Abstract
Mucosal (nasal or oral) administration of anti-CD3 mAb is effective in ameliorating animal models of autoimmunity (experimental autoimmune encephalomyelitis, diabetes, and lupus) by inducing LAP+ regulatory T cells. We tested this approach in an arthritis model using type II collagen. We found that nasal anti-CD3 was more effective than oral anti-CD3 in attenuating the development of arthritis. Nasal anti-CD3 induced a LAP+ regulatory T cell that secreted high levels of IL-10 and suppressed collagen-specific T cell proliferation and anti-collagen Ab production. However, neither nasal nor oral anti-CD3 attenuated disease when given to animals with ongoing arthritis, and this was associated with a lack of induction of LAP+ regulatory T cells. We found, however, that coadministration of a novel emulsome adjuvant, which enhances Th2 responses, resulted in the induction of LAP+ regulatory T cells and suppression of ongoing arthritis by both nasal and oral anti-CD3. Suppression of arthritis by mucosal anti-CD3 was associated with less joint damage, a decrease of TNF-α and IFN-γ mRNA expression in joints, and a reduction in anti-collagen Abs. These results demonstrate that mucosal anti-CD3 therapy may serve as a therapeutic approach in arthritis and that the biologic effect is enhanced by an emulsome-based adjuvant.
Autoimmune processes in rheumatoid arthritis may be related to immune responses to joint-specific autoantigens such as type II collagen (CII). This is demonstrated in mice that develop polyarthritis with pathological symptoms similar to those observed in humans following immunization with CII (1, 2). Strategies to target autoreactive T cells and induce immune tolerance are being developed for the treatment of autoimmunity. We have been investigating immune modulation of autoimmune and inflammatory diseases by mucosal (nasal and oral) administration of protein Ags and of anti-CD3 mAb designed to induce regulatory T cells (3–7) and have identified a regulatory T cell that expresses surface latency-associated peptide (LAP). LAP is the N-terminal domain of the TGF-β precursor peptide and remains noncovalently associated with the TGF-β peptide after cleavage, forming the latent TGF-β complex. We have found that oral and nasal anti-CD3 led to the generation of LAP+ regulatory T cells that suppress experimental autoimmune encephalomyelitis (8), type I diabetes (9), type II diabetes (10), and lupus (11, 12). Others have found that oral anti-CD3 inhibits the development of atherosclerosis in mice by inducing regulatory T cells (13). In the current study, we investigated whether mucosal anti-CD3 could affect the development of arthritis and suppress ongoing disease in an animal model of collagen-induced arthritis (CIA).
We found that suppression of arthritis was linked to the induction of LAP+ regulatory T cells and that nasal anti-CD3 was more effective than oral anti-CD3 in preventing arthritis. Coadministration of a novel adjuvant emulsome, which biases toward a Th2 anti-inflammatory response, was needed for the effective treatment of ongoing arthritis by both nasal and oral anti-CD3. Our results demonstrate a new approach for the treatment of arthritis that decreases levels of TNF-α in the joints and identifies an adjuvant that enhances the effects of mucosal anti-CD3 and the induction of LAP+ regulatory T cells.
Materials and Methods
Mice
DBA/1 male mice 4- to 6-wk-old were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed in a pathogen-free environment, and the animal protocols were approved according to the guidelines of the Committee on Animals of Harvard Medical School.
Ags and Abs
Chicken CII was purchased from Chondrex (Redmond, WA). Emulsome was obtained from GSK Biologicals (Laval, Quebec, Canada). Fluorescent-labeled anti-mouse Abs used in flow cytometry (FACS) were CD4-specific (RM4-5), CD25-specific (PC61), IL-10–specific (JES5-16E3), 7-amino-actinomycin D (7AAD), and streptavidin-allophycocyamin (all from BD Biosciences, San Jose, CA). Affinity-purified biotinylated goat LAP-specific polyclonal Ab was from R&D Systems (Minneapolis, MN). We used CD16/CD32-specific Ab (BD Biosciences) for FcγR blocking.
Mucosal administration of anti-CD3 and induction of arthritis
Mice were nasally treated three times with 0.5 μg hamster IgG CD3-specific F(ab′)2 Ab (anti-CD3, clone 145-2C11; BioExpress, Kaysville, UT) or hamster IgG control F(ab′)2 Ab (IC; Jackson ImmunoResearch Laboratories, West Grove, PA) dissolved in 10 μl PBS every other day. For oral treatment, mice were fed 5 μg IC or anti-CD3 five times on consecutive days. For prevention of CIA, 2 d after the last nasal or oral treatment mice were shaved on the back and immunized with 100 μg CII emulsified in CFA (Sigma-Aldrich, St. Louis, MO) divided equally in five intradermal injection sites. Three weeks after the primary CII immunization, mice received a booster injection of 100 μg soluble CII i.p. We began observing the mice 7 d after the booster immunization. In therapeutic studies, we induced arthritis by CII immunization. Immediately after the booster immunization, mice received nasal or oral IC or anti-CD3 emulsified in 5% emulsome as indicated above. Mice were then followed for 100 d. To assess arthritis, mice were scored on a scale of 0–4: 0, no signs of disease; 1, erythema and mild swelling of the tarsus; 2, moderate erythema and swelling of the tarsus and ankles; 3, severe swelling of the tarsus and ankles; and 4, ankylosis and bone deformity. The arthritic score for each mouse was the combined score of all four paws.
T cell proliferation
Cervical (superficial and deep), mesenteric lymph node cells or splenocytes were cultured in triplicate at 0.5 × 106/well in the presence of 100 μg CII in 96-well round bottom microtiter plates (Corning Glass, Corning, NY) for 72 h at 37°C with 5% CO2 in a humidified incubator. Tissue culture medium was RPMI 1640 supplemented with 1% L-glutamine, 1% penicillin/streptomycin (BioWhittaker, Walkersville, MD), and 10% FCS. Cultures were pulsed with 1 μCi tritiated thymidine (PerkinElmer, Wellesley, MA) for the last 12 h. Tritiated thymidine incorporation was measured using a liquid scintillation beta counter (Wallac; PerkinElmer). Cell proliferation was expressed in δ CPM (Δ CPM).
Histology
Mouse paws were fixed in 10% formalin (Fisher Scientific, Pittsburgh, PA). Before H&E staining, paws were embedded in paraffin (Tissue-Tek, Tor-rance, CA), and 10 μM sections were cut on a cryostat. Slides were examined in a blinded fashion.
Cytokine detection
The level of cytokines produced in vitro by cell cultures was determined using BD OptEIA ELISA set and reagent set B (BD Biosciences). Samples were tested in duplicate using manufacturer’s recommended assay procedures. For the detection of cytokines, cell culture supernatants were harvested at different time points (40 h for IL-2, IL-4, IL-6, IL-10, and IFN-γ or 72 h for TGF-β).
Flow cytometry
Cells were washed with PBS containing 1% BSA (PBS/BSA; Bio-Whittaker). FcγRs were blocked by incubation with anti-CD16/CD32 Ab for 30 min at 4°C. Cells were washed twice before being stained with fluorescent-labeled anti-mouse Abs (1 μg/106 cells/test) or relevant IC Ab for 30 min at 4°C in dark. After staining, cells were washed again with PBS/BSA before FACS (FACScan; BD, Franklin Lakes, NJ). Intracellular staining was carried out as described previously (12). All FACS data were analyzed using FlowJo software (Tree Star, Ashland, OR).
ELISA for serum anti-CII Abs
Abs were measured as described previously (14). Briefly, chicken CII at 5 μg/ml in 0.1 M sodium bicarbonate (pH 9.2) was coated onto 96-well plates overnight at 4°C. Plates were washed with PBS and incubated for 2 h at room temperature with PBS containing 1% BSA, 0.5 μl/ml Tween 20, and 2.5% normal goat serum to neutralize nonspecific binding sites. Plates were washed with PBS, and serum samples in different dilutions were added (50 μl/well). For the detection of total IgG, IgG1, IgG2a, or IgG2b Abs, 50 μl/well HRP-conjugated rat anti-mouse Ab (BD Biosciences) at 0.001 μg/ml was added and incubated at room temperature for 1 h.
Polymerase chain reaction
Skin was removed from paws before being homogenized in TRIzol (Sigma-Aldrich). RNA was extracted from joint homogenate using RNAeasy columns (Qiagen, Valencia, CA). cDNA was transcribed as recommended (Applied Biosystems, Foster City, CA). RT-PCR was carried out using cDNA from joint homogenates. All primer/probe mixtures were obtained from Sigma-Genosys (The Woodlands, TX). PCR products were visualized on 1% agarose gel. TaqMan analysis was performed on AB 7500 Fast System (Applied Biosystems). Gene expression was normalized to the expression of β-actin. All primer/probe mixtures were obtained from Applied Biosystems.
Statistical analysis
Statistical differences in cell proliferation, cytokine production, and serum Ab levels were derived from Student t test. The Wilcoxon rank-sum test was used for all of the pairwise group comparisons. A p value <0.05 was considered significant.
Results
Mucosal anti-CD3 prior to induction of CIA attenuates disease
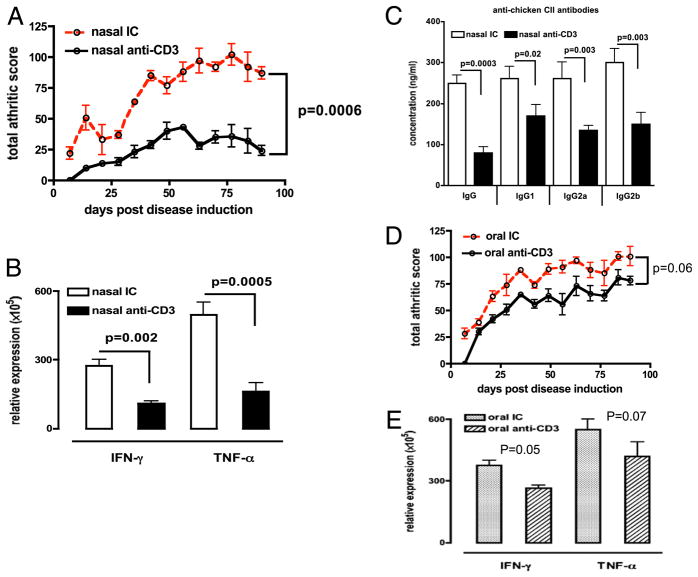
We administered 0.5 or 5 μg anti-CD3 nasally or orally, respectively, in accordance with doses used in our previous studies (11, 12). We used the F(ab′)2 fragment of anti-CD3 Ab as done in previous studies (12). DBA/1 mice received IC or anti-CD3 nasally (Fig. 1A) or orally (Fig. 1E) before immunization with CII. As shown in Fig. 1A, nasal anti-CD3 significantly lowered the severity and incidence (Table I) of arthritis compared with nasal IC in mice. In addition, we found significantly lower mRNA expression of the proinflammatory cytokines IFN-γ (p = 0.002) and TNF-α (p = 0.0005) in joints of nasal anti–CD3-treated mice (Fig. 1B) and decreased levels of IgG and IgG subclass Abs to chicken CII in anti–CD3-treated animals (Fig. 1C). Oral anti-CD3 given before the induction of CIA showed a trend toward disease attenuation but did not achieve statistical significance (p = 0.06) (Fig. 1D) and less prominent changes in cytokine expression in joints (Fig. 1E) compared with nasal anti-CD3 (Fig. 1B). No changes in serum Ab levels were observed in oral anti–CD3-treated animals (data not shown).
FIGURE 1.
Nasal anti-CD3 suppresses CIA in DBA/1 mice. A, DBA/1 mice (eight per group) received three doses of 0.5 μg IC or anti-CD3 Ab nasally every other day before immunization with CII. Arthritis was scored as indicted in Materials and Methods. Total scores of each group at each time point are presented. Error bars represent SEM of three experiments. B, Joint homogenates from nasal IC (open bars) or nasal anti-CD3 (filled bars) mice (eight per group) were used in quantitative PCR for IFN-γ and TNF-α mRNA expression. C, Serum (eight per group) was collected 100 d postdisease induction. The concentration of anti-chicken collagen II Abs was calculated using standard curves derived from known concentrations of IgG and each IgG subclass Abs with HRP-conjugated anti-mouse Ab. D, DBA/1 mice (eight per group) received five consecutive doses of 5 μg IC or anti-CD3 Ab orally before immunization with CII. Error bars represent SEM of three experiments. E, Joint homogenates from oral IC (dotted bars) or oral anti-CD3 (slashed bars) mice (eight per group) were used in quantitative PCR for IFN-γ and TNF-α mRNA expression.
Table I.
Incidence of arthritis following nasal anti-CD3
| Nasal IC | Nasal Anti-CD3 | |
|---|---|---|
| Severe arthritis (clinical score >3) | 8 | 1* |
| Mild arthritis (clinical score <2) | 0 | 3* |
| No arthritis | 0 | 4* |
DBA/1 mice (eight per group) received three doses of 0.5 μg IC or anti-CD3 Ab nasally every other day before immunization with CII. Arthritis was scored as indicted in Materials and Methods. Incidence of arthritis at 100 d postdisease induction in each treatment group is shown. Data were analyzed by χ2 test.
p < 0.05 compared with IC-treated controls.
Nasal anti-CD3 suppresses Ag-specific T cell proliferation and induces IL-10 production
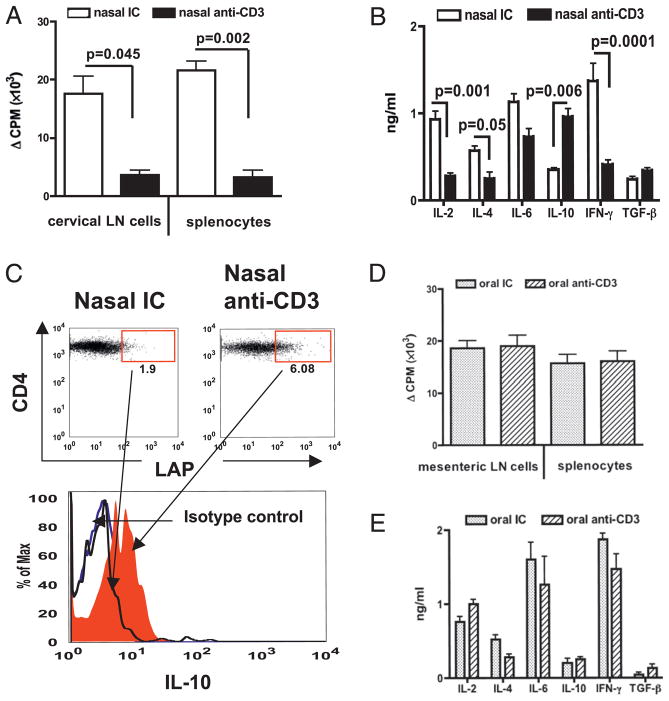
We next examined the effect of nasal anti-CD3 on Ag-specific T cell proliferation and cytokine production. We found a significant reduction in T cell proliferation to CII both in cervical lymph node (p = 0.045) and spleen (p = 0.002) (Fig. 2A) demonstrating that T cell regulation occurred both locally and systemically following nasal anti-CD3 treatment. We did not observe apoptosis in CD4+ T cells from cervical lymph nodes or spleens (data not shown) as has been reported for i.v. anti-CD3 treatment (15). In addition, we observed a significant (p = 0.001) decrease in IL-2 production (Fig. 2B) and downregulation of both Th1 (IFN-γ) and Th2 (IL-4) type cytokine production (Fig. 2B), which correlates with our finding of reduced Th1- and Th2-linked IgG subclass Abs in serum of nasal anti–CD3-treated mice (Fig. 1D). In accordance with our previous findings (11, 14, 16), we found a high level of IL-10 production by T cells of nasal anti–CD3-treated mice (Fig. 2B, 2C). We did not observe changes in proliferation (Fig. 2D) or cytokine production (Fig. 2E) by splenocytes or mesenteric lymph node cells after oral anti-CD3 treatment.
FIGURE 2.
T cell hyporesponsiveness and cytokine production. A, DBA/1 mice (three per group) received three doses of 0.5 μg IC (open bars) or anti-CD3 (filled bars) Ab nasally every other day before immunization with CII. Seven days after CII immunization, cervical lymph node cells or splenocytes were cultured in vitro with CII (100 μg/ml). B, Splenocyte culture supernatants were used in ELISA. C, Cervical lymph node cells were surface stained with CD4 and LAP and intracellularly stained with IL-10 Abs. IL-10 in gated CD4+LAP+ T cells is shown in histogram. D, DBA/1 mice (three per group) received five consecutive doses of 5 μg IC or anti-CD3 Ab orally before immunization with CII. Seven days after CII immunization, mesenteric lymph node cells or splenocytes were cultured in vitro with CII (100 μg/ml). E, Splenocyte culture supernatants were used in ELISA. These experiments were repeated once with similar results.
Nasal anti-CD3 induces LAP+ T cells
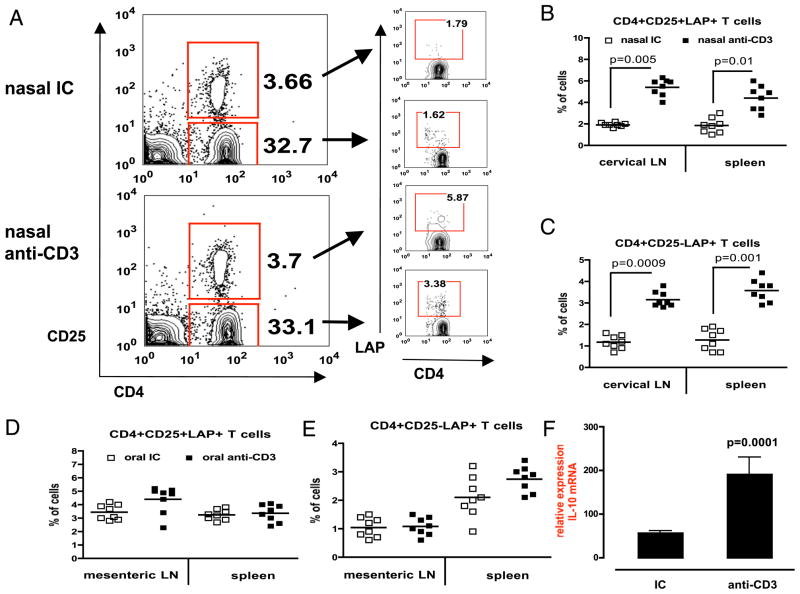
We have previously shown that nasal or oral anti-CD3 induced a LAP+ regulatory T cell that suppresses experimental autoimmune encephalomyelitis (8), type I and II diabetes (9, 10), and lupus (11, 12) in mice. Thus, we investigated LAP expression following nasal anti-CD3 in DBA/1 mice. We found an upregulation of LAP expression in both CD4+CD25− and CD4+CD25+ T cells in cervical lymph node of mice nasally treated with anti-CD3 (Fig. 3A). We carried out comparative studies of LAP expression in individual mice (eight per group) treated with nasal anti-CD3 and found that there was a significant upregulation of LAP+ T cells in both cervical lymph nodes and spleen (Fig. 3B, 3C). This correlates well with our findings of local and systemic downregulation of proliferative responses to CII (Fig. 2A), indicating that LAP+ T cells are associated with suppression of Ag-specific immune responses. Oral anti-CD3 did not upregulate LAP expression on T cells in mesenteric lymph nodes or spleen (Fig. 3D, 3E). As shown in Fig. 2C that nasal anti-CD3 generated IL-10–producing CD4+LAP+ T cells, we investigated a role of IL-10 in local immune response in target tissue. We examined IL-10 mRNA expression in joints of mice 100 d postnasal treatment. Fig. 3F shows that nasal anti-CD3 but not IC led a significant increase in IL-10 mRNA expression. This suggests that nasal anti-CD3 attenuated CIA development that is associated with an induction of IL-10–producing LAP+ regulatory T cells.
FIGURE 3.
Induction of LAP+ regulatory T cells. A, DBA/1 mice (eight per group) were nasally treated with 0.5 μg IC or anti-CD3 before induction of CIA. Mice were followed for 100 d, at which point cervical lymph nodes were stained with anti-mouse CD4, CD25, and LAP Abs (left panel). LAP expression on live 7AAD−CD4+CD25+ or CD25− T cells is shown in the right panel. Representative FACS plots of two independent experiments are shown. Percentages of CD4+CD25+LAP+ (B) or CD4+CD25−LAP+ (C) T cells in cervical lymph node and spleen of individual mice are shown. Each symbol represents one mouse. Mean values are represented by cross bars. CD4+CD25+LAP+ (D) or CD4+CD25−LAP+ (E) T cells in mesenteric lymph node and spleen of mice fed five consecutive doses of 5 μg IC or anti-CD3 Ab are shown. F, Joint homogenates from nasal IC or nasal anti-CD3 mice (eight per group) was used in quantitative PCR for IL-10 mRNA expression.
Both nasal and oral anti-CD3 suppresses ongoing arthritis when given together with emulsome
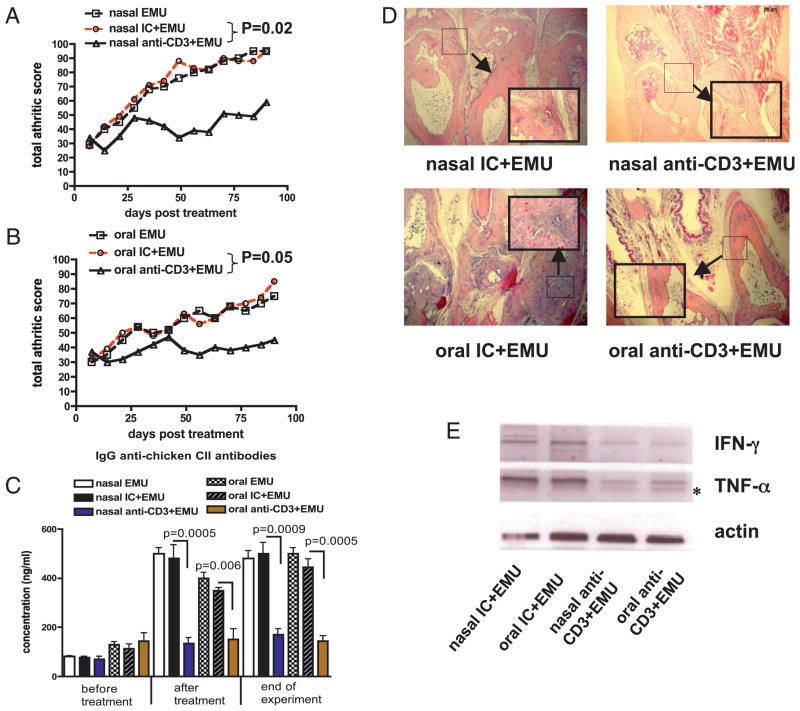
To test the therapeutic effect of mucosal anti-CD3, we treated animals with ongoing arthritis after disease induction with nasal or oral anti-CD3. We found no effect on the severity of arthritis or immune responses including serum anti-collagen Abs or induction of LAP+ regulatory T cells with either mucosal route (data not shown). These results suggest that induction of LAP+ regulatory T cells may be influenced by ongoing inflammation. To test this hypothesis, we coadministered an adjuvant that preferentially induces anti-inflammatory Th2 responses (17) to determine whether it restored the effect of nasal and oral anti-CD3 in mice with ongoing arthritis. We found that adding the emulsome to nasal anti-CD3 significantly attenuated (p = 0.02) ongoing arthritis for as long as 100 d after disease induction (Fig. 4A, Table II). A similar effect was observed when emulsome was added to oral anti-CD3 (p = 0.05) (Fig. 4B, Table II). No effect was observed with the emulsome alone or given with IC Ab.
FIGURE 4.
Suppression of ongoing CIA by mucosal anti-CD3 and emulsome. A, DBA/1 mice (eight per group) received three doses of 0.5 μg IC or anti-CD3 in emulsome or emulsome alone nasally every other day after induction of CIA. Arthritis was scored as indicted in Materials and Methods. Total scores of each group at each time point are presented. B, DBA/1 mice (eight per group) received five consecutive doses of 5 μg IC or anti-CD3 in emulsome or emulsome alone orally after induction of CIA. C, Serum was collected before mucosal treatment, after mucosal treatment and at the end of experiment. The concentration of anti-chicken collagen II Abs was calculated using standard curves derived from known concentrations of IgG Abs with HRP-conjugated anti-mouse Ab. D, One hundred days after mucosal treatment, whole foot was embedded in paraffin, and joint pathology was examined by H&E staining. A selected region showing muscle and cartilage is enlarged at a magnification of ×40 and shown in a window. E, RNA was extracted from joint homogenates, and cDNA was used in RT-PCRs for detection of IFN-γ and TNF-α expression. These experiments were repeated once with similar results. Asterisk indicates a nonspecific band.
Table II.
Incidence of arthritis following mucosal anti-CD3 given with emulsome
| Nasal EMU | Nasal IC + EMU | Nasal Anti-CD3 + EMU | Oral EMU | Oral IC + EMU | Oral Anti-CD3 + EMU | |
|---|---|---|---|---|---|---|
| Severe arthritis (clinical score >3) | 8 | 8 | 2* | 5 | 7 | 1* |
| Mild arthritis (clinical score <2) | 0 | 0 | 6* | 3 | 1 | 7* |
DBA/1 mice (eight per group) received three doses of 0.5 μg IC or anti-CD3 in emulsome or emulsome alone nasally every other day or five consecutive doses of 5 μg IC or anti-CD3 in emulsome or emulsome alone orally after induction of CIA. Arthritis was scored as indicted in Materials and Methods. Incidence of arthritis at 100 d postdisease induction in each treatment group is shown. Data were analyzed by χ2 test.
p < 0.05 compared with IC-treated controls.
We next measured the effect of emulsome given together with mucosal anti-CD3 on collagen Abs, joint destruction, and the induction of LAP+ T cells. We found that both nasal and oral anti-CD3 given together with emulsome decreased anti-CII Abs when measured at the end of the experiment (100 d after disease induction; Fig. 4C).
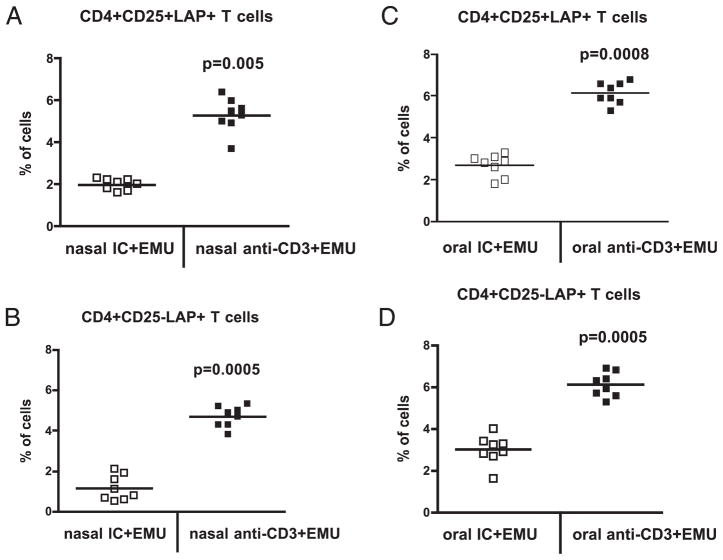
Joint destruction characterized by inflammatory hyperplasia, muscle fiber thinning, and bone erosion was prevented by nasal or oral anti-CD3 plus emulsome (Fig. 4D). This was associated with a downregulation of mRNA expression of IFN-γ and TNF-α in the joints (Fig. 4E). Emulsome alone given nasally or orally did not affect these parameters (data not shown). We hypothesized that the effect of emulsome was related to the induction of LAP+ regulatory T cells by mucosal anti-CD3. To test this, we examined LAP expression on CD4+CD25− and CD4+CD25+ T cells in spleens of mice at 100 d after mucosal anti-CD3 and emulsome combination therapy. We found that anti-CD3 plus emulsome given either nasally (Fig. 5A, 5B) or orally (Fig. 5C, 5D) sig-nificantly increased the percentage of LAP+CD4+CD25− and LAP+CD4+CD25+ T cells compared with emulsome given together with IC. Thus, the therapeutic effect of mucosal anti-CD3 plus emulsome in arthritic mice is associated with the induction of LAP+ regulatory T cells.
FIGURE 5.
Induction of LAP+ regulatory T cells following mucosal anti-CD3 and emulsome. DBA/1 mice (eight per group) were nasally treated with 0.5 μg or fed 5 μg IC or anti-CD3 and emulsome after induction of CIA. Mice were followed for 100 d, and at which point splenocytes were stained with 7AAD, anti-mouse CD4, CD25, and LAP Abs. 7AAD−CD4+ live T cells were gated, and percentages of CD4+CD25+LAP+ or CD4−CD25−LAP+ T cells in spleen of individual mice are shown. A and B represent mice treated nasally. C and D represent mice treated orally. Each symbol represents one mouse. Mean values are represented by cross bars. These experiments were repeated once with similar results.
Discussion
We show in this paper that nasal anti-CD3 attenuated the development of CIA in DBA/1 mice and that the therapeutic effect was associated with an induction of LAP+ regulatory T cells. Nasal anti-CD3 given prior to the induction of CIA attenuated the development of disease and downregulated anti-collagen Ab production as well as IFN-γ and TNF-α mRNA expression in joints. Previously, we also observed decreases in Ab production and cytokine secretion in lupus-prone mice treated with nasal anti-CD3 (11). This effect was mediated by IL-10–secreting LAP+ regulatory T cells that controlled the differentiation and expansion of follicular helper T cells leading to downregulation of B cell activation (11). In the current study, our finding of a significant decrease in collagen-specific T cell proliferation following nasal anti-CD3 suggests that a similar regulatory mechanism may exist in animal models of arthritis. In support of this view, we found that the effect of nasal anti-CD3 was accompanied by a significant increase in IL-10 production and-LAP+ regulatory T cell differentiation.
In contrast, we did not find a significant effect of oral anti-CD3 in preventing CIA or inducing immunomodulatory effects, although we did observe a trend in reduction of disease severity. The reason for differences in nasal verses oral anti-CD3 in preventing arthritis is not clear. It could relate to dosing, because we did not test a wide range of doses given orally in the collagen model. It is also possible that tolerogenic mediators, which are functional in the upper airways, may be defective in the gut mucosa of DBA/1 mice. Consistent with this view, we have found increased apoptosis of CD11c+ dendritic cells in mesenteric lymph nodes of DBA/1 mice (H.Y. Wu, unpublished data).
Induction of tolerance prior to disease induction in animal models may not be applicable to clinical situations in which patients have already developed disease. Thus, we tested the therapeutic effect of mucosal anti-CD3 following CIA induction in DBA/1 mice. Contrary to the positive effects seen with anti-CD3 treatment before CIA induction, we observed no effect on disease or immune measures including serum anti-collagen Abs and the induction of LAP+ regulatory T cells when mice were treated with nasal or oral anti-CD3 after CIA induction. We hypothesized that the lack of effect in mice with ongoing disease may be due to the inhibitory effect of systemic inflammation on LAP+ regulatory T cell generation by mucosal anti-CD3. A shift in immune response toward an inflammatory Th1 phenotype during the development of arthritis in mice (18) and in arthritic joints of patients with rheumatoid arthritis (19) has been reported.
To address this issue, we investigated the coadministration of emulsome, an adjuvant that biases toward Th2-type immune responses (17). Emulsome is a novel lipoidal particulate vehicle with features intermediate between liposomes and oil-in-water emulsions (17, 20). Emulsome has a bioadhesive polymer that confers mucoadhesive properties to the lipoidal particles to improve attachment to mucosal surfaces. Feeding of emulsome suppressed IFN-γ production by T cells following in vitro Ag stimulation (R. Maron, unpublished data). Therefore, we tested the effect of a combination therapy with mucosal anti-CD3 and emulsome in mice following CIA induction. We found that nasal or oral emul-some restored the suppressive effects of anti-CD3 and attenuated ongoing disease in mice. A combination of mucosal anti-CD3 and emulsome suppressed the production of anti-CII Abs and improved the severity of joint pathology by reducing inflammatory cytokines in joints. This was associated with an upregulation of LAP+ T cells indicating that, indeed, induction of LAP+ regulatory T cells by mucosal anti-CD3 was associated with suppression of disease processes. Therefore, dampening inflammatory responses by emulsome promotes the generation of LAP+ regulatory T cells by mucosal anti-CD3. To our knowledge, this is the first study that shows a combination of anti-CD3 and adjuvant is efficacious in controlling ongoing arthritis in mice. This puts forward the possibility of a combinational therapy in other autoimmune diseases where therapeutic efficacy is hindered by systemic inflammation during active disease. In this regard, we have found that administration of β-glucosylceramide enhances the effect of oral anti-CD3 in a model of type II diabetes (10).
Recent studies have demonstrated that systemic injection of anti-CD3 in DBA/1 mice suppressed CIA by expanding CD4+/CD8+ CD25+Foxp3+ regulatory T cells (21). This may be due to an initial deletion of pathogenic T cells, thus allowing the expansion of Foxp3+ regulatory T cells (22). In addition, CD8+CD25+Foxp3+ regulatory T cells have been induced after i.v. anti-CD3 in humans (23). We have recently characterized human LAP+ regulatory T cells (24). In contrast to systemic delivery, mucosal administration of anti-CD3 induced CD4+LAP+ regulatory T cells without significant upregulation of Foxp3 expression (8, 11, 12). Furthermore, we observed neither deletion of T cells nor downregulation of CD3 expression following mucosal anti-CD3. Thus, different mechanisms are involved in the induction of regulatory T cell by mucosal verses systemic delivery of anti-CD3 (25).
We previously demonstrated that nasal or oral administration of CII in DBA/1 mice before induction of CIA suppressed IFN-γ production by CII-specific pathogenic T cell as well as TNF-α and IL-6 mRNA expression in joints of orally tolerized mice (26). Disease suppression was associated with a shift in immune responses from a Th1 to a Th2 phenotype. Interestingly, immune deviation from Th1 to Th2 responses was also observed in previous nasal tolerance studies using histone peptides in animal models of lupus (14). More recently, Broere et al. (27) demonstrated that oral or nasal administration of proteoglycan induced functional IL-10–producing CD4+ regulatory T cells in the cartilage proteoglycan-induced chronic arthritis model. Both nasal and oral application of human proteoglycan before induction of disease suppressed arthritis severity and incidence (27). In this study, we show that mucosal anti-CD3 not only attenuated the development of CIA when given in a preventive regimen but also arrested ongoing disease and inflammatory processes by inducing LAP+ regulatory T cells. Similarly, mucosal anti-CD3 in lupus prone mice attenuated disease development and suppressed ongoing disease that was mediated by LAP+ regulatory T cells (11, 12).
We performed our experiments with a F(ab′)2 Ab to eliminate any potential side effects related to the Fc portion of the molecule that might occur after multiple administrations of the Ab mucosally. We observed no mitogenic effect of nasal hamster CD3-specific F(ab′)2 Ab in mice, and no evidence of cytokine release syndrome (wasted appearance and ruffled fur) after multiple mucosal administrations. Furthermore, we did not observe an anti-globulin response against anti-CD3 in mice mucosally treated with anti-CD3 (data not shown). Mucosal administration of CD3-specific Ab is applicable in chronic therapy and would not be expected to have side effects including cytokine release syndromes and antiglobulin responses.
In summary, we have shown that mucosal anti-CD3 Ab attenuates the development of CIA in DBA/1 mice. Nasal anti-CD3 suppresses CIA when given prior to disease induction and both nasal and oral anti-CD3 suppresses ongoing disease when given with an emulsome adjuvant. Disease suppression was associated with a downregulation of collagen-specific T cell response and anti-collagen Ab production as well as joint inflammation. This was accompanied by the induction of LAP+ regulatory T cell. In a phase I trial, we have shown that oral administration of OKT3 mAb to human subjects is safe and induces a dose-dependent immunologic effect in T cells and den-dritic cells (28). This approach is thus applicable for testing as a mucosal, noninvasive therapy for rheumatoid arthritis.
Acknowledgments
This work was supported by National Institutes of Health Grants AI435801 and NS38037 (to H.L.W.).
We thank Dr. B.H. Waksman for critical assessment of the manuscript.
Abbreviations used in this paper
- 7AAD
7-aminoactinomycin D
- CIA
collagen-induced arthritis
- CII
type II collagen
- IC
isotype control
- LAP
latency-associated peptide
Footnotes
Disclosures
H.L.W. is a consultant for Nasvax. The other authors have no financial conflicts of interest.
References
- 1.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuart JM, Huffstutter EH, Townes AS, Kang AH. Incidence and specificity of antibodies to types I, II, III, IV, and V collagen in rheumatoid arthritis and other rheumatic diseases as measured by 125I-radioimmunoassay. Arthritis Rheum. 1983;26:832–840. doi: 10.1002/art.1780260703. [DOI] [PubMed] [Google Scholar]
- 3.Wu HY, Weiner HL. Oral tolerance. Immunol Res. 2003;28:265–284. doi: 10.1385/IR:28:3:265. [DOI] [PubMed] [Google Scholar]
- 4.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci USA. 1991;88:10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- 7.Frenkel D, Huang Z, Maron R, Koldzic DN, Hancock WW, Moskowitz MA, Weiner HL. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10–producing CD4+ T cells. J Immunol. 2003;171:6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- 8.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+CD25−LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 10.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10–secreting CD4+CD25−LAP+ regulatory T cell and is associated with down-regulation of IL-17+CD4+ICOS+CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu HY, Center EM, Tsokos GC, Weiner HL. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25−LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus. 2009;18:586–596. doi: 10.1177/0961203308100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki N, Yamashita T, Takeda M, Shinohara M, Nakajima K, Tawa H, Usui T, Hirata K. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 2009;120:1996–2005. doi: 10.1161/CIRCULATIONAHA.109.863431. [DOI] [PubMed] [Google Scholar]
- 14.Wu HY, Ward FJ, Staines NA. Histone peptide-induced nasal tolerance: suppression of murine lupus. J Immunol. 2002;169:1126–1134. doi: 10.4049/jimmunol.169.2.1126. [DOI] [PubMed] [Google Scholar]
- 15.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci USA. 1994;91:123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu HY, Monsonego A, Weiner HL. The mechanism of nasal tolerance in lupus prone mice is T-cell anergy induced by immature B cells that lack B7 expression. J Autoimmun. 2006;26:116–126. doi: 10.1016/j.jaut.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Kretschmar M, Amselem S, Zawoznik E, Mosbach K, Dietz A, Hof H, Nichterlein T. Efficient treatment of murine systemic infection with Candida albicans using amphotericin B incorporated in nanosize range particles (emulsomes) Mycoses. 2001;44:281–286. [PubMed] [Google Scholar]
- 18.Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 19.Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–1969. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 20.Lowell GH, Kaminski RW, VanCott TC, Slike B, Kersey K, Zawoznik E, Loomis-Price L, Smith G, Redfield RR, Amselem S, Birx DL. Proteosomes, emulsomes, and cholera toxin B improve nasal immunogenicity of human immunodeficiency virus gp160 in mice: induction of serum, intestinal, vaginal, and lung IgA and IgG. J Infect Dis. 1997;175:292–301. doi: 10.1093/infdis/175.2.292. [DOI] [PubMed] [Google Scholar]
- 21.Notley CA, McCann FE, Inglis JJ, Williams RO. ANTI-CD3 therapy expands the numbers of CD4+ and CD8+ Treg cells and induces sustained amelioration of collagen-induced arthritis. Arthritis Rheum. 2010;62:171–178. doi: 10.1002/art.25058. [DOI] [PubMed] [Google Scholar]
- 22.Chatenoud L. Immune therapies of autoimmune diseases: are we approaching a real cure? Curr Opin Immunol. 2006;18:710–717. doi: 10.1016/j.coi.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi R, Farez MF, Wang Y, Kozoriz D, Quintana FJ, Weiner HL. Cutting edge: human latency-associated peptide+ T cells: a novel regulatory T cell subset. J Immunol. 2010;184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu HY. Induction of mucosal tolerance in SLE: a sniff or a sip away from ameliorating lupus? Clin Immunol. 2009;130:111–122. doi: 10.1016/j.clim.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia G, Komagata Y, Slavin AJ, Maron R, Weiner HL. Suppression of collagen-induced arthritis by oral or nasal administration of type II collagen. J Autoimmun. 1999;13:315–324. doi: 10.1006/jaut.1999.0320. [DOI] [PubMed] [Google Scholar]
- 27.Broere F, Wieten L, Klein Koerkamp EI, van Roon JA, Guichelaar T, Lafeber FP, van Eden W. Oral or nasal antigen induces regulatory T cells that suppress arthritis and proliferation of arthritogenic T cells in joint draining lymph nodes. J Immunol. 2008;181:899–906. doi: 10.4049/jimmunol.181.2.899. [DOI] [PubMed] [Google Scholar]
- 28.Ilan Y, Zigmond E, Lalazar G, Dembinsky A, Ben Ya’acov A, Hemed N, Kasis I, Axelrod E, Zolotarov L, Klein A, et al. Oral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cells. J Clin Immunol. 2010;30:167–177. doi: 10.1007/s10875-009-9323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]