Abstract
The effect of conversion from cyclosporine-steroid immunosuppression to the new agent FK506 was studied in 96 liver allograft recipients who were experiencing graft dysfunction or cyclosporine toxicity. Patients were stratified according to the cause of graft dysfunction that ultimately led to conversion to FK506. Response to FK506 introduction was monitored pathologically and biochemically. The outcome of a switch from CsA to FK506 was highly favorable in patients experiencing acute and the early stages of chronic rejection, despite optimal conventional therapy. Patients with later stages of chronic rejection did not respond to conversion to FK506 and most eventually lost their liver grafts in this process. Patients in whom we had difficulty separating chronic rejection from chronic persistent or low-grade chronic active hepatitis were mostly unaffected by conversion to FK506. Active hepatitis was a poor indication for conversion, because most of the patients experienced graft failure or died from liver failure. As a group, there was no statistically significant change in renal function 180 days after conversion to FK506. These findings expand the experience with FK506 in human liver allograft recipients.
The new immunosuppressant FK506 was first used in humans for the treatment of acute and even relatively advanced chronic liver graft rejection (1–3). This report is of a much longer experience and with longer follow-up. An attempt was made to correlate the histopathologic findings and biochemical parameters before and after FK506 was given as a “rescue” agent. FK506 was offered to patients whose grafts became dysfunctional despite standard CsA/steroid immunosuppression.
MATERIALS AND METHODS
Patient selection
The clinicopathologic profile was reviewed of all liver allograft recipients who were switched from CsA/steroid to FK506 therapy during the period of 03/01/89 (first day of use in humans) until 12/15/89. During this time, 136 patients were converted. Ninety-six who met the following criteria were selected for more detailed study on the basis of the following: (1) biochemical graft dysfunction, as defined by elevated liver function tests (>50% over normal values) without evidence of mechanical causes (e.g., biliary obstruction or vascular occlusion; (2) availability of a liver biopsy within 7 days before the switch, with at least one follow-up biopsy. The other 38 cases were excluded because a preswitch biopsy was not obtained (n=22), because no follow-up biopsy was available (n=10), because the drug switch was at the time of transplantation (n=7), or because diagnosis causing the decision for the drug change was erroneous (n=1).
The age, sex, duration of CsA therapy, previous liver transplant(s) and cause of previous graft failure(s) in the 96 patients studied are shown in Table 1. The case mix was similar to that in our group from throughout the years (4). Eighteen of the 96 patients qualified for inclusion on grounds of minor hepatic dysfunction, but had other and more important justifications for a drug change such as steroid or cyclosporine toxicity. These patients had generally less-advanced histopathologic alterations in the liver grafts than the others. This subgroup of 18 patients will not be discussed further because neither the minimally perturbed hepatic function tests nor the histopathologic findings was significantly altered by the change in therapy.
Table 1.
Profile of patients who were switched from CsA to FK506
| A. | |
|---|---|
| Total number of patients | 96 |
| Male/female | 57/39 |
| Average age (yrs. [range]) | 42 (1–74) |
| Average days on CSA prior to the switch (range) | 640 (4–2402) |
| B. | |
| Graft number at the time of conversion | No. patients |
| First | 75 |
| Second | 14 |
| Third | 5 |
| Fourth | 2 |
| C. | |
| Causes of prior graft failure(s) under CsAa | No. grafts |
| Acute cellular rejection | 2 |
| Chronic rejection | 14 |
| Primary dysfunction/preservation injury | 11 |
| Bile duct complication | 1 |
| Hepatic artery thrombosis | 2 |
| Total | 30 |
Causes of prior graft failures under CsA, in patients who were switched to FK506 with a nonprimary graft in place. For example, if a patient had lost a prior graft to chronic rejection under CsA, and was recapitulating the course in a second graft, requiring conversion to FK506, the cause of failure in the first graft is listed as chronic rejection.
The final decision to convert from CsA to FK506 was ultimately made by the clinicians. The liver function tests, the clinical reason for conversion and clinicopathologic profile of the patient prior to the switch were recorded (Table 2). The patients were followed until 9/1/90, graft failure, or death, whichever came first. The details of therapy during the drug switch and after have been reported elsewhere (1,2, 5). In essence, cyclosporine was stopped at the time FK506 was started, and prior steroid doses were reduced or discontinued. Azathioprine was also discontinued. The factor of hepatic dysfunction in reducing the beginning and maintenance doses of FK506 from the standard of 0.3 mg/kg/day has been emphasized in recent studies.4 Failure to make those reductions based on nephrotoxicity, neurotoxicity, and plasma monitoring of FK506 levels is a source of potential tragedy since the ability to metabolize this drug is inversely related to the quality of liver function.4 For those patients who survived with the same graft until 9/1/90, liver function studies were recorded at 30, 60, 90, and 180 days after conversion (5). The normal range for liver and kidney function tests is as follows: total bilirubin(TB)* <1.5 mg/dl; alanine aminotransferase <40 IU/ml; aspartate aminotransferase <40 IU/ml; alkaline phosphatase <130 IU/ml; gamma glutamyl transpeptidase(gGTP) <120 IU/ml; and creatinine <1.5 mg/dl.
Table 2.
Clinicopathologic profile of patients at the time of conversion from CsA to FK506a
| Clinical reason for conversion | No. pts. | Biopsy diagnosis | No. pts. | Days of CsA therapy (range) |
|---|---|---|---|---|
| Rejection | 76 | Acute or chronic | 18 | 173 (10–1401) |
| CR/hep.b | 33 | 624 (10–2402) | ||
| 20 | 905 (170–2089) | |||
| CsA/steroid side effects or other reason | 14 | Otherc | 18 | 270 (4–1797) |
| Predominantly hepatitis | 6 | Hepatitis | 7 | 672 (91–1743) |
| Totals | 96 | 96 | 640 (4–2402) |
Pre-FK506 laboratory values (mean [range]): total bilirubin (mg/dl) 7.1 (0.3–39.4); AST (IU/L) 195 (13–1040); ALT (IU/L) 300 (9–1229); AP (IU/L) 381 (56–1730); gGTP (IU/L) 951 (51–5830); BUN (mg/dl) 37 (8–115); creatinine (mg/dl) 1.8 (0.3–5.5).
CR/hep = overlap between chronic rejection and chronic persistent or low-grade chronic active hepatitis.
Other includes clinically suspected rejection, not verified pathologically, preservation injury, and mild portal inflammation.
Pathologic studies
All posttransplant pathology specimens taken from these patients were reviewed by a single pathologist (A.J.D.). Standard histopathologic criteria for the diagnosis of acute cellular rejection - and nonrejection-related syndromes were employed (4) on H&E-stained, 6-μ sections. Biopsies were considered adequate if 4 or more portal triads were identified.
An attempt was made to temporally categorize the changes associated with “chronic” rejection, based on our previous experience (4). A diagnosis of “early” chronic rejection was made when there was evidence of lymphocytic bile duct damage in 50% or more of the triads, bile duct loss limited to less than 25% of the triads, and no cholestasis or other lobular changes. A pathologic diagnosis of “late” chronic rejection was defined by duct loss in more than 50% of the triads, lymphocytic duct damage in the remaining bile ducts, and hepatocanalicular cholestasis and other lobular changes associated with “chronic” rejection (4).
The biopsies were rereviewed without knowledge of the original disease, clinical course, or liver biochemical profile for assessment of the effect of FK506. However, the pathologist was aware of the temporal sequence of the biopsies and the date of FK506 introduction. The effect of FK506 on hepatic morphology was graded as “improved,” “worsened,” or “no change” based on an overall assessment of the severity of portal and/or lobular inflammation, bile duct damage, hepatocyte necrosis, and architectural distortion.
RESULTS
Entry diagnosis as a function of the original disease
The entry or Pre-FK506 diagnosis, as a function of the original disease is shown in Table 3. A biopsy diagnosis of either hepatitis or overlapping chronic rejection and chronic hepatitis was more prevalent in patients with hepatitis B-induced, autoimmune, NANB-induced, or cryptogenic cirrhosis prior to transplantation. Rejection was a more common reason for conversion in the other patients.
Table 3.
Entry diagnosis (reason for switch) as a function of the original liver disease
| Original disease No. (% of total) | Entry diagnosis (No. [%]) |
||||
|---|---|---|---|---|---|
| ACRa | CR | CR/hep. | Hep. | Other | |
| CC/NANB: 26 (27%) | 2 (8) | 12 (46) | 7 (27) | 4 (15) | 1 |
| CAH-B: 9 (9%) | 0(0) | 2 (22) | 4 (44) | 0(0) | 3 |
| PSC: 14 (15%) | 5 (36) | 6 (43) | 1 (7) | 0(0) | 2 |
| PBC: 8 (8%) | 2 (25) | 2 (25) | 1 (13) | 0 (0) | 3 |
| Met/other: 12 (12%) | 3 (25) | 4 (33) | 3 (25) | 1 (8) | 1 |
| Alcoholic: 17 (18%) | 4 (24) | 4 (24) | 3 (18) | 1 (6) | 5 |
| Biliary atresia: 5 (5 %) | 2 (40) | 3 (60) | 0(0) | 0(0) | 0 |
| Carcinoma: 2 (2%) | 0 | 0 | 0 | 0 | 2 |
| Autoimmune CAH: 3 (3%) | 0 | 0 | 1 (33) | 1 (33) | 1 |
| Total | 18 | 33 | 20 | 7 | 18 |
CC/NANB = cyptogenic cirrhosis/non-A, non-B hepatitis; CAH-B-hepatitis B virus-induced cirrhosis; PSC = sclerosing cholangitis; PBC = primary biliary cirrhosis; Met/other = metabolic and other; ACR = acute cellular rejection; CR = chronic rejection; CR/hep. = overlapping chronic rejection and chronic hepatitis; Hep. = chronic hepatitis.
Patient and graft status after conversion
The patient and graft status as of 09/01/90 as a function of the entry diagnosis is shown in Table 4. The original disease had no statistically significant effect on patient or graft survival after conversion (data not shown). However, the rate of subsequent graft failure was slightly higher in patients with cryptogenic or non-A-, non-B-induced cirrhosis as their original disease. In patients with functioning grafts, the average interval between the immediate pre-FK506 biopsy and the latest post-FK506 follow-up biopsy was 123 days. However, the average clinical follow-up was 314 days. Graft failure under FK506 occurred at an average of 82 days after conversion (range 11–279 days) and was most common in patients with an entry diagnosis of “late” chronic rejection. A more detailed description of graft failures and deaths is given later.
Table 4.
Patient and graft status as of 9/01/90 as a function of the pre-FK506 diagnosis (top)
| Entry diagnosis | Functioning | Failed | Died | Total |
|---|---|---|---|---|
| Acute rejection | 14 | 1 | 3 | 18 |
| Chronic rejection | 22 | 11 | 0 | 33 |
| CR/hep. | 16 | 4 | 0 | 20 |
| Hepatitis | 2 | 1 | 4 | 7 |
| Other | 13 | 1 | 4 | 18 |
| Total | 67 | 18 | 11 | 96 |
Effect of conversion to FK506
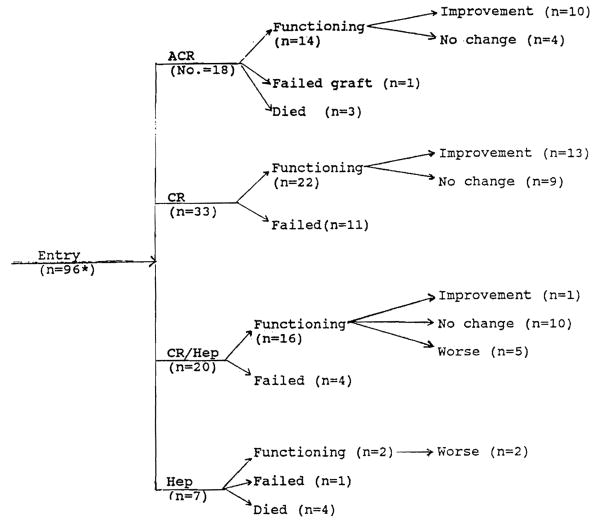
It should be noted that many of the post-FK506 liver biopsies did not coincide with dates on which tests used for assessing liver injury occurred. This fact probably accounts for some of the discrepancies noted between the pathologic and biochemical response to conversion. A flow diagram showing the outcome and pathologic response after conversion from CsA to FK506 is shown in Figure 1. A more detailed discussion of each clinicopathologic indication for conversion is given below.
Figure 1.
Histologic evaluation of biopsies from patients after conversion to FK506. Eighteen patients were switched for reasons other than significant graft dysfunction. There were minimal pathologic alterations before and after conversion to FK506. They are not included here.
Acute cellular rejection
Eighteen of the patients were switched because of acute cellular rejection, which varied histologically from mild to severe. All but two of the 18 patients had received additional steroid therapy (n=12) and/or OKT3 (n=7), prior to conversion. The two exceptions were converted primarily because of renal dysfunction but had coexistent rejection. Although the average time on CsA prior to the switch was 219 days, 12 of the 18 patients were switched in the first five weeks after transplantation. Two of these patients died within two weeks after conversion because of sepsis. Another died of recurrent hepatoma, and a fourth patient required a second liver because the primary graft failed from chronic rejection. Ten of the 14 remaining functional grafts showed histologic as well as biochemical normalization (Table 5).
Table 5.
Comparison of laboratory values in patients with acute cellular rejection before and 180 days after conversion to FK506
| Pre-FK506a (mean [range]) | Values at 180 daysa (mean [range]) | |
|---|---|---|
| CsA (days) | 219 (12–1401) | — |
| FK506 (days) | 343 (266–547) | — |
| TB (mg/dl) | 8.3 (0.6–30.3) | 0.5b (0.1–1.2) |
| AST (IU/L) | 96 (24–210) | 65c (21–141) |
| ALT (IU/L) | 219 (34–1229) | 82c (13–152) |
| ALP (IU/L) | 215 (56–429) | 164c (74–448) |
| gGTP (IU/L) | 284 (71–750) | 123c (22–440) |
| BUN (mg/dl) | 38 (5–101) | 36c (14–84) |
| CR (mg/dl) | 2.0 (0.1–5.0) | 1.9c (0.4–3.5) |
Patient with functioning grafts as of 9/01/90 (n=14).
P<0.05 (paired t test) compared with pre-FK506 values.
P>0.05 (paired t test) compared with pre-FK506 values.
No change in the histologic findings were observed in the remaining four, although some biochemical improvement was seen (Table 5). In the 4 patients with functioning grafts but no change on biopsy, two had a pathologic follow-up of less than two weeks. Mild central venulitis developed in the third patient, 70 days after beginning FK506, although the portal infiltrate had diminished. In the fourth, mild portal inflammation with focal duct damage was still present 66 days after conversion.
“Chronic” rejection
Twenty-two of the 33 (67%) patients switched for chronic rejection have functioning grafts as of 09/01/90. None of the patients switched for this indication has died, but 11 required retransplantation. Liver injury parameters were significantly worse in patients with chronic rejection whose grafts failed compared with those whose grafts were successfully rescued (Table 6). Thirteen of the 22 patients with intact grafts have shown histologic improvement, and 10 of these have also shown biochemical improvement. In the remaining 9, while no significant histologic difference was seen following conversion, 6 had a lowering of liver function abnormalities by 180 days.
Table 6.
Comparison of laboratory values in patients with chronic rejection as the indication for FK506 switch separated into those who failed and were rescued, as well as average liver biochemistries in patients at 180 days with currently functioning grafts
| Pre-FK506 average values (range) |
Average values at 180 daysa | ||
|---|---|---|---|
| Failed grafts | Functioning grafts | ||
| CsA (days) | 572d (23–2158) | 825 (27–2402) | — |
| FK506 (days) | 47 (11–180) | 316 (268–426) | — |
| TB (mg/dl) | 21c (0.9–39.4) | 4.3 (0.3–20) | 1.1b (0.2–7.0) |
| AST (IU/L) | 282d (48–803) | 204 (26–618) | 97b (8–310) |
| ALT (IU/L) | 412e (123–964) | 297 (13–697) | 126b (4–536) |
| ALP (IU/L) | 666d (399–1359) | 478 (65–1702) | 326e (41–985) |
| gGTP (IU/L) | 1661d (244–4076) | 1316 (138–5332) | 677b (35–3200) |
| BUN (mg/dl) | 33 (9–85) | 33 (8–75) | 35e (10–117) |
| CR (mg/dl) | 1.3 (0.3–4.7) | 1.8 (0.5–5.1) | 1.8e (0.3–4.6) |
In patients with functioning grafts (n=11 for failed grafts; n=22 for functioning grafts).
P<0.05 (paired t test) compared with pre-FK values in functioning grafts.
P<0.05 (t test) compared with pre-FK506 values in patients with functioning grafts.
P>0.05 (t test) compared with pre-FK506 values in patients with functioning grafts.
P>0.05 (paired t test) compared with pre-FK506 values in functioning grafts.
The histopathology present in liver biopsies from patients who ultimately were FK506 treatment failures manifested a later stage of chronic rejection as compared with those who have maintained viable grafts.
All but four of the patients who failed a rescue attempt had pre-FK506 biopsies that were characterized by bile duct loss in 50% or more of the triads (Fig. 2). Other stigmata of late chronic rejection, including mild portal inflammation, cholestasis, hepatocyte dropout in zone 3 of the acinus, and perivenular fibrosis were also seen in the grafts that failed to respond. Although no single histopathologic parameter was able to predict a nonresponse to therapy, the combination of a TB >20 mg/dl combined with greater than 50% duct loss on biopsy was highly predictive of nonresponse.
Figure 2.
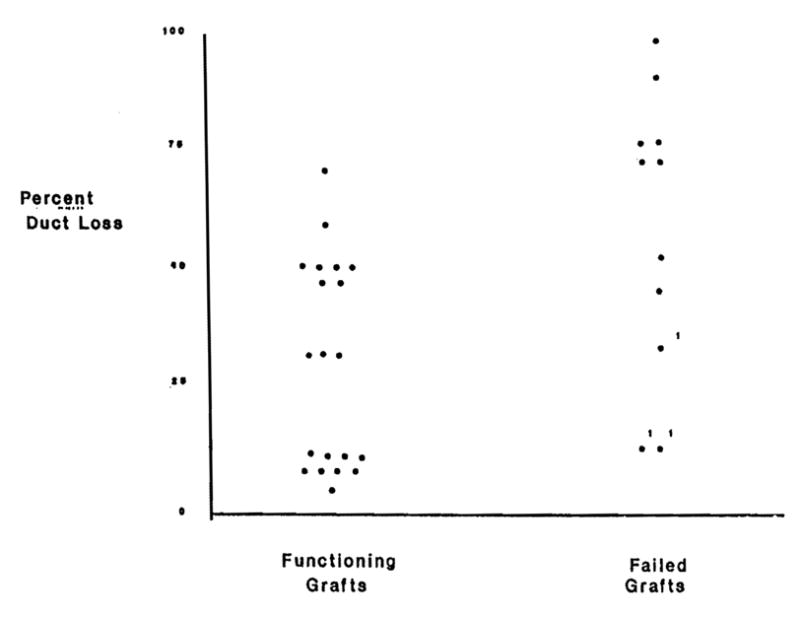
Percent bile duct loss in biopsies taken prior to conversion to FK506.
In three of the four patients with milder changes on entry, the ultimate cause of graft failure was not chronic rejection. One of the patients initially responded to FK506 but lost his graft because of arterial thrombosis at the suture line. Another patient normalized her liver function only to lose the graft, 6 months after conversion, to a combination of hepatitis and rejection. Examination of the failed graft in this patient revealed duct loss limited to 30% of the triads, centrilobular perivenular hepatocyte dropout, and fibrosis. Biliary sludge with ascending cholangitis was the ultimate cause of graft failure in the third patient.
Entry liver biopsies from patients with functioning grafts 180 days after conversion to FK506 showed less severe damage from chronic rejection. Ongoing lymphocytic bile duct damage was present in biopsies from all patients but duct loss was seen in 50% or less of the triads in all but two cases (Fig. 2). The coexistent lobular changes mentioned above were less frequent. Because of the possibility of sampling errors, it was not possible to state that bile duct regrowth occurred with rescue, but in several patients the percent of duct loss was less in follow-up biopsies. A comparison of the degree of duct loss between the successful rescues and the treatment failures is illustrated graphically in Figure 2.
Most often histologic improvement was marked by a decrease in portal inflammation and accompanying bile duct damage. This was particularly true if there had been a moderate portal infiltrate before conversion to FK506. In other cases, portal inflammation remained stable but bile duct damage decreased. Paradoxically, in some cases the cholestasis present in biopsies increased shortly after the initiation of FK506, only to resolve later.
Resolution of the biochemical abnormalities in patients with chronic rejection who have responded to FK506 occurred over a period of weeks to months. Dwindling serum bilirubin values and a loss of clinical jaundice were often the first signs of success. Dramatic declines were also observed in the canalicular enzymes (gGTP and ALP), although many of the patients still had elevations of these two enzymes 180 days after conversion (Table 6). Overall, there was a statistically significant (P<0.05) decrease in total bilirubin, AST, ALT, and gGTP. The levels of AP were also lower, but the difference did not reach statistical significance.
Overlapping chronic rejection and hepatitis
Sixteen of the 20 (80%) patients switched because of an entry biopsy diagnosis of overlapping chronic rejection and chronic persistent or low-grade chronic active hepatitis have retained their grafts. None of the patients in this group died. Four required retransplantation—one because of chronic rejection, one from hepatitis, one from a combination of chronic rejection and hepatitis, and one because of severe atherosclerotic narrowing of an arterial graft as well as hepatitis. In general, the pathologic alterations and liver function abnormalities prior to FK506 in this group were persistent but were not severe (Table 7).
Table 7.
Comparison of laboratory values in patients with an entry diagnosis of chronic rejection/CPH, and low-grade CAH beforehand and at 180 days following FK506 conversion
| Pre-FK506a values (mean [range]) | Values at 180 daysa (mean [range]) | |
|---|---|---|
| CsA (days) | 731 (170–1555) | — |
| FK506 (days) | 299 (264–344) | — |
| TB (mg/dl) | 1.1 (0.3–2.1) | 0.6b (0.1–1.3) |
| AST (IU/L) | 173 (21–411) | 85b (23–210) |
| ALT (IU/L) | 291 (45–805) | 110b (22–256) |
| ALP (IU/L) | 142 (81–308) | 154c (105–293) |
| gGTP (IU/L) | 413 (68–1540) | 309c (18–907) |
| BUN (mg/dl) | 26 (11–55) | 32c (12–92) |
| CR (mg/dl) | 1.6 (0.9–2.8) | 2.1b (1.0–4.4) |
In patients with functioning grafts as of 9/01/90 (n=16).
P<0.05 (paired t test) compared with pre-FK506 values.
P>0.05 (paired t test) compared with pre-FK506 values.
Histologically, most patients (ten) in this group have stayed the same or showed less portal inflammation and duct damage after conversion to FK506. Despite the histologic assessment in this group, statistically significant (P<0.05) lowering of the total bilirubin, AST, and AL T values were observed 180 days after conversion. An overall worsening of the pathologic findings was seen in 5 patients. In 4, the deterioration was a result of increased hepatitic activity, that assumed a “cholestatic” or chronic active pattern. The former was characterized by lobular disarray and steatosis, hepatocyte swelling and spotty necrosis, cholestasis, and a conspicuous paucity of inflammation. These changes were accompanied by cholangiolar proliferation; acute cholangiolitis; and a mild mixed, but often neutrophilic, portal and periportal infiltrate. In the last patient, both hepatitis and rejection activity increased.
Hepatitis
Of the seven patients with hepatitis prior to FK506 conversion, four have died, one lost his graft, and only 2 have functional grafts after 180 days. One of the latter patients showed both biochemical and histologic deterioration, due to rejection rather than hepatitis. This same patient had also lost a prior graft to chronic rejection.
The last patient deserves a special description. The etiology of his original disease was never ascertained, with features of both autoimmune chronic active hepatitis and posttransfusional (HCV antibody-positive) cirrhosis. After transplantation he experienced a three-year course of chronic active hepatitis, which on biopsy always had a heavy plasmacytic infiltrate, typical of autoimmune chronic active hepatitis. Although his bilirubin was normal at the time of conversion, he had elevations of AST and ALT 6-11 times normal with near-normal ALP and gGTP. However, his liver biopsy demonstrated early cirrhosis. Six months after starting FK506, his liver function tests were normal. The last liver biopsy, which was obtained 89 days after conversion, showed less inflammation but persistent fibrosis.
Analysis of grafts failing under FK506
In most instances, the entry diagnosis explained why the graft failed (Table 8). Only one patient, whose ultimate cause of graft failure was chronic rejection despite conversion to FK506, would not have been considered for retransplantation if FK506 had not been available. Only one patient with an entry diagnosis of acute cellular rejection did not respond to conversion, leading to a need for retransplantation. In this patient, the lesion had progressed to chronic rejection when the graft was finally removed.
Table 8.
Cause of graft failure under FK506 as a function of the reason for conversion from CsA
| Cause of graft failure | Entry diagnosis |
|||||
|---|---|---|---|---|---|---|
| ACR | CR | CR/hep. | Hep. | Other | Total | |
| Acute rejection | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic rejection | 1 | 7 | 2 | 0 | 0 | 10 |
| Hepatitis | 0 | 0 | 1 | 1 | 0 | 2 |
| Biliary tract problem | 0 | 2a | 0 | 0 | 0 | 2 |
| Hepatic artery thrombosis | 0 | 1b | 0 | 0 | 0 | 1 |
| Other | 0 | 1c | 1d | 0 | 1e | 3 |
| Totals | 1 | 11 | 4 | 1 | 1 | 18 |
Examination of the failed graft revealed biliary sludge in the deep hilar region and focal duct loss without arteriopathy.
The patient initially responded to FK506, but the graft failed because of thrombosis at the arterial suture line.
The cause of failure is uncertain. Severe sclerosis of terminal hepatic venules and early cirrhosis were found in the failed graft.
There was severe atherosclerosis of arterial graft and hepatitis.
There was a mistaken pathologic diagnosis; a portal vein-bile duct fistula was found in failed graft that was missed on pre-FK biopsy.
Nonetheless, there were several exceptions in which the diagnosis at entry was not the cause of graft failure (Table 8). In 2/11 cases a pre-FK biopsy diagnosis of chronic rejection was not confirmed on examination of the failed graft. These inconsistencies were most likely the result of an erroneous pre-FK506 pathologic diagnosis. Biliary sludge was found in the deep hilar bile ducts in both grafts at retransplantation and was likely the major cause of failure. Portal fibrosis, focal small bile duct loss involving less than 25% of the triads, and cholestasis were seen in the subcapsular region, without obliterative arteriopathy. In a third case the cause of ultimate graft loss was uncertain. Severe sclerosis of the terminal hepatic venules and an early cirrhosis with prominent cholangiolar proliferation were the striking features. In a fourth graft, the early stages of chronic rejection were confirmed in the failed graft, but they appeared to have been lost because of an unrecognized arterial thrombus at the suture line.
Questions of toxicity
Acute FK506 nephrotoxicity (a reversible rise in serum creatinine) was obvious in the first patients treated and was observed in the majority of patients (1,5,6). This was most severe when intravenous FK506 was used in patients with severe hepatic dysfunction resulting in extremely high drug levels. As reported previously, creatinine regularly rises after switching from cyclosporine to FK506 and falls toward baseline values after weeks of therapy. This crossover nephrotoxicity was attenuated in patients starting FK506 orally and at doses less than the standard 0.15 mg/kg given twice per day, which was our initial practice.
Renal function in 25 of the 96 patients was studied with sequential iothalomete clearance for glomerular filtration rate and hippuran clearance for effective renal plasma flow. Baseline studies obtained within one week of initiating therapy and at 120 days, respectively, were: ERPF 250.3±114 to 209±159.8 ml/min and GFR 51.1±14.8 to 48.5±27.4 ml/min. The filtration fraction (GFR/ERPF) did not consistently change after conversion. Since ERPF and GFR fell proportionately (causing little change in filtration fraction) no clear inferences can be made as to whether FK506 causes its effects primarily by vasoconstriction as cyciosporine does. Long term follow-up of these studies are in progress.
Serum creatinine preconversion was 1.8±0.9 mg/dl (Table 9) and at one year 1.9±0.9 mg/dl. Peak creatinine (2.5±1.4 mg/dl) for the group occurred at week 4 and progressively fell thereafter. In most cases the fall in creatinine was preceded by a reduction in drug dosage. In the first patients treated, cyclosporine was continued during the initial days of conversion. This resulted in a dramatic rise in creatinine but did not require dialysis in any case. This was certainly due to the combined nephrotoxicity of FK506 and cyclosporine.
Table 9.
Serum creatinine values as a function of the duration of CsA therapy, immediately before and 180 days after the switch to FK506 (only patients with functioning grafts)
| Number of patients | CsA (days) | Pre-FK506 creatininea | Creatininea after 180 days of FK506 therapy |
|---|---|---|---|
| 15 | <100 | 1.9±1.7 (0.1–5.5) | 1.6b ±0.5 (0.4–2.3) |
| 4 | 100–200 | 2.0±1.8 (0.5–4.7) | 1.6b ±1.0 (0.9–3.0) |
| 7 | 200–300 | 2.5±1.7 (0.7–5.1) | 2.0b±1.2 (0.3–3.6) |
| 42 | >300 | 1.7±0.6 (0.6–3.1) | 2.0b±0.9 (0.4–4.6) |
| Total 68 | 1.8±0.9 (0.1–5.5) | 1.9b ±0.9 (0.3–4.6) |
Values are given in mg/dl.
P>0.05 (paired t test) compared with pre-FK506 values.
No renal biopsies were performed either prior to the conversion or after, precluding morphologic analysis of the possible effects of FK506 on the native kidney. Such an analysis would require both preconversion biopsies and follow-up biopsies since the magnitude of cyclosporine-induced interstitial fibrosis was likely to be substantial.
Deaths under FK506
Eleven patients died while being treated with FK506 at an average of 95 days after starting FK506 therapy (range 7–272 days). Six of the eleven died from sepsis, three during treatment for acute cellular rejection. Another died from recurrent hepatoma. Three of the patients died because of liver failure from hepatitis. In two, hepatitis (type B and presumed non-A-non-B) was diagnosed by biopsy prior to the switch to FK506. One patient died unexpectedly after approximately one year. However, she had been switched back to cyclosporine from FK506 6 months prior to her death because of availability of the FK506 during the early phase of our trials.
Vascular morphology
These studies were largely limited to examination of failed allografts and liver allograft biopsies. Two allografts contained vascular lesions that were not readily explainable on the basis of rejection-related vasculopathy. Focal arterial medial necrosis was seen in one graft that failed because of presumed non-A, non-B hepatitis. Focal arterial medial scarring (possibly healed necrosis) was seen in another liver that failed primarily because of severe narrowing of a free arterial graft supplying a liver that had hepatitis.
Hepatic morphology
There were two morphologic patterns of hepatic injury that could possibly be associated with the treatments used. The first is probably best described as a cholestatic hepatitis. It was characterized by hepatocellular swelling, hepatocanalicular cholestasis, lobular disarray, spotty acidophilic necrosis, cholangiolar proliferation, acute cholangiolitis, and a mild mixed portal infiltrate. It was most common in patients with cryptogenic cirrhosis, or non-A-non-B CAH/cirrhosis as their original disease, although all were not hepatitis C virus antibody-positive (data not shown). Because of the prior history of hepatitis in these patients, it was assumed that graft malfunction was virus-related. Consequently, discontinuance of FK506 was not considered.
The second pattern of liver injury was centrilobular hepatocellular dropout, congestion with hemorrhage and mononuclear inflammation—and, ultimately, sclerosis of the terminal hepatic venules. This lesion was present in some of the biopsies from 18 patients after the switch to FK506. However, in 11 of them, the same change was present while the patient was on CsA, at which time it was considered a manifestation of rejection. This particular lesion worsened in some of the biopsies taken after conversion to FK506 in 7 of the patients. The relative contribution of a concomitant reduction in steroids to the evolution of any of these changes, if any, cannot be directly addressed in this patient population beyond noting that a nearly universal strategy was to reduce or stop steroids after the change from CsA to FK506.
Malignancies
One patient developed a secondary neoplasm after conversion to FK506. An IgA lambda monoclonal and monomorphic lymphoproliferative disorder was discovered on gastric biopsy. The lesion resolved promptly after lowering the FK506 dosage.
DISCUSSION
The data generated from this study could not be used for a comparison of FK506 with continuation of conventional therapy. The latter option had already failed, particularly in patients who had lost previous grafts from chronic rejection and who were recapitulating the same course. A randomized experiment in any of the patients was considered inhumane. Nevertheless, the experience yielded valuable information.
FK506 provided more potent immunosuppression than that previously in effect, even though steroids were reduced or stopped and azathioprine was always discontinued. It was most effective for the treatment of patients experiencing acute cellular or the early stages of chronic rejection, under optimal standard CsA and adjuvant immunosuppression. FK506 was less effective in patients in whom we were unable to confidently separate chronic rejection from a chronic persistent or low-grade chronic active hepatitis as the cause of graft dysfunction. These patients had a mixed response pathologically, but most were unaffected, although a biochemical response was seen in many. Those with hepatitis alone fared poorly after conversion to FK506. The mixed or poor results obtained with patients experiencing what appeared to be an overlap of chronic rejection and hepatitis, or hepatitis alone, suggests that this should not be a prime consideration for conversion. Furthermore, the observed biochemical response could conceivably be the result of a natural resolution of hepatitic disease activity.
In all of the cohorts, biochemical improvement was not uncommon even though this was not mirrored in the histopathology. One possible reason for this discrepancy was the difference in time between the last biopsy and liver function monitoring. Patients were not routinely subjected to biopsy 180 days after conversion to FK506 if liver function tests were normal. Therefore, pathologic analysis alone may have underestimated the response.
Renal functions were presented in this study only as a backdrop to events occurring in the liver grafts. However, it is noteworthy that the results achieved were not at the expense of renal function. Although renal function temporarily worsened at the time of the drug switch, average creatinine values 180 days after FK506 conversion were lower than pre-FK506 levels in patients who had received CsA for less than 300 days. A slight increase in serum creatinine was noted in those who had received CsA for a longer period. Neither change was statistically significant. The possibility that longer CsA treatment had led to permanent renal structural damage (7,8) must be considered.
Hepatotoxicity of FK506 could not be clearly evaluated in this patient population. It is possible but improbable that FK506 could be associated with a cholestatic hepatitis or terminal hepatic vein phlebitis. Neither of the above lesions appeared to result from a direct hepatotoxic effect of FK506. There was no direct association of these injury patterns with serum FK506 levels or FK506 dosage (data not shown). In addition, similar, if not identical, lesions were often present in the same patients prior to FK506 introduction and have been described in other liver allograft recipients treated with cyclosporine (9) or azathioprine (10). These observations are contrary to the temporal eligibility requirement for an adverse drug reaction (11). Whether the concomitant reduction in steroids will effect these manifestations of rejection will require further study.
We are hesitant also to ascribe the arterial necrosis seen in two failed allografts to drug toxicity since it is commonly seen in rejecting liver allografts under different immunosuppression or in untreated animals (12, 13). Such questions are best addressed in patients in whom the target organ is not an allograft (i.e., liver studies in heart or kidney recipients).
Our data suggest that FK506 is more potent than other current agents, although an alternative explanation could be offered for our results. Based on in vitro data (4), it could be speculated that conversion to a new immunosuppressant after the development of rejection affects a clone of lymphocytes that was selected because of its resistance to the original agent or regimen. Direct comparison, as is now being done in a randomized trial should yield answers, as well as quantification of side-effects. These comparisons of FK506 with conventional immunosuppression in historical control patients already have placed FK506 in favorable light (14).
In summary, a clinicopathologic analysis was carried out on 96/136 patients who were switched from standard CsA to FK506 immunosuppression. The majority of the patients whose dysfunction was ascribable to acute cellular or the early stages of chronic rejection despite conventional therapy responded favorably to the conversion, along with reduction or discontinuance of steroid therapy and stopping of other adjuvant therapy. Nonrejection causes of graft dysfunction were mostly unaffected pathologically by FK506, except for progressive hepatitis, which proved to be a poor indication for conversion. Transient FK506 nephrotoxicity was observed at the time of drug switch and this was severe in some patients, but in the whole group there was no significant change in mean serum creatinine after 180 days of FK506. The results in the larger collection of patients with much longer follow-up are similar to those reported in the first clinical trials of FK506 in humans (1,2).
Acknowledgments
We thank Dr. Ed Yunis for access to the pediatric cases; Dr. Ron Jaffe for review of the manuscript, Greg Sysyn for collecting patient data, and Mary Ann Mient for her excellent editorial assistance.
Footnotes
Abu-Elmagd K, Fung JJ, Alessiani M, et al. The effect of graft function on FK506 plasma levels, doses, and renal function: with particular reference to the liver. (Manuscript submitted for publication.)
Abbreviations: gGTP, gamma glutamyl transpeptidase; TB, total bilirubin.
References
- 1.Starzl TE, Todo S, Fung JJ, Demetris AJ, Venkataramanan R, Jain A. FK506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung JJ, Todo S, Jain A, et al. Conversion from cyclosporine to FK506 in liver allograft recipients with cyclosporine-related complications. Transplant Proc. 1990;22:6. [PMC free article] [PubMed] [Google Scholar]
- 3.Demetris AJ, Fung JJ, Todo S, et al. Pathologic observations in human allograft recipients treated with FK506. Transplant Proc. 1990;23:25. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Demetris AJ. Liver transplantation: a 31 year perspective. Curr Probl Surg. 1990;28:51. doi: 10.1016/0011-3840(90)90021-v. [DOI] [PubMed] [Google Scholar]
- 5.Fung JJ, Todo S, Tzakis A, et al. Use of FK506 in the treatment of liver allograft rejection. Transplant Proc. (in press) [Google Scholar]
- 6.McCauley J, Fung JJ, Jain A, Todo S, Starzl TE. The effects of FK506 on renal function after liver transplantation. Transplant Proc. 1990;22:17. [PMC free article] [PubMed] [Google Scholar]
- 7.Klintmalm GBG, Iwatsuki S, Starzl TE. Nephrotoxicity of cyclosporine A in liver and kidney transplant patients. Lancet. 1981;2:470. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams R, Blackburn A, Neuberger J. Long term use of cyclosporine in liver grafting. Q J Med. 1987;57:897. [PubMed] [Google Scholar]
- 9.Ludwig J, Gross JB, Perkins JD, Moore SB. Persistent centrilobular necrosis in hepatic allografts. Hum Pathol. 1990;21:656. doi: 10.1016/s0046-8177(96)90013-1. [DOI] [PubMed] [Google Scholar]
- 10.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, editor. Experience in hepatic transplantation. Philadelphia: Saunders; 1969. p. 422. [Google Scholar]
- 11.Irey NS. Tissue reactions to drugs. Am J Pathol. 1976;82:613. [PMC free article] [PubMed] [Google Scholar]
- 12.Todo S, Ueda Y, Demetris AJ, et al. Immunosuppression of canine, monkey and baboon allografts by FK506: with special reference to synergism with other drugs and to tolerance induction. Surgery. 1988;104:239. [PMC free article] [PubMed] [Google Scholar]
- 13.Ochiai T, Sakamoto K, Gunji Y, et al. Effects of combination treatment with FK506 and cyclosporine on survival time and vascular changes in renal-allograft-recipient dogs. Transplantation. 1989;48:193. doi: 10.1097/00007890-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney and thoracic organ transplantation under FK506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]