This report is concerned with a further evaluation of auxiliary canine homotransplantation of the liver and with the attempted application of such a procedure for the treatment of three patients.
Methods
There were 33 mongrel dogs weighing 17.7 to 22.2 kg (average, 17.5 kg) (39 to 49 lb [average, 38.5 lb]) used as recipients. The experiment was completed in 11 animals. The other 22 died early of anesthetic, technical, and septic complications and are excluded from further discussion. In the 11 test dogs the donors (weight 9.5 to 20 kg [21 to 44 lb]) were smaller than their recipients, the average weight being 13.6 kg (30 lb). The general surgical techniques and the studies obtained postoperatively were similar to those described in previous publications.1, 2 The average ischemia time for the cooled homografts was 44 minutes. Postoperatively, 2 to 8 mg/kg azathioprine were administered daily.
All animals received a completely diverting portacaval shunt. Immediately thereafter, the homotransplantation was performed. The animals are grouped into two categories.
Group 1 (Eight Animals)
Both of the inflowing vessels to the homograft were proved to be patent by premortem angiograms and by autopsy studies (Fig 1, A).
Fig 1.
Experimental design of auxiliary canine homotransplantation of the liver, in conjunction with host portacaval shunt. (A) Group 1 Series: Note that the homograft receives a double blood supply, the portal inflow being of systemic venous origin. Except for the host portacaval shunt, the procedure is essentially that described by Welch?. (B) Group 2 Series: In these dogs the portal venous anastomosis clotted. Thus, both the host liver and homograft received only an arterial blood supply.
Group 2 (Three Animals)
The inferior venacaval to portal venous anastomosis was shown to be occluded (Fig 1, B). In these experiments, both the autologous liver and the homograft had only an arterial blood supply.
Results
General Observations
There was no significant difference in the postoperative clinical course between the two groups of animals. Dogs of group 1 lost an average of 2.4 kg (5.3 lb) compared to 3.6 kg (7.9 lb) in group 2. All animals had transient rises in Serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) with return to normal for varying periods before death or sacrince; changes in alkaline phosphatase were similar but did not return to normal. Progressive anemia was invariably noted.
Group 1 Animals
Terminally two of the eight animals became jaundiced, with maximum bilirubins of 5.1 and 8.2 mg/100 cc, respectively. The dogs lived for 24 to 150 days (average 58 days). Two were killed in apparent good health; two died of unknown causes; and four died of infection.
At autopsy the host livers appeared somewhat small but were otherwise normal. Similarly, some degree of gross atrophy affected the homografts. The weights of the homografts and the autologous livers (24 to 150 days after transplantation) are listed in Table 1. Marked homograft atrophy was present in only one animal. Since the donor animals were generally smaller than the recipients, these postmortem liver measurements were related to the total body weights of the respective animals at the time of transplantation. The weights of the homografts in relation to the original donor body weights averaged 2.03%. The mean ratio of autologous liver weights to the original recipient body weights was 1.79%. Since the predicted liver weights per total body weight ratio should be approximately 2.80%, 3, 4 it seems likely that both organs underwent relative atrophy, the host liver slightly more than the homograft.
Table 1.
Dogs With Patent Portal Venous and Hepatic Arterial Inflow to the Homograft
| Dog No. | Survival, Days | Weight of Transplant, Gm | Weight of Host Liver, Gm |
|---|---|---|---|
| 1 | 63 | 460 | 260 |
| 2 | 150 | 215 | 240 |
| 3 | 39 | 300 | 376 |
| 4 | 35 | 26 | 496 |
| 5 | 24 | 284 | 264 |
| 6 | 57 | 460 | 435 |
| 7 | 29 | 335 | 370 |
| 8 | 63 | 360 | 500 |
| Average | 58 | 305 | 368 |
Autopsy transplant liver weight per donor body weight: 2.03%.
Autopsy host liver weight per host body weight at time of operation: 1.79 %.
Histopathology of Group 1 Animals
The eight host livers examined at autopsy, 24 to 150 days after operation, all showed atrophy of the centrilobular hepatocytes accompanied by collapse of the central part of the lobular reticulin framework (Fig 2). Similar changes were present in the one biopsy that was obtained at 35 days. Seven of the eight livers contained moderate to large quantities of hemosiderin in the Kupffer cells. Centrilobular cholestasis with bile “thrombi” was present in four of the livers.
Fig 2.
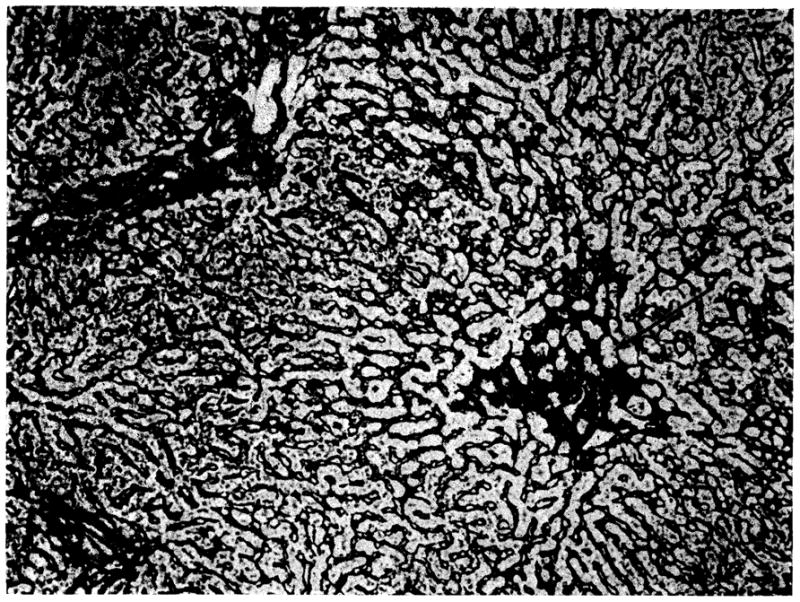
Own liver of dog 8 from group 1. Atrophy of the centrilobular hepatocytes has permitted collapse of the central part of the lobular reticulin framework (arrow) (reticulin stain, × 80).
In six of the eight transplanted livers examined at autopsy there was absence of hepatocytes and complete collapse of the lobular reticulin. The liver cells were replaced by mesenchymal cells. Portal tracts and central canals were crowded together. Areas of hemorrhage and cellular infiltration, either mononuclear or polymorphonuclear, were frequent. Of the two less severely affected livers, one from a dog dying at 24 days (dog 5) showed necrosis of the centrilobular and midzonal hepatocytes together with collapse of the central part of the lobular reticulin framework, but no cellular infiltration or cholestasis. The other, from an animal killed at 63 days (dog 8), showed atrophy of the centrilobular hepatocytes, increased central and portal connective tissue, and bands of reticulin which linked portal tracts and cut across lobules producing pseudolobules. A small number of mononuclear cells were present in the portal tracts of this homograft, but only about 10% of these possessed pyroninophilic cystoplasm. There was cholestasis, some proliferation of bile ductules in the portal areas, and much hemosiderin in macrophages. Similar, though less severe, changes were present in this animal’s homograft when it was biopsied at 35 days (Fig 3).
Fig 3.
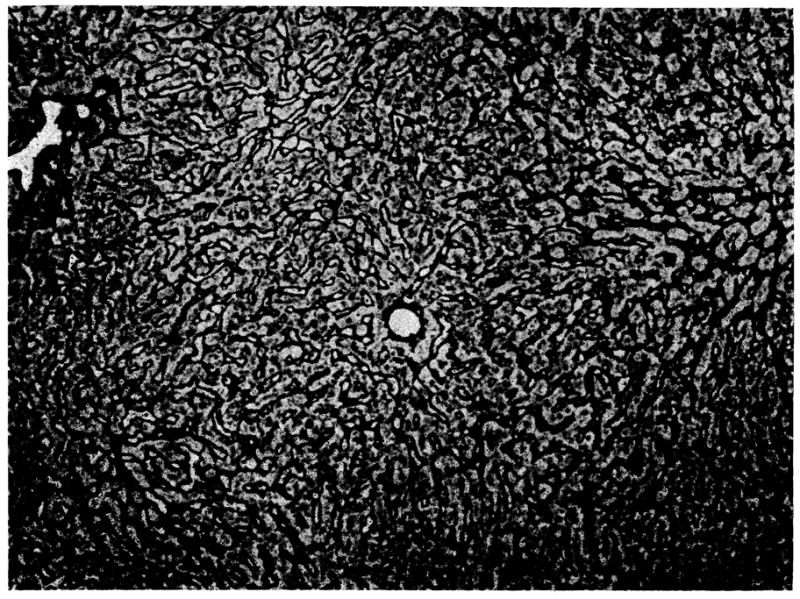
Biopsy at 35 days of auxiliary hepatic homograft from dog 8 (group 1). The lobular reticulin framework is normal. Twenty-eight days later this liver showed increased central and portal connective tissue and some pseudolobules (reticulin stain, × 80).
Group 2 (Three Animals)
None of the animals became jaundiced at any time; two were killed after 136 and 105 days and the third died of pneumonia after 73 days.
Two of the three host livers were small but were otherwise normal. The homograft livers showed a marked degree of atrophy but maintained normal gross architecture (Table 2). The weights of the homografts in relation to the original donor body weights averaged 0.72%, whereas the average weight ratio of autologous livers to the original recipient body weights was 1.52%. When compared to predicted weights, both sets of livers showed atrophy, affecting the transplant more than the host organ.
Table 2.
Dogs With Patent Hepatic Arterial Supply Only
| Dog No. | Survival, Days | Weight of Transplant, Gm | Weight of Host Liver, Gm |
|---|---|---|---|
| 1 | 136 | 82 | 232 |
| 2 | 73 | 36 | 150 |
| 3 | 105 | 150 | 410 |
| Averages | 105 | 89 | 264 |
Autopsy transplant liver weight per donor body weight: 0.72 %.
Autopsy host liver weight per host body weight at time of operation: 1.52 %.
Histopathology of Group 2 Animals
The three host livers examined at autopsy, 73 to 136 days after operation, all showed atrophy of hepatocytes, most prominent centrally but present to a lesser degree in the other zones of the lobules. This was accompanied by collapse of the central reticulin and accumulation of fat in the hepatocytes. Similar changes were present in the two biopsies that were examined at 35 days. Cholestasis was present in two livers.
In two of the three auxiliary transplants the hepatocytes had disappeared leaving only mesenchymal cells and the collapsed, condensed lobular framework of the liver. The third transplant, from a dog (1) killed at 136 days, showed increased reticulin and fibrous tissue in the portal areas and around the central veins. Connective tissue strands joined portal tracts and cut across lobules forming pseudolobules. Many mononuclear cells were present in the portal tracts. The cytoplasm of about 30% of these was pyronin-positive, and several of the cells were in mitosis. Macrophages in the portal areas contained large amounts of hemosiderin. Several of the hepatic arteries were narrowed by fibrous intimal thickening. The biopsy obtained from this liver at 35 days showed more cellular infiltration and less arterial damage (Fig 4).
Fig 4.
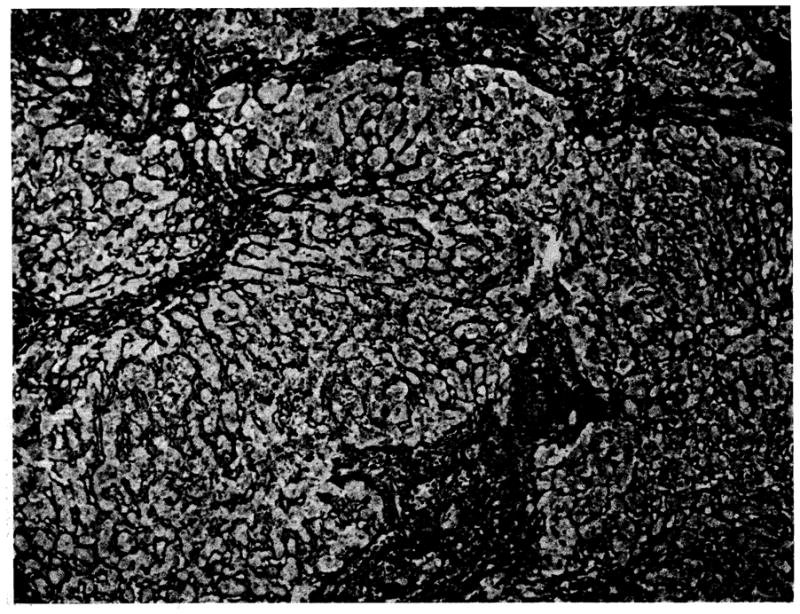
Biopsy at 35 days of auxiliary hepatic homograft from dog 1 (group 2). The normal lobular architecture has been disrupted by bands of connective tissue which link portal tracts and some central veins. There is increased reticulin in the portal areas and around the central veins (reticulin stain, × 80).
Report of Cases
Case 1
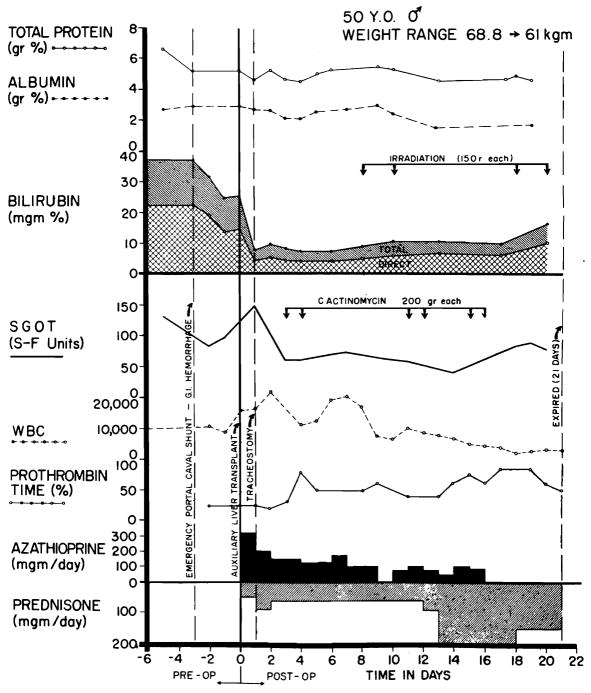
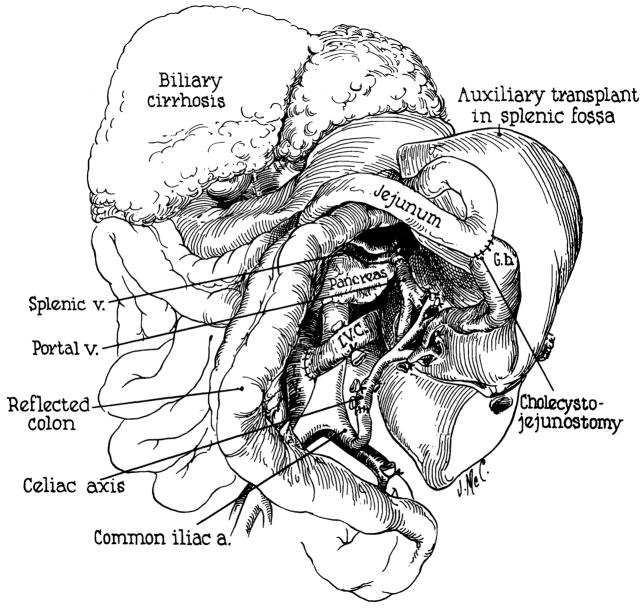
A 50-year-old man was known to have alcoholic cirrhosis since 1957. In October 1964, he developed intermittent tarry stools and jaundice. Physical examination after admission on Jan 26, 1965, revealed a tremulous, icteric, wasted man with an increased abdominal venous pattern, an enlarged tender liver, ascites, palmar erythema, bilateral varicose veins, and peripheral edema. Hematocrit reading was 42%; leukocyte count, 5,100 cu mm; total serum bilirubin. 22.5 mg/100 cc with 14.5 mg/100 cc direct; total serum protein, 6.6 gm/100 cc; albumin, 2.69 gm/100 cc; γ-globulin, 1.68 gm/100cc; alkaline phosphatase, 7.55 Bessey Lowry units; SGOT, 240 Sigma-Frankel units; prothrombin time, 61%; 4+ urine bile; blood urea nitrogen (BUN), 11 mg/100 cc; and fasting blood sugar, 90 mg/100 cc. There was progressive deterioration of liver function with increasing mental confusion; bilirubin rose to almost 40 mg/100 cc (Fig 5). On the 22nd hospital day, he began to bleed massively from esophageal varices. An emergency end-to-side portacaval shunt was performed with prompt cessation of the hemorrhage. He became disoriented on the second postoperative day.
Fig 5.
Clinical course of patient 1. Note the sharp fall in serum bilirubin after auxiliary homotransplantation, as well as the sustained improvement in prothrombin time. Later, the bilirubinemia slowly increased at a time when rejection was thought to be present. Severe leukopenia ensued late in the second week and necessitated withdrawal of azathioprine.
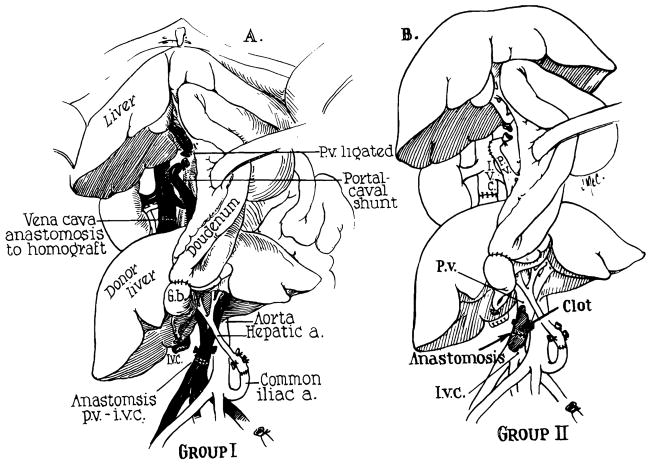
Three days after performance of the portacaval shunt, a cadaveric auxiliary hepatic homograft was placed in the right paravertebral gutter. Both donor and recipient were A positive blood type. The donor was a 79-year-old man who died after a cardiac arrest in the operating room. External and internal cardiac massage were carried out for 105 minutes before death was pronounced. Total body perfusion was then started with an electrolyte-primed extracorporeal circuit into which a cooling coil was incorporated.5 After 108 minutes of extracorporeal perfusion, the liver was removed and further perfused with intraportal cooled lactated Ringer’s solution. Forty-seven minutes later the homograft was revascularized in the recipient, attaching the celiac axis to the side of the terminal aorta. Portal venous inflow was from the distal end of the transected terminal inferior vena cava. Hepatic venous outflow was provided by anastomosing the suprahepatic donor vena cava to the proximal end of the transected recipient vena cava. Roux-en-Y internal biliary drainage to the jejunum was established.
A tracheostomy was performed immediately after surgery because of severe respiratory distress. Postoperatively, there was an almost immediate clearing of his sensorium. Bilirubin and prothrombin times were strikingly improved (Fig 5); other liver functions were not markedly altered. His immunosuppressive regimen is depicted in Fig 5.
During most of the next three weeks, he remained lethargic but oriented. There were numerous post-operative complications including several bouts of gastrointestinal hemorrhage, urinary retention, reversible failure, electrolyte abnormalities, and purulent tracheobronchitis due to Pseudomonas aeruginosa and Staphylococcus aureus. In addition, homograft rejection was diagnosed and treated on the eighth postoperative day, after which there was slow deterioration of function. Progressive leukopenia was abserved from the 11th day onward. Death occurred on the 22nd day after transplantation.
At autopsy, the immediate cause of death was thought to be necrotizing bronchopneumonia with multiple pulmonary abscesses. The homograft was grossly well preserved, weighing 1,400 gm. His own cirrhotic liver weighed 1,600 gm. Six liters of ascites were present.
Histologically the portal tracts, particularly the smaller ones, were infiltrated by mononuclear cells (Fig 6). Between 5% to 10% of the invaders were primitive “blast”-like lymphoid cells with pyroninophilic cytoplasm. Several of the smaller hepatic artery branches showed foci of fibrinoid necrosis in their walls. A few of the larger bile ducts and many of the smaller ducts contained bile casts and there was prominent centrilobular cholestasis. The hepatocytes were depleted of glycogen and the centrilobular cells contained excess lipofuscin and some bile pigment. Those hepatocytes immediately adjacent to the central vein were atrophic. There was also a slight increase in portal connective tissue, and many of the large branches of the hepatic artery showed reduplication of the internal elastic lamina sometimes accompanied by fibroelastic intimal thickening. These latter changes had in all probability occurred in the donor before the liver was transplanted.
Fig 6.
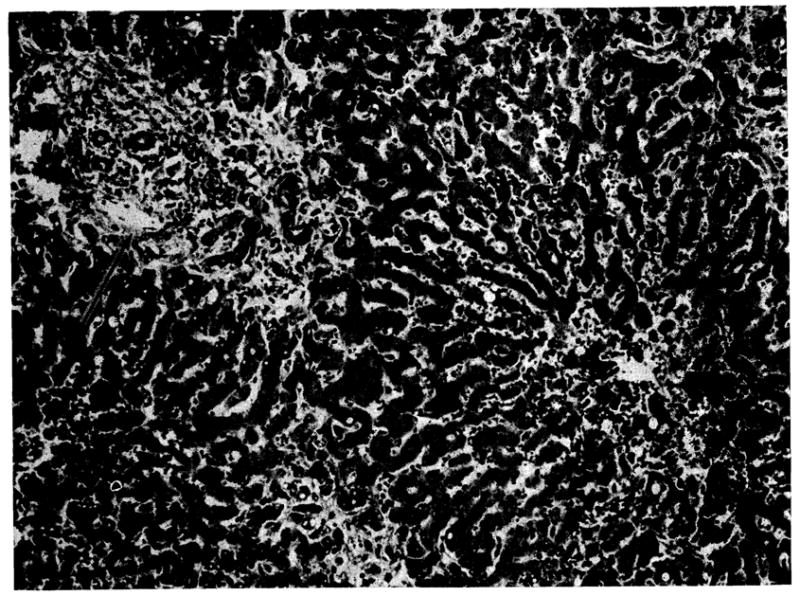
Auxiliary human hepatic homograft 22 days after transplantation (case 1). The portal tract (arrow) is infiltrated by mononuclear cells and several of the smaller bile ducts contain casts. There is central cholestasis, excess lipofuscin in the central hepatocytes, and atrophy of the liver cells adjacent to the central vein (lower right) (hematoxylin and eosin, ×80).
Case 2
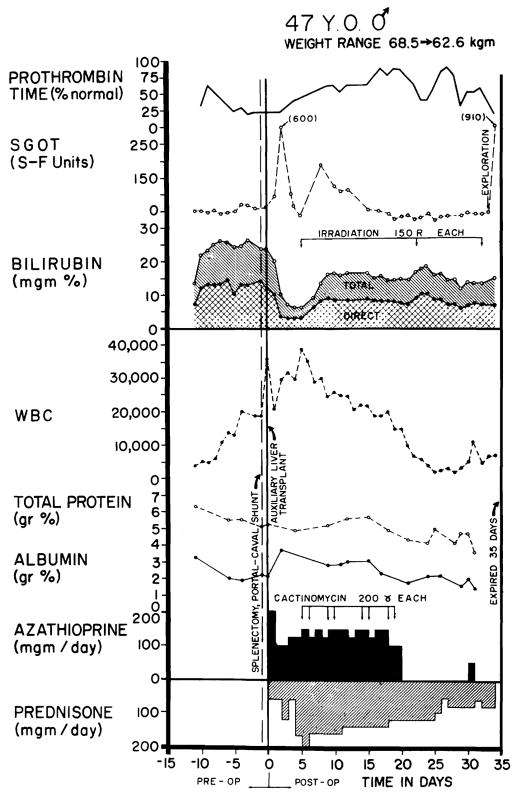
A 47-year-old white man had a diagnosis of alcoholic cirrhosis established by percutaneous liver biopsy in November 1964. He was admitted May 28, 1965, with a one-month history of jaundice, anorexia, night sweats, and progressive anemia. He had a large, tender liver, an enlarged spleen, moderate ascites, spider angiomata, palmar erythema, mental confusion, and 2+ guaiac positive stools. The hematocrit reading was 33%; reticulocyte count, 21.3%; total serum bilirubin, 22.2 mg/100 cc with 12.8 mg/100 cc direct; cholesterol, 250 mg/100 cc; total protein, 6.2 gm/100 cc; albumin, 3.10 gm/100 cc; γ-globulin, 1.28 gm/100 cc; SGOT, 190 SF units; alkaline phosphatase, 9.25 Bessey Lowry units; prothrombin time, 32% increasing to 38% after parenteral phytonadione; fasting blood sugar, 102 mg/100 cc; and BUN, 4 mg/100 cc. During the first month of hospitalization his condition deteriorated with an increase in the serum bilirubin (to 27 mg/100 cc), ascites, abdominal pain, pleural effusion, and the development of fever of unknown origin. After five weeks he suddenly became stuporous prompting consideration of auxiliary homotransplantation.
In preparation for this, a side-to-side portacaval shunt (Fig 7) and splenectomy were performed on July 4, 1965, at which time he was found to have 5,000 cc of ascitic fluid and portal hypertension. On the following day a cadaveric hepatic homograft was transplanted into his right iliac fossa by a slightly different method (Fig 8) than that used in case 1. The donor was a 12-year-old boy who died from cerebral trauma. Both donor and recipient were O blood type. The liver was cooled with cold lactated Ringer’s following 40 minutes of external cardiac massage. Arterial revascularization was completed three hours after the donor death, and the portal inflow was completed 45 minutes later.
Fig 7.
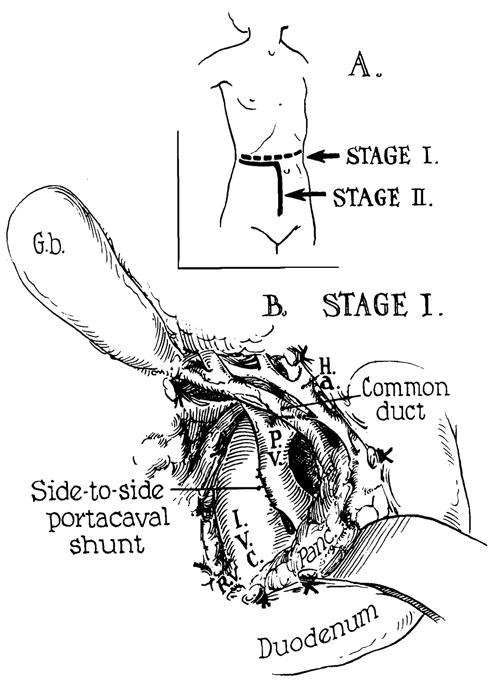
Human auxiliary liver homotransplantation. At the first stage, portacaval shunt was performed transabdominally for emergency control of variceal hemorrhage. Stage 2 was the actual transplantation, one day later. (A) Incisions: Note that part of the stage 1 incision was reused for stage 2. (B) Completed side-to-side portacaval shunt.
Fig 8.
Actual auxiliary transplantation (Stage 2). (A) Prepared operative field before receipt of homograft. (B) Completed operation. Note that the right external iliac vein provides the portal venous inflow. Hepatic venous outflow is into the transected vena cava.
The immunosuppressive regimen is summarized in Fig 9, as well as the postoperative liver functions. Bilirubin fell to a low of 4.5 mg/100 cc. A rejection crisis was diagnosed at four days, after which jaundice deepened. Nevertheless, he was able to resume a full diet and his general condition was excellent until progressive leukopenia developed late in the third postoperative week. Subsequently he had intermittent episodes of fever, gastrointestinal bleeding, and confusion. On the 31st postoperative day he was reexplored for persistent gastrointestinal bleeding; vagotomy and pyloroplasty were performed although a localized site of bleeding was not identified. Terminally he continued to bleed, became anuric and hypotensive, and developed radiographic evidence of bilateral pneumonitis. He died at 34 days.
Fig 9.
Clinical course of patient 2. As in case 1 (Fig 5) the early rise in SGOT is probably ascribable to ischemic injury to the homograft. Note the abrupt fall in serum bilirubin and the improvement in prothrombin time. Rejection was diagnosed on day 4, after which serum bilirubin rose but never to the preoperative level. The inexorable leukopenia which began during the third post-operative week was apparently the most important cause of ultimate failure; the patient died of sepsis.
At autopsy, the patient was shown to have Pneumocystis carinii and cytomegalic inclusion infestation of the lungs. Most of the small bowel and colon were denuded of epithelium secondary to invasive monilial enterocolitis; this was apparently responsible for the hemorrhage. The homograft weighed 1,100 gm; the patient’s own cirrhotic liver weighed 1,300 gm. Histologically, there was hemorrhagic necrosis of the centrilobular hepatocytes, accompanied by collapse and condensation of the centrilobular reticulin (Fig 10). In the middle and peripheral zones of the lobules, the hepatocytes contained much fat. There was marked centrilobular and midzonal cholestasis with large bile “thrombi.” Many of the Kupffer cells contained bile pigment and small quantities of hemosiderin. There were casts in several of the bile ducts and cytomegalic inclusion disease affected many of the biliary epithelial cells. There was no cellular infiltration and the branches of the portal and central veins and of the hepatic artery were all normal.
Fig 10.
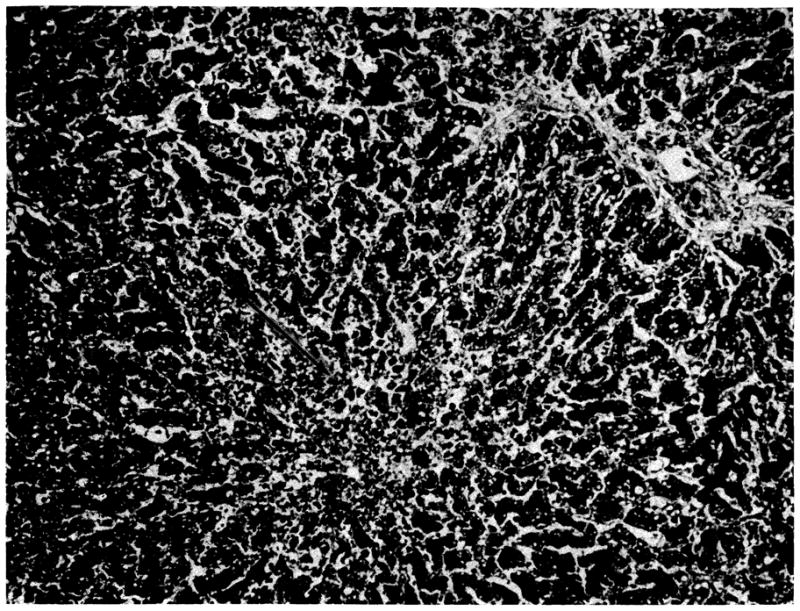
Auxiliary human hepatic homograft 34 days after transplantation (case 2). The centrilobular hepatocytes are necrotic (arrow) and there are fat droplets in the liver cells in the rnidzonal and peripheral parts of the lobule. There is no cellular infiltration (hematoxylin and eosin, ×80).
Case 3
A 16-month-old female infant with proved biliary atresia had an unsuccessful attempt at auxiliary hepatic homotransplantation on Nov 3, 1965. She had a total bilirubin of 21 mg/100 cc, prothrombin time of 70%, total serum protein of 6.8 gm/100 cc, and alkaline phosphatase of 26.4 Bessey Lowry units. Both donor and recipient were A blood type. The donor was a 7-month-old female infant with Crabbe’s disease, who during the last hour of life became markedly hypothermic and had no detectable peripheral blood pressure or pulse. After death, external cardiac massage was carried out for 45 minutes, until intraportal perfusion with cold lactated Ringer’s solution was begun and continued for the ensuing 90 minutes.
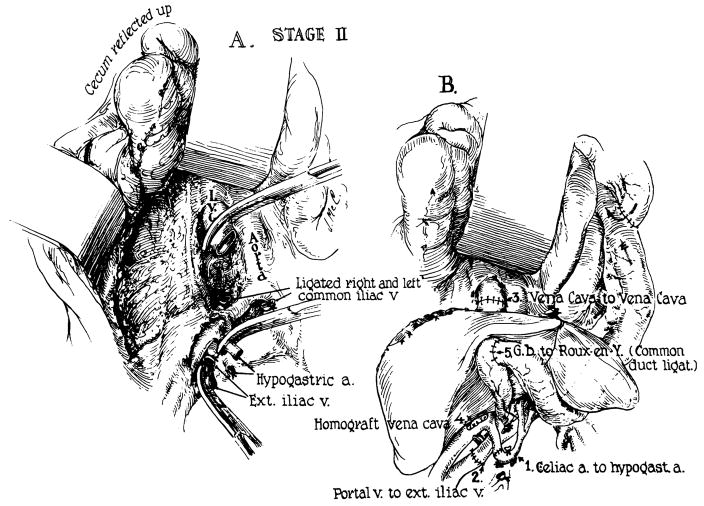
Thirty-six hours before transplantation, the recipient patient underwent an abdominal exploratory operation and the splenic vessels, lower inferior vena cava, and the left common iliac artery were skeletonized. At the definitive operation, the wound was reopened, the spleen removed, and the homograft attached as depicted in Fig 11. Since portal hypertension was present, it was expected that the splenic vein would provide retrograde flow to the homograft. The time from donor death to revascularization was 153 minutes. The auxiliary liver was at first pink and well vascularized but after about 30 minutes it became cyanotic. It was discovered that the origin of the left common iliac artery had kinked due to compression within the overcrowded abdomen; the distal vessels had clotted. After division of the lowest two lumbar arteries, the arterial reanastomosis was performed. However, the homograft now had multiple cyanotic areas with islands of intervening viable tissue. An immediate liver scan was obtained with radioactive gold (198Au) which failed to show any radioactivity in the homograft. Accordingly, the transplant was immediately removed. Thirty minutes later, during closure, the child had a cardiac arrest from which she could not be resuscitated.
Fig 11.
Technique used for attempted transplantation in case 3. Note that the homograft is facing medially. The splenic vein and common iliac artery are anastomosed to the homograft portal vein and hepatic artery, respectively. Note also the angulation near the origin of the left cornmon iliac artery. This was responsible for the technical failure of the transplant.
The excised honiograft was mottled in appearance. The anastomoses were patent. Histologically, many of the branches of the portal vein and hepatic artery were thrombosed and there were multiple areas of hemorrhagic infarction. Hemorrhages were present in and around the portal areas and the sinusoids were dilated and filled with blood. There was no cellular infiltration.
Comment
Auxiliary liver transplantation, a procedure first described by Welch,6,7 subsequently studied in dogs by many others,1,2,8–13 and first applied clinically by Absolon,14 may ultimately find a role in the treatment of hepatic failure due to benign diseases. Such an operation has certain attractive features. Recipient hepatectomy is avoided and the patient is not deprived of whatever function might remain in his own diseased liver.
Unfortunately, the use of an auxiliary liver introduces physiologic problems quite apart from rejection. A second liver arterialized from the aortoiliac system and provided with a portal venous inflow from the distal vena cava undergoes striking atrophy in the immunosuppressed dog beginning within two weeks.1 If, alternatively, most of the splanchnic venous flow is diverted through the “heterotopic liver” Marchioro showed that the homograft atrophy is prevented and instead there is shrinkage of the host liver.2 Apparently the splanchnic venous blood contains a hepatotropic substance3,15 essential for maintenance of the morphologic and functional integrity of hepatic tissue. That organ which has first exposure to splanchnic venous blood operates at a physiologic advantage; the other organ becomes atrophic.
Clinically, provision of a splanchnic venous inflow for the homograft may often not be technically feasible. The animal studies herein reported were designed to test a compromise procedure in which neither the homograft nor the autologous liver was directly perfused with nonhepatic splanchnic venous blood. Instead, the autologous liver received only an arterial blood supply. In contrast, the homograft had the additional advantage of also having a portal venous inflow even though this was of systemic venous origin.
The results of these experiments indicate that host portacaval shunt does, in fact, have some protective affect upon the homograft, a possibility first suggested by the experiments of Marchioro2 and Thomford.11 A similar opinion was recently expressed by Tretbar and his associates on the basis of experiments resembling those of the present study,13 but since maximum survival in their dogs was only 37 days, it might have been argued that the time of follow-up was too short to permit valid conclusions.
Even though the magnitude of the atrophic process in the homograft seemed to be reduced, with observations for as long as 150 days, the results in the present study indicate that it is not prevented. Furthermore, the ultimate degree of injury was often severe, and far more extensive than in either orthotopic homografts16 studied at a comparable time or in auxiliary homografts provided with nonhepatic splanchnic venous inflow.2 The operation is thus an imperfect one even under these circumstances. In the three experiments in which both the homograft and the autologous liver had only an arterial supply, the degree of transplant atrophy was even more striking as might be predicted since only the transplant is subject to the ravages of rejection.
In the clinical cases, it might be expected that the environment for the homograft would be even more favorable because of the further loss of competitive potential by the diseased host liver. Whether this was actually true is impossible to say inasmuch as death occurred at too early a time to assess the development of atrophic changes. The degree of histologic injury was, however, somewhat greater than in previously examined human orthotopic liver homografts.17
In view of these incompletely understood physiologic considerations, the future value of such auxiliary operations is uncertain. In addition, technical problems constitute a further hazard, as exemplified in case 3. These derive from the necessity of placing a large additional organ into an abdomen which may already be overcrowded by hepatomegaly or splenomegaly. At least eight other attempts at auxiliary hepatic homotransplantation are known to have been made in various centers; in some of these unreported cases, similar mechanical problems were encountered.
Finally, the clinical potential of liver homotransplantation by any heterotopic method or with an orthotopic procedure will be limited as long as dependence is placed upon presently available immunosuppressive regimens. The two patients in the present study who survived operation both died as the direct consequence of drug toxicity and resultant sepsis. The deficiencies of current therapy and suggestions for future improvements are discussed elsewhere.18
Summary
In normal dogs, receiving immunosuppressive therapy, auxiliary liver homografts undergo rapid atrophy if they are not provided with a portal venous inflow from the splanchnic bed. In 11 dogs, the influence of host portacaval shunt in preventing this transplant shrinkage was studied. In eight experiments, the homograft received both an hepatic arterial inflow, as well as a portal supply with venous blood returning from the hind quarters; in the other three, only an arterial supply was present. In all 11 dogs, the host liver had solely an arterial supply. The animals were followed for 24 to 150 days.
The results indicate that host portacaval anastomosis has a modest protective effect upon the homograft. Transplant atrophy was partially prevented when the homograft had a double blood supply; it was not influenced when both the homograft and the host liver each had only an arterial supply. In all cases moderate atrophy was observed in the autologous livers, suggesting that the protective effect was at least partly at the expense of injury to the host hepatic tissue. The long-term poor preservation of the homografts emphasize that the operation is not ideal from a physiologic point of view.
Using operations designed to provide the homografts with a physiologic advantage, three attempts at clinical auxiliary transplantation were made. Two adult patients with cirrhosis survived for 22 and 34 days after operation, both eventually dying of sepsis. In these cases, there was unequivocal evidence of homograft function. A third attempt in a child with congenital biliary atresia resulted in technical failure. The specific deficiencies of auxiliary transplantation procedures are discussed.
Acknowledgments
This study was supported by grants AM 06283, AM 06344, HE 07735, AM 07772, AI 04152, FR 00051, and FR 00069 from the US Public Health Service and by a grant from the Medical Research Council of Great Britain.
Footnotes
Read before the 23rd Annual Meeting of the Central Surgical Association, Chicago, March 3–5, 1966.
Generic and Trade Names of Drugs
Azathioprine—Imuran
Prednisone—Deltasone, Deltra, Meticorten, Paracort
References
- 1.Starzl TE, et al. Immunosuppression After Experimental and Clinical Homotransplantation of the Liver. Ann Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchioro TL, et al. Physiological Requirements for Auxiliary Liver Transplantation. Surg Gynec Obstet. 1965;121:17. [PMC free article] [PubMed] [Google Scholar]
- 3.Marchioro TL, et al. The Specific Role of Splanchnic Venous Blood in the Maintenance of Hepatic Structure and Function. to be published. [Google Scholar]
- 4.Spector WD, editor. Handbook of Biological Data. Philadelphia: W. B. Saunders Co; 1956. [Google Scholar]
- 5.Marchioro TL, et al. The Use of Extra-corporeal Perfusion for Obtaining Postmortem Grafts. Surgery. 1963;54:900. [PMC free article] [PubMed] [Google Scholar]
- 6.Welch CS. A Note on Transplantation of the Whole Liver in Dogs. Transplant Bull. 1955;2:54. [Google Scholar]
- 7.Goodrich EO, Jr, et al. Surgery. 1956;39:244. [PubMed] [Google Scholar]
- 8.Hagihara P, Absolon KB. Experimental Studies on Homologous Heterotopic Liver Transplantation. Surg Gynec Obstet. 1964;119:1297. [PubMed] [Google Scholar]
- 9.Mehrez IO, et al. Homotransplantation of the Canine Liver: A New Technic. Ann Surg. 1964;159:416. doi: 10.1097/00000658-196403000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sicular A, et al. Studies of Rejection of the Homotransplanted Canine Liver. Surg Forum. 1963;14:202. [PubMed] [Google Scholar]
- 11.Thomford NR, Shorter RG, Hallenbeck GA. Homotransplantation of the Canine Liver: Survival and Histology With and Without Azathioprine. Arch Surg. 1965;90:527. doi: 10.1001/archsurg.1965.01320100071012. [DOI] [PubMed] [Google Scholar]
- 12.Paronetto F, et al. Immunologic Observations on Homografts: I. The Canine Liver. Transplantation. 1965;3:303. doi: 10.1097/00007890-196505000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Tretbar LL, Beven EG, Hermann RE. Homotransplantation of an Auxiliary Dog Liver Into the Pelvis: Effect of Portacaval Shunt in the Prevention of Liver Atrophy. Surg Forum. 1965;16:219. [PubMed] [Google Scholar]
- 14.Absolon KB. Personal communication to the author. Nov 27, 1964.
- 15.Marchioro TL, et al. The Specific Influence of Non-hepatic Splanchnic Venous Blood Flow Upon the Liver. Surg Forum. 1965;16:280. [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, et al. Factors Determining Short- and Long-Term Survival After Orthotopic Liver Homotransplantation in the Dog. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Marchioro TL, Porter KA. Progress in Homotransplantation of the Liver. In: Welch Claude., editor. Advances in Surgery. Year Book Medical Publishers, Inc; Chicago: 1966. to be published. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Marchioro TL, Faris TD. Liver Transplantation. Ann Intern Med. 1966;64:473. doi: 10.7326/0003-4819-64-2-473. [DOI] [PubMed] [Google Scholar]