Abstract
The Ficini [2 + 2] cycloaddition using N-sulfonyl substituted ynamides is described, featuring the utility of CuCl2 and AgSbF6 as catalysts. This work represents the first success example of ynamides participating in a thermal [2 + 2] cycloaddition with enones.
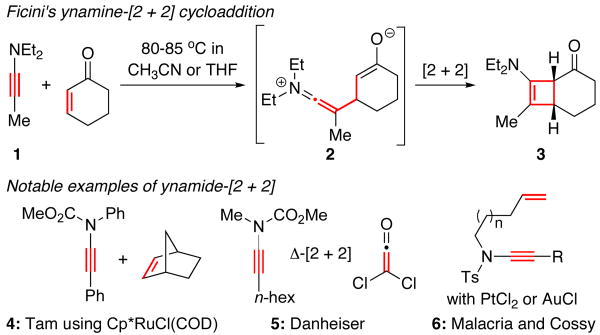
More than 40 years ago, Ficini1 disclosed perhaps the most useful carbon-carbon bond forming reaction involving ynamines2: a thermally driven stepwise [2 + 2] cycloaddition3 of ynamine [1] with cyclic enones, leading to the formation of cyclobutenamine 3 [Scheme 1].4-6 In the last 15 years, ynamides have emerged as a superior synthetic equivalent of ynamines.7,8 Beautiful chemistry in the area of [2 + 2] cycloadditions has followed by way of Tam's Ru-catalyzed ynamide-[2 + 2] cycloaddition of norbornene,9 Danhesier's thermal cycloaddition of ketenes,10 and formal [2 + 2] processes through enyne cycloisomerizations using platinum or gold catalysts developed by Malacria11 and Cossy.12 However, a thermally driven stepwise [2 + 2] cycloaddition in a Ficini manner using ynamides remained elusive.13 Our own efforts in trying to develop this cycloaddition reaction lasted for 13 years. We report here our first success in a Ficini [2 + 2] cycloaddition of ynamides.
Scheme 1.
Ficini's Ynamine-[2 + 2] Cycloadditions.
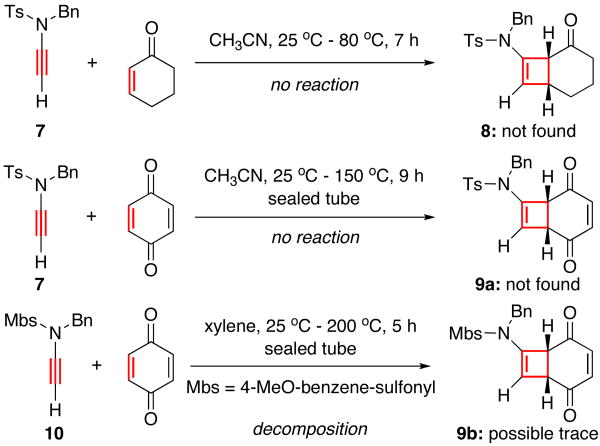
Over the last 15 years, we failed numerous attempts at succeeding Ficini [2 + 2] cycloaddition of ynamides using lactam or oxazolidinone substituted ynamides under thermal and/or Lewis-acidic consitions.14 In the current pursuit of this cycloaddition, we chose to employ N-sulfonyl substituted ynamides because the nitrogen pair of the sulfonamido group is more delocalized toward the alkyne.15 Therefore, N-sulfonyl substituted ynamides possess enhanced nucleophilicity over simple amide or urethane substituted ynamides, and they are also less stable than amide or urethane substituted ynamides.
However, to our disappointment, N-sulfonyl substituted ynamides such as 7 and 10 did not undergo any desired thermal cycloaddition [Scheme 2]. Even when we used quinone and adopt the more electron-rich para-methoxy benzensulfonyl group [Mbs] as shown in ynamide 10, no appreciable amount of the desired cycloadduct 9b was observed, thereby further underscoring the superior stability of ynamides over ynamines.
Scheme 2.
Thermal Ficini [2 + 2] Cycloadditions of Ynamide.
Our next best option would appear to again involve Lewis acids, which had not been successful over the years when using lactam or oxazolidinone substituted ynamides.14 More specifically, our efforts were derailed when using Lewis acids because hydro-halogenations of ynamides, leading to alpha-halogenated enamides, was a serious competing pathway.14,16,17 In addition, when hydro-halogenation is not competing, possible hydrolysis under these suitable Lewis acids represents another challenge associated with ynamides. Consequently, much of ynamide chemistry7a has been limited to halo-substituted Lewis acids that do not involve metals such as Mg, Ti, Sn, Si, B, Al, or In [i.e., CuX2 or ZnX2 is feasible], or Lewis acids with OTf serving as the counter anion. As a result, we screened a small sample of Lewis acids as summarized in Table 1.
Table 1.
A Cu(II)-Catalyzed Ynamide-[2 + 2] Cycloaddition.
 | ||||||
|---|---|---|---|---|---|---|
| entry | R = | solvent | catalyst [mol %] | temp [°C] | time [h]a | yield [%]b |
| 1 | 10: H | CH3CN | In(OTf)2 [30] | - 15 | 1 | --c |
| 2 | 10: H | CH3CN | Sc(OTf)3 [30] | - 15 | 1 | --c |
| 3 | 10: H | CH3CN | Cu(OTf)2 [10] | 25 – 80 | 4 | --d |
| 4 | 10: H | CH3CN | AgSbF6 [10] | 0 – 80 | 5 | --d |
| 5 | 10: H | CH3CN | AgSbF6 [10] | 50 – 120 | 2 | --d |
| 6 | 10: H | CH2Cl2 | CuCl2/AgSbF6 [20/42] | - 78 – 25 | 10 | ≤ 5d,e |
| 7 | 12: Me | CH2Cl2 | CuCl2/AgSbF6 [20/60] | - 40 | 1 | 72 |
| 8 | 12: Me | CH2Cl2 | CuCl2/AgSbF6 [20/60] | - 15 | 1 | 77 |
| 9 | 12: Me | CH2Cl2 | CuCl2/AgSbF6 [20/60] | 0 | 1 | 76 |
Time for syringe pump addition of a solution of 10 [or 12] and enone.
Isolated yields.
Hydrolysis of 10 was the major outcome.
No reaction – recovered starting material 10.
Polymerization was the major outcome in addition to hydrolysis.
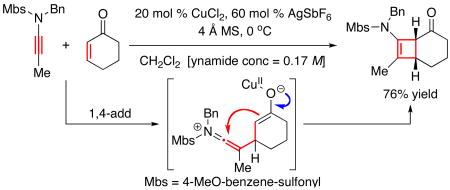
Initial failure is quite evident in entries 1-6 when using ynamide 10. However, after observing trace amount of the possible product 11 when using CuCl2 and AgSbF4 [entry 6], we speculated that 10 was polymerizing under these reactions conditions. Therefore, we turned to ynamide 12 with a Me group as the terminal-substitution. Gratifyingly, we found that cycloadduct 1318 could be attained in good yields at three different low temperatures within an hour [entries 7-9]. This result represents the first successful Ficini [2 + 2] cycloaddition using ynamides. Cycloadduct 13 was unambiguously assigned using X-ray [Figure 1]. It is noteworthy that the amido-cyclobutene motif is quite robust. The pericyclic ring-opening does not occur readily since the allowed thermal conrotatory ring-opening would lead to a trans-cycloalkenone.19
Figure 1.
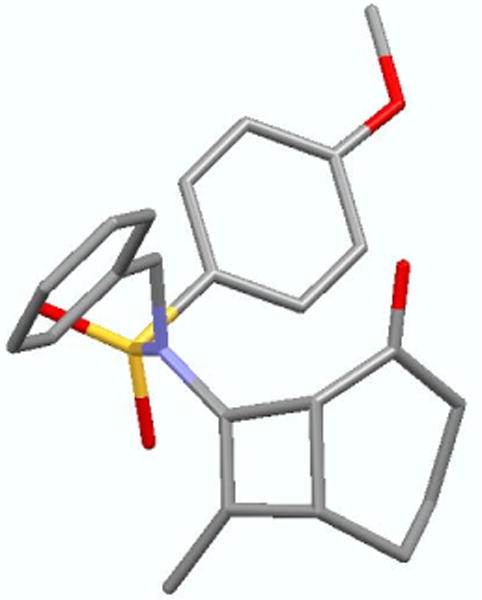
X-Ray Structure of the [2 + 2] Cycloadduct 13.
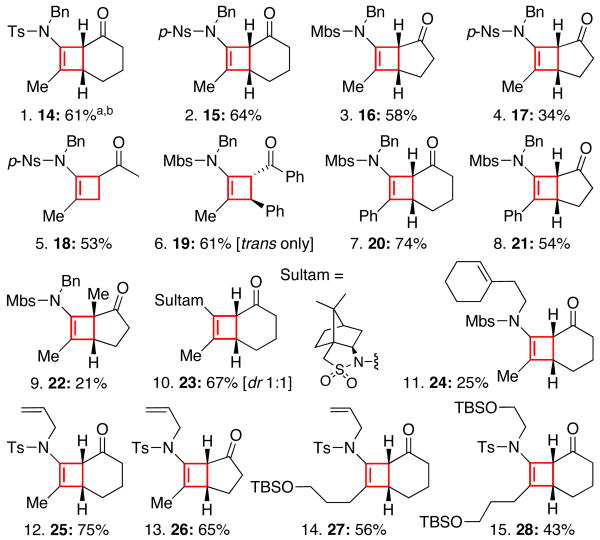
The generality of this cycloaddition could be established from examples shown in Figure 2. Several features are: (a) The N-sulfonyl group does not need to be Mbs [entries 1, 2, and 10]; (b) acyclic enones are also suitable [entries 5 and 6];20 (c) the alkyne substitutions [entries 7, 8, 14, and 15] and substitutions on the nitrogen atom [entries 11-15] can be varied, which should significantly enhance the potential applications of these cycloadducts.
Figure 2.
Scope of the Ynamide-[2 + 2] Cycloaddition.
a. All reactions were carried out in anhyd CH2Cl2 [ynamide conc = 0.17 M] using 4 Å MS, 20 mol % of CuCl2, and 60 mol % of AgSbF6; CuCl2 and AgSbF6were premixed at RT for 1 h prior to the addition of a respective ynamide and enone [1.20 equiv] as a combined solution via a syringe pump over 1 h at 0 °C; the reaction was stirred for an additional 30 min to 1 h before isolation. b. Isolated yields.
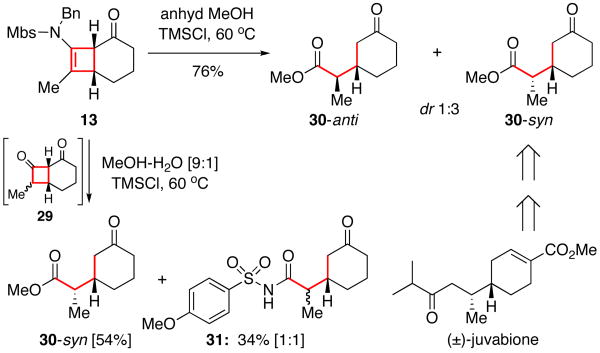
Moreover, the [2 + 2] cycloadducts such as 13 could be subjected to hydrolytic conditions and further undergo retro-Claisen via the intermediacy of diketone 29 [Scheme 3], leading to keto-ester 30.21 Intriguingly, while anhydrous conditions led to 30 in 76% yield, when using MeOH-H2O as solvent, keto-imide 3122 was found in addition to 30. Ficini also observed ketoamide formation but only under neutral or basic hydrolytic conditions, and its formation likely proceeded through an aminal intermediate.1,4,23 The modest syn-selectivity was also reported in Ficini's related work,4,23 and the saponified 30-syn was used by Ficini in their synthesis of (±)-juvabione.24
Scheme 3.
Stereoselective Hydrolysis of the Cycloadduct 13.
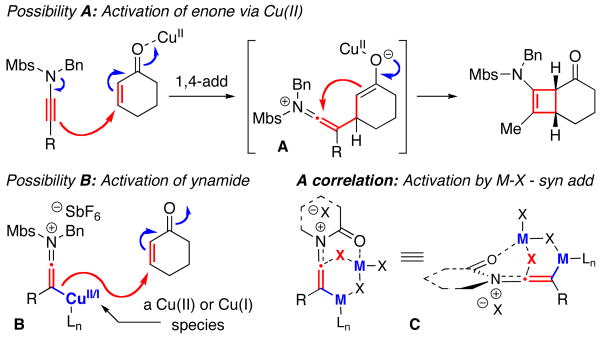
Lastly, a simple and straightforward mechanistic consideration would be that this is step-wise cycloaddition with a nucleophilic 1,4-addition by the ynamide onto the enone activated via the cationic Cu(II) catalyst [see Possibility A in Figure 3]. However, there may be another possibility. That is, the cationic Cu(II) species is activating the alkyne [Possibility B], leading to an intermediate that could participate in a cuprate-like 1,4-addition. While we are not sure of the oxidation state of such copper species, this proposed possibility resonates with our earlier proposal of the intermediacy of C to explain the exclusive syn addition of “H-X” [hydro-halogenation] to ynamides that was observed when using catalysts such as MgX2,14 TiCl4,16 or Rh(I)Cl(Ph3P)3.17 We are currently exploring such a mechanistic possibility.
Figure 3.
Mechanistic Considerations.
We have uncovered here the Ficini [2 + 2] cycloaddition using ynamides. These reactions could be catalyzed using CuCl2 and AgSbF6. Efforts are underway to develop synthetic applications of this cycloaddition reaction.
Supplementary Material
Acknowledgments
We thank NIH [GM066055] for funding. We thank Dr. Vic Young of the University of Minnesota for providing X-ray structural analysis. We also thank Professor Steve Burke of University of Wisconsin for valuable discussions.
Footnotes
Supporting Information Available: Experimental procedures as well as NMR spectra, and characterizations are available for all new compounds and free of charge via Internet http://pubs.acs.org.
References
- 1.For a seminal review on Ficini [2 + 2] cycloaddition using ynamines, see: Ficini J. Tetrahedron. 1976;32:1448.
- 2.For two other comprehensive reviews on ynamine chemistry, see: Himbert G. In: Methoden Der Organischen Chemie (Houben-Weyl) Kropf H, Schaumann E, editors. Georg Thieme Verlag; Stuttgart: 1993. pp. 3267–3443.Zificsak CA, Mulder JA, Hsung RP, Rameshkumar C, Wei LL. Tetrahedron. 2001;57:7575.
- 3.For a review on thermal [2 + 2] cycloaddition reactions, see: Baldwin JE. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, Pattenden G, editors. Vol. 5. Pergamon Press; New York: 1991. p. 63.
- 4.Ficini J, Krief A. Tetrahedron Lett. 1969;10:1431.Ficini J, Touzin AM. Tetrahedron Lett. 1972;13:2093.Ficini J, Touzin AM. Tetrahedron Lett. 1972;13:2097.Ficini J, Touzin AM. Tetrahedron Lett. 1974;15:1447.Tetrahedron Lett. 1977;18:1931. (f) For cycloadditions to quinone, see: Ficini J, Krief A. Tetrahedron Lett. 1967;8:2497.
- 5.For related examples that were contemporary, see: Franck-Neuman M. Tetrahedron Lett. 1966;7:341.Grubbs RH. PhD Dissertation. Columbia University; 1968. Kuehne ME, Linde H. J Org Chem. 1972;37:4031.
- 6.For Ficini's later work, see: Ficini J, Guingant A, d'Angelo J, Stork G. Tetrahedron Lett. 1983;24:907.Ficini J, Krief A, Guingant A, Desmaele D. Tetrahedron Lett. 1981;22:725.
- 7.For comprehensive reviews on chemistry of ynamides, see: DeKorver KA, Li H, Lohse AG, Hayashi R, Shi Z, Zhang Y, Hsung RP. Chem Rev. 2010;110 doi: 10.1021/cr100003s. ASAP.Evano G, Coste A, Jouvin K. Angew Chem Int Ed. 2010;49:2840. doi: 10.1002/anie.200905817.
- 8.For recent examples, see: Li H, Antoline JE, Yang JH, Al-Rashid ZF, Hsung RP. New J Chem. 2010;34:1309. doi: 10.1039/c0nj00063a.Kramer S, Madsen JLH, Rottländer M, Skrydstrup T. Org Lett. 2010;12:2758. doi: 10.1021/ol1008685.Banerjee B, Litvinov DN, Kang J, Bettale JD, Castle SL. Org Lett. 2010;12:2650. doi: 10.1021/ol1008679.Gourdet B, Rudkin ME, Lam HW. Org Lett. 2010;12:2554. doi: 10.1021/ol100769p.Jia W, Jiao N. Org Lett. 2010;12:2000. doi: 10.1021/ol1004615.DeKorver KA, Hsung RP, Lohse AG, Zhang Y. Org Lett. 2010;12:1840. doi: 10.1021/ol100446p.Burley GA, Davies DL, Griffith GA, Lee M, Singh K. J Org Chem. 2010;75:980. doi: 10.1021/jo902466f.Yamasaki R, Terashima N, Sotome I, Komagawa S, Saito S. J Org Chem. 2010;75:480. doi: 10.1021/jo902251m.
- 9.(a) Riddell N, Villeneuve K, Tam W. Org Lett. 2005;7:3681. doi: 10.1021/ol0512841. [DOI] [PubMed] [Google Scholar]; (b) Cockburn N, Karimi E, Tam W. J Org Chem. 2009;74:5762. doi: 10.1021/jo9010206. [DOI] [PubMed] [Google Scholar]
- 10.Kohnen AL, Mak XY, Lam TY, Dunetz JR, Danheiser RL. Tetrahedron. 2006;62:3815. doi: 10.1016/j.tet.2005.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marion F, Coulomb J, Courillon C, Fensterbank L, Malacria M. Org Lett. 2004;6:1509. doi: 10.1021/ol049530g.Marion F, Coulomb J, Servais A, Courillon C, Fensterbank L, Malacria M. Tetrahedron. 2006;62:3856. Also see: Soriano E, Marco-Contelles J. J Org Chem. 2005;70:9345. doi: 10.1021/jo0514265.
- 12.(a) Couty S, Meyer C, Cossy J. Angew Chem Int Ed. 2006;45:6726. doi: 10.1002/anie.200602270. [DOI] [PubMed] [Google Scholar]; (b) Couty S, Meyer C, Cossy J. Tetrahedron. 2009;65:1809. [Google Scholar]
- 13.For a beautiful equivalent of this reaction using ynol-ethers and AgNTf2, see: Sweis RF, Schramm MP, Kozmin SA. J Am Chem Soc. 2004;126:7442. doi: 10.1021/ja048251l.
-
14.Mulder JA, Kurtz KCM, Hsung RP, Coverdale HA, Frederick MO, Shen L, Zificsak CA. Org Lett. 2003;5:1547. doi: 10.1021/ol0300266. [DOI] [PubMed] [Google Scholar];
- 15.While sulfonamides [R1(SO2)-N(H)R2 are more acidic than amides R1CO2N(H)R2 in general because of the overall stability different between the respective conjugate bases [as one referee kindly pointed out], sulfonyl substituted ynamides [or enamides] are more reactive and less stable than simple amide or urethane substituted ynamides [or enamide]. The nitrogen lone pair in the former is more delocalized into the alkyne [or alkene motif], and more into the carbonyl group in the latter. Likewise but in a reverse sense, for iminium ion chemistry, sulfonyl substituted iminium species are more stable and less reactive than straight N-Acyl iminium ions because the nitrogen lone pair in the former is more involved in the pi-donation to the carbocation. See: Royer J, Bonin M, Micouin L. Chem Rev. 2004;104:2311. doi: 10.1021/cr020083x.
- 16.Kurtz KCM, Hsung RP, Zhang Y. Org Lett. 2006;8:231. doi: 10.1021/ol052487s. [DOI] [PubMed] [Google Scholar]
- 17.For alpha-halogenations of ynamides observed using Pd(0) and Rh(I), see: Tracey MR, Zhang Y, Frederick MO, Mulder JA, Hsung RP. Org Lett. 2004;6:2209. doi: 10.1021/ol0493251.Oppenheimer J, Johnson WL, Tracey MR, Hsung RP, Yao PY, Liu R, Zhao K. Org Lett. 2007;9:2361. doi: 10.1021/ol0707362.
- 18.See Supporting Information.
- 19.Ficini J, Dureault A. Tetrahedron Lett. 1977;18:809. Also see: Büchi G, Burgess EM. J Am Chem Soc. 1960;82:4333.Corey EJ, Bass JD, Le Mahisu R, Mitra RB. J Am Chem Soc. 1964;86:5570.
-
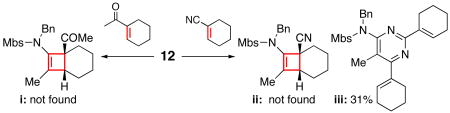
20.Conjugation appears to be a key, as cyclohexenyl methyl ketone did not give i when reacted with ynamide 12. On the other hand, cyclohexenyl nitrile gave a completely different product pyrimidine iii, thereby suggesting a cyclotrimerization process. Regiochemistry of iii was assigned using nOe [see SI].
- 21.Mikami K, Terada M, Nakai T. J Org Chem. 1991;56:5456. [Google Scholar]
- 22.Keto-imide 31 could be further hydrolyzed to 30-syn and 30-anti in 1:1 ratio using the same conditions.
- 23.(a) Ficini J, Guingant A. Nouv J Chim. 1980;4:421. [Google Scholar]; (b) Ficini J, Desmaele D, Touzin AM. Tetrahedron Lett. 1983;24:1025. [Google Scholar]; (c) Ficini J, Eman A, Touzin AM. Tetrahedron Lett. 1976;17:679. [Google Scholar]
- 24.Ficini J, d'Angelo J, Noiré J. J Am Chem Soc. 1974;96:1213. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.