, GRK2 and RGS4 immunoblot. Each lane contains 50 μg of lysate protein.
In contrast, co-expression of stabilized RGS4 (RGS4-C2S) inhibited the agonist-induced reduction in AGS3-RLuc·Gαil-YFP BRET (Fig. 6, B and C). RGS4-C2S did not alter basal AGS3-RLuc·Gαil-YFP BRET (Fig. 6A). The effect of RGS4 on the reduction in AGS3-RLuc·Gαil-YFP BRET observed in the presence of agonist was dependent upon its GAP activity as it was not observed with a GAP-deficient RGS4 mutant (N128A) or with Gαil-YFP rendered insensitive to RGS4 by the G184S substitution (65–67) (Fig. 6C and supplemental Fig. S5). These data indicate that nucleotide exchange and hydrolysis play a role in the agonist-induced reduction in AGS3-RLuc·Gαil-YFP BRET (Fig. 6, B and C).
Interaction of AGS3 and Gαi1 in a Receptor Signaling Complex
We next asked if the AGS3·Gαil complex is in spatial proximity to the receptor and if receptor activation altered the subcellular distribution of AGS3 or Gαil. Co-expression of AGS3-RLuc and α2A-AR-Venus did not exhibit any apparent specific BRET (Fig. 7A). However, co-expression of Gαi1 or Gαi3 revealed specific BRET between AGS3-RLuc and α2A-AR-Venus (Fig. 7, A–C). The AGS3-RLuc·α2A-AR-Venus BRET was dependent upon the amount of co-expressed Gαi1, it was inhibited by receptor activation with the agonist UK-14304 and the effect of agonist was blocked by pertussis toxin treatment (Fig. 7B and supplemental Fig. S6). The AGS3-RLuc·α2A-AR-Venus BRET was not observed with co-expression of Gαi3-G202T or Gαi3-Q204L (Fig. 7C), which is consistent with the minimal BRET observed for AGS3-RLuc·Gαi1-G202T-YFP or -Gαi1-Q204L-YFP (Fig. 2B) further confirming the specificity of the observed signals. The α2-AR antagonist rauwolscine reversed the ongoing effect of agonist on Gαi1-dependent AGS3-RLuc·α2A-AR-Venus BRET (Fig. 7D). The AGS3-RLuc·α2A-AR-Venus BRET was dependent upon interaction of AGS3 with Gαi, as it was not observed with the AGS3-Q/A-RLuc variant (Fig. 8). Gαi-dependent BRET was also observed when AGS3-RLuc was co-expressed with the YFP-tagged α2B-adrenergic and μ-opioid receptors and the BRET was reduced upon agonist treatment (Fig. 8). The differences in the magnitude of AGS3-receptor net BRET for the α2A-AR, α2B-AR, and μ-opioid receptors likely reflect varying spatial orientations of energy acceptor and donor within a signaling complex. This series of experiments involved similar expression levels of the different receptors as determined by relative fluorescent units (supplemental Fig. S7). A lower level of Gαi-dependent BRET was observed upon co-expression of the β2-AR-Venus and AGS3-RLuc, but the signal was not regulated by the β-AR agonist isoproterenol (Fig. 8).
FIGURE 7.
Gαi-dependent and agonist-sensitive interaction between AGS3 and the α2-adrenergic receptor. A, HEK-293 cells were transfected with a fixed amount of pRLuc::AGS3 (10 ng) and increasing amounts of α2A-AR-Venus plasmid (0–750 ng) in the absence or presence of pcDNA3::Gαi1 (1 μg). Cells were incubated with vehicle or UK-14304 (10 μm) and BRET assays were performed (n = 5) as described under “Experimental Procedures.” Cells were exposed to agonist for 2 min. B, HEK-293 cells were transfected with pRLuc::AGS3 (10 ng) and α2A-AR-Venus plasmid (750 ng) with increasing amounts of pcDNA3::Gαi1 (0–1 μg). One group of cells was incubated with pertussis toxin (100 ng/ml) for 16 h before the experiment. Control cells (n = 3) and pertussis toxin-pretreated cells (n = 3) were incubated with UK-14304 (10 μm) and BRET assays were performed. Cells were exposed to agonist for 2 min. Results are expressed as the mean ± S.E. *, p < 0.05 compared with vehicle for each amount of pcDNA3::Gαi1. **, p < 0.05 compared with UK-14304 treated for each amount of pcDNA3::Gαi1. C, net BRET signal generated from HEK-293 cells transfected with pRLuc::AGS3 (10 ng), α2A-AR-Venus (250 ng), and pcDNA3::Gαi3, pcDNA3::Gαi3-G202T, or pcDNA3::Gαi3-Q204L (1 μg) (n = 5). Inset, Gαi3 immunoblot. Each lane contains 50 μg of lysate protein. D, reversal of agonist-mediated effects on Gαi-dependent AGS3-RLuc·α2A-AR-Venus BRET by rauwolscine. Cells were transfected with pRLuc::AGS3 (10 ng), 250 ng of α2A-AR-Venus plasmid, and 1 μg of pcDNA3::Gαi1. BRET measurements were obtained twice from three sets of tubes. One set of tubes contained UK-14304 (1 μm). In a second set of tubes, UK-14304 (1 μm) and rauwolscine (10 μm) were added at the same time. In a third set of tubes, samples were first incubated with UK-14304 (1 μm) for 2 min and the first BRET measurement was performed and then rauwolscine (10 μm final) was added and the incubation continued for 1 min before the second BRET measurement. *, p < 0.05 compared with UK-14304 (n = 4). **, p < 0.05 compared with the initial BRET measurement for UK-14304 alone after a 2-min incubation with agonist (n = 4).
FIGURE 8.
AGS3-RLuc interaction with G-protein-coupled receptors. HEK-293 cells were transfected with pRLuc::AGS3-Q/A (10 ng) and α2A-AR-Venus plasmid (250 ng) or pRLuc::AGS3 (10 ng) and α2A-AR-Venus plasmid (50 ng), pEYFP-N1::α2B-AR (750 ng), pEYFP-N1::μ-OR (750 ng), or β2-AR-Venus plasmid (1 μg) in the presence and absence of pcDNA3::Gαi1 (750 ng) (n = 4). pcDNA3::Gαi1 (750 ng)-transfected cells were also incubated with agonist for 2 min (UK-14304, 10 μm for α2A-AR-Venus and α2B-AR-YFP; DAMGO, 10 μm for μ-OR-YFP; isoproterenol, 100 μm for β2-AR-Venus). BRET assays were performed as described under “Experimental Procedures.” *, **, and ***, p < 0.05 compared with the corresponding vehicle control (n = 4).
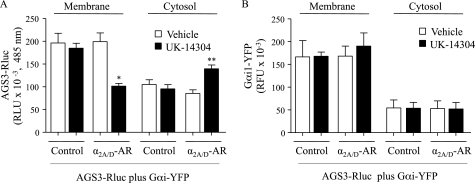
Thus, it appears as if the receptor-mediated regulation of the AGS3-Gαi module occurs at the cell cortex. However, it is not known if the agonist-induced reduction in AGS3-RLuc·Gαil-YFP BRET involves conformational changes within an AGS3·Gαi complex that alter the spatial relationship between energy acceptor and donor or whether there is dissociation of AGS3 and Gαi. As a first step to address this issue, we determined the effect of receptor activation on the relative distribution of AGS3-RLuc and Gαi1-YFP in membrane and cytosol fractions. Cells were incubated with vehicle or agonist and then lysed and centrifuged to obtain crude membrane and cytosol fractions. Receptor activation decreased the amount of AGS3-RLuc in the membrane fraction and increased its presence in the cytosol fraction (Fig. 9A). The agonist-induced redistribution of AGS3-GFP from the cell cortex was also observed by fluorescent microscopy, and this redistribution was also reversed by the antagonist rauwolscine.5 In contrast, receptor activation did not alter the distribution of Gαi1-YFP in the membrane or cytosol fractions (Fig. 9B). These data indicate that upon receptor activation, AGS3 and Gαi physically dissociate with AGS3 released into the cytosol and Gαi retained at the cell cortex. This observation and the reversibility of the agonist-induced effects upon addition of antagonist (Fig. 5B) also indicate that the agonist-induced reduction of AGS3-RLuc·Gαil-YFP BRET is not likely the result of displacement of Gαil-YFP bound to AGS3 by endogenous non-YFP-tagged Gαi/αo.
FIGURE 9.
Subcellular distribution of AGS3-RLuc and Gαi1-YFP. HEK-293 cells were transfected with pRLuc::AGS3 (10 ng), pcDNA3::Gαi1-YFP (750 ng), and/or pcDNA3::α2A/D-AR (1 μg). Cells were incubated with vehicle or UK-14304 (10 μm) and lysed to obtain membrane and cytosol fractions. Luminescence (A) and fluorescence (B) were measured in samples containing 50 μg of protein as described under “Experimental Procedures.” Results are expressed as the mean ± S.E. of three independent experiments. * and **, p < 0.05 compared with corresponding vehicle control. RLU, relative luminescence units. RFU, relative fluorescent units (excitation, 485 nm; emission, 535 nm).
DISCUSSION
The regulatory mechanisms operational with AGS proteins revealed unexpected diversity in the “G-switch” signaling mechanism expanding the functional roles of G-protein subunits and opening up new avenues for therapeutic manipulation of G-protein signaling. However, we do not fully understand the mechanisms of signal input to AGS proteins and what may be “downstream” of Gα and Gβγ functioning in this context. The current manuscript presents a platform to address these questions for AGS3 and other Group II AGS proteins and indicates that the AGS3-Gαi signaling cassette is part of a larger signaling complex regulated by a cell-surface seven-transmembrane span receptor.
Interaction of AGS3 and Gαi
The interaction of AGS3 with Gαi as measured by BRET is specific as it was not observed with AGS3 mutants incapable of binding Gαi and the BRET is sensitive to spatial positioning of the acceptor and donor. The magnitude of the AGS3-RLuc·Gαi1-YFP net BRET is at least 10 times larger than the BRET observed when AGS3 was tagged at the carboxyl terminus with eGFP, YFP, or Venus and co-expressed with Gαi1-RLuc (luciferase inserted at residue 122). The larger net BRET signal generated for AGS3-Gαi1 interactions when AGS3 served as the donor (AGS3-RLuc and Gαi-YFP) as compared with the signal observed with Gαi1 as the donor (Gαi1-RLuc and AGS3-Venus) likely reflects the docking of multiple (up to 4) acceptors (Gαi1-YFP) in the vicinity of one donor molecule (AGS3-RLuc) (63, 64). Indeed, the donor/acceptor ratio required to achieve 50% of the maximal BRET signal was significantly left shifted for AGS3-YFP·Gαi1-RLuc versus AGS3-RLuc·Gαi1-YFP and the magnitude of the BRET signal obtained when Gαi1 served as the donor (Gαi1-RLuc and AGS3-Venus) was similar to that obtained for AGS3-RLuc and Gαi1-YFP when the AGS3-RLuc donor contained only one intact GPR motif (Figs. 1B and 2C). These observations are consistent with the interpretation that multiple acceptors bound to the GPR motifs (Gαi1-YFP) receive energy from AGS3-RLuc.
Further analysis of the role of individual GPR motifs in AGS3 indicated that the magnitude of the AGS3-RLuc·Gαi1-YFP BRET was generally proportional to the number of GPR motifs, but that the relative contributions of specific motifs were not equal. Among the four GPR motifs, elimination of G-protein binding to GPR3 or GPR4 alone elicited the largest reductions in AGS3-RLuc·Gαil-YFP BRET. However, neither the third nor the fourth GPR motif alone was sufficient to support robust BRET to Gαi as reflected in the minimal AGS3-RLuc·Gαil-YFP BRET observed for AGS3-RLuc containing the Q/A mutation in GPR1–3 or GPR1,2,4 (Fig. 2C). Furthermore, there are likely complex interactions among the four GPR motifs and the bound Gαi that cooperatively coordinate conformational adaptations within the complex (68, 69). For example, disruption of GPR1 alone did not alter AGS3-RLuc·Gαil-YFP BRET. However, when the Q/A mutation is introduced into GPR2, GPR3, or GPR4, then disruption of GPR1 reduces AGS3-RLuc·Gαil-YFP BRET. Overall, these data are consistent with the hypothesis that the sequential binding of Gαi to AGS3 elicits conformational changes in AGS3 and that the spatial orientation of acceptor and donor is dependent upon the number of Gαi bound to AGS3.
Regulation of AGS3-Gαi by a Seven-transmembrane Span Receptor
Our data suggest that the interaction of Gαi with AGS3 stabilizes the presence of AGS3 at the cell cortex as part of a larger signaling complex and that activation of specific cell-surface receptors alter this signaling complex such that AGS3 dissociates from Gαi and the cell cortex. A similar regulatory mechanism may be operative for the GPR-containing protein AGS4 (70). Gαi remains at the cell membrane ready to rebind AGS3 once receptor stimulation is terminated. Indeed, the agonist-induced reduction in AGS3-RLuc·Gαil-YFP BRET is immediately reversed by the antagonist rauwolscine. The AGS3 “released” from the putative signaling complex following receptor activation may engage other signaling proteins in the cytosol and the Gαi retained at the cell membrane would be available for engaging effector or entering into a cycle of receptor-G-protein coupling. Receptor activation thus likely regulates AGS3-RLuc·Gαil-YFP positioned at the cell cortex and accessible to the receptor rather than any AGS3-RLuc·Gαil-YFP that may exist in the cytosol. It is not clear if the AGS3-RLuc·Gαil-YFP BRET is the reflection of a stable complex or a complex where there is a certain, steady state, dynamic rate of association and dissociation of AGS3 and Gαil that involves a basal nucleotide exchange and hydrolysis cycle.
The receptor may directly couple to the AGS3-Gαi module and act as a guanine nucleotide exchange factor in a manner analogous to receptor-mediated activation of Gαβγ. Alternatively, the agonist-induced regulation of the AGS3-Gαi interaction may result from receptor coupling to heterotrimeric Gαiβγ. A third possibility is that the receptor couples to both AGS3-Gαi and heterotrimeric Gαβγ within a larger signaling complex. It is also not clear as to whether heterotrimeric Gαiβγ and AGS3-Gαi define separate pools of Gαi within the cell or if there is dynamic equilibrium among heterotrimeric Gαiβγ and AGS-Gαi within a common pool of G-protein subunits that is adjusted to a new set point upon receptor activation.
Sequestration of Gβγ by GRK-CT suggests that Gβγ is not a key player in the agonist-induced regulation of the AGS3-Gαi interaction. However, the effect of RGS4 to inhibit agonist-mediated effects on AGS3-RLuc·Gαil-YFP BRET certainly implicates the Gα activation-deactivation cycle (nucleotide exchange and hydrolysis) in the regulatory process. How the Gα activation-deactivation cycle is involved in this regulation is not clear.
Three hypotheses present themselves. Agonist activation of receptor coupling to Gαβγ could initiate a signaling cascade through Gαi that results in altered AGS3-Gαi interaction. In this situation, signal termination may be accelerated by RGS4 reducing the impact of agonist on AGS3-RLuc-Gαil-YFP BRET. Alternatively, agonist activation of receptor coupling to AGS3-RLuc·GαiGDP-YFP would initiate nucleotide exchange resulting in the generation of GαiGTP-YFP and dissociation of AGS3-RLuc from Gαi1-YFP accounting for the reduction in AGS3-RLuc·Gαil-YFP BRET and the release of AGS3-RLuc from the membrane. The reduction of the AGS3-RLuc·Gαil-YFP BRET following agonist activation of receptor coupling to AGS3-RLuc·GαiGDP-YFP may actually involve an altered steady state that is regulated by the rate at which bound GTP is hydrolyzed to form GαiGDP-YFP that could re-associate with AGS3-RLuc. Introduction of RGS4 would accelerate nucleotide hydrolysis and the formation of GαiGDP-YFP for subsequent “re-association” with AGS3-RLuc resulting in an apparent reduction in the effect of agonist on AGS3-RLuc·Gαil-YFP BRET. A third hypothesis postulates that there is a dynamic equilibrium between heterotrimeric Gαiβγ and AGS3-Gαi with a common pool of G-protein subunits. Agonist-induced coupling of the receptor to Gαβγ would adjust this equilibrium such that the binding of GαilGDP-YFP to AGS3-RLuc is reduced. Introduction of RGS4 would accelerate nucleotide hydrolysis altering the equilibrium such that more GαiGDP-YFP is bound to AGS3-RLuc accounting for the reduced effect of agonist on AGS3-RLuc·Gαil-YFP BRET.
Our data do not allow us to conclude if the Gα-dependent BRET observed for AGS3 and a cell-surface receptor actually reflects direct binding of the receptor to the AGS3-Gαi module as opposed to simply existing in the same microenvironment. If the receptor directly couples to AGS3-Gαi, then as the effect of receptor activation on AGS3-RLuc·Gαil-YFP BRET is pertussis toxin sensitive, the conformational mechanism by which an activated receptor leads to activation of an AGS3·Gαi complex is postulated to be similar to that by which a receptor activates a Gαβγ complex. However, although Gβγ and GPR motifs both stabilize the GDP-bound conformation of Gα, it is not known if Gαi complexed with AGS3 is a substrate for pertussis toxin as is the case when it is complexed with Gβγ (71). The direct coupling of a G-protein-coupled receptor to a GPR·Gαi complex would have broad implications for signal diversity and the development of “pathway targeted” ligands (72) as suggested for receptor systems coupling to β-arrestin and Gαβγ (73). Receptor coupling to Gαβγ, GPR-Gαi, and β-arrestin offers substantial flexibility for systems to adapt to physiological and pathological challenges and orchestrate complex behaviors.
Supplementary Material
Acknowledgments
We thank Heather Bainbridge for technical assistance. We thank Dr. Thomas W. Gettys (Pennington Biomedical Research Center, Baton Rouge, LA) for Gαi3 antisera, Drs. Guangyu Wu and Chumin Dong for the YFP-tagged α2B-adrenergic receptor, Dr. Gregory G. Tall (University of Rochester School of Medicine and Dentistry, Rochester, NY) for pcDNA3.1::Gαi1-YFP, Dr. Atsushi Miyawaki (Laboratory for Cell Function and Dynamics, Saitama, Japan) for pNPY-Venus-N1, Dr. John D. Hildebrandt (Medical University of South Carolina, Charleston, SC) for Gβ-antisera, Dr. Ron Taussig (University of Texas, Southwestern Medical Center, Dallas, TX) for RGS4 antisera, Dr. Richard Neubig (University of Michigan, Ann Arbor, MI) for pcDNA3::RGS4-C2S, Dr. Jeffrey Benovic (Thomas Jefferson University, Philadelphia, PA) for pcDNA3::GRK2-CT, Dr. Zach Ma (University of California, Santa Barbara) for AGS3 antisera, and Dr. Lakshmi A. Devi (Mount Sinai Medical Center, New York) for FLAG-tagged and YFP-tagged μ-opioid receptors. We thank Dr. Andrew Gelasco (Medical University of South Carolina) for assistance with the initial measurement of luminescence spectra.
This work was supported, in whole or in part, by National Institutes of Health Grants NS24821 (to S. M. L.), DA025896 (to S. M. L.), GM086510 (to J. B. B.) and a grant from the Canadian Institutes of Health Research (to M. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
AGS3-RLuc expression levels were ∼20–30% of endogenous AGS3, and AGS3-RLuc·Gαi-YFP BRET is observed at levels of Gαi1-YFP expression that are comparable with endogenous Gαi1/2 (supplemental Fig. S1). Our experience indicates that the level of transfected GPCRs achieved with such transfection procedures are generally 5–20 times higher than the levels of endogenous receptor depending upon the cell type, the properties of the specific receptor, and the amount of plasmid transfected.
S. Oner and S. M. Lanier, manuscript in preparation.
- GPR
- G-protein regulatory motif
- RGS
- regulator of G-protein signaling
- BRET
- bioluminescence resonance energy transfer
- RLuc
- Renilla luciferase
- μ-OR
- μ-opioid receptor.
REFERENCES
- 1.Sato M., Blumer J. B., Simon V., Lanier S. M. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 151–187 [DOI] [PubMed] [Google Scholar]
- 2.Blumer J. B., Smrcka A. V., Lanier S. M. (2007) Pharmacol. Ther. 113, 488–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takesono A., Cismowski M. J., Ribas C., Bernard M., Chung P., Hazard S., 3rd, Duzic E., Lanier S. M. (1999) J. Biol. Chem. 274, 33202–33205 [DOI] [PubMed] [Google Scholar]
- 4.Cismowski M. J., Takesono A., Ma C., Lizano J. S., Xie X., Fuernkranz H., Lanier S. M., Duzic E. (1999) Nat. Biotechnol. 17, 878–883 [DOI] [PubMed] [Google Scholar]
- 5.Sato M., Cismowski M. J., Toyota E., Smrcka A. V., Lucchesi P. A., Chilian W. M., Lanier S. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao X., Cismowski M. J., Sato M., Blumer J. B., Lanier S. M. (2004) J. Biol. Chem. 279, 27567–27574 [DOI] [PubMed] [Google Scholar]
- 7.De Vries L., Fischer T., Tronchère H., Brothers G. M., Strockbine B., Siderovski D. P., Farquhar M. G. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 14364–14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Q., Stukenberg P. T., Macara I. G. (2001) Nat. Cell Biol. 3, 1069–1075 [DOI] [PubMed] [Google Scholar]
- 9.Gotta M., Ahringer J. (2001) Nat. Cell Biol. 3, 297–300 [DOI] [PubMed] [Google Scholar]
- 10.Willard F. S., Kimple R. J., Siderovski D. P. (2004) Annu. Rev. Biochem. 73, 925–951 [DOI] [PubMed] [Google Scholar]
- 11.Yu F., Morin X., Cai Y., Yang X., Chia W. (2000) Cell 100, 399–409 [DOI] [PubMed] [Google Scholar]
- 12.Schaefer M., Shevchenko A., Knoblich J. A. (2000) Curr. Biol. 10, 353–362 [DOI] [PubMed] [Google Scholar]
- 13.Weiss T. S., Chamberlain C. E., Takeda T., Lin P., Hahn K. M., Farquhar M. G. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14961–14966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson Y. K., Bernard M. L., Ma H., Hazard S., 3rd, Graber S. G., Lanier S. M. (2000) J. Biol. Chem. 275, 33193–33196 [DOI] [PubMed] [Google Scholar]
- 15.Pace A. M., Wong Y. H., Bourne H. R. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 7031–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natochin M., Lester B., Peterson Y. K., Bernard M. L., Lanier S. M., Artemyev N. O. (2000) J. Biol. Chem. 275, 40981–40985 [DOI] [PubMed] [Google Scholar]
- 17.Kimple R. J., De Vries L., Tronchère H., Behe C. I., Morris R. A., Gist Farquhar M., Siderovski D. P. (2001) J. Biol. Chem. 276, 29275–29281 [DOI] [PubMed] [Google Scholar]
- 18.Kimple R. J., Kimple M. E., Betts L., Sondek J., Siderovski D. P. (2002) Nature 416, 878–881 [DOI] [PubMed] [Google Scholar]
- 19.Sachdev P., Menon S., Kastner D. B., Chuang J. Z., Yeh T. Y., Conde C., Caceres A., Sung C. H., Sakmar T. P. (2007) EMBO J. 26, 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C., Sato M., Lanier S. M., Smrcka A. V. (2007) J. Biol. Chem. 282, 19938–19947 [DOI] [PubMed] [Google Scholar]
- 21.Lee M. J., Dohlman H. G. (2008) Curr. Biol. 18, 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Marcos M., Ghosh P., Farquhar M. G. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3178–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tall G. G., Krumins A. M., Gilman A. G. (2003) J. Biol. Chem. 278, 8356–8362 [DOI] [PubMed] [Google Scholar]
- 24.Siderovski D. P., Diversé-Pierluissi M., De Vries L. (1999) Trends Biochem. Sci. 24, 340–341 [DOI] [PubMed] [Google Scholar]
- 25.An N., Blumer J. B., Bernard M. L., Lanier S. M. (2008) J. Biol. Chem. 283, 24718–24728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston C. A., Hirono K., Prehoda K. E., Doe C. Q. (2009) Cell 138, 1150–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas C. J., Tall G. G., Adhikari A., Sprang S. R. (2008) J. Biol. Chem. 283, 23150–23160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopein D., Katanaev V. L. (2009) Mol. Biol. Cell 20, 3865–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Q., Taylor L., Compton D. A., Macara I. G. (2002) Curr. Biol. 12, 1928–1933 [DOI] [PubMed] [Google Scholar]
- 30.Bowman S. K., Neumüller R. A., Novatchkova M., Du Q., Knoblich J. A. (2006) Dev. Cell 10, 731–742 [DOI] [PubMed] [Google Scholar]
- 31.Blumer J. B., Bernard M. L., Peterson Y. K., Nezu J., Chung P., Dunican D. J., Knoblich J. A., Lanier S. M. (2003) J. Biol. Chem. 278, 23217–23220 [DOI] [PubMed] [Google Scholar]
- 32.Wiser O., Qian X., Ehlers M., Ja W. W., Roberts R. W., Reuveny E., Jan Y. N., Jan L. Y. (2006) Neuron 50, 561–573 [DOI] [PubMed] [Google Scholar]
- 33.Willard F. S., Zheng Z., Guo J., Digby G. J., Kimple A. J., Conley J. M., Johnston C. A., Bosch D., Willard M. D., Watts V. J., Lambert N. A., Ikeda S. R., Du Q., Siderovski D. P. (2008) J. Biol. Chem. 283, 36698–36710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaefer M., Petronczki M., Dorner D., Forte M., Knoblich J. A. (2001) Cell 107, 183–194 [DOI] [PubMed] [Google Scholar]
- 35.Sanada K., Tsai L. H. (2005) Cell 122, 119–131 [DOI] [PubMed] [Google Scholar]
- 36.Zheng Z., Zhu H., Wan Q., Liu J., Xiao Z., Siderovski D. P., Du Q. (2010) J. Cell Biol. 189, 275–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechler T., Fuchs E. (2005) Nature 437, 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess H. A., Röper J. C., Grill S. W., Koelle M. R. (2004) Cell 119, 209–218 [DOI] [PubMed] [Google Scholar]
- 39.Bowers M. S., Hopf F. W., Chou J. K., Guillory A. M., Chang S. J., Janak P. H., Bonci A., Diamond I. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12533–12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao L., McFarland K., Fan P., Jiang Z., Ueda T., Diamond I. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7877–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groves B., Gong Q., Xu Z., Huntsman C., Nguyen C., Li D., Ma D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 18103–18108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumer J. B., Lord K., Saunders T. L., Pacchioni A., Black C., Lazartigues E., Varner K. J., Gettys T. W., Lanier S. M. (2008) Endocrinology 149, 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattingre S., De Vries L., Bauvy C., Chantret I., Cluzeaud F., Ogier-Denis E., Vandewalle A., Codogno P. (2003) J. Biol. Chem. 278, 20995–21002 [DOI] [PubMed] [Google Scholar]
- 44.Groves B., Abrahamsen H., Clingan H., Frantz M., Mavor L., Bailey J., Ma D. (2010) PLoS One 5, e8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadella R. B., Jr., Iia G., Kwon M., Akbulut T., Qian F., Sedlic F., Wakatsuki T., Sweeney W. E., Wilson P. D., Lanier S. M., Park F. (2010) J. Am. Soc. Nephrol. 8, 1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gotta M., Dong Y., Peterson Y. K., Lanier S. M., Ahringer J. (2003) Curr. Biol. 13, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 47.Schaefer M., Knoblich J. A. (2001) Exp. Cell Res. 271, 66–74 [DOI] [PubMed] [Google Scholar]
- 48.Fan P., Jiang Z., Diamond I., Yao L. (2009) Mol. Pharmacol. 76, 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao L., McFarland K., Fan P., Jiang Z., Inoue Y., Diamond I. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 8746–8751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato M., Gettys T. W., Lanier S. M. (2004) J. Biol. Chem. 279, 13375–13382 [DOI] [PubMed] [Google Scholar]
- 51.Ma H., Peterson Y. K., Bernard M. L., Lanier S. M., Graber S. G. (2003) Biochemistry 42, 8085–8093 [DOI] [PubMed] [Google Scholar]
- 52.Jahangeer S., Rodbell M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 8782–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson S. K., Gilman A. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. (2002) Nat. Biotechnol. 20, 87–90 [DOI] [PubMed] [Google Scholar]
- 55.Rios C., Gomes I., Devi L. A. (2006) Br. J. Pharmacol. 148, 387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bodenstein J., Sunahara R. K., Neubig R. R. (2007) Mol. Pharmacol. 71, 1040–1050 [DOI] [PubMed] [Google Scholar]
- 57.Galés C., Rebois R. V., Hogue M., Trieu P., Breit A., Hébert T. E., Bouvier M. (2005) Nat. Methods 2, 177–184 [DOI] [PubMed] [Google Scholar]
- 58.Blumer J. B., Chandler L. J., Lanier S. M. (2002) J. Biol. Chem. 277, 15897–15903 [DOI] [PubMed] [Google Scholar]
- 59.Galés C., Van Durm J. J., Schaak S., Pontier S., Percherancier Y., Audet M., Paris H., Bouvier M. (2006) Nat. Struct. Mol. Biol. 13, 778–786 [DOI] [PubMed] [Google Scholar]
- 60.Audet N., Galés C., Archer-Lahlou E., Vallières M., Schiller P. W., Bouvier M., Pineyro G. (2008) J. Biol. Chem. 283, 15078–15088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pizzinat N., Takesono A., Lanier S. M. (2001) J. Biol. Chem. 276, 16601–16610 [DOI] [PubMed] [Google Scholar]
- 62.Vural A., Oner S., An N., Simon V., Ma D., Blumer J. B., Lanier S. M. (2010) Mol. Cell Biol. 30, 1528–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernard M. L., Peterson Y. K., Chung P., Jourdan J., Lanier S. M. (2001) J. Biol. Chem. 276, 1585–1593 [DOI] [PubMed] [Google Scholar]
- 64.Adhikari A., Sprang S. R. (2003) J. Biol. Chem. 278, 51825–51832 [DOI] [PubMed] [Google Scholar]
- 65.Lan K. L., Sarvazyan N. A., Taussig R., Mackenzie R. G., DiBello P. R., Dohlman H. G., Neubig R. R. (1998) J. Biol. Chem. 273, 12794–12797 [DOI] [PubMed] [Google Scholar]
- 66.DiBello P. R., Garrison T. R., Apanovitch D. M., Hoffman G., Shuey D. J., Mason K., Cockett M. I., Dohlman H. G. (1998) J. Biol. Chem. 273, 5780–5784 [DOI] [PubMed] [Google Scholar]
- 67.Srinivasa S. P., Watson N., Overton M. C., Blumer K. J. (1998) J. Biol. Chem. 273, 1529–1533 [DOI] [PubMed] [Google Scholar]
- 68.Du Q., Macara I. G. (2004) Cell 119, 503–516 [DOI] [PubMed] [Google Scholar]
- 69.Nipper R. W., Siller K. H., Smith N. R., Doe C. Q., Prehoda K. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14306–14311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oner S. S., Maher E. M., Breton B., Bouvier M., Blumer J. B. (2010) J. Biol. Chem. 285, 20588–20594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casey P. J., Graziano M. P., Gilman A. G. (1989) Biochemistry 28, 611–616 [DOI] [PubMed] [Google Scholar]
- 72.Kenakin T. (2007) Mol. Pharmacol. 72, 1393–1401 [DOI] [PubMed] [Google Scholar]
- 73.Gesty-Palmer D., Flannery P., Yuan L., Corsino L., Spurney R., Lefkowitz R. J., Luttrell L. M. (2009) Sci. Transl. Med. 1, 1ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.