Abstract
Five patients had complete cadaveric small bowel transplants under FK506 immunosuppression, one as an isolated graft and the other 4 in continuity with a liver. Three were children and two were adults. The five patients are living 2–13 months posttransplantation with complete alimentation by the intestine. The typical postoperative course was stormy, with sluggish resumption of gastrointestinal function. The patient with small intestinal transplantation alone had the most difficult course of the five, including two severe rejections, bacterial and fungal translocation with bacteremia, renal failure with the rejections, and permanent consignment to renal dialysis. The first four patients (studies on the fifth were incomplete) had replacement of the lymphoreticular cells in the graft lamina propria by their own lymphoreticular cells. Although the surgical and aftercare of these patients was difficult, the eventual uniform success suggests that intestinal transplantation has moved toward becoming a practical clinical service.
Until recently, death or graft loss after clinical intestinal transplantation usually was caused by failure to control rejection and/or the inability to prevent an attack on the host by graft lymphoid tissue (1). A stimulus for continued trials was provided in 1987 by the prolonged survival without rejection or GVHD of a patient whose functioning multivisceral graft contained the entire small bowel and other intraabdominal hollow viscera (2). Using a variation of the multivisceral operation in which the liver and small intestine constituted the graft, Grant et al. (3) achieved complete rehabilitation of a 41-year-old woman who is still alive after 33 months (Personal communication, W. Wall, April 1991). These patients and a handful of others with isolated intestinal grafts (4-6) or short segments of duodenum and jejunum in cluster grafts (7,8) were treated with cyclosporine-based immunosuppression.
In several animal intestinal transplant models (9-12), FK506 has provided results superior to cyclosporine, prompting us to institute a clinical intestinal transplant trial. We report here 5 consecutive cases—one with small intestinal transplantation alone and the other 4 with an intestine-liver combination. All 5 patients are alive after 2–13 months and nutritionally supported entirely by their intestinal grafts.
MATERIALS AND METHODS
Two of the recipients were young adults and 3 were children. Four of the 5 patients also had liver failure after 30–52 months of parenteral hyperalimentation. The recipient of the isolated intestine lost his small bowel 6 months before transplantation and still had good (although not normal) liver function. These and other features of the 5 cases are summarized in Table 1.
Table 1.
Clinical features
| Preoperative TPM | Liver failure | Transplantation | Postoperative course | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (years) |
Sex | Cause of short gut syndrome |
Remaining intes- tine |
Duration (months) |
Complication | Total bilirubin (mg/dl) |
Portal hy- pertension |
Date | Graft | ICU Stay (weeks) |
Closure of stomas (weeks) |
Hospital stay (weeks) |
Discontinuation of TPN (weeks) |
Graft func- tion |
Survival (days) |
Comment |
| 1 | 31.1 | M | Gunshot injury of SMA |
Transverse colon- rectum |
6 | Systemic sepsis, cholecystitis |
1.1 | No | 5/2/90 | Small bowel | 2 | 8 (proximal), 16 (distal) |
36 | 36 | Satisfactory | 394 | Fully active but on hemodi- alysis |
| 2 | 2.3 | F | Necrotizing enterocolitis |
Transverse colon- rectum |
38 | Systemic sepsis, thrombophlebitis, cholelithiasis |
15.1 | Yesa | 7/24/90 | Small bowel Liver |
0.7 | 8 (both) | 29 | 8 | Good | 311 | Fully active |
| 3 | 26.7 | F | SMA thrombo- sis |
Jejunum (20 cm), ileum (10 cm), whole colon |
30 | Systemic sepsis, thrombophlebitis (extensive) |
18.1 | No | 8/3/90 | Small bowel Liver |
0.6 | 10 (both) | 21 | 15 | Good | 301 | Fully ac- tive, re- quired femoral artery graft |
| 4 | 4.3 | M | Gastroschisis | Jejunum, (10 cm) transverse co- lon-rectum |
52 | Systemic sepsis, thrombophlebitis, cholelithiasis |
16.4 | Yesa | 11/24/90 | Small bowel Liver |
0.4 | 8 (both) | 17 | 16 | Good | 188 | Fully active |
| 5 | 2.8 | M | Intestinal atre- sia |
Transverse colon- rectum |
33 | Systemic sepsis, thrombophlebitis |
42.0 | Yesa | 3/24/91 | Small bowel Liver |
6 | – | >8 | 6 | Good | 67 | In hospital |
Hypersplenism in 2 and variceal hemorrhage in all 3.
The donors
The donors were ABO-identical with the recipient and were either size-matched (2 examples) or significantly smaller (Table 2). HLA matching was random and uniformly poor (Table 2). Donor and recipient were the opposite sex in 3 of the 5 cases. The principles of the donor operations that allow flexibility of planning are described elsewhere (8). An attempt was made at selective bacterial decontamination. Limited core cooling by aortic infusion of a limited amount (1000 ml maximum) of UW solution was used in all but one donor whose uninfused intestine was placed in an ice bath. No attempt was made to alter the graft lymphoreticular tissue with ALG or other modalities. In the last 3 cases, the contents of the intestine were entrapped by stapling the proximal jejunum and terminal ileum and carried with the specimen throughout the preservation and implantation; intraluminal washing was performed on the grafts for the first 2 patients.
Table 2.
Demographics of donors and recipients
| HLAa | Preservation of graftsb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Body weight (kg) |
Sex | Blood type | A | B | DR | BW | Solution for vascular perfusion |
Solution for luminal ir- rigation |
Cold ischemia time (hr) |
|
| 1 | Recipient | 86 | M | 0 | 28,31 | 35,51 | 2,5 | 4,6 | |||
| Donor | 54 | F | 0 | 1,3 | 62,70 | 5 | 6 | None | LR | 10.5 | |
| 2 | Recipient | 12.4 | F | 0 | 19,24 | 13,35 | 5,7 | 4,6 | |||
| Donor | 12.0 | F | 0 | 2,28 | 57,60 | 6,7 | 4,6 | UW | LR | 8.9 | |
| 3 | Recipient | 54.2 | F | B | 2 | 7,55 | 2,5 | 6 | |||
| Donor | 45.5 | M | B | 2,30 | 7,18 | 1,11 | 6 | UW | None | 7.7 | |
| 4 | Recipient | 19.6 | M | A | 1,2 | 44,57 | – | 4 | |||
| Donor | 9.1 | F | A | 1,11 | 8,35 | 3,4 | 6 | UW | None | 2.9 | |
| 5 | Recipientc | 12.8 | M | 0 | 2,31 | 18,55 | 4,6 | 6 | |||
| Donor | 13 | M | 0 | 2,34 | 44,45 | 2,8 | 4,6 | UW | None | 10.6 | |
The monoclonal antibodies in italics were used to identify HLA phenotypes of lymphocytes in peripheral blood and intestinal biopsies.
UW (University of Wisconsin solution), LR (lactated Ringer’s solution).
Positive cytotoxic crossmatch with DTT.
Recipient operations
Vascularization: The technique for isolated intestinal transplantation was similar to that originally used by Lillehei et al. (13) in dogs more than 30 years ago, except that arterialization was with a free segment of donor iliac artery that was interposed between the superior mesenteric artery of the intestinal graft and the recipient aorta. In this case (patient 1), the distal stump of the recipient superior mesenteric vein was found after a tedious dissection and anastomosed to the graft SMV.
The 4 liver-intestine transplantations varied in detail but followed the principles described elsewhere (8), which derived in turn from the experimental canine procedure of multivisceral transplantation (14). A typical reconstruction is shown in Figure 1, including the use of an interposition arterial graft that was used in all cases from the recipient aorta to the graft arterial supply. Other specific details of revascularization included (1) The use of the piggy-back venous outflow (15) in all 4 liver-intestine cases with preservation of the intrahepatic inferior vena cava; and (2) portacaval shunting prior to the anhepatic phase of the liver-intestine transplantations to prevent acute congestion of the residual splanchnic bed. The recipient portal vein was later detached and anastomosed to the portal vein or superior mesenteric vein of the allograft in 2 of the 4 patients, thereby assuring transhepatic delivery of pancreaticoduodenosplenic affluent from the native organ. In the other 2 patients, the portacaval shunt was retained permanently. The spleen had been removed at an earlier operation in one recipient. It was kept in 2 others and removed in the fourth in order to make more room for the liver-intestinal graft.
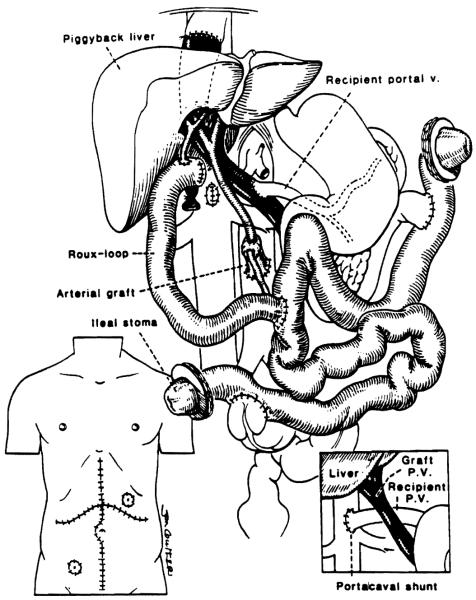
Figure 1.
Liver plus small bowel transplantation. Native liver is replaced by the piggyback technique with the recipient’s portal blood drainage into the graft portal vein or into the inferior vena cava (inset—right). Two ends of the intestinal graft are exteriorized by chimney enterostomy at the left upper and right lower quadrant of the abdomen (inset—left) .
Biliary drainage: No procedure was needed in the patient with intestinal transplantation only. Biliary drainage for the liver-intestine recipients was through an isolated bowel conduit taken from the proximal end of the jejunum and emptied at a lower point into the jejunum (Fig. 1).
Gastrointestinal reconstruction: Continuity was restored in stages in all 5 patients with construction of proximal (graft jejunostomy) and distal (ileostomy) vents (Fig. 1). Because end-to-side anastomoses to the duodenum (or jejunal stump) and colon (or, in one case, stump of ileum) were performed primarily (Fig. 1), the vents allowed early alimentation—or, alternatively, decompression on a moment-to-moment basis. Then 8–16 weeks later when intestinal motility was adequate, the vent chimneys were excised at a second operation.
Immunosuppression
FK506 was given intravenously at first, at 0.10–0.15 mg/kg/day and enterally later at a starting dose of 0.3 mg/kg/day in divided doses, as described elsewhere for simple liver transplantation (16) (Figs. 2 and 3). Maintenance doses usually were lower. Prednisone was given at the outset in all but case 5 and later stopped in each of the children (Fig. 3). Drug therapy was changed from intravenous to enteral (Table 1) when jejunostomy feeding and ultimately oral intake were possible (Figs. 2 and 3). Patient 5 is still being fed and given oral medications through a nasogastric tube with its tip advanced into the graft jejunum.
Figure 2.
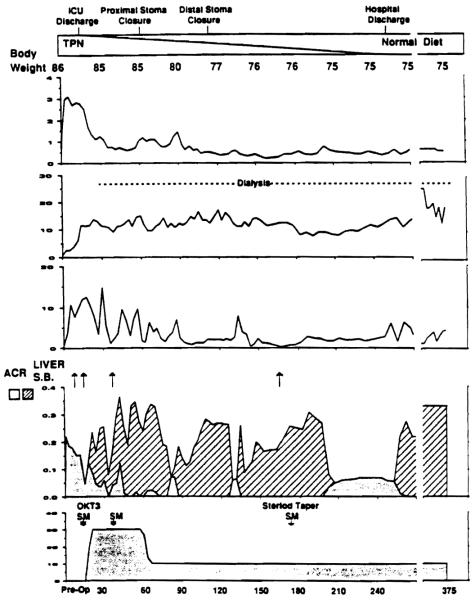
Clinical course of patient 1 who received an isolated small bowel graft. He had a stormy course in the immediate postoperative period, with severe rejection, bacteremia and renal failure requiring dialysis. (TPN, total parenteral nutrition; arrows = ACR (acute cellular rejection) and SM (solumedroll, boluses; SB, small bowel.
Figure 3.
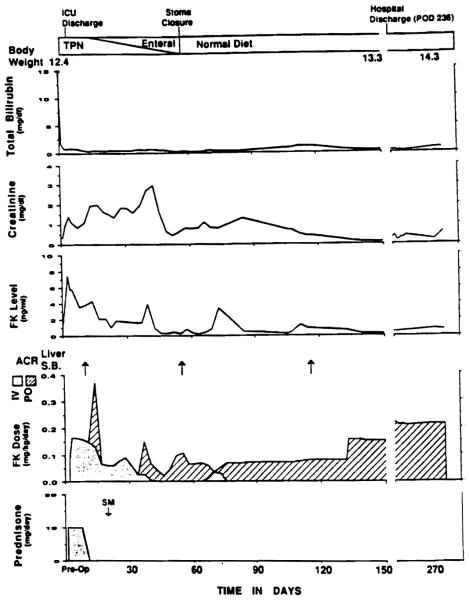
Clinical course of patient 2 who received a liver–plus–small bowel graft. The first episode of intestinal graft rejection (POD 18) was treated by augmentation of the FK dose. Note the rapid decline of the total bilirubin.
Management of immunosuppression was greatly facilitated by serial biopsies of both the upper and lower graft stomas, and of the liver in cases 2–5. These biopsies also were used for studying the graft lymphoreticular and epithelial phenotypes for their donor-recipient specificity as described elsewhere (17).
Bacterial translocation
Frequent blood and stool cultures were obtained and the results compared for similarity and dissimilarity of the flora. Selective decontamination was used for 4 weeks with a combination of polymyxin E, gentamycin/tobramycin, and mycostatin/amphotericin B. Vancomycin was added at the time of positive blood cultures.
Nutrition
Adequacy of intestinal function was judged principally by the ability to hold or gain weight, and to maintain serum protein concentrations. D-xylose absorption tests (18) were performed sporadically.
RESULTS
Survival and hospitalization
The 5 patients are alive, and only the last one (2 months postoperatively) is in the hospital. The first 4 recipients were hospitalized for 4–9 months. primarily because of difficulties in weaning from intravenous to enteral feeding. Restoration of reliable intestinal motility was slow, necessitating frequent switches from parenteral to enteral feeding and back before management could be stabilized. This required 8–36 weeks in patients 1–4. The longest interval of 36 weeks was in the patient who received intestine alone (Table 1). By the end of these times, all food and medications were given by the enteral route. The enteral doses of FK506 and steroids were not different from those in patients with liver transplantation alone (Figs. 2 and 3).
Bacterial translocation
Patients 1, 2, and 4 had the same microorganisms (Candida albicans, Enterococcus fecium/Fecalis, cagula-negative Staphylococcus, and indifferent combinations) in the intestine and blood simultaneously for 3–7 days at 2. 2.5, and 0.6 months postoperatively, respectively. Only one of these incidents was associated with rejection.
Graft function
Liver: None of the recipients of the liver-intestinal grafts (cases 2–5) has been jaundiced for more than a few days. However, patient 2 had continuous hypoalbuminemia (<2 g%) until the third postoperative month. The cause was suspected to be the portacaval shunt used to drain the venous outflow of the recipient stomach, duodenum, and pancreas (Fig. 1, insert). However, the hypoalbuminemia (now >4g%) resolved without treatments and did not recur, despite an unequivocal hepatic rejection (confirmed by biopsy) at 176 days.
Patient 4 had transient jaundice at 3 months when rejection was diagnosed by liver biopsies. The jaundice resolved with increased immunosuppression.
Intestine: After resuming full alimentation, all 5 intestinal recipients have either maintained or gained weight. Patient 1 had a weight loss from 86 to 75 kg during the first 9 postoperative months, but after diet was started and hyperalimentation stopped, weight stabilized and slowly increased. All other patients gained weight (Fig. 4).
Figure 4.
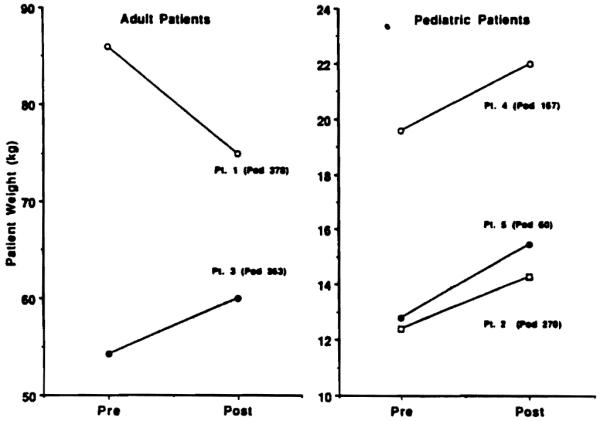
All but one patient gained body weight, from 5 to 21%, at 2–10 months postoperatively. They are supported entirely by their intestinal transplants.
D-xylose absorption in the recipient of the isolated intestine (patient 1) was determined frequently and was normal until after a severe intestinal rejection at day 166. Three weeks later, absorption was severely depressed (Fig. 5, left). The latest D-xylose absorptions in the first three liver-intestine recipients (cases 2–4) ranged from normal to slightly depressed (Fig. 5, right).
Figure 5.
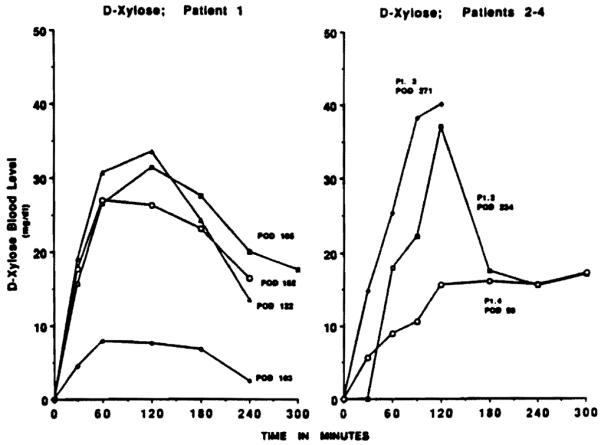
D-xylose absorption tests in 4 small bowel recipients. Absorption in patient 1 (left) was normal until 4 months postoperatively, but was suppressed shortly after when he had drug-noncompliant rejection at 166 days. D-xylose absorption in patients 2 and 3 was normal at 8–9 months postoperatively, and was satisfactory in patient 4. Patient 5 has not been tested yet.
Incidence of rejection
The diagnosis of rejection of either the intestine or liver was made histopathologically at the times indicated in Figures 2 and 3. Most of the biopsies (all summarized in Table 3) were free of unequivocal rejection—and, even when present, the tissue diagnosis did not correlate well with clinical events unless the degree of rejection was classified as prominent.
Table 3.
Histological monitoring of graft rejection and treatment
| Histological degree of rejection | Treatment of rejection [postoperative days] |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Grafts | Number of biopsies |
||||||
| None-to-minimal | Mild | Prominent | Bolus | Recycle | OKT3 | |||
| 1 | Small bowel | 17 | 21, 28, 58, 71, 78, 91, 126, 150, 176, 195, 215, 245, 378 |
7,36 | 14, 166 | 3 [14, 36, 166] |
1 [166] |
1 [14] |
| 2 | Small bowel | 8 | 20, 31, 66, 99, 224, | 10, 55, ll6 | – | 1 [20] |
– | – |
| Liver | 4 | 57, 98, 226 | – | 176 | 1 [230] |
– | – | |
| 3 | Small bowel | 8 | 10, 15, 56, 104, 107, 285 |
25, 35 | – | 1 [35] |
– | – |
| Liver | 6 | 8, 15, 22, 39, 77, | 236 | – | 4 [11, 18, 107, 236] |
– | – | |
| 4 | Small bowel | 8 | 8, 10, 15, 43, 54, 114, 166 |
70 | – | – | – | – |
| Liver | 6 | 15 | 74, 167 | 79, 88, 112 | 4 [11, 82, 118, 120] |
– | – | |
| 5 | Small bowel | 4 | 46, 54 | 11 | 20 | 2 [11, 21] |
– | – |
| Liver | 4 | 21, 23, 46, 54 | – | – | – | – | – | |
The most serious intestinal rejection was in the patient who received an intestine only (case 1). At 14 days, he became hypotensive and acidotic when both graft stomas turned cyanotic. Stomal biopsies showed severe rejection that was reversed with augmented FK506 and prednisone. At 166 days after transplantation after temporary discharge from the hospital, and concomitant with drug noncompliance, he developed the same hypotensive syndrome and ileus. Endoscopy showed mucosal sloughing, and biopsies revealed severe rejection and intramural microabscesses. The process was reversed with immunosuppression.
In the 4 recipients of a liver-intestine graft, both organs had relatively few diagnoses of unequivocal rejection (Table 3). Neither the liver nor the intestine appeared to be more or less favored relative to the other.
Graft lymphoreticular repopulation
In the first four cases the HLA phenotypes of the lymphoid tissue of the lamina propria became those of the recipient after 54–86 days. During the same time, donor mononuclear cells were found in all of the recipients’ peripheral blood. The details of these studies have been published elsewhere (17). Studies in patient 5 are still in progress.
DISCUSSION
This experience has demonstrated the inherent feasibility and practicality of small bowel transplantation in humans, a procedure which was first attempted in humans by Lillehei et a1. almost 25 years ago (19). As with the liver at an earlier time (20), the exploitation of intestinal transplantation awaited better immunosuppression before emancipation from the “forbidden” organ category. Liver transplantation became a service with the advent of cyclosporine, and now intestinal transplantation may come into wide use because of FK506 whose qualities have been shown by direct comparison to be superior to cyclosporine in rat intestinal transplant experiments (10, 11).
Most of the other lessons from the early days of liver transplantation appear also to be applicable to the intestine including the infectious implications of rejection. With its rejection, the hepatic graft becomes a sieve for bacteria translocated from the intestine (21, 22). Paradoxically, such hepatic graft based infections could be prevented or treated only by the heavier immunosuppression that itself contributes to systemic susceptibility to infection. The therapeutic philosophy of preventing bacterial translocation in this way is even more applicable to the intestinal graft in which the foremost requirement is maintenance of an intact mucosal barrier. Using cyclosporine-based immunosuppression in multivisceral recipients, it was not possible to interdict bacterial leakage, with the consequence that there was repeated bacteremia or fungemia with the same microorganisms found in the intestinal content (2). Of the 5 presently reported patients under FK506, 3 had this complication, but it could be controlled with adjustments of immunosuppression combined with efficient antibiotic treatment.
Prevention of GVHD is also dependent upon highly effective immunosuppression. Clinical GVHD was not seen in any of our 5 recipients despite severe histoincompatibility and the appearance of donor mononuclear cells in the peripheral blood of all (17). At the same time, the lymphoreticular cells in the lamina propria of the graft were being replaced by lymphoreticular cells of the recipient. Rapid cell migration and repopulation of lymphoreticular cells in chronically tolerated piglet intestinal grafts under cyclosporine were noted by Arnaud-Battandier et a1. (23) in 1985, and by Jaffe et a1. (24) in the intestinal component of a multivisceral graft that also was the site of lymphoproliferative lesions of recipient origin. Clark et al. (25) and Lear et al. (26) showed striking cell migration in rat intestinal recipients.
In most of these reports, the cell repopulation was equated more with rejection or GVHD than with graft acceptance (24-26). The significance of the cell repopulation phenomenon and its indispensability for graft acceptance was first described by Murase et a1. in rats (12) and by Iwaki et al. in the first 3 patients of the present human series (17). By this process, the transplanted intestine becomes a “composite” organ within a few weeks but with retention of donor-specific epithelium.
Studies are in progress to learn the fate of the donor lymphoreticular cells that leave the graft and enter the circulation. Lear et a1. (26) described the major movement of passenger (presumably T) cells into thymus-dependent host lymphoid tissue, and warned that GVHD could actually be promoted by effective immunosuppression that would allow these cells to become established. The phenomenon of donor cell peripheralization and replacement with recipient cells is probably generic, occurring in all kinds of grafts, although it is most easily studied in the intestine because of the intestine’s rich lymphoid constituency. For example, macrophage repopulation of the liver has been known for almost two decades (27) and similar replacement of the lymphoreticular cells has been described in heart-lung grafts (28).
What was made clear by Murase’s rat experiments (12) and by Iwaki’s study of the presently reported patients (17) is that the relocated cells can be functionally inert, causing neither rejection nor GVHD, provided that the conditions of induction and maintenance immunosuppression are propitious. We have speculated that the cell exchange occurs most reliably in the presence of a normal microenvironment. If so, we have pointed out that the common practices may be ill-conceived, damaging the graft lymphoid deposits with irradiation, antilymphoid globulins, chemotherapy, or other measures (8). No such graft pretreatment was employed in any of our 5 cases.
Even at a technical surgical level, other widely accepted assumptions about intestinal transplantation come into question. For example, the preservation of the intestine was with an extremely simple technique in which the graft was cooled by immersion in an ice bath, or with just enough intraarterial UW solution to cause blanching of the capillary bed. Instead of washing out the succus entericus of the intestine, this was kept with the specimen throughout in 3 of the 5 cases by stapling shut the intestinal graft at the upper and lower ends. There were no consequent infections from this practice.
In the recipient an attempt was made to restore normal anatomic relationships between the residual recipient organs and the transplanted or native liver, and above all to direct the venous affluent from the pancreas, intestine, and other splanchnic viscera through the retained or concomitantly engrafted new liver. The complex metabolic and immunologic interrelationships of the various intraabdominal organs are discussed elsewhere (8,29).
The function of the transplanted intestine alone or with accompanying liver has been satisfactory in all five patients, with eventual complete independence from parenteral nutrition. Since resuming full alimentation, all 5 patients have maintained (1 case) or gained weight (4 cases). Reliable absorption studies with D-xylose were difficult to obtain under these clinical circumstances, and the results did not correlate well with common-sense clinical assessments of the state of nutrition.
The achievement of alimentation was a slow process, requiring from 6 weeks to 9 months. By the time this was achieved, the doses of oral FK506, steroids, and other medications were in the same range as with other kinds of transplantation, indicating the efficient absorption of these medications. Earlier in the postoperative course, the transplanted and denervated intestine was prone to ileus, making it essential to have proximal and distal enteric vents either for decompression or as an entry for graded feedings. There are theoretical reasons for early resumption of alimentation, but practical reasons for doing this with extreme caution.
The question remains whether intestinal transplantation alone is a more or less difficult procedure than combined liver-intestine transplantation. The experimental work of Murase et al. (12, 30) and others (8) has supported the concept that the liver may shield the intestine from immunologic attack, but this advantage is relative only. The long survival of patient 1 in our series and that of the recipients of DeItz et al. (6) and Ricour and Goulet et al. (5) have demonstrated the feasibility of the technically less draconian procedure of isolated intestinal transplantation.
In our patient 1, severe but reversible rejection after 2 weeks and again at 5½ months after isolated small bowel transplantation caused a shock syndrome, with a third space fluid accumulation in the graft, as well as bacteremia and candidemia that led to permanent secondary loss of renal function. However, the intestinal changes were reversible, including healing and regeneration of ulcerative and sloughed intestinal epithelium. The power of the reparative process has been seen even more dramatically in a duodenal jejunal intestinal segment that was part of an upper abdominal cluster graft that recovered to a normal state after being almost completely denuded of epithelium as a consequence of rejection (8, 31).
Table 4.
Donor and recipient lymphocytes in peripheral blood and in the intestinal graft
| Donor lymphocytes in peripheral blood |
Repopulation of recipient lymphocytes in small bowel graft |
|||
|---|---|---|---|---|
| Patient | ||||
| Highest pro- portion (%) |
Postoperative days of disappearance |
Postoperative days |
Proportion of replacement (%) |
|
| 1 | 8.9 | 45 | 72 | 100 |
| 2 | 11.6 | 54 | 86 | 100 |
| 3 | 11.4 | 12 | 77 | 100 |
| 4 | NDa | NDa | 54 | >80 |
Analysis not completed.
Acknowledgments
We thank Mrs. Terry Mangan, Mr. Steve Miller, and Miss Sally Wagner for their help in preparing the manuscript.
Appendix
ORAL DISCUSSION
DR. A. LANGNAS (Omaha, Nebraska): You indicated that there were a number of episodes of bowel rejection. How did you document and classify this? How did you adjust your therapy?
DR. TODO: The most important diagnostic tool for the small bowel recipient is the inspection of the stoma. Cyanotic change is the first sign of rejection. When this is noted, we perform endoscopy through the stoma to obtain biopsy material for histologic confirmation. Later, after the stomas have been closed the situation might be more difficult—however, we have observed only one episode of rejection in that setting. This patient had an episode at 166 days, the result of drug-noncompliance. He had severe abdominal pain and diarrhea. At colonoscopy we saw sloughing of the mucosa, and biopsy showed the denuding of the mucosal layers.
DR. LANGNAS: Have you seen any findings in the submucosa such as the vasculitis described in a number of small animal models?
DR. TODO: Yes, we observed slight arteritic changes in the intestinal wall in one patient when a biopsy was taken at the operation for stomal closure about 4 months after transplantation.
DR. ALSINA (Hartford, Connecticut): I would like to know if you are planning to use portal venous drainage in the future. The issue as to whether this is beneficial or necessary has not been settled. I also have a comment. We have finished a small bowel transplant study in outbred pigs and have observed more graft-versus-host reaction in the native tissues when using very low doses of cyclosporine in combination with azathioprine and prednisone.
DR. TODO: Responding to your comment first, in our laboratory we also saw the GVH disease when the animals were treated with subtherapeutic immunosuppression, but we didn’t see any GVH diseases in our five clinical small bowel recipients. Concerning the first question, we believe that venous drainage from the graft should be with a portal anastomosis, since it is physiological and may also have metabolic and immunological advantages.
DR. HARDY (New York, New York): I wonder whether you could comment on two issues: One is the question that you alluded to suggesting a certain exchange in migration of lymphocytes, so that the donor lymphocytes end up in the blood and recipient lymphocytes end up in the small bowel, an expected phenomenon. A recent article by your group suggested that there is some magic about this in terms of protection of the bowel. Perhaps you could comment on those observations in relation to other people’s experience with pretreating the donor in clinical situations with massive doses of antilymphoid preparations. and in experimental situations with various antilymphoid drugs, as well as low-dose radiation. My second question concerns GVHD. We all know that a small amount of graft-versus-host disease may help the graft to survive. You have an excellent model for studying this in men. Does it help?
DR. TODO: Although exchange of donor and recipients lymphocytes could have been expected, this phenomenon was documented clinically only recently in hepatic and small bowel allografts by our group and the Canadian group. This suggests that pretreatment of the donor or small bowel graft to avoid GVH disease is probably unnecessary. However, we do not know the value of pretreatment as a strategy to reduce graft antigenicity, such as by passenger leukocyte depletion—this is our current laboratory interest.
DR. D. GRANT (London Ontario Canada): We have three patients who are well with liver–small bowel grafts at two and half years, one and a half years, and three months after transplantation. These patients were treated with cyclosporine. I’m wondering if you think that your success is due to the combined liver graft, or whether you think it’s due to the potency of FK506. Are you prepared to proceed with isolated small bowel grafting using the FK506?
DR. TODO: Regarding successful combined liver and small bowel graft, I think both factors contributed to the result. As you described in the Lancet, and has been described also by our group and others, the liver seems to have a protective effect on other organs when transplanted together. The potency of FK506 should not be ignored, since 2 out of 5 recipients were treated virtually only with FK506. We plan more isolated small bowel transplantations using FK506; we additionally have several ongoing animal studies designed to support the clinical program.
DR. HARDY: Dr. Grant, do you still pretreat your donors with massive doses of OKT3 or a similar preparation?
DR. GRANT: We have, but I’m not sure that it is necessary.
DR. FRANK GUTTMAN (Montreal, Quebec, Canada): I would like to comment on the liver–small bowel experience in rats. I was privileged a few weeks ago to see a manuscript from Revillon’s group in Paris. They have the experience of nine clinical small bowel transplantations in children. They have completed a study in a rat combination where the liver is accepted between the two strains without any immunosuppression, but the bowel is not. However, if they do a liver transplant and 17 days later carry out a bowel transplant, again without immunosuppressive drugs, tolerance of the small bowel was observed.
DR. HARDY: Can you identify the specific rat combinations?
DR. GUTTMAN: It was Rt1A and C or D, something like that.
DR. HARDY: This phenomenon, protection by the liver, has in many instances been rat-specific. I would warn those who wish to repeat the experiment to be aware of the specific shown combinations.
Footnotes
Presented at the 17th Annual Meeting of the American Society of Transplant Surgeons, May 29–31, 1991, Chicago, IL.
This work was supported by Research Grants from the Veterans Administration and by Project Grant DK 29961 from the National Institutes of Health, Bethesda, Md.
Department of Pathology.
REFERENCES
- 1.Kirkman RT. Small bowel transplantation. Transplantation. 1984;37:429. doi: 10.1097/00007890-198405000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449. [PMC free article] [PubMed] [Google Scholar]
- 3.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder P, Goulet O, Lear PA. Small bowel transplantation: European experience [Letter] Lancet. 1990;336:110. doi: 10.1016/0140-6736(90)91621-g. [DOI] [PubMed] [Google Scholar]
- 5.Goulet O, Revillon Y, Jan D, et al. Small-bowel transplantation in children. Transplant Proc. 1990;22:2499. [PubMed] [Google Scholar]
- 6.Deltz E, Schroeder p, Gebhardt H, et al. Successful clinical small bowel transplantation: report of a case. Clin Transplant. 1989;3:89. [Google Scholar]
- 7.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335. [PMC free article] [PubMed] [Google Scholar]
- 9.Murase N, Kim D, Todo S, Cramer DV, Fung J, Starzl TE. Induction of liver, heart, and multivisceral graft acceptance with a short course of FK 506. Transplant Proc. 1990;22:74. [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffman AL, Makowka L, Banner B, et al. The use of FK 506 for small intestine allotransplantation: inhibition of acute rejection and prevention of fatal graft-versus-host disease. Transplantation. 1990;49:483. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K, Stangl MJ, Todo S, Langrehr JM, Starzl TE, Schraut WH. Successful orthotopic small bowel transplantation with short term FK 506 immunosuppressive therapy. Transplant Proc. 1990;22:78. [PMC free article] [PubMed] [Google Scholar]
- 12.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 13.Lillehei RC, Goott B, Miller FA. The physiologic response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzakis A, Todo S, Starzl TE. Piggyback orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. 1991;337:818. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breiter HC, Craig RM, Levee G, Atkinson AJ. Use of kinetic methods to evaluate d-xylose malabsorption in patients. J Lab Clin Med. 1988;112:533. [PubMed] [Google Scholar]
- 19.Lillehei RC, Idezuki Y, Feemster JA, et al. Transplantation of stomach, intestine, and pancreas: experimental and clinical observations. Surgery. 1967;62:721. [PubMed] [Google Scholar]
- 20.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brettschneider L, Tong JL, Boose DS, et al. Specific bacteriologic problems after orthotopic liver transplantation in dogs and pigs. Arch Surg. 1968;97:313. doi: 10.1001/archsurg.1968.01340020177021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starzl TE. Infectious complications, excluding partial hepatic gangrene. In: Starzl TE, editor. Experience in hepatic transplantation. Saunders; Philadelphia: 1969. p. 329. [Google Scholar]
- 23.Arnaud-Battandier F, Salmon H, Vaiman M, et al. Small intestinal allotransplantation in swine with cyclosporine treatment: studies of the intestinal lymphoid populations. Transplant Proc. 1985;17:1440. [Google Scholar]
- 24.Jaffe R, Trager JDK, Zeevi A, Sonmez-Alpan E, Duquesnoy R. Multivisceral intestinal transplantation: surgical pathology. Pediatr Pathol. 1989;9:633. doi: 10.3109/15513818909022372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark CLI, Cunningham AJ, Crane PW, Wood RFM, Lear PA. Lymphocyte infiltration patterns in rat small-bowel transplants. Transplant Proc. 1990;22:2460. [PubMed] [Google Scholar]
- 26.Lear PA, Cunningham AJ, Clark CLI, Crane PW, Wood RFM. What role for passenger leucocytes in small-bowel allografts? Transplant Proc. 1990;22:2463. [PubMed] [Google Scholar]
- 27.Porter KA. Pathology of the orthotopic homograft and heterograft. In: Starzl TE, editor. Experience in hepatic transplantation. Saunders; Philadelphia: 1969. p. 422. [Google Scholar]
- 28.Fung JJ, Zeevi A, Kaufman C, et al. Interactions between bron-choalveolar lymphocytes and macrophages in heart-lung transplant recipients. Hum Immunol. 1985;14:287. doi: 10.1016/0198-8859(85)90236-8. [DOI] [PubMed] [Google Scholar]
- 29.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr Probl Surg. 1983;20:687. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of the multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880. [PMC free article] [PubMed] [Google Scholar]
- 31.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]