Abstract
Sixteen episodes of cytomegalovirus (CMV) disease occurred in 10 of 41 children undergoing intestinal transplantation from 1990 to 1995. Stratification of CMV disease by donor (D)/recipient (R) serological status was as follows: 3 of 8, D+/R−; 3 of 9, D+/R+; 4 of 9, D−/R+; and 0 of 15, D−/R−. Treatment resulted in resolution of CMV disease in 93.3% of episodes. No deaths attributable to CMV disease occurred in this series. CMV in D+/R− children resulted in more extensive and persistent disease. However, patient and graft survival rates were similar in the different D/R subgroups and between children with and without CMV disease. Cumulative dose of steroid boluses (relative risk [RR]. 1.59; 95% confidence interval [CI]. 1.14–2.21) and history of steroid recycles (RR, 2.72; 95% CI, 1.21–6.13) were associated with CMV disease. These results suggest that although CMV-associated morbidity in pediatric intestinal transplant recipients was substantial, it was not associated with an increased rate of mortality or graft loss, even among high-risk D+/R− patients.
Although the intestine was one of the first organs to be transplanted experimentally, the clinical evolution of intestinal transplantation has remained hindered by technical, immunologic, and infectious complications [1–3]. Recently, with advances in surgical and clinical management and the use of tacrolimus (FK-506) as the principal immunosuppressive drug, actuarial patient and graft survivals at 24 months post-transplantation have exceeded 74% and 63%, respectively [4]. Infections, however, continue to be a significant cause of morbidity.
We have previously reported high rates of late mortality and graft loss due to cytomegalovirus (CMV) infection in our overall experience with adult and pediatric intestinal transplant recipients [5]. The occurrence of CMV infection following intestinal transplantation in children, however, may have differing epidemiological and behavioral characteristics, as has been seen with other organ recipients. A retrospective analysis of our experience with CMV after intestinal transplantation in children is the basis of this report.
Materials and Methods
The medical records of children who received either isolated small bowel (SB), liver-small bowel (L-SB), or multivisceral transplants between 1990 and 1995 at the Children’s Hospital of Pittsburgh were systematically reviewed, and data were collected regarding clinical course, virological studies, biopsy results, immunosuppression, and outcome, with use of standardized definitions. The donor and recipient surgical procedures were as previously described [6].
Immunosuppressive regimens and graft surveillance
Immunosuppressive regimens varied slightly over the study period and have been reported elsewhere [7]. All children received a combination of tacrolimus and steroids. In addition, all but the first eight intestinal graft recipients received prostaglandin E1 until intravenous tacrolimus was withdrawn. A low dose of azathioprine was added for selected patients, in cases of tacrolimus-induced renal toxicity or to enhance baseline immunosuppression in cases of recurrent rejection. Suspected or proven rejection was initially treated with bolus steroids and optimization of tacrolimus levels. A steroid recycle was used in cases of more severe rejection or when bolus therapy was inadequate. OKT3 was used for steroid refractory rejection.
Surveillance endoscopy was performed and mucosal biopsies were performed twice weekly during the initial transplant admission and thereafter whenever clinically indicated. The histologic definition of rejection has been described elsewhere [8].
Diagnosis of CMV
Serological studies for CMV from the donor and recipient were performed preoperatively. All patients received CMV-negative blood products during the transplantation operation and throughout their posttransplantation course. Blood, urine, and respiratory specimens for CMV cultures were obtained as part of the routine evaluation of fever. In addition, all endoscopic specimens were evaluated histologically for the presence of CMV, as defined by the presence of inclusions or by immunoperoxidase staining.
Definition of CMV infection
CMV infection was defined by (1) the CMV-positivity of a culture specimen from any site after transplantation or (2) seroconversion in a previously seronegative patient. Isolation of CMV was attempted by shell vial assay [9] and by standard culture. CMV IgG was measured by a commercially available assay [10] (FIAX; Whittaker Bio-products, Walkersville. MD).
CMV disease was defined by the presence of symptoms attributable to CMV infection in a patient with a positive culture (of blood, urine, respiratory secretions, or tissue) or histologically positive biopsy tissue (documented by the presence of inclusions or by immunofluorescent staining), in the absence of another pathogen to explain these symptoms. CMV enteritis was diagnosed by histologic evidence of CMV within the gastrointestinal tract in association with fever, abdominal bloating, and diarrhea or an increase in stool or stomal output. CMV hepatitis was diagnosed by the presence of fever, elevated transaminase levels, and characteristic histopathology.
CMV pneumonia was diagnosed when patients had clinical and/or radiographic evidence of lower respiratory tract disease and a culture-positive bronchoalveolar lavage specimen. Recurrence of CMV disease was defined as a new episode of disease after at least 1 month of negative histopathology and/or virology. Persistent CMV disease was defined by the continuous presence of CMV in serial histologic specimens for >1 month.
CMV prophylaxis and treatment
The prophylactic strategy against CMV evolved during the study period. Twelve patients who underwent transplantation between July 1990 and July 1992 received prophylaxis with oral acyclovir (800 mg/m2) four times a day for 12 weeks after transplantation. Twenty-nine patients who received transplants between July 1992 and July 1995 received intravenous ganciclovir (10 mg/[kg · d], divided into two doses) for the first 2 weeks after transplantation. Twenty of the 29 patients subsequently received oral acyclovir for the next 10 weeks following intestinal transplantation. Three of these 29 were given concomitant CMV-specific hyperimmunoglobulin (100 mg/[kg · d]) (Cytogam; Medimmune, Gaithersburg, MD), according to the previously published protocol for prevention of CMV disease in adult liver transplant recipients [11].
Episodes of CMV disease were treated with intravenous ganciclovir alone or in combination with CMV-specific hyperimmunoglobulin (100 mg/kg, three times per week) until resolution of clinical symptoms and histologically proven elimination of inclusion bodies. For patients with persistent CMV disease, CMV-specific hyperimmunoglobulin was administered between one and four times per month until resolution of CMV disease. Immunosuppression was maintained at a therapeutic baseline and reduced only when clinical status deteriorated.
Statistical methods
Incidence and outcome of CMV were evaluated for all pediatric intestinal transplantation recipients. Patient survival was calculated from the date of transplantation until death, and graft survival from the date of transplantation until retransplantation or death. CMV-disease-free survival was calculated from the date of transplantation until diagnosis of CMV disease. Survival curves were generated by the Kaplan-Meier method and compared by the log-rank (Mantel-Cox) test. Wilcoxon’s rank-sum test was used to compare the median number of acute allograft rejection episodes between groups. Pearson’s χ2 test or Fisher’s exact test was used to compare proportions.
Analyses were also performed according to donor/recipient (D/R) serological status (i.e., D−/R−, D+/R−, D−/R+, or D+/R+). Cox’s proportional hazards model was used to analyze risk factors for CMV disease, rejection, and mortality. Cox’s model was used to compute relative risk and 95% confidence intervals. The cumulative dose of steroids and number of steroid recycles were incorporated into the Cox’s model as time-dependent covariates. Also incorporated as time-dependent covariates were the incidences of rejection and CMV disease.
Results
Patient population
During the study period, 41 children received 44 intestinal transplants at our center. There were 10 isolated SB, 27 L-SB, and 7 multivisceral transplants. Five patients also received a concomitant donor bone marrow infusion, and 20 received an allograft colon; these latter sets of patients were distributed among all three recipient cohorts. There were 19 males and 22 females with ages ranging between 0.5 and 18.1 years (mean, 4.2 years).
CMV infection and disease
The incidence and recurrence rate of CMV disease, according to D/R CMV serostatus of the patients, are shown in table 1. CMV disease occurred in 10 (24%) of the 41 children. None of the 15 patients in the D−/R− cohort developed CMV infection or disease. In contrast, 3 of 8 D+/R−, 3 of 9 D+/R+, and 4 of 9 D−/R+ patients developed CMV disease, resulting in a combined incidence for these three cohorts of 10 of 26 (38%). CMV disease was initially seen a median of 53.5 days (range, 9–121 days) posttransplantation, while the second episode occurred a median of 225 days (range, 85–1,350 days) following transplantation.
Table 1.
Incidence, recurrence rate, and outcome of cytomegalovirus disease in pediatric intestinal transplant recipients, according to donor/recipient serostatus.
| Serostatus | No. of patients | Incidence: no. (%) developing CMV disease | No. of episodes |
No. of patients with CMV disease at time of death | |
|---|---|---|---|---|---|
| CMV disease | Recurrent CMV disease | ||||
| D−/R− | 15 | 0 | 0 | 0 | 0 |
| D+/R− | 8 | 3 (37) | 5 | 2 | 0 |
| D+/R+ | 9 | 3 (33) | 4 | 1 | 1 |
| D−/R+ | 9 | 4 (44) | 7 | 2 | 0 |
NOTE. CMV = cytomegalovirus; D = donor; R = recipient.
The intestinal allograft was affected in 90% of patients who developed CMV disease and in 11 (69%) of the 16 episodes of CMV disease. The diagnosis of CMV in the intestinal allograft was based on histology in 10 instances and isolation of CMV from a gastric biopsy specimen in the remaining episodes. CMV also invaded the native stomach, duodenum, and colon in 20% of children with CMV disease, although this occurred only in D+/R− children.
The distribution of CMV disease by site of involvement was as follows: intestinal allograft enteritis (10), allograft gastritis (1), native gastroduodenitis (2), native colitis (2), and pneumonitis (3). CMV hepatitis was not seen, even though the liver was part of the composite allograft in 73% of these patients. The three episodes of pneumonitis were relatively mild and did not result in major changes in the clinical status of the patients.
Recurrent CMV disease was observed in 5 of the 10 patients with symptomatic CMV: 4 patients had 2 episodes and 1 patient had 3 episodes. Three episodes of recurrent CMV disease occurred in the gastrointestinal tract. Of the two remaining recurrences, one consisted of a febrile illness associated with a positive buffy coat culture for CMV and the other involved the lung. Persistent CMV disease occurred in 2 patients (including 1 of the 5 patients with recurrent disease), who were in the D+/R− subgroup; resolution of disease occurred for both patients after 7 months of therapy.
Asymptomatic CMV infection occurred in 1 D+/R+ and 2 D−/R+ recipients who had previously had 1 episode each of CMV disease.
Because the cohort of 15 D−/R− intestinal transplantation recipients had no CMV infection or disease, subsequent analysis of CMV disease was restricted to the 26 at-risk children who either were seropositive pretransplantation or had a seropositive donor (D+/R−, D−/R+, or D+/R+). The episodes of CMV disease among the 26 at-risk children were distributed among allograft types as follows: SB, 3 of 4; L-SB, 5 of 19; and multivisceral, 2 of 3. Although determination of differences in the incidence of CMV disease between allograft types is limited by the small number of transplant recipients, a trend toward a lower incidence of CMV disease occurred among L-SB recipients (5 of 19), in comparison with recipients of an isolated SB (3 of 4) (P = .103, Fisher’s exact test). No relationship was found between age, donor sex, or recipient sex and the occurrence of CMV disease.
The incidence of CMV disease in patients receiving ganciclovir prophylaxis was 6 of 19 (33%), vs. 4 of 7 (57%) in those receiving only oral acyclovir (P = .547, Fisher’s exact test). Among children receiving intravenous ganciclovir prophylaxis, the rate of CMV disease was no different in those receiving ganciclovir alone than in those treated with ganciclovir followed by oral acyclovir (2 of 5 vs. 2 of 11; P = .547, Fisher’s exact test). Two patients who received ganciclovir and CMV-specific hyperimmunoglobulin (one D+/R− and the other D−/R+) developed CMV disease, while a third patient (D+/R+) treated with this regimen did not develop symptomatic CMV disease.
Pathology of CMV disease
Histologically, numerolls inclusion bodies were usually detected. Although most CMV-infected cells had characteristic nuclear and/or cytoplasmic inclusion bodies, an atypical appearance was noted in some patients with CMV disease. This atypical appearance was characterized by the presence of a hyperchromatic smudged nucleus that sometimes protruded into the lumen of the gland. In addition, we noted muscle cells with large hyperchromatic and irregular nuclei, as well as endothelial cells with a single large or many smaller eosinophilic bodies (figure 1A). The use of immunoperoxidase stain for CMV was helpful in confirming the diagnosis of CMV within these atypical cells. Associated inflammatory changes included cryptitis (figure 1B) and gland damage, sometimes with prominent apoptosis, as well as variable chronic inflammation.
Figure 1.
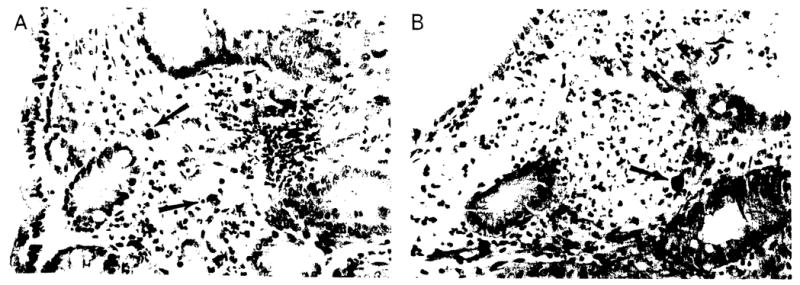
A. Colonic mucosa with numerous cells infected with cytomegalovirus in a pediatric patient following intestinal transplantation. The morphology varied from diagnostic inclusions, to cells resembling ganglion cells, to some with dense eosinophilic granules or bodies (arrows). B. Cryptitis with large hyperchromatic cell (arrow). Such cells in these patients should always raise the suspicion of viral infection (hematoxylin/eosin stain; original magnification, × 400).
Outcome of CMV disease
Successful clinical management of CMV disease was accomplished in 90% of patients with CMV disease and in 93% of episodes. Management typically consisted of administration of intravenous ganciclovir alone or in combination with CMV-specific hyperimmunoglobulin. Baseline immunosuppression was maintained unless there was clinical deterioration. Ganciclovir was used for 14–28 days in all but the two patients with persistent CMV disease.
The median time to resolution (defined by the absence of inclusions in serial gastrointestinal biopsy specimens) after diagnosis was 20 days (range, 8 days to 7 months). Three patients with a history of CMV disease died. One D+/R+ patient died of severe intestinal allograft rejection, with evidence of persistent CMV infection at the time of death; two patients (one D+/R+ and the other D+/R−) died of postransplantation lymphoproliferative disease, without evidence of symptomatic CMV disease at the time of death.
The actuarial rates of patient and graft survival were compared between the different D/R CMV serological subgroups by the Kaplan-Meier method. No statistically significant difference was observed in the 2-year patient or graft survival rates among the four subgroups or between any pair of serological subgroups (D−/R− patient survival, 53%, and graft survival, 53%; D+/R+, 67% and 67%; D+/R−, 50% and 46%; and D−/R+, 53% and 53%). Similarly, the 2-year patient survival rates were compared between patients with and without CMV disease. A survival rate of 61 % was found among patients with CMV disease, vs. a 56% survival rate among children without CMV disease (P = NS). The 2-year survival rate of each of these two groups of children did not differ from that of the D−/R− cohort, which was 57%.
Interaction of rejection and CMV
One or more episodes of rejection occurred in 14 of 16 patients with CMV disease, vs. 8 of 10 patients with no history of CMV disease (P = NS). The relative risk of developing rejection among patients with and without CMV disease was 0.79 (95% CI, 0.15–4.10). Similarly, a history of rejection did not appear to be a risk factor for CMV disease (RR; 1.19; 95% CI, 0.30–4.73). However, rejection was diagnosed histologically a mean of 4.7 days (range, 0–11 days) before the diagnosis of CMV disease in 13 of 16 (81%) of the CMV disease episodes observed in this study. Among the 10 episodes of CMV disease involving the intestinal allograft, all were immediately preceded by or occurred concomitantly with an episode of rejection.
The role of augmented immunosuppression in the incidence of CMV disease was also evaluated. The cumulative dose of steroid boluses and recycles given was associated with an increased incidence of CMV disease (respectively: RR, 1.59, and 95% CI, 1.14–2.21; RR, 2.72, and 95% CI, 1.21–6.13). Five patients were receiving azathioprine. The effect of OKT3 on the incidence of CMV disease was also explored in the 26 at-risk patients. Four of nine children receiving OKT3 developed CMV disease, compared with six of 17 of these at-risk children who did not receive OKT3 (P = NS).
Discussion
Infection with CMV has long been recognized as a major cause of morbidity and mortality following solid-organ transplantation, since the first reports of such infection in human transplant recipients [12–16]. Our initial report of CMV disease among adult and pediatric intestinal transplant recipients identified a high incidence, rate of recurrence, and rate of intractability of CMV disease [17], compared with those of CMV disease in other organ transplant recipients. However, in this current report, we identified an incidence of CMV disease of 24% among pediatric intestinal transplant recipients, which is similar to the incidence observed among children receiving liver allografts [16] and half of what we previously observed in our adult intestinal transplant recipients (46%) [5].
This decreased rate in children is likely explained by the large number of D−/R− pediatric intestinal transplant recipients at our center and contrasts with our adult intestinal transplantation experience, in which transplantation among D−/R− patients has rarely occurred. The overall recurrence rate of CMV disease found in this study was 50%, which is similar to the rate of recurrence found in adult intestinal transplant recipients and is within the range found in recipients of other solid organs (6%–59%) [18].
CMV involvement of the gastrointestinal tract was frequently noted in this study. This may be explained by a higher tropism for CMV in gastrointestinal epithelial cells [19] and endothelial cells [20] and by the large number of donor lymphocytes, monocytes, and polymorphonuclear leukocytes [21]. The intestinal allograft was affected in 90% of our children with CMV disease and in 68.7% of the CMV disease episodes. Involvement of the native gastrointestinal tract was observed only in CMV D+/R− patients. CMV hepatitis was not seen, even though the liver was part of the composite allograft in nearly 75% of these patients.
Augmentation of immunosuppression for the treatment of rejection may interfere with the host mechanisms against CMV infections, specifically by decreasing the number of cytotoxic T lymphocytes and natural killer cells [22, 23]. In addition, steroid therapy has been associated with CMV inclusions in the gastrointestinal tract [24, 25]. Similar to findings in our previous report of adult and pediatric intestinal transplant recipients [5, 17], the cumulative doses of steroid boluses and recycles were associated with a higher incidence of CMV in this experience with children.
Transplant rejection has been suggested as a risk factor for the development of CMV disease [26]. However, despite the fact that histologic findings suggestive of rejection were found shortly before the majority of episodes of CMV disease in our patients, rejection was not a risk factor for CMV disease in this study (RR, 1.4). The fact that rejection was not identified as a risk factor despite being present just prior to >80% of episodes of CMV disease may be explained by the relatively high frequency of allograft rejections in all of our patients during the period of peak risk for CMV disease. Given this high frequency of rejection, the relatively small sample size available for analysis in this study may not have the power to identify differences between children with and without rejection.
Finally, the presence of CMV has been suggested to be a risk factor for rejection after transplantation [27, 28]. We were not able to show an increased incidence of rejection after CMV disease in our patients.
Management of CMV disease with ganciclovir alone or in combination with CMV-specific hyperimmunoglobulin while maintaining baseline immunosuppression resulted in resolution of >90% of the episodes of CMV disease observed in this study. Maintenance of immunosuppression at baseline levels prevented the development of rebound rejection and the subsequent need for augmentation of immunosuppressive therapy. Although most episodes of CMV disease resolve within 14–28 days of antiviral therapy, long-term therapy (7 months) with subsequent “weaning” was required in two D+/R− children.
The use of foscarnet in conjunction with ganciclovir might provide more potent anti-CMV therapy [29, 30] for patients with persistent CMV disease and shorten the course of their disease. However, its use was avoided in our children with CMV disease because of the additive nephrotoxicity of tacrolimus and foscarnet observed in our adult population.
The experiences at several centers have suggested that the use of intravenous ganciclovir is effective prophylaxis against CMV in recipients of solid organs [31–33]. In our global experience (with adults and children) with intestinal transplantation, we have noted that the frequency of CMV disease with and without ganciclovir prophylaxis was similar. In this pediatric experience, it appears that ganciclovir prophylaxis alone may be more effective in preventing CMV disease than the exclusive use of oral acyclovir.
Only 6 of 19 patients receiving ganciclovir prophylaxis developed disease (31.5%), compared with 4 of 7 patients (57%) who received prophylaxis with oral acyclovir alone. Although this increase was not significant, it is possible that more experience and multicenter trials may provide better insights into effective prophylaxis against CMV disease after intestinal transplantation. Similarly, recently presented data regarding the prolonged use of oral ganciclovir suggest the potential utility of this prophylactic strategy [34]. This approach (alone or in combination with use of high-titer immunoglobulin) is worthy of study in recipients of intestinal transplantation and may result in decreased rates of CMV disease in this population.
The most effective strategy in the prevention of CMV disease is the transplantation of seronegative allografts into seronegative recipients (D−/R−). This was exemplified by our 15 D−/R− patients who remained CMV-free. Because the donor organ is a common inoculation source in seronegative recipients after transplantation, it has been reported that patients in the serological cohort D+/R− are at the greatest risk for development of CMV disease after transplantation of an intestine [17] as well as other types of allografts [16]. However, we found an equal frequency and rate of recurrence of disease in each of the D+/R−, D+/R+, and D−/R+ groups. However, there was a trend toward more persistent CMV disease involving the allograft in D+/R− children. It is of interest that these persistent episodes were not always symptomatic, but they did take up to 7 months to resolve histologically. Children in this group were the only ones to have CMV disease of the native gastrointestinal tract (66% of patients).
We did not find significant differences in the survival rates of patients with and without CMV disease, nor did we find a difference in survival between patients with CMV disease and children belonging to the D−/R− subgroup. Likewise, there were no significant differences in patient or graft survival rates among different CMV-serological-status subgroups, including patients in the subgroup D+/R−. This contrasts with our previously reported combined experience with adults and children [17]. This is partially explained by the fact that there were no deaths directly attributable to CMV disease in this study.
In summary, there appears to be less CMV-associated morbidity and mortality in children undergoing intestinal transplantation than in adult intestinal-transplant recipients at our center. The high rates of morbidity and mortality previously reported with regard to our global experience with CMV disease following intestinal transplantation in adults and children had led us to recommend the use of CMV-negative donors for CMV-seronegative intestinal transplant recipients.
This recommendation remains reasonable for recipients of isolated intestinal transplants in whom transplantation is not performed on an emergent basis. However, given the reasonable outcome of D+/R− children reported in this study, for pediatric patients urgently awaiting composite intestinal allografts because of concomitant liver failure induced by total parenteral nutrition, we recommend using CMV-positive donors when seronegative donors are not available.
Acknowledgments
Grant support: Veterans Administration and National Institutes of Health (DK 29961), Bethesda, Maryland; in part by Fondo de Investigacion Sanitaria de la Seguridad Social (95/5071)
References
- 1.Lillehei RC, Gott B, Miller FA. The physiological response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543–60. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA., Jr Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Grant D. Intestinal transplantion: current status. Transplant Proc. 1989;21(1 Pt 3):2869–71. [PubMed] [Google Scholar]
- 4.Abu-Elgmad K, Todo S, Tzakis A, et al. Three years’ clinical experience with intestinal transplantation. J Am Coll Surg. 1994;179:385–400. [PMC free article] [PubMed] [Google Scholar]
- 5.Furukawa H, Manez R, Kusne S, et al. Cytomegalovirus disease in intestinal transplantation. Transplant Proc. 1995;27:1357–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa H, Abu Elgmad K, Reyes J, et al. Technical aspects of intestinal transplantion. In: Braverman MH, Tawes RL, editors. Surgical technology international II. San Francisco: Surgical Technology International; 1993. pp. 165–70. [PubMed] [Google Scholar]
- 7.Todo S, Tzakis A, Abu-Elgmad K, Reyes J, Starzl TE. Current status of intestinal transplantation. Advances in Surgery. 1994;27:295–316. [PMC free article] [PubMed] [Google Scholar]
- 8.White F, Reyes J, Jaffe R, Yunis E. Pathology of intestinal transplantation in children. Am J Surg Pathol. 1995;19:687–98. doi: 10.1097/00000478-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Dunn DL, Mayoral JE, Gillingham KJ, et al. Treatment of invasive cytomegalovirus disease in solid organ transplant patients with ganciclovir. Transplantation. 1991;51:98–106. doi: 10.1097/00007890-199101000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Hopson DK, Niles AC, Murray PR. Comparison of the Vitek Immunodiagnostic assay system with three immunoassay systems for detection of cytomegalovirus specific immunoglobulin G. J Clin Microbiol. 1992;30:2893–5. doi: 10.1128/jcm.30.11.2893-2895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snydman DR, Werner BG, Dougherty NN, et al. Cytomegalovirus immune globulin prophylaxis in liver transplantation: a randomized, double blind, placebo-controlled trial. The Boston Center for Liver Transplantation. CMV IG Study Group. Ann Intern Med. 1993;119:984–91. doi: 10.7326/0003-4819-119-10-199311150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rifkind D, Starzl TE, Machioro L, et al. Transplantation pneumonia. JAMA. 1964;189(11):114–8. doi: 10.1001/jama.1964.03070110010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers JD, Ljungrnan P, Fisher LD. Cytomegalovirus excretion as a predictor of cytomegalovirus disease after marrow transplantation: importance of cytomegalovirus viremia. J Infect Dis. 1990;162:373–80. doi: 10.1093/infdis/162.2.373. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Dummer JS, Kusne S, et al. Infections with cytomegalovirus and other herpesviruses in 121 liver transplant recipients: transmission by donated organ and the effect of OKT3 antibodies. J Infect Dis. 1988;158:124–31. doi: 10.1093/infdis/158.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smyth RL, Scott JP, Borysiewicz LK, et al. Cytomegalovirus infection in heart-lung transplant recipients: risk factors, clinical associations, and response to treatment. J Infect Dis. 1991;164:1045–50. doi: 10.1093/infdis/164.6.1045. [DOI] [PubMed] [Google Scholar]
- 16.Strata RJR, Shatter MS, Margin RS. Clinical patterns of cytomegalovirus disease after liver transplantation. Arch Surg. 1989;124:1443–9. doi: 10.1001/archsurg.1989.01410120093018. [DOI] [PubMed] [Google Scholar]
- 17.Manez R, Kusne S, Green M, et al. Incidence and risk factors associated with the development of cytomegalovirus disease after intestinal transplantation. Transplantation. 1995;59:1010–4. doi: 10.1097/00007890-199504150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falagas ME, Snydman DR. Recurrent cytomegalovirus disease in solid organ transplant recipient. Transplant Proc. 1995;27(5 suppl 1):34–7. [PubMed] [Google Scholar]
- 19.Myerson D, Hackman RC, Nelson JA, Ward DC, McDougall JK. Widespread presence of histologically occult cytomegalovirus. Human Pathol. 1984;15:430–9. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- 20.Roberts WH, Sneddon JM, Waldman J, Stephens RE. Cytomegalovirus infection of gastrointestinal endothelium demonstrated by simultaneous nucleic acid hybridization and immunochemistry. Arch Pathol Lab Med. 1989;113:461–4. [PubMed] [Google Scholar]
- 21.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in smail bowel transplant. Lancet. 1991;337:818–9. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasternack MS, Medearis DN, Jr, Rubin RH. Cell-mediated immunity in experimental cytomegalovirus infections: a perspective. Rev Infect Dis. 1990;12(suppl 7):S720–6. doi: 10.1093/clinids/12.supplement_7.s720. [DOI] [PubMed] [Google Scholar]
- 23.Rubin RH. Impact of cytomegalovirus infection on organ transplant recipients. Rev Infect Dis. 1990;12(suppl 7):S754–66. doi: 10.1093/clinids/12.supplement_7.s754. [DOI] [PubMed] [Google Scholar]
- 24.Orloff JJ, Saito R, Lasky S, Dave H. Toxic megacolon in cytomegalovirus colitis. Am J Gastroenterol. 1989;84:794–7. [PubMed] [Google Scholar]
- 25.Henson D. Cytomegalovirus inclusion bodies in the gastrointestinal tract. Arch Pathol. 1972;93:477–82. [PubMed] [Google Scholar]
- 26.Kaplan ChS, Petersen EA, lcenogle TB, et al. Gastrointestinal cytomegalovirus infection in heart and heart-lung transplant recipients. Arch Intern Med. 1989;149:2095–100. [PubMed] [Google Scholar]
- 27.Reinke P, Fietze E, Odde-Hakim S, et al. Late acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet. 1994;44:1737–8. doi: 10.1016/s0140-6736(94)92887-8. [DOI] [PubMed] [Google Scholar]
- 28.O’Grady JG, Alexander GJ, Sutherland S, et al. Cytomegalovirus infection and donor/recipient HLA antigen: interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet. 1988;2:302–5. doi: 10.1016/s0140-6736(88)92356-2. [DOI] [PubMed] [Google Scholar]
- 29.Walton RCh, Whitcup SM, Mueller BU, Lewis LL, Pizzo PA, Nussenblatt RB. Combined intravenous ganciclovir and foscarnet for children with recurrent cytomegalovirus retinitis. Ophthalmology. 1995;102:1865–70. doi: 10.1016/s0161-6420(95)30782-8. [DOI] [PubMed] [Google Scholar]
- 30.Butler KM, De Smet MD, Husson RH, et al. Treatment of aggressive cytomegalovirus retinitis with ganciclovir in combination with foscarnet in a child with human immunodeficiency virus. J Pediatr. 1992;120:483–6. doi: 10.1016/s0022-3476(05)80926-6. [DOI] [PubMed] [Google Scholar]
- 31.Freise CE, Pons V, Lake J, et al. Comparison of three regimens for cytomegalovirus prophylaxis in 147 liver transplant recipients. Transplant Proc. 1991;23(1 Pt 2):1498–500. [PubMed] [Google Scholar]
- 32.Merigan TC, Renlun DG, Keay S, et al. A controlled trial of ganciclovir to prevent cytomegalovirus disease after heart transplantation. N Engl J Med. 1992;326:1182–6. doi: 10.1056/NEJM199204303261803. [DOI] [PubMed] [Google Scholar]
- 33.Green M, Reyes J, Nour B, et al. Randomized trial of ganciclovir followed by high-dose oral acyclovir vs ganciclovir alone in the prevention of cytomegalovirus disease in pediatric liver transplant recipients: preliminary analysis. Transplant Proc. 1994;26:173–4. [PubMed] [Google Scholar]
- 34.Pescovitz M, Gane E, Saliba F, et al. Efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus (CMV) disease in liver transplant recipients [abstract no H4]. Program and abstracts of the 36th Inter-science Conference on Antimicrobial Agents and Chemotherapy; New Orleans. Washington, DC: American Society for Microbiology; 1996. p. 163. [Google Scholar]


