Abstract
Recent advances in catheter-based optical coherence tomography (OCT) have provided the necessary resolution and acquisition speed for high-quality intravascular imaging. Complications associated with clearing blood from the vessel of a living patient have prevented its wider acceptance. We identify a surgical application that takes advantage of the vascular imaging powers of OCT but that circumvents the difficulties. Coronary artery bypass grafting (CABG) is the most commonly performed major surgery in America. A critical determinant of its outcome has been postulated to be injury to the conduit vessel incurred during the harvesting procedure or pathology preexistent in the harvested vessel. As a test of feasibility, intravascular OCT imaging is obtained from the radial arteries (RAs) and/or saphenous veins (SVs) of 35 patients scheduled for CABG. Pathologies detected by OCT are compared to registered histological sections obtained from discarded segments of each graft. OCT reliably detects atherosclerotic lesions in the RAs and discerns plaque morphology as fibrous, fibrocalcific, or fibroatheromatous. OCT is also used to assess intimal trauma and residual thrombi related to endoscopic harvest and the quality of the distal anastomosis. We demonstrate the feasibility of OCT imaging as an intraoperative tool to select conduit vessels for CABG.
Keywords: conduit quality, optical coherence tomography, cardiac surgery, (CABG) coronary artery bypass grafting, endothelial integrity, graft failure
1 Introduction
Coronary artery bypass grafting (CABG) is the most common major surgical procedure in the United States with over 300,000 cases performed each year. To restore blood flow to the affected myocardium, a vessel from another part of the body is procured to create a bypass around a critically stenosed coronary artery. The internal thoracic artery (ITA) remains the conduit of first choice due to its superior long-term patency, which is primarily a result of near perfect integrity of its inner blood-contacting lining, the “intima.”1 However, almost all patients referred for CABG require additional grafts to provide a complete revascularization. This necessitates the harvest of other vessels, most commonly the saphenous vein (SV) and/or radial artery (RA). These conduits have higher rates of intimal irregularities2 and early graft occlusion compared3,4 to the ITA.
Strategies aimed at screening the intimal quality of potential conduits as a means of improving bypass graft patency would be clinically beneficial since graft failure is associated with increased morbidity and mortality and often requires reoperation.5,6 Intraoperative assessment of a conduit is currently limited to gross inspection by the surgeon for externally apparent abnormalities such as lacerations, branch avulsions, or varicosities with no effort to assess the intima. However, injury to this inner vascular layer is more likely to directly influence the risk of early failure by hampering the antiinflammatory and antithrombotic role of the endothelium.7 Since the vascular endothelium is also the body’s major source of endogenous vasodilators such as prostacyclin and nitric oxide, atherosclerotic plaque or traumatic injury may increase the risk of postoperative spasm, particularly for the RA graft.8 In a prospective analysis of patients who ultimately developed graft failure, our group found that the degree of endothelial cell disruption detected in surplus segments of the SV graft immediately after harvest was directly associated with the risk of failure.2
“Bench-to-bedside” translation of our understanding that endothelial integrity plays a key role in conduit selection practices has been hindered by the lack of a convenient means to objectively assess endothelial quality in the operating room setting. Immunohistochemistry (IHC) remains the “gold standard” for analyzing vascular endothelium, but cannot be obtained in real time and is insensitive to heterogeneous abnormalities of the endothelium throughout the conduit. Intravascular ultrasound9 (IVUS) and high-resolution computed tomography (CT) scanning10 can provide a more global method for screening the entire in situ RA graft in real time, but have insufficient resolution for detecting most intimal abnormalities.
Catheter-based optical coherence tomography (OCT) is an emerging imaging technology capable of an axial resolution in the range of 2 to 15 μm, at least a 10-fold improvement11 over IVUS. The feasibility of OCT for visualization of coronary plaques in patients was first demonstrated12 in 2002. OCT has been applied for plaque and thrombus characterization, determining the risk of plaque rupture by macrophage detection, and therapeutic guidance of coronary interventions for stent visualization.13,14 The superior resolution of OCT versus IVUS enables more precise evaluation of stent deployment.15–17
Despite a wide array of advantages, the adoption of OCT has been slow in the cardiology field. The problem with intracoronary OCT imaging in live patients is that blood must be flushed out of the coronary artery for the image to be obtained. This requires that a proximal segment of the vessel be occluded with a high-pressure balloon for approximately 30 s. Such localized pressure can injure the endothelium and increase the risk of subsequent thrombosis. Second, periods of coronary occlusion may not be safe in unstable patients. Finally, because of the finite distance between the balloon and imaging positions, plaques close to branch points cannot be imaged.
The challenge has been to identify applications that will optimally utilize the strength of catheter-based OCT without these limitations. In this paper, we propose and present preliminary human data on a novel cardiac application for OCT: intraoperative screening of harvested conduits CABG procedures. Graft imaging during CABG is performed in a segment of vessel that is exsanguinated and bathed in crystalloid preservation solution. Consequently, there is no need for the endothelium to be subjected to the potentially damaging pressure of an inflated intraluminal balloon. In this paper, we report the feasibility of OCT for real-time analysis of luminal abnormalities within bypass conduits that result from atherosclerosis, trauma (e.g., intimal tear, medial dissection), or retention of thrombi.
2 Methods
2.1 Subject Enrollment and Study Design
Following IRB (Institutional Review Board) approval (protocol H25350), all clinical subjects in whom the RA and/or SV was considered as a conduit between March and December 2006 provided informed consent before enrollment into a prospective observational study assessing the feasibility of OCT for evaluating bypass conduits. A total of 27 RA and 33 SV conduits were evaluated from 35 patients.
2.2 Surgical Technique
CABG was performed via a median sternotomy and the left ITA, SV, and/or RA were harvested in all patients. RAs were procured using either endoscopic (56%) or pedicle (44%) techniques as described previously,18 whereas all SVs were harvested endoscopically (VasoView6; Guidant Systems, Inc., Minneapolis, Minnesota). Endoscopic harvest was initiated by inflating a tourniquet while exsanguinating the leg or arm with an Esmark bandage. A longitudinal 3-cm skin incision was made over the distal portion of the vessel and a trochar port inserted with the balloon inflated to establish a seal, which is necessary to create CO2 pressure (8 to 10 mm Hg) within a subcutaneous tunnel. Anterior and posterior exposure around the vessel was created by blunt dissection with endoscopic visualization. Division of branches was performed with minimal tension using bipolar electrocautery. Proximal RA or SV ligation was performed through a separate stab incision. The vessel was then removed from the tunnel and flushed with a plasmalyte solution containing glyceryl trinitrate and verapamil19.
2.3 OCT Analysis of Bypass Grafts
Conduits were imaged using OCT in situ prior to harvest and ex vivo after harvest (LightLab Imaging, Inc., Westford, Massachusetts) (Fig. 1). The in situ OCT examination was performed by inserting a 1.2 F (0.4-mm) imaging probe (ImageWire®, LightLab Imaging, Westford, Massachusetts) into the exsanguinated vessel. Additional clearance of blood from the vessel was facilitated by infusing heparinized saline during imaging. Vessels were imaged at a rate of 0.5 mm/s and data were processed using proprietary software according to the principles of OCT imaging described elsewhere.20 Ex vivo examination was performed in a similar manner, except that one end of the vessel was occluded with a spring-loaded vascular clip to allow for gentle distension of the vessel during imaging (Fig. 1). Plaques visualized by cross-sectional OCT imaging were categorized as fibrous, fibrocalcific, or fibroatheromatous, based on the American Heart Associations scientific statement for advanced coronary lesions,21–23 and intimal disease was quantified by intimal-medial ratio, as previously described.24 Harvesting injury was categorized as mild when intimal disruption was restricted to the ostium of branch points and severe when the tear affected the luminal surface. Intraluminal thrombus was identified as a lobulated mass with high signal intensity and characteristic radial shadowing, as previously described.22,25
Fig. 1.
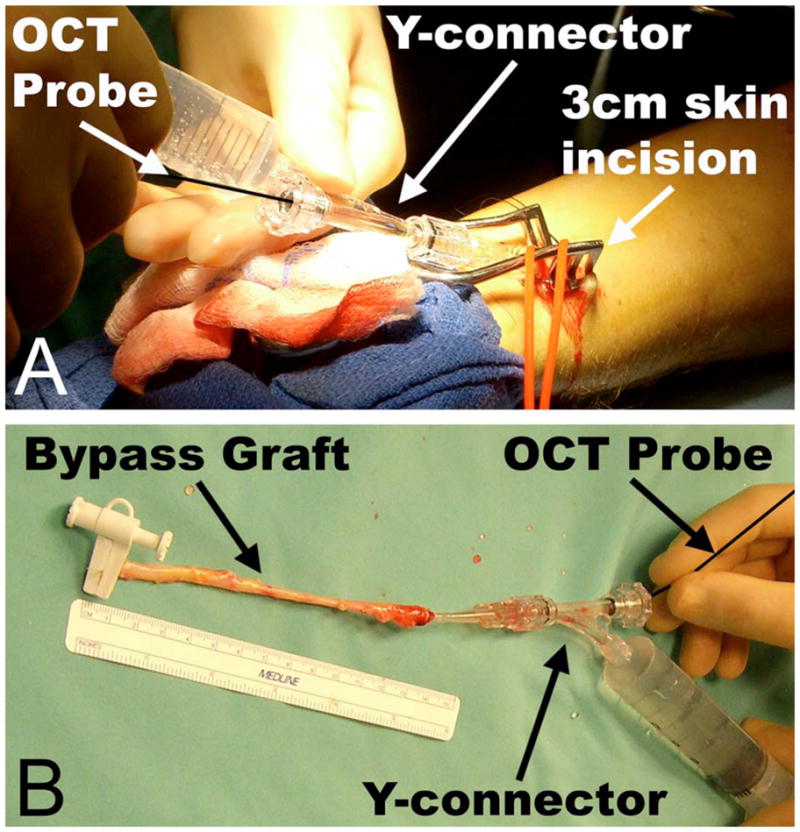
Schematic of in situ and ex vivo scanning protocol. In situ OCT examination (A) was performed prior to any surgical manipulation by exsanguinating the forearm using a tourniquet, inserting the OCT probe into the distal RA, and advancing it proximally. Gentle Hank’s balanced salt solution (HBSS) infusion via a Y-connector connected to the cannula was used to inflate the vessel to facilitate optimal imaging. This examination identified preexisting intimal pathology, such as atherosclerotic plaque. The excised RA underwent a second ex vivo OCT examination (B) to assess any damage incurred during harvest.
In a subset of SVs (n=3), the OCT imaging wire was introduced via a small venotomy in the body of the graft and advanced into the distal anastomosis. This allowed for imaging of the patency of this graft-to-coronary connection, as previously described using coronary ultrasound.26
2.4 Histological Analyses
Biopsy specimens for histological processing were procured from discarded conduit segments. To exactly register the OCT images with the corresponding histological sections, the vessel site at which the biopsy specimen was obtained was marked externally at the location of the catheter, visualized by the rotating infrared light at the catheter tip. These “image-guided” biopsy specimens were then stored in solution before being embedded and frozen in cutting compound (Tissue-Tek O.C.T., Redding, California). Frozen sections were analyzed via van Giesen staining (elastin) to visualize the internal (IEL) and external elastic lamina (EEL), and the intimal-medial ratio was measured. Selected frozen sections were also analyzed for the presence of macrophages with anti-CD68 mAb (Invitrogen, Carlsbad, California), as previously described.27
2.5 Tissue Factor Activity
Selected vessel segments were incubated at 37°C in a custom designed chamber containing Tris buffer (pH 7.4), 50 mM CaCl2, 2 U/ml Factor VII, and 2 U/ml Factor X (American Diagnostica, Stamford, Connecticut). After 60 min, reaction was stopped by adding 25 mM EDTA. This incubation solution was combined with Tris buffer (pH 8.6) and 5 mM chromogenic FXa substrate (Spectrozyme FXa, American Diagnostica, Stamford, Connecticut) in a 96-well plate, and incubated for 60 min at 37°C. The absorption of the reaction buffer was assessed at 405 nm and then compared with a standard curve to determine tissue factor activity.
2.6 Statistics and Data Analyses
Bland-Altman analysis was used to verify the degree of agreement for measurements of intimal-medial ratio calculated via OCT analysis versus histological examination. Reproducibility of plaque characterization via OCT was determined by defining interobserver κ correlation coefficients. Statistical analysis was performed with the InStat statistical package (GraphPad Software, San Diego, California) with consultation of a biostatistician. Although LightLab Imaging Inc. participated in the study design and execution, none of the sponsors of the study had a role in the decision to publish these data.
3 Results and Discussion
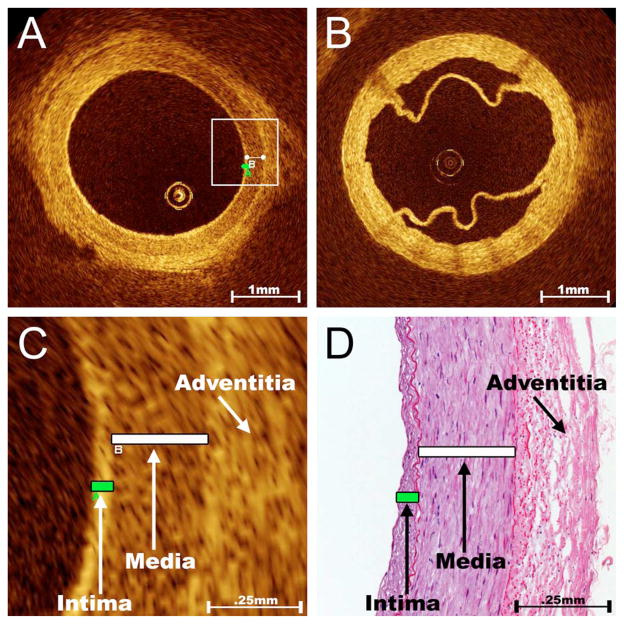
OCT imaging of bypass conduits showed that the RAs and SVs possess unique imaging characteristics based on the respective compositions of their vasculature walls. Three distinct tissue layers are imaged for the normal RA: an inner high-intensity band of varying thickness representing the vascular intima, a thicker low-intensity band representing the smooth muscle of the media, and an outer heterogeneous band of higher signal intensity representing the connective tissue of the adventitia (Fig. 2). These three layers are demarcated by well-developed internal and external elastic laminae in the RA, but are less discernable in OCT images of the SV, which has a poorly developed internal elastic lamina (Fig. 2). Due to the excellent resolution of tissue layers in the RA, intimal hyperplasia in this vessel is easily detected by OCT. RA intimal-medial ratios measured by OCT showed a strong correlation with the analysis of registered histologic sections (R =0.88, p<0.001, Fig. 2) and a small average discrepancy and consistent variation (−0.07±0.22) as determined by Bland-Altman analysis. Our experience corroborates other reports12–17,21,22 concluding that OCT is ideally suited for evaluating the intimal surface of blood vessels.
Fig. 2.
Normal appearance of RA and SV on OCT imaging. As shown in these representative cross-sectional OCT images, the optical reflectance properties of the normal RA (A) illustrate three distinct layers that correspond to the intima, media, and adventitia; however, the differentiation of vascular tissue layers is more difficult in the SV (B). The appearance of a normal valve can also be appreciated here. Although the penetration of OCT is limited to ~1 mm, its resolution is better than 20 μm and therefore ideally suited for evaluating the intimal surface of blood vessels. This unsurpassed imaging resolution yields RA intimal and medial thickness measurements via OCT (C) that compare favorably to the analysis of registered histological sections (D).
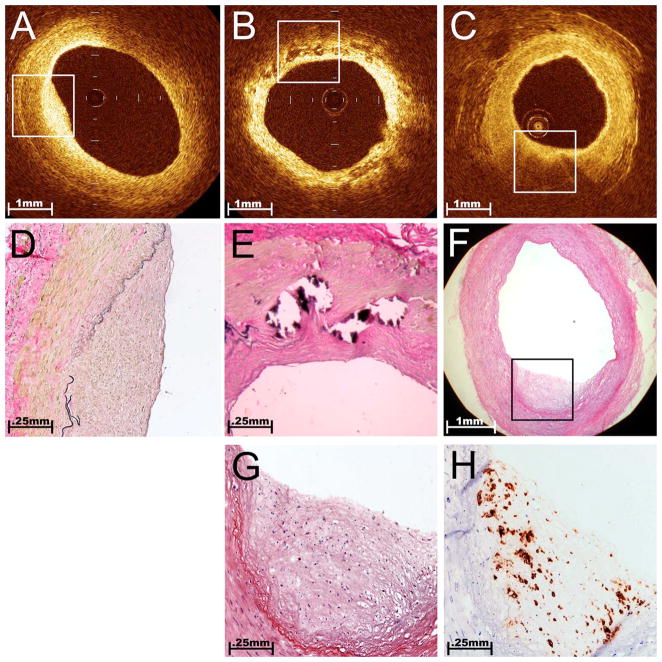
While patients referred for CABG obviously have atherosclerotic lesions in their coronary arteries, plaques can also occur throughout their vasculature, including the RAs. The main finding of our study was that OCT imaging easily and quickly elucidates RA atherosclerosis. A unique advantage when using this imaging modality during ex vivo applications is the ease of confirming imaging findings by histological correlations. The OCT probe’s IR light transilluminates the vessel wall, enabling an exact registration with biopsies obtained from surplus segments of the graft. Further characterization of the plaques as fibrous or more complicated fibrocalcific and fibroatheromatous plaques (Fig. 3) was accomplished with strong interobserver agreement (κ correlation >0.80 for each). The characterization of fibrocalcific or fibroatheromatous plaque in the RA is particularly important because it marks a more severe form of intimal disease. Several recent studies have suggested that intimal quality may relate to the risk of postoperative graft failure.28,29 Intimal calcification, in particular, has been widely viewed as a contraindication for use of this graft.30 Fibroatheromas may be of even greater concern since recent data from our group suggests that this particular finding in a procured RA correlates with an increased risk for postoperative vessel spasm.31
Fig. 3.
Atherosclerotic plaques in the RA. Using histological sections that were registered to the areas of OCT images, we established that OCT was able to differentiate plaque morphology within RA conduits on a microscopic scale. A fibrous plaque [(A) and (D)] is demonstrated by a homogeneous region of signal-rich intensity that is eccentrically located on the interior of the vessel. The additional ability to discriminate the underlying media and adventitia layers illustrates that light penetration is effective in this area. In this example, the boundary between the bright intima and darker media is difficult to discern, but the external elastic membrane that demarcates the boundary between the media and adventitia appears as a sharp line. A fibrocalcific plaque (B) is recognized by discrete areas of poor signal intensity with well-demarcated borders that represent areas of intimal calcification. Here, the concentrically thickened intimal layer is seen as a bright inner band, consistent with the fibrotic neointimal tissue shown in the corresponding histological section (E). A fibroatheroma [(C) and (F)] appeared as shadowed areas capped by a bright overlying layer. High attenuation of light within dense atheromatous tissue creates a shadowing artifact that limits penetration into the deeper media and adventitia layers. Lipid-laden foam cells (macrophages), which create bright localized reflections with spoke-like shadows on OCT imaging, were identified by histological appearance (G) and CD68 IHC (H). Note the relatively low density of foam cells in the middle of the plaque and the corresponding region of preserved signal penetration in the OCT image (C).
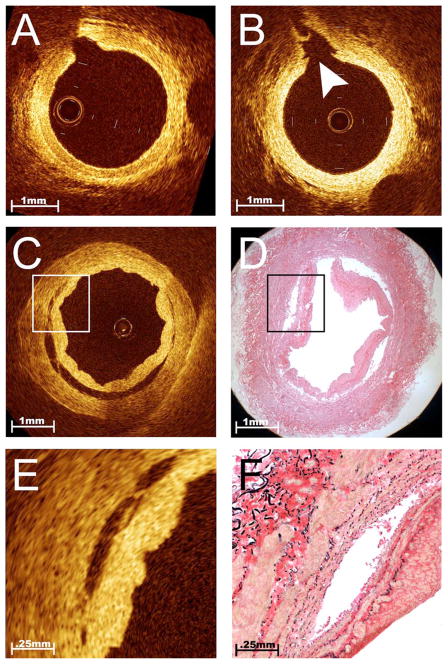
Intimal trauma induced during the harvesting procedure can be detected by comparing in situ and ex vivo OCT images. Procurement-related injury to the conduit is thought to strongly influence early patency, particularly for SV grafts.2 Minor intimal damage (confined to the ostia of branch points) (Fig. 4) was a unique finding in endoscopically harvested RAs, not found after harvest using the traditional “no-touch” technique. Although less common than RA trauma, medial dissections were initially noted on OCT screening and then confirmed by histological correlation within endoscopically harvested SVs (Fig. 4). This severe injury was also found to be associated with an increase in local tissue factor activity as compared to adjacent areas with intact intima (3.71 versus 0.76 U/cm2). Given the key role that tissue factor plays in thrombosis,32 this finding suggests that OCT may identify specific areas within the conduit that are likely to be more thrombogenic when exposed to the coronary blood stream after grafting. Previous investigations have suggested that endoscopic harvest does not affect intimal integrity in the RA Ref. 33 or SV Ref. 34, but have been limited to analyses of discarded segments. Given the focal nature of intimal trauma, these studies may not have had sufficient sensitivity to determine the consequences of endoscopic harvest. The concerning pathologies in endoscopically harvested conduits, as detected by OCT imaging in this study, are more in line with recent evidence from a large, multicenter graft patency trial, showing that endoscopic harvest was an independent predictor of bypass graft failure.35 Undoubtedly, OCT can provide a quality assurance tool for addressing these controversies and directing the development of improved conduit harvesting methods and devices.
Fig. 4.
Intimal trauma associated with endoscopic harvest. Serial OCT imaging of bypass conduits before and after harvest provided the opportunity to evaluate the safety of novel techniques for vessel procurement such as endoscopic harvest. Unlike traditional open harvesting techniques, this method requires the creation of dissection planes around the vessel pedicle using a blunt-tipped conical cannula. While this harvesting step is performed under endoscopic guidance, it creates tension at branch points that does not occur with the open method. This representative example of in situ OCT images obtained from a RA before (A) and after (B) blunt dissection illustrates a unique pattern of vessel injury to the intimal layer, localized within the ostia of branch points (B, arrowhead). Although less common than trauma in the RA, severe dissections were occasionally imaged within the endoscopically harvested SV [(C) and (E)]. This incidentally discovered finding was confirmed by comparison to histology [(D) and (F)] and was associated with increased local tissue factor activity, suggesting that this vessel segment was likely to be highly thrombogenic if used as a bypass graft.
Ex vivo OCT imaging also revealed a high incidence of retained clot within SVs (Fig. 5). All veins in this study were harvested using an endoscopic technique and therefore were exposed to a period of pressurized CO2 insufflation, which is required to facilitate endoscopic visualization. A side effect of this pressurization is that the vein is compressed leading to stagnation of intraluminal blood that may promote clot formation. These clots might serve as nidus to activate the coagulation cascade and cause acute graft failure. While intraluminal thrombi have been noted anecdotally by surgeons in the past, the use of OCT in this study enabled the first true appreciation of total clot burden contained within these endoscopically harvested bypass grafts.20,36 The ability of OCT to accurately quantify the volume of retained clot provides a highly sensitive endpoint for identifying potential risk factors and testing strategies for preventing thrombus formation.37
Fig. 5.
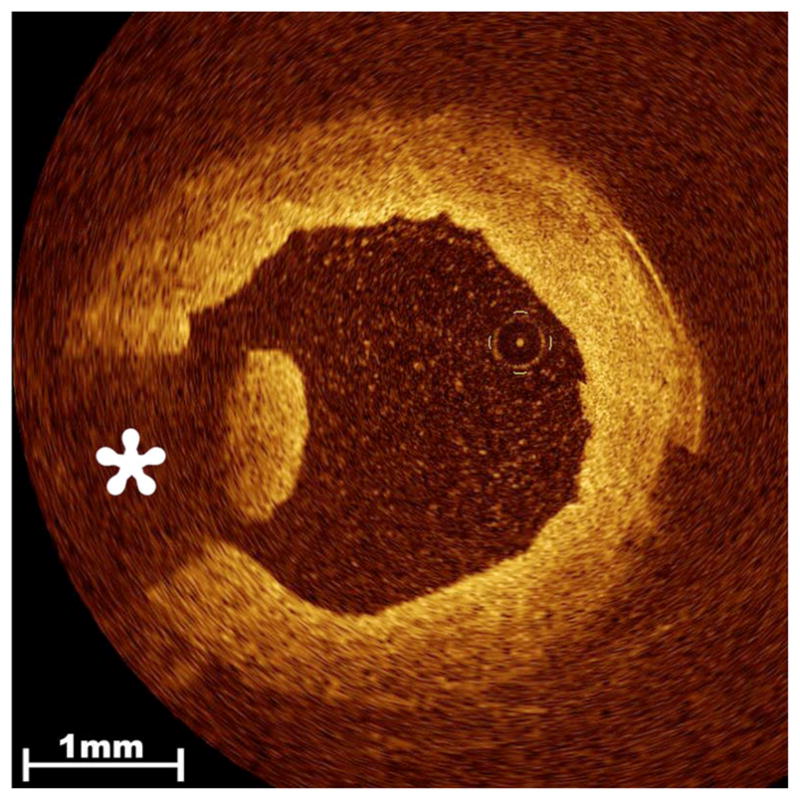
Thrombus in the endoscopically harvested SV. Residual clot strands that remain within the lumen of the procured vessel are readily detected by OCT imaging and range in severity from a single minute strand to near occlusive thrombus. Clot appears as an intraluminal lobulated mass with high signal intensity that produces characteristic radial signal attenuation (asterisk) due to the presence of entrapped red blood cells.
OCT is used widely in ophthalmology,38 but its application to vascular imaging has not been met with enthusiasm. Up to now, clinical research into catheter-based OCT has been devoted primarily to imaging plaques within the coronary arteries.12–15,17,21,22 However, a problem with intracoronary OCT imaging in live patients is that blood must be flushed out of the coronary artery for the image to be obtained. This requires brief occlusion of the coronary artery with an inflated balloon, risking endothelial injury and ischemia. Imaging of bypass conduits provides a more convenient and advantageous application of this powerful technology. Unlike the coronary artery, the limb can be exsanguinated with a tourniquet without subjecting the vessel to the effects of an occlusive balloon. Since ischemic periods of up to a few hours are well-tolerated in the extremities,39,40 a 3 to 5 min period of limb ischemia to perform these examinations was not a concern. In addition, the ability to fully flush all blood out of the vessel produced very high quality OCT images as compared to coronary applications in which the image is often compromised by the presence of residual blood (Fig. 6).
Fig. 6.
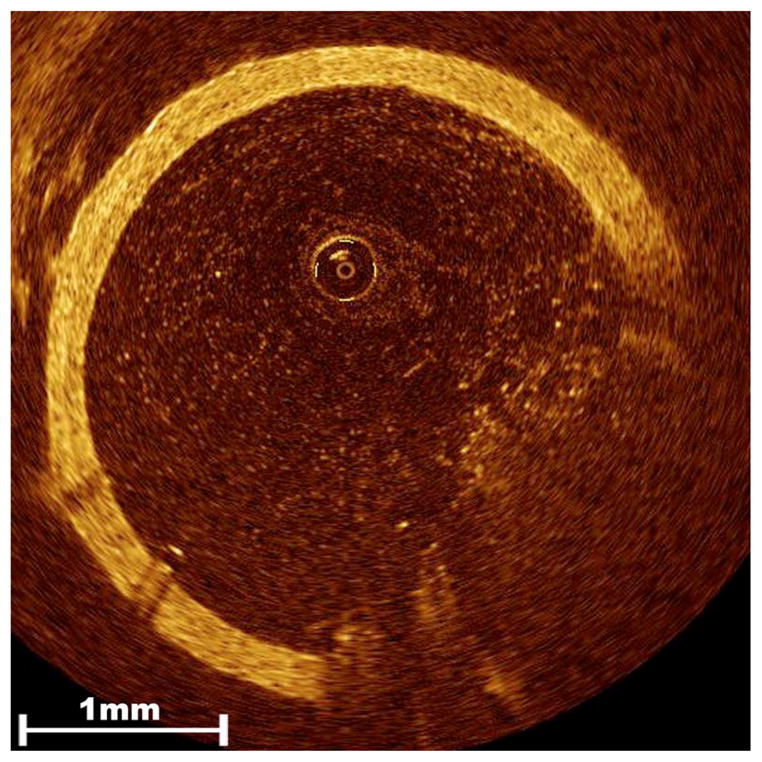
Poor image quality due to residual blood. When blood is not completely flushed out of the vessel prior to OCT imaging, it can severely compromise resolution of relevant vascular structures. This was not an issue with our specific application since bypass grafts could be fully exsanguinated and thoroughly flushed with saline without risk of harm to the patient.
One of the most promising applications of OCT in cardiac surgery is for evidence-based “targeted conduit selection.” The average lengths of harvested conduit in this study were 16.2±1.6 cm for RAs and 27.2±7.3 cm for SVs, while the average length discarded was 4.9±3.1 cm. This means that length in excess of that required for grafting was consistently harvested. OCT imaging enables comprehensive assessment of the bypass conduit in real time, prior to grafting into the coronary circulation. Therefore, the surgeon is able to exclude regions of conduit considered less optimal (i.e., containing a fibroatheroma or thrombus). In the event of severe preexisting intimal disease throughout the conduit, the ability to conduct in situ OCT scanning enables rejection of the entire vessel length prior to harvest, sparing the patient potential morbidity associated with removing a vessel that is not subsequently utilized. Thus, targeted conduit selection using OCT imaging has the potential to improve graft patency and patient outcomes through utilization of bypass grafts with the highest quality intima possible.
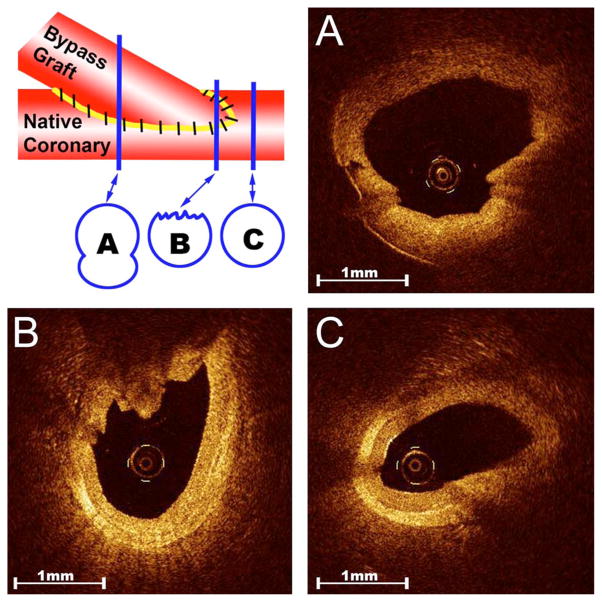
Another potential application of OCT toward the assessment of bypass conduits is the imaging of the distal anastamosis (i.e., the location where the bypass graft is sutured to the native coronary vessel). A poor-quality anastomosis can reduce blood flow in the graft and increase the risk of occlusion.41 A similar technique has been described using 13-MHz epicardial ultrasound,26 but the resolution offered by OCT is vastly superior and enables detection of much more subtle defects. OCT is unique in that it enables high-resolution visualization of every aspect of the suturing (Fig. 7) and can be used to determine if low flow is in fact due to a poor surgical technique that necessitates revision. In addition, this application would further enhance the value of OCT as a quality-control device, particularly in the training of novice coronary surgeons.
Fig. 7.
Evaluation of anastomosis of bypass graft to coronary artery. Note the ability of OCT to image distinct components of the anastomosis: (A) midanastomosis, (B) toe, and (C) native coronary. OCT imaging enables for complete and precise analysis of anastomosis quality.
4 Conclusion
Cardiac surgery, and CABG in particular, is a field the truly requires innovation if it is to succeed into the future. Recent discoveries about the importance of intimal quality for bypass graft patency have a tremendous potential to improve outcomes, but not without a suitable means of real-time assessment. OTC has the potential to fill this niche. Despite the vastly superior resolution of OCT as compared to other currently available imaging techniques, it has not yet been widely accepted for intravascular imaging. These data provide support for the initiation of appropriately powered clinical trials to confirm the relationship between intimal quality identified by OCT and bypass graft patency. In this example of “bench-to-bedside” translational research, we identify a novel application that exploits OCT’s high spatial resolution and real-time imaging capabilities while circumventing the problems associated with clearing blood from the coronary vessel. It is also an application with high impact in terms of the potential number of patients affected and the ability to alter clinical outcomes.
Acknowledgments
RSP is supported by grants from Guidant Corporation, American Heart Association (0435318N), an Intramural Grant at the University of Maryland, a pilot grant from the Tobacco Restitution Fund at the University of Maryland, NHLBI (RO1 HL084080-01A1), and LightLab Imaging, Inc. (supplies for the performance of OCT only). CMT is supported by a VA Merit Review grant and a RO1 grant from NINDS.
Contributor Information
Emile N. Brown, University of Maryland, School of Medicine, Department of Cardiac Surgery, 22 South Greene Street N4W94, Baltimore, Maryland 21201
Nicholas S. Burris, University of Maryland, School of Medicine, Department of Cardiac Surgery, 22 South Greene Street N4W94, Baltimore, Maryland 21201
Junyan Gu, University of Maryland, School of Medicine, Department of Cardiac Surgery, 22 South Greene Street N4W94, Baltimore, Maryland 21201.
Zachary N. Kon, University of Maryland, School of Medicine, Department of Cardiac Surgery, 22 South Greene Street N4W94, Baltimore, Maryland 21201
Patrick Laird, University of Maryland, School of Medicine, Department of Cardiac Surgery, 22 South Greene Street N4W94, Baltimore, Maryland 21201.
Seeta Kallam, University of Maryland, School of Medicine, Department of Cardiac Surgery, 22 South Greene Street N4W94, Baltimore, Maryland 21201.
Cha-Min Tang, University of Maryland, School of Medicine, Department of Neurology, 22 South Greene Street, Baltimore, Maryland 21201.
Joseph M. Schmitt, LightLab Imaging, 1 Technology Park Drive, Westford, Maryland 01886
Robert S. Poston, University of Maryland, School of Medicine, Department of Cardiac Surgery, 22 South Greene Street N4W94, Baltimore, Maryland 21201
References
- 1.Cameron A, Davis KB, Green G, Schaff HV. Coronary bypass surgery with internal-thoracic-artery grafts–effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. doi: 10.1056/NEJM199601253340402. [DOI] [PubMed] [Google Scholar]
- 2.Manchio JV, Gu J, Romar L, Brown JM, Gammie J, Pierson RN, III, Griffith BP, Poston RS. Disruption of graft endothelium correlates with early failure after off-pump coronary artery bypass surgery. Ann Thorac Surg. 2005;79:1991–1998. doi: 10.1016/j.athoracsur.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Khan NE, De Souza A, Mister R, Flather M, Claque J, Davies S, Collins P, Wang D, Sigwart U, Pepper J. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21–28. doi: 10.1056/NEJMoa031282. [DOI] [PubMed] [Google Scholar]
- 4.Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L, Pierson RN, III, Griffith BP. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006;131:122–130. doi: 10.1016/j.jtcvs.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Campeau L, Enjalbert M, Lespérance J, Bourassa MG. Course of angina 1 to 12 years after aortocoronary bypass surgery related to changes in grafts and native coronary arteries. Can J Surg. 1985;28:496–498. [PubMed] [Google Scholar]
- 6.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 7.Chester AH, Yacoub M. Vascular reactivity and endothelial function of bypass grafts. Curr Opin Cardiol. 1990;5:733–736. doi: 10.1097/00001573-199012000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kawano H, Ogawa H. Endothelial dysfunction and coronary artery spasm. Curr Drug Targ Cardiovasc Haematol Disord. 2004;4:23–33. doi: 10.2174/1568006043481301. [DOI] [PubMed] [Google Scholar]
- 9.Oshima A, Takeshita S, Kozuma K, Yokoyama N, Motoyoshi K, Ishikawa S, Honda M, Oga K, Ochiai M, Isshiki T. Intravascular ultrasound analysis of the radial artery for coronary artery by-pass grafting. Ann Thorac Surg. 2005;79:99–103. doi: 10.1016/j.athoracsur.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 10.Feuchtner GM, Smekal A, Friedrich GJ, Schachner T, Bonatti J, Dichtl W, Deutschmann M, Zur Nedden D. High-resolution 16-MDCT evaluation of radial artery for potential use as coronary artery bypass graft: a feasibility study. Am J Roentgenol. 2005;185:1289–1293. doi: 10.2214/AJR.04.0945. [DOI] [PubMed] [Google Scholar]
- 11.Brezinski ME, Tearney GJ, Bouma BE, Boppart SA, Hee MR, Swanson EA, Southern JF, Fujimoto JG. Imaging of coronary artery microstructure (in vitro) with optical coherence tomography. Am J Cardiol. 1996;77:92–93. doi: 10.1016/s0002-9149(97)89143-6. [DOI] [PubMed] [Google Scholar]
- 12.Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 13.Pinto TL, Waksman R. Clinical applications of optical coherence tomography. J Interv Cardiol. 2006;19:566–573. doi: 10.1111/j.1540-8183.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 14.Low AF, Tearney GJ, Bouma BE, Jang IK. Technology insight: optical coherence tomography—current status and future development. Nat Clin Pract Cardiovasc Med. 2006;3:154–162. doi: 10.1038/ncpcardio0482. [DOI] [PubMed] [Google Scholar]
- 15.Grube E, Gerckens U, Buelesfeld L, Fitzgerald PJ. Images in cardiovascular medicine. Intracornary imaging with optical coherence tomography: a new high-resolution technology providing striking visualization in the coronary artery. Circulation. 2002;106:2409–2410. doi: 10.1161/01.cir.0000037784.22657.6e. [DOI] [PubMed] [Google Scholar]
- 16.Bouma BE, Tearney GJ, Yabushita H, Shishkov M, Kauffman CR, DeJoseph Gauthier D, MacNeill BD, Houser SL, Aretz HT, Halpern EF, Jang IK. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart. 2003;89:317–320. doi: 10.1136/heart.89.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meissner OA, Rieber J, Babaryka G, Oswald M, Reim S, Siebert U, Redel T, Reiser M, Mueller-Lisse U. Intravascular optical coherence tomography: comparison with histopathology in atherosclerotic peripheral artery specimens. J Vasc Interv Radiol. 2006;17:343–349. doi: 10.1097/01.RVI.0000195324.52104.00. [DOI] [PubMed] [Google Scholar]
- 18.Reyes AT, Frame R, Brodman RF. Technique for harvesting the radial artery as a coronary artery bypass graft. Ann Thorac Surg. 1995;59:118–126. doi: 10.1016/0003-4975(94)00673-U. [DOI] [PubMed] [Google Scholar]
- 19.He GW, Rosenfeldt FL, Angus JA. Pharmacological relaxation of the saphenous vein during harvesting for coronary artery bypass grafting. Ann Thorac Surg. 1993;55:1210–1217. doi: 10.1016/0003-4975(93)90036-h. [DOI] [PubMed] [Google Scholar]
- 20.Burris NS, Schwartz K, Tang CM, Jafri MS, Schmitt J, Kwon MH, Toshinaga O, Gu J, Brown JM, Brown EN, Pierson RN, III, Poston RS. Cathether-based infrared light scanner as a tool to assess conduit quality in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;133:419–427. doi: 10.1016/j.jtcvs.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 21.Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 22.Manfrini O, Mont E, Leone O, Arbustini E, Eusebi V, Virmani R, Bugiardini R. Sources of error and interpretation of plaque morphology by optical coherence tomography. Am J Cardiol. 2006;98:156–159. doi: 10.1016/j.amjcard.2006.01.097. [DOI] [PubMed] [Google Scholar]
- 23.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 24.Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary intima-media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J. 2005;69:903–907. doi: 10.1253/circj.69.903. [DOI] [PubMed] [Google Scholar]
- 25.Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006;97:1713–1717. doi: 10.1016/j.amjcard.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Budde RP, Meijer R, Dessing TC, Borst C, Gründeman PF. Detection of construction errors in ex vivo coronary artery anastomoses by 13-MHz epicardial ultrasonography. J Thorac Cardiovasc Surg. 2005;129:1078–1083. doi: 10.1016/j.jtcvs.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Janssen L, Chu FV. Atherosclerosis of radial arterial graft may increase the potential of vessel spasm in coronary bypass surgery. J Thorac Cardiovasc Surg. 2005;130:1477–1478. doi: 10.1016/j.jtcvs.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 29.Kim YO, Choi YJ, Kim JI, Kim YS, Kim BS, Park CW, Song HC, Yoon SA, Chang YS, Bang BK. The impact of intima-media thickness of radial artery on early failure of radiocephalic arteriovenous fistula in hemodialysis patients. J Korean Med Sci. 2006;21:284–289. doi: 10.3346/jkms.2006.21.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruengsakulrach P, Brooks M, Sinclair R, Hare D, Gordon I, Buxton B. Prevalence and prediction of calcification and plaques in radial artery grafts by ultrasound. J Thorac Cardiovasc Surg. 2001;122:398–399. doi: 10.1067/mtc.2001.114096. [DOI] [PubMed] [Google Scholar]
- 31.Burris NS, Brown EN, Laird P, Schmitt J, Brown J, Poston RS. Lipid Content of the Grafted Radial Artery Increases the Risk of Postoperative Spasm. presented at Int. Soc. of Minimally Invasive Cardiac Surgeons 10th Anniversary Scientific Mt; 2007. [Google Scholar]
- 32.Muluk SC, Vorp DA, Severyn DA, Gleixner S, Johnson PC, Webster MW. Enhancement of tissue factor expression by vein segments exposed to coronary arterial hemodynamics. J Vasc Surg. 1998;27:521–527. doi: 10.1016/s0741-5214(98)70327-1. [DOI] [PubMed] [Google Scholar]
- 33.Shapira OM, Eskenazi BR, Anter E, Joseph L, Christensen TG, Hunter CT, Lazar HL, Vita JA, Shemin RJ, Keaney JF., Jr Endoscopic versus conventional radial artery harvest for coronary artery bypass grafting: functional and histologic assessment of the conduit. J Thorac Cardiovasc Surg. 2006;131:388–394. doi: 10.1016/j.jtcvs.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 34.Kiaii B, Moon BC, Massel D, Langlois Y, Austin TW, Willoughby A, Guiraudon C, Howard CR, Guo LR. A prospective randomized trial of endoscopic versus conventional harvesting of the saphenous vein in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2002;123:204–212. doi: 10.1067/mtc.2002.118682. [DOI] [PubMed] [Google Scholar]
- 35.Magee MJ, Alexander JH, Hafley G, Ferguson TB, Gibson CM, Harrington RA, Peterson ED, Califf RM, Kouchoukos N, Herbert MA, Mack MJ. Saphenous vein graft failure and clinical outcomes following on-pump and off-pump coronary artery bypass: findings from prevent IV. presented at Southern Thorac. Surg. Assn. 53rd Ann. Mt; 2006. [DOI] [PubMed] [Google Scholar]
- 36.Burris NS, Schwartz K, Brown JM, Kwon MH, Pierson RN, III, Griffith BP, Poston RS. Incidence of residual clot strands in saphenous vein grafts after endoscopic harvest. Innovat Technol Techniq Cardiothorac Vasc Surg. 2006;1:323–327. doi: 10.1097/IMI.0b013e31802f4399. [DOI] [PubMed] [Google Scholar]
- 37.Brown EN, Kon ZN, Tran R, Burris NS, Gu J, Laird P, Brazio PS, Kallam S, Schwartz K, Bechtel L, Joshi A, Zhang S, Poston RS. Strategies to reduce intraluminal clot formation in endoscopically-harvested saphenous veins. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2007.07.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa RA, Skaf M, Melo LA, Calucci D, Cardillo JA, Castro JC, Huang D, Wojtkowski M. Retinal assessment using optical coherence tomography. Prog Retin Eye Res. 2006;25:325–353. doi: 10.1016/j.preteyeres.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Santavirta S, Kauste A, Rindell K. Tourniquet ischaemia: clinical and biochemical observations. Ann Chir Gynaecol. 1978;67:210–213. [PubMed] [Google Scholar]
- 40.Enger EA, Jennische E, Medegård A, Haljamäe H. Cellular restitution after 3 h of complete tourniquet ischemia. Eur Surg Res. 1978;10:230–239. doi: 10.1159/000128012. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe JA. The coronary artery bypass conduit: II. Assessment of the quality of the distal anastomosis. Ann Thorac Surg. 2001;72:S2253–2258. doi: 10.1016/s0003-4975(01)03298-2. [DOI] [PubMed] [Google Scholar]