Abstract
We retrospectively analyzed 42 hepatitis C virus (HCV)-infected patients who underwent cadaveric liver transplantation under two strategies of immunosuppression: (1) daily tacrolimus (TAC) throughout and an initial cycle of high-dose prednisone (PRED) with subsequent gradual steroid weaning, or (2) intraoperative antithymocyte globulin (ATG) and daily TAC that was later space weaned. After 36 ± 4 months, patient and graft survival in the first group was 18/19 (94.7%) with no examples of clinically serious HCV recurrence. In the second group, the three-year patient survival was 12/23 (52%), and graft survival was 9/23 (39%); accelerated recurrent hepatitis was the principal cause of the poor results. The data were interpreted in the context of a recently proposed immunologic paradigm that is equally applicable to transplantation and viral immunity. In the framework of this paradigm, the disparate hepatitis outcomes reflected different equilibria reached under the two immunosuppression regimens between the relative kinetics of viral distribution (systemically and in the liver) and the slowly recovering HCV-specific T-cell response. As a corollary, the aims of treatment of the HCV-infected liver recipients should be to predict, monitor, and equilibrate beneficial balances between virus distribution and the absence of an immunopathologic antiviral T-cell response. In this view, favorable equilibria were accomplished in the nonweaned group of patients but not in the weaned group. In conclusion, since the anti-HCV response is unleashed when immunosuppression is weaned, treatment protocols that minimize disease recurrence in HCV-infected allograft recipients must balance the desire to reduce immunosuppression or induce allotolerance with the need to prevent antiviral immunopathology.
In hepatic transplant recipients whose chronic liver disease had been caused by hepatitis B virus (HBV), accelerated recurrence of chronic hepatitis1 was almost universal2 until the development of HBV-specific antiviral therapy.3 More recently, chronic hepatitis C virus (HCV) has emerged as the leading indication for liver transplantation worldwide. Without treatment comparable to that for HBV, disease recurrence in HCV-infected recipients has reached epidemic proportions and threatens to swamp liver centers.4 Donor and recipient risk factors that contribute to posttranspiant HCV recurrence have been identified,5,6 but there has been no consensus about optimal immunosuppression for these patients.7-9
We addressed the dilemma of optimal immunosuppression with a retrospective analysis of 42 patients with chronic HCV hepatitis who underwent liver replacement under alternative management strategies during 2001-2002. The outcomes were remarkably different with the two strategies of immunosuppression. The data available in these patients were too incomplete to independently formulate a mechanism-based explanation for the difference. However, here we describe and discuss the results in our patients from the point of view of a previously proposed immunologic paradigm that takes into account antigen kinetics, the antigen-specific T-cell responses to the viral and donor antigens, and the susceptibility of the respective responses to the different immunosuppressive regimens.10-12 The courses of 51 uninfected liver recipients treated with one or the other strategy during the same period were similarly analyzed.
Patients and Methods
Patient Populations
The 42 patients comprised all adult primary cadaveric liver recipients whose transplantations were for chronic HCV hepatitis between August 2001 and August 2002, except for 6 who were excluded because of HIV co-infection. Only one of the 42 donors had evidence of a prior HCV infection by serologic testing. With the objective of facilitating natural tolerance mechanisms,12 23 of these recipients were lymphoid-depleted with antithymocyte globulin (ATG, thymoglobulin) prior to allograft revascularization and treated after transplantation with tacrolimus (TAG) monotherapy from which weaning was ultimately attempted.13 The other 19, including the only recipient of a liver from a donor with positive HCV serology, were immunosuppressed continuously with TAG and decremental doses of prednisone (PRED).
Both protocols of immunosuppression were judged by the University of Pittsburgh Institutional Review Board to be within the boundaries of standard treatment and then remanded to the Presbyterian University Hospital Innovative Practices Committee and to the Pharmacy & Therapeutic Committee with approval by both. The protocol used in individual cases was selected by combined patient and surgeon choice. The decision was strongly influenced by the time available for a preoperative ATG infusion and by specific potential contraindications for the ATG medication (e.g., hepatopulmonary syndrome). All patients provided informed consent. In addition, separate informed consent was obtained for studies of immune variables not routinely obtained in our conventional practice. In compliance with our long-standing institutional policy, data integrity and safety and efficacy monitoring were assured by establishment of a formal review every week of all cases.
During the same one-year period beginning in August 2001, all 51 adult primary cadaveric liver recipients who were not infected with HCV were treated with one or the other immunosuppression protocol. None of these donors had positive HCV serology or other evidence of a primary infection. Thus, 4 non-randomized groups could be defined by the choice of immunosuppression and the presence or absence of preexisting HCV disease: HCV-positive/TAC-PRED (n = 19), HCV-positive/ATG-TAC (n = 23), HCV-negative/TAC-PRED (n = 28), and HCV-negative/ATG-TAC (n = 23).
Donor and recipient factors that have been reported to influence liver transplant outcome, with or without HCV, are summarized for the 4 groups in Table 1. The conclusions reached in the evaluation of the results were not changed when adjusted for differences in the various donor and recipient characteristics.
Table 1.
Population Characteristics
| HCV Positive |
HCV Negative |
|||
|---|---|---|---|---|
| TAC-PRED | ATG-TAC | TAC-PRED | ATG-TAC | |
| No. of patients | 19 | 23 | 28 | 23 |
| Recipient age (yr) | 51.3 ± 6.1 | 50.8 ± 7.2 | 55.9 ± 9.0 | 54.6 ± 8.3 |
| Female (%) | 26 | 43 | 39 | 57 |
| African-American (%) | 0 | 0 | 0 | 4 |
| Mean MELD Score at Tx | 16.4 ± 5.5 | 13.4 ± 4.4 | 19.4 ± 7.9 | 16.0 ± 5.5 |
| 1 Donor age (yr) | 44.1 ± 16.7 | 47.9 ± 16.3 | 44.1 ± 19.4 | 41.3 ± 18.3 |
| Female (%) | 47 | 61 | 29 | 48 |
| African-American (%) | 16 | 14 | 9 | 4 |
| Cold ischemic time (hours) | 10.3 ± 2.4 | 9.9 ± 2.2 | 11.3 ± 3.2 | 9.1 ± 2.2 |
| Mean HLA mismatch | 4.4 ± 1.0 | 4.4 ± 1.1 | 4.7 ± 1.0 | 4.9 ± 0.8 |
Abbreviations: HCV, hepatitis C virus; TAC, tacrolimus; PRED, prednisone; ATG, antithymocyte globulin; MELD, Model for End-Stage Liver Disease; Tx, transplantation; HLA, human leukocyte antigen.
An analysis of variance by ANOVA revealed that significant differences in the population parameters of the 4 cohorts were limited to the MELD Score (P = 0.009) and ischemic time (P = 0.024). The conclusions reached in the evaluation of the results were not changed when adjusted for these population differences.
Immunosuppression
TAC-PRED
TAG was administered twice daily from the first postoperative day onward with a target 12-hour trough blood concentration of 10 ng/mL. After the first several months, lower trough levels were considered acceptable. At one and two years, the trough levels averaged 8.6 ± 3.1 and 6.6 ± 3.0 ng/mL, respectively. Steroid treatment was begun intraoperatively with an infusion of 1 or 2 g methylprednisolone, followed by a 3- to 5-day postoperative course in which the daily methylprednisolone (or oral PRED) quantities were reduced stepwise from 200 to 20 mg. With further gradual steroid weaning, 34 (74%) of the patients were off PRED or in the range of 5-7.5 mg/day by the end of 3 months. At 12 months, 29 (63%) of the 46 surviving patients were steroid free, and at 24 months, 36 (84%) of the 43 survivors were off steroids.
ATG-TAC
The patients were infused with approximately 5 mg/kg rabbit ATG with co-infusion of 1-2 g methylprednisolone. On the first postoperative day, TAC monotherapy was begun in the same way as in the TAC-PRED groups. Neither PRED nor other agents (e.g., mycophenolate mofetil, sirolimus, or muromonab-CD3 [OKT3]) were added, singly or in combination, unless there was a breakthrough rejection. After 4 to 6 months, patients who had been on stable tacrolimus monotherapy for at least 2 months had extension of the interval between TAG doses (“spaced weaning”) to once a day, every other day, or longer if this was compatible with stable graft function.
Viral and Donor Leukocyte Antigen Loads
HCV RNA concentrations were performed in our hospital using the Roche Amplicor HCV Monitor 2.0 (Roche Diagnostics, Basel, Switzerland) and other comparable assays, or at a reference laboratory using the Heptimax test (Quest Diagnostics, Teterboro, NJ). Samples were obtained as clinically indicated rather than by protocol. Details are provided in Results.
In 20 of the patients immunosuppressed with ATG-TAC, donor leukocyte macro- or microchimerism was assessed in peripheral venous blood with previously described flow cytometric, cytospin, and/or polymerase chain reaction (PCR) techniques.14 Confirming earlier observations,14-16 there was a declining incidence of blood macro- or microchimerism between 1 and 12 months.
Distinction of Rejection and HCV Recurrence
The differential diagnosis of rejection and HCV recurrence was made by strict adherence to previously described histopathologic criteria17 in biopsies obtained because of abnormalities in liver function tests. The courses of all patients not biopsied were reviewed and found to have no evidence of serious biochemical abnormalities. In the treatment-defined HCV subgroups, the number of biopsies in patients treated with ATG-TAC averaged 6.6 ± 3.2 vs. 3.1 ± 2.2 in those treated with TAC-PRED. For final diagnosis of individual biopsies, pathologists correlated the histopathologic findings with those in previous biopsies and with clinical observations including response to immunosuppression and HCV RNA levels. Thirty-one histological parameters including liver fibrosis (i.e., none, mild, moderate, or severe) were scored for each allograft biopsy.17 Scoring for hepatitis activity index and staging fibrosis were done for allograft biopsies diagnosed as recurrent HCV.
Statistical Analysis
An honest brokering system approved by the University of Pittsburgh IRB was used for data management. Data were extracted, reviewed, augmented (where required) for accuracy and completeness, and de-identified for statistical analysis. Differences in means and standard deviations calculated from participants' characteristics by treatment group were evaluated for statistical significance using χ2 tests for categorical comparisons and t tests and one-way analysis of variance for continuous comparisons. Kaplan-Meier survival curves were generated and evaluated for significance using a log-rank test for patient survival, graft survival, rejection, and time to HCV recurrence. Cox proportional hazards models were used to adjust survival comparisons for relevant covariates. A P value of <0.05 two sided was considered significant. The conclusions reached in the evaluation of the results using unadjusted comparisons were not affected by adjusting for differences in the patient and donor populations.
Results
Patient and Graft Survival
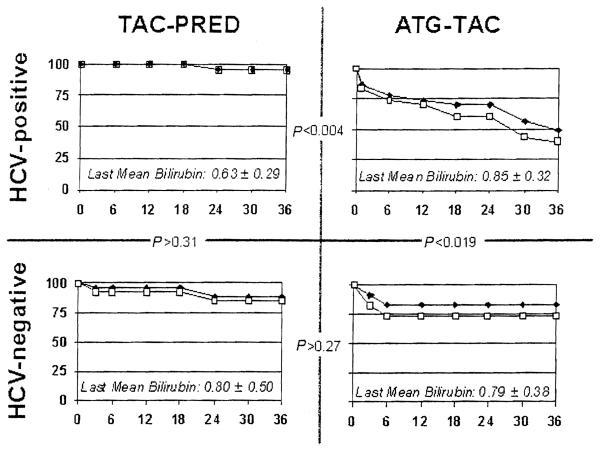
All patients were followed to the time of death or to March 1, 2005. The three-year survival of HCV-infected recipients and of their primary grafts is 94.7% with the TAC-PRED strategy (Fig. 1A). The patient survival with the ATG-TAC protocol is 52% and graft survival is 39% (P < 0.004) (Fig. 1B). The postoperative day and proximate cause of all graft losses and patient deaths in both groups are summarized in Table 2. The higher loss rate during the first month in the ATG-TAC group was not obviously associated with recurrent HCV disease. However, 9 of the 11 subsequent failures were directly attributable to recurrent chronic hepatitis. In contrast, there were no examples of disease recurrence that led to hepatic insufficiency in the patients immunosuppressed with the TAC-PRED strategy. The mean serum bilirubin in the surviving patients who still bear their primary grafts was not significantly different in the two treatment groups (see subscripts in Fig. 1).
Figure 1.
Patient and graft survival (months). Patient (black diamonds) and graft (gray squares) survival to three years for HCV-infected liver recipients (top panels) and uninfected recipients (bottom panels) under the TAC-PRED and ATG-TAC immunosuppression regimens. The P values calculated by log-rank between the charts show that all other survival results were similar except in HCV-positive recipients under ATG-TAC immunosuppression. The current mean serum bilirubin of surviving primary grafts in the different groups (subscripts) is not significantly different. Horizontal axis in months.
Table 2.
Time (Days) and Causes of Graft Loss in HCV-Infected and Uninfected Recipients Under Two Kinds of Immunosuppression*
| Immune Suppression |
n | Primary Dysfunction or Nonfunction |
Recurrent HCV | Non-HCV Sepsis |
Other |
|---|---|---|---|---|---|
| HCV-Infected | |||||
| TAC-PRED | 19 | None | None | None | 681† |
| ATG-TAC | 23 | 1, 2, 10, 19 | 110, 308, 411, | None | 111‡, |
| 508, 761, 797, | 932§ | ||||
| 865, 874, 1,077 | |||||
| Not HCV-infected | |||||
| TAC-PRED | 28 | 5 | NA | 35, 493 | 579 | |
| ATG-TAC | 23 | 2, 9, 9 | NA | 75, 171 | 180¶ |
Abbreviations: HCV, hepatitis C virus; TAC, tacrolimus; PRED, prednisone; ATG, antithymocyte globulin.
The bolded numbers indicate current patient survival after retransplantation.
Disseminated metastases from small hepatoma in excised native liver.
Motor vehicle accident.
Atherosclerotic heart disease.
Gunshot suicide.
Laceration femoral artery during radiographic procedure.
After the first 9 days, patient and graft survival in the contemporaneous uninfected patients was similar under both kinds of immunosuppression (Fig. 1C-D) with causes of failure that were much the same (Table 2).
Time of HCV Recurrence or Rejection
Recurrent Disease
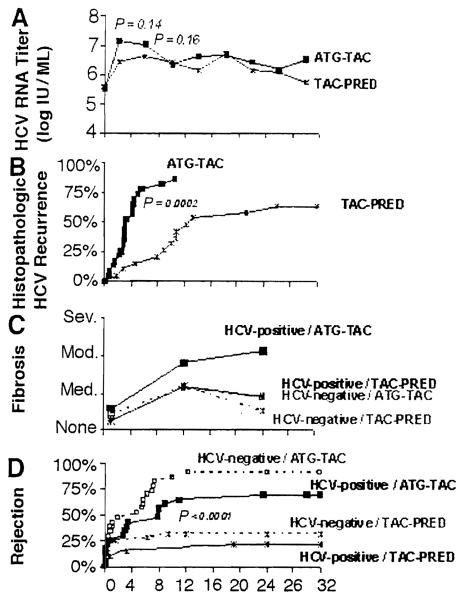
Prior to transplantation, serum HCV RNA concentrations were comparable (and low) in both immunosuppression-defined groups of infected patients (Fig. 2A and Table 3). Afterwards, there was a rapid rise that was greater and longer lasting under ATG-TAC (Fig. 2A). The viral load out to three years has remained 10-fold or more higher than before transplantation in both immunosuppression groups. When immunosuppression was augmented in response to the diagnosis of rejection, there were secondary increases. Conversely, treatment with pegylated interferon and ribavirin frequently resulted in a reduction in HCV level, most markedly in a small number of patients with HCV other than genotype 1. As previously reported,18,19 there was no apparent association of antiviral treatment with rejection.
Figure 2.
The effect of two strategies of immunosuppression (TAC-PRED and ATG-TAC) in HCV-mfected liver recipients (continuous lines of A-D). The dashed lines in C and D also depict the hepatic fibrosis scores and time to first rejection in noninfected contemporaneous liver recipients. Note that severe hepatic fibrosis occurred only in the HCV-infected patients treated with ATG-TAC (C). Horizontal axis in months.
Table 3.
Comparison of the Patients in the Two Immunosuppression-Defined HCV-Infected Subpopulations Whose Grafts Survived >30 Days
| ATG-TAC (original n = 23) |
TAC-PRED (original n = 19) |
|
|---|---|---|
| Grafts surviving at 1 month | 19 | 19 |
| HCV genotype 1 | 14/17 (82%) | 11/14 (79%) |
| HCV titer (log10 IU/mL) | ||
| Pretransplant titer | 4.97 ± 1.04 | 4.35 ± 1.34 |
| Maximum titer first year | 6.8 ± 0.78 | 6.1 ± 1.09 |
| Posttransplant antiviral treatment | ||
| Number treated within 2 years | 10/19 | 8/19 |
| Onset treatment (months) | 6.2 ± 2.3 | 12.4 ± 4.8 |
| Duration treatment (months) | 15.2 ± 9.1 | 13.1 ± 3.0 |
| Time to spaced dose weaning (months) | 4.4 ± 1.5 | n/a |
| Acute rejection | ||
| Preweaning | 3/16 | n/a |
| Postweaning | 10/16 | n/a |
| Hepatic staging fibrosis (0-6 scale) | ||
| Preweaning* | 0.6 ± 0.81 | n/a |
| Postweaning† | 2.3 ± 1.19 | n/a |
| Hepatitis activity index (0-18 scale) | ||
| Preweaning* | 3.2 ± 2.56 | n/a |
| Postweaning† | 4.8 ± 1.94 | n/a |
| Later antirejection treatment | ||
| Received steroid bolus after 1 year (n) | 8/19 | 3/19 |
| Received alemtuzumab rescue (n) | 8/19 | 1/19 |
| Time to alemtuzumab rescue (months) | 11.9 ±4.2 | 10.5 |
Abbreviations: ATG, antithymocyte globulin; TAG, tacrolimus; PRED, prednisone; HCV, hepatitis C virus.
From biopsy 0 to 2 months before the start of spaced-dose weaning.
From biopsy 3 to 5 months after the start of spaced-dose weaning.
Antiviral Therapy
The policy at our institution in 2001-2002 was to consider HCV-infected liver recipients for interferon-based therapies on an individualized basis. Treatment was deferred until recovery from the surgery and stabilization of immunosuppression, and was not given in the presence of acute graft rejection and infections or in patients with no biochemical, clinical, or histopathologic evidence of liver damage. Treatment was not given to any patient during the first 12 postoperative weeks. Once initiated, the protocol called for a 48-week course.
Of the 23 patients immunosuppressed with ATG-TAC, 10 were treated at some time between 3 and 24 months compared with 8 of the 19 in the TAC-PRED group. All but 4 of the 18 treated patients were given one subcutaneous dose per week of 1 μg/kg PEG-Intron (Schering-Plough, Kenilworth, NJ) in combination with daily oral doses of 800 mg ribavirin. The starting dose of ribavirin was adjusted to as low as 200 mg per day (e.g., in response to anemia) or escalated when feasible up to 1,200 mg per day. Erythropoietin and filgrastim were administered as needed for anemia and leucopenia, respectively. In the 4 exceptional patients (all ATG-TAC), interferon treatment was initiated with three subcutaneous doses per week of 3 million units IntronA (Schering-Plough), combined with the ribavirin schedule as described above. After 4 and 12 weeks, two of the 4 patients were switched to one dose per week PEG-Intron. The other two were switched after 6 and 11 months to 180 μg per week subcutaneous Pegasys (Roche Pharmaceuticals).
Histopathologic evidence of disease recurrence in HCV-infected recipients occurred much earlier under ATG-TAC than with TAC-PRED immunosuppression (Fig. 2B). By two years, the degree of fibrosis (mild, moderate, or severe), regardless of its location, was greater in those treated with ATG-TAC (Fig. 2C, solid lines).
Rejection
Clarification of the extent to which the fibrosis was due to disease recurrence required analyses of rejection. The histopathologic diagnosis of rejection was made earlier and more frequently in patients treated with ATG-TAC than in those under TAC-PRED in both the study group of HCV-infected patients and in the contemporaneous HCV-free recipients (Fig. 2D). Hepatic fibrosis scores of the uninfected recipients treated with ATG-TAC were no worse than those of the infected and uninfected recipients treated with TAC-PRED (Fig. 2C, compare solid and dashed lines). There were no graft losses or late chronic rejections under either kind of immunosuppression regardless of the HCV status.
Temporal Associations
In HCV-infected recipients whose grafts survived more than a month (19 in each treatment group), the virus genotype, HCV titer before transplantation, and the maximum titer during the first two years were similar in the ATG-TAC and TAC-PRED groups (Table 3). The incidence and duration of antiviral treatment were also similar, but the onset of this treatment was earlier in the ATG-TAC patients because of their propensity for earlier recurrence. The higher incidence of acute rejection in weaned patients who originally were treated with ATG-TAC resulted in a large percentage of patients who were then “rescued” with delayed alemtuzumab (Campath). In these recipients, however, an increase in hepatitis activity and staging fibrosis already was present by the time of the alemtuzumab rescue (Table 3). Thus, the primary risk factor for recurrence was the weaning, rather than the rescue therapy.
Maintenance Immunosuppression
ATG-TAC
Two of the 19 surviving HCV-free patients treated with ATG-TAC have been off all immunosuppression for more than a year. Ten more are on spaced doses of tacrolimus monotherapy: every other day (n = 2), three times per week (n = 5), and two times per week (n = 3). Of the other 7, 5 are on daily tacrolimus monotherapy and only 2 receive an additional immunosuppressant.
One of the 11 HCV-infected patients who still survive after ATG-TAC treatment has been off all immunosuppression for more than a year. Five others are on spaced doses of tacrolimus only: three times per week (n = 1), two times per week (n = 1), one time per week (n = 1), and every two weeks (n = 2). Of the other 5, three are on daily tacrolimus monotherapy and two receive an additional immunosuppressant.
TAC-PRED
No attempt was made to space wean tacrolimus in either the infected or uninfected patients treated with the TAC-PRED protocol. The greater long-term exposure to a calcineurin-inhibitor drug was reflected in the 43 currently surviving recipients of these two groups by a 9.3% incidence of renal dialysis or transplantation and a 29% incidence of hypertension vs. rates of 3.3% and 16% in the 30 survivors treated with ATG-TAC.
Discussion
Although it may be assumed that HCV recurred promptly in every patient,20 clinically significant manifestations were almost completely avoided out to three years in our HCV-infected liver recipients who were immunosuppressed throughout with daily doses of tacrolimus to which prednisone was added in large doses for the first 5 postoperative days and slowly weaned thereafter. The competing strategy of intraoperative lymphoid depletion, steroid avoidance, and later weaning from tacrolimus monotherapy clearly promoted life-threatening HCV disease recurrence. An explanation of the disparate results begins with consideration of basic mechanisms.
The presence of a surgically revascularized organ transplant is recognized only when the graft's passenger leukocytes migrate to host lymphoid organs where a donor-specific cytolytic T lymphocyte (CTL) response is induced. In the same way, a pathogen-specific response is induced by the hematogenous spread to lymphoid organs of the noncytopathic HCV.10,21-23 The activated CTL then target cells displaying complexes of major histocompatibility complex (MHC) molecules plus peptides derived from the specific antigen.24,25 The targeted cells of the hepatitis victim are heavily concentrated in the liver. With transplantation, all of the allogeneic cells of an organ may be targets. In most experimental liver transplant models and in humans, the donor-specific CTL of a nonimmunosuppressed recipient acutely reject the peripheralized donor leukocytes as well as the “nonself” parenchymal cells of the source hepatic allograft. Thus, rejection of the constituent cells of a liver allograft is comparable to the destruction of HCV-infected liver cells during a bout of acute infectious hepatitis. In either case, the live antigen may persist in small quantities in protected niches, periodically move to lymphoid organs, and stimulate continued antigen-specific immunity.11,12,21
Alternatively, the T-cell response may be exhausted and deleted during the first few weeks of maximum antigen distribution.15,16,21,26 Perpetuation of the resulting nonreactive state depends on the persistence of sufficient antigen with access to host lymphoid organs.11,12,21 Residual antigen is therefore a two-edged sword, the effects of which may range from ongoing low-grade immunity (perhaps equivalent in some cases to memory) to the other extreme of durable deletional tolerance. Between these extremes, various balances may be reached between the amount of mobile cells expressing “nonself” antigen and the number of antigen-specific CTL.
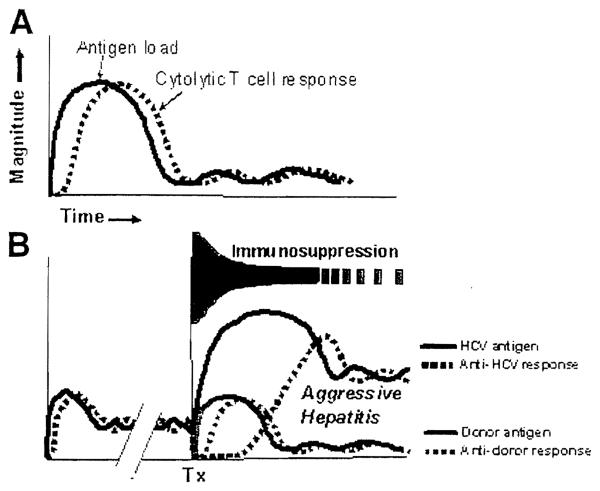
In our reference immunologic paradigm, these balances are manifested by a diversity of transplant outcomes and by analogous virus carrier syndromes, all of which represent different degrees of partial tolerance.11,12 Such immunologic “compromises” include chronic allograft rejection and its analog, chronic hepatitis. From this perspective, it is apparent that the typical HCV-infected patient who comes to hepatic replacement after years or decades of slowly evolving liver damage is variably tolerant to the virus at the time strong transplant-related immunosuppression is started. In contrast, the allograft induces a de novo donor-specific response (Fig. 3).
Figure 3.
(A) Interrelation of noncytopathic antigen quantity and the antigen-specific cytolytic T-cell (CTL) response after a primary HCV infection or, alternatively, after primary liver transplantation. (B) Liver transplantation (Tx) in a patient with chronic aggressive hepatitis long after the viral/CTL balance shown in panel A is reached. The potential effect of immunosuppression on the quantity of the preexisting viral antigen, the de novo transplant antigen, and their respective CTL responses are shown schematically and described in the text. Horizontal axis = time.
Both immunosuppression strategies used in our infected patients clearly disequilibrated the preexisting balance between HCV-specific CTL and virus quantity. However, the resulting viremia was succeeded by severe bridging fibrosis only in the lymphoid-depleted patients who were systematically space weaned. With weaning of these recipients, large numbers of infected liver cells expressing HCV peptides apparently were destroyed by reemerging HCV-specific CTL (Fig. 3). Whether infected or not, the lymphoid depleted and weaned patients had a high incidence of rejection, which was caused by the separate and distinct donor-specific CTL. In the majority of uninfected recipients treated with ATG-TAC, exhaustion and deletion of the antidonor response was manifested by the ability to variably space wean without graft loss or chronic rejection. Whereas the comparably treated HCV-infected recipients suffered an exorbitant mortality from accelerated disease recurrence, the rate-spaced weaning that was accomplished without chronic rejection in those who remained was similar to that in the uninfected recipients.
Why didn't accelerated HCV disease recurrence develop under TAC-PRED despite the proviral effect of the large initial amount of perioperative and postoperative steroids? This can be explained as follows. In these patients, the relatively light double drug immunosuppression reached by the sixth postoperative day (i.e., twice daily tacrolimus doses and prednisone doses of 20 mg/day) was further reduced only by cautious steroid weaning. The continuous immunosuppression presumably controlled the independent donor-specific and HCV-specific CTL responses, allowing establishment of stable balances between these CTL and the respective antigens.
It should be emphasized that the TAC-PRED protocol employed in these patients was identical to the steroid-sparing strategy used when tacrolimus was introduced clinically27,28 and before that when cyclosporine was the baseline agent.29 When the historical strategy was systematically applied in individual centers, 5- and 10-year survivals were essentially the same as in uninfected patients.30,32 The dire consequences of HCV infection subsequently documented in multicenter registries coincided with a worldwide movement to more complex regimens in which three or more drugs were administered for protracted periods from the time of transplantation.4,5,7,33 Although this kind of heavy prophylactic therapy greatly reduces the incidence of acute rejection, we previously have suggested that the resulting excessive immunodepression can subvert the seminal mechanism of allograft-induced tolerance (i.e., clonal exhaustion-deletion) and thereby contribute to long-term immunosuppression dependence.11,12
How these multiagent protocols also may promote accelerated HCV disease recurrence in the same way as in our ATG-TAC cohort of recipients can be readily envisioned. Both the ATG-TAC protocol and the regimens of multiple-drug prophylactic immunosuppression cause viremia. In turn, the magnitude and duration of viremia has been associated with the onset and severity of disease recurrence.34 Such observations have prompted speculation by some authorities that the normally noncytopathic HCV may become cytotoxic.35 However, in the paradigm used to interpret our clinical results, the viremia that is inevitable with any effective immunosuppression is neither cytotoxic nor the critical factor per se.
Instead, the treatment outcome depends on the effect of the immunosuppression on the overall balance between virus distribution and immune responsiveness.12 When high-dose multiple-drug strategies are instituted at the time of transplantation, reduction and/or discontinuance of one or more constituents of the “cocktail” ultimately becomes obligatory to avoid the lethal risk of prolonged general immunodepression. The cells of the transplanted liver, which presumably have been heavily infested in proportion to the extent of the immunosuppression-induced viremia, are then targeted by the slowly recovering HCV-specific CTL as well as by the slowly recovering donor-specific CTL whose efficient activation → exhaustion-deletion has been precluded by the early heavy treatment.12 If recovery of the independent HCV-directed and graft-directed CTL responses occurs out of phase and the isolated diagnosis of HCV recurrence is made at this juncture, the instinctive decision to abruptly reduce immunosuppression may make the recurrence worse in the same way as occurred with spaced weaning in the ATG-TAC cohort (Fig. 3).
Thus, the optimum immunosuppression for HCV-infected liver recipients is contingent on its timing and continuity, not the avoidance of a specific drug or drugs (including steroids and the lymphoid-depleting agents). In this view, the aims of treatment of a recipient with HCV disease should be to predict, monitor, and equilibrate beneficial balances between virus distribution and the absence of an immunopathological anti-HCVT-cell response. The TAC-PRED protocol exemplifies how these objectives are achievable. The initial very short course of high doses of preemptive prednisone protects the graft from irreversible damage from acute rejection, followed by extremely cautious and gradual steroid weaning. If over-immunosuppression is scrupulously avoided after the first few days, interference with donor-specific tolerogenesis may be minimized, allowing low levels of maintenance immunosuppression to be promptly reached.12,13
Other protocols with different drug combinations that include antilymphoid antibodies may yield good results if these therapeutic principles are applied. For example, Eason et al. observed no degradation of results after a mean follow-up of 18.5 months in a large series of HCV-infected patients treated perioperatively with ATG and managed thereafter with an absolutely steroid-free posttransplant regimen of daily tacrolimus that was never weaned.36 Although the results with the generalizable approach to immunosuppression exemplified by our TAC-PRED group of recipients can be gratifying, the limitations of the strategy are obvious. These include a lifetime commitment to a relatively fixed level of daily immunosuppression and the epidemiologic implications of producing HCV carrier recipients. Further improvements will require the same steps that led to the control of HBV. The highest public health priority is the development of an effective vaccine to protect health care providers and others at risk. For infected liver transplant candidates, the foremost objective is containment of the viral load with yet to be developed HCV-specific drugs: e.g., the protease inhibitor described by Reiser et al.37 An alternative option is the development of better neutralizing anti-HCV antibodies.38,39
Abbreviations
- HCV
hepatitis C virus
- TAC
tacrolimus
- PRED
prednisone
- ATG
antithymocyte globulin
- HBV
hepatitis B virus
- CTL
cytotoxic T lymphocyte
References
- 1.Corman JL, Putnam CW, Iwatsuki S, Redeker AG, Porter KA, Peters RL, et al. Liver allograft. Its use in chronic active hepatitis with macronodular cirrhosis, hepatitis B surface antigen. Arch Surg. 1979;114:75–78. doi: 10.1001/archsurg.1979.01370250077016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13:619–626. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim WR, Poterucha JJ, Kremers WK, Ishitani MB, Dickson ER. Outcome of liver transplantation for hepatitis B in the United States. Liver Transpl. 2004;10:968–974. doi: 10.1002/lt.20217. [DOI] [PubMed] [Google Scholar]
- 4.Curry MP. Hepatitis B and hepatitis C viruses in liver transplantation. Transplantation. 2004;78:955–963. doi: 10.1097/01.tp.0000140927.63952.58. [DOI] [PubMed] [Google Scholar]
- 5.Berenguer M, Crippin J, Gish R, Bass N, Bostrom A, Netto G, et al. A model to predict severe HCV-related disease following liver transplantation. Hepatology. 2003;38:34–41. doi: 10.1053/jhep.2003.50278. [DOI] [PubMed] [Google Scholar]
- 6.Neumann UP, Berg T, Bahra M, Puhl G, Guckelberger O, Langrehr JM, Neuhaus P. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004;77:226–231. doi: 10.1097/01.TP.0000101738.27552.9D. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner RH, Sorrell MF, Villamil F, the International Liver Transplantation Society Experts Panel, editors. Report of the first International Liver Transplantation Society expert panel consensus conference on liver transplantation and hepatitis C. Liver Transpl. 2003;9(Suppl 3):S1–S119. doi: 10.1053/jlts.2003.50268. Liver transplantation and hepatitis C: a single topic and consensus development symposium. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Luna H, Vargas HE. Management of hepatitis C virus infection in the setting of liver transplantation. Liver Transpl. 2005;11:479–489. doi: 10.1002/lt.20424. [DOI] [PubMed] [Google Scholar]
- 9.Everson GT. Impact of immunosuppressive therapy on recurrence of hepatitis C. Liver Transpl. 2002;8(Suppl 1):S19–S27. doi: 10.1053/jlts.2002.35852. [DOI] [PubMed] [Google Scholar]
- 10.Zinkernagel RM, Hengartner H. T-cell mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol Today. 1994;15:262–268. doi: 10.1016/0167-5699(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Zinkernagel RM. Transplantation tolerance from a historical perspective. Nature Reviews. Immunology. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metes D, Logar A, Rudert WA, Zeevi A, Woodward J, Demetris AJ, et al. Four-color flow cytometric analysis of peripheral blood donor cell chimerism. Hum Immunol. 2003;64:787–795. doi: 10.1016/s0198-8859(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 17.Demetris AJ, Eghtesad G, Marcos A, Ruppert K, Nalesnik MA, Randhawa P, et al. Recurrent hepatitis C in liver allografts: prospective assessment of diagnostic accuracy, identification of pitfalls, and observations about pathogenesis. Am J Surg Pathol. 2004;28:658–669. doi: 10.1097/00000478-200405000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain A, Demetris AJ, Manez R, Tsamanadas AC, Van Thiel D, Rakela J, et al. Incidence and severity of acute allograft rejection in liver transplant recipients treated with alfa interferon. Liver Transpl Surg. 1998;4:197–203. doi: 10.1002/lt.500040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakil AO, McGuire B, Crippin J, Teperman L, Demetris AJ, Conjeevaram H, et al. A pilot study of interferon alfa and ribavirin combination in liver transplant recipients with recurrent hepatitis C. Hepatology. 2002;36:1253–1258. doi: 10.1053/jhep.2002.36162. [DOI] [PubMed] [Google Scholar]
- 20.Konig V, Bauditz J, Lobeck H, Lusebring R, Neuhaus P, Blumhardt G, et al. Hepatitis C virus reinfection in allografts after orthotopic liver transplantation. Hepatology. 1992;16:1137–1143. doi: 10.1002/hep.1840160506. [DOI] [PubMed] [Google Scholar]
- 21.Zinkernagel RM, Moskophidis D, Kundig T, Oehen S, Pircher H, Hengartner H. Effector T-cell induction and T-cell memory versus peripheral deletion of T cells. Immunol Rev. 1993;133:199–223. doi: 10.1111/j.1600-065x.1993.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 22.Zinkernagel RM. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 23.Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H. Antigen localization regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 24.Doherty PC, Zinkernagel RM. A biological role for the major histocompatibility antigens. Lancet. 1975;i:1406–1409. doi: 10.1016/s0140-6736(75)92610-0. [DOI] [PubMed] [Google Scholar]
- 25.Zinkernagel RM, Doherty PC. The discovery of MHC restriction. Immunol Today. 1997;18:14–17. doi: 10.1016/s0167-5699(97)80008-4. [DOI] [PubMed] [Google Scholar]
- 26.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 27.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todo S, Fung JJ, Starzl TE, Tzakis A, Demetris AJ, Kormos R, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Klintmalm GBG, Porter KA, Iwatsuki S, Schroter GPJ. Liver transplantation with use of cyclosporin A and prednisone. N Engl J Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah G, Demetris AJ, Gavaler JS, Lewis JH, Todo S, Starzl TE, Van Thiel DH. Incidence, prevalence, and clinical course of hepatitis C following liver transplantation. Gastroenterology. 1992;103:323–329. doi: 10.1016/0016-5085(92)91130-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, et al. Long-term survival after liver transplantation in 4000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghobrial RM, Steadman R, Gornbein J, Lassman C, Holt CF, Chen P, et al. A 10-year experience of liver transplantation for hepatitis C: analysis of factors determining outcome in over 500 patients. Ann Surg. 2001;234:384–393. doi: 10.1097/00000658-200109000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berenguer M, Prieto M, San Juan F, Rayon JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;236:202–210. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 34.Charlton M, Seaberg E, Wiesner R. Predictors of patients and graft survival following liver transplantation for hepatitis C. Hepatology. 1998;28:823–830. doi: 10.1002/hep.510280333. [DOI] [PubMed] [Google Scholar]
- 35.Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl. 2003;9:S28–S34. doi: 10.1053/jlts.2003.50248. [DOI] [PubMed] [Google Scholar]
- 36.Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE., Jr Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75:1396–1399. doi: 10.1097/01.TP.0000062834.30922.FE. [DOI] [PubMed] [Google Scholar]
- 37.Reiser M, Hinrichsen H, Benhamou Y, Reesink HW, Wedemeyer H, Avendano C, et al. Antiviral efficacy of NS3-serine protease inhibitor BILN-2061 in patients with chronic genotype 2 and 3 hepatitis C. Hepatology. 2005;41:832–835. doi: 10.1002/hep.20612. [DOI] [PubMed] [Google Scholar]
- 38.Klenerman P. Commentary: T cells get by with a little help from their friends. Eur J Immunol. 2004;34:313–316. doi: 10.1002/eji.200324844. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann MF, Hunziker L, Zinkernagel RM, Storni T, Kopf M. Maintenance of memory CTL responses by T helper cells and CD40-CD40 ligand: antibodies provide the key. Eur J Immunol. 2004;34:317–326. doi: 10.1002/eji.200324717. [DOI] [PubMed] [Google Scholar]