Abstract
One thousand one hundred and twenty-eight candidates for liver transplantation were stratified into five urgency-of-need categories by the United Network for Organ Sharing (UNOS) criteria. Most patients of low-risk UNOS 1 status remained alive after 1 yr without transplantation; the mortality while waiting was 3% after a median of 229.5 days. In contrast, only 3% of those entered at the highest risk UNOS 5 category survived without transplantation; 28% died while waiting, the deaths occurring at a median of 5.5 days. The UNOS categories in between showed the expected gradations, in which at each higher level fewer patients remained as candidates throughout the 1-yr duration of study while progressively more died at earlier and earlier times while waiting for an organ. In a separate study of posttransplantation survival during the same time period, the best postoperative results were in the lowest-risk UNOS 1 and 2 patients (88% combined), and the worst results were those in UNOS 5 (71%). However, a relative risk cross-analysis showed that a negative benefit of transplantation may have been the result in terms of 1-yr survival for the low-risk elective patients, but that a gain in life extension was achieved in the potentially lethal UNOS categories 3, 4 and 5 (greatest for UNOS 3). These findings and conclusions are discussed in terms of total care of patients with liver disease, and in the context of organ allocation policies of the United States and Europe.
Many of the earlier presentations at this Consensus Development Conference have focused on specific diseases and the wisdom of performing liver replacement for disorders that are apt to recur (such as cancer, hepatitis and alcoholic cirrhosis). Others have discussed limiting the operation to prime-of-life candidates, with exclusion of the very young or aged. Expansion of the donor pool has been proposed in the “splitting” of livers that are shared between two candidates or in the use of liver fragments from living donors. These subjects have been judiciously addressed in the context of organ shortage and allocation, leaving the all-important questions of patient disease severity and complexity almost untouched. To focus on this problem, Bronsther et al. (1) have analyzed elsewhere and we present here in more detail the dynamics in a collection of more than a thousand adult patients who were given candidacy in our program. The results may be useful in policy determination about organ allocation and in definition of the role of liver transplantation in the total armamentarium available to treat hepatic diseases.
MATERIALS AND METHODS
Case Material
The records were reviewed of 1,208 consecutive adult patients who became transplant candidates at the University of Pittsburgh Medical Center between January 1, 1989, and December 31, 1990. The patients were stratified at the time of candidacy activation into one of the five United Network of Organ Sharing (UNOS) categories, defined as follows: (1) working; (2) home (many still working) but requiring close medical supervision, sporadic hospital care or both; (3) hospital-bound continuously or most of the time; (4) intensive care unit-bound; and (5) UNOStat, meaning a life expectancy of only a few days without transplantation. In 80 cases, chart data were insufficient for accurate classification. The remaining, 1, 128 patients constituted the study population. Of these, 129 (11.4%) were activated to candidacy at lowest risk category 1, 160 (14.2%) were category 5 and the remainder fell in between (Table 1). Hepatic diagnoses were parenchymal diseases with cirrhosis (62%), cholestatic disorders (18%), malignancy without or usually with cirrhosis (10%), inborn metabolic errors (3%) and miscellaneous conditions including fulminant liver failure (7%).
Table 1.
UNOS status at entry to waiting list
| UNOS status | Frequency (%) |
|---|---|
| 1 | 129 (11.4) |
| 2 | 203 (18.0) |
| 3 | 408 (36.2) |
| 4 | 228 (20.2) |
| 5 | 160 (14.2) |
| TOTAL | 1, 128 (100.0) |
Evaluation of Candidate Stability
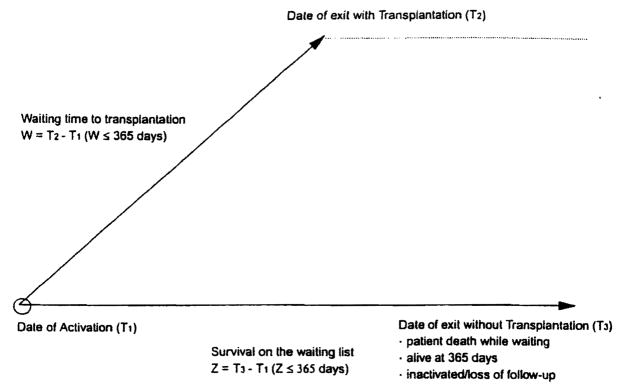
All 1,128 patients were followed on the waiting list for 1 yr unless their observation was terminated during this time for reasons of death or transplantation. The date of entry on the waiting list was designated T1 for everyone. The date of exit was called T2 in the event of transplantation. The exit of nontransplant patients was designated T3, a value that was 365 days for those who survived without transplantation for the entire year and less than this for those who died while waiting for a graft. The interrelationship of these three time points is summarized in Figure 1. For those who came to transplantation during the year, the waiting time (W) was calculated with the formula W = T2 – T1. Survival while waiting for those without transplantation (Z) was calculated as Z = T3 – T1. From these data, the median times were determined for exit, transplantation or death while waiting with each UNOS entry category. To capture disease progression while waiting, we recorded each patient’s UNOS status when they exited the waiting list (at time point T2 or T3).
Fig. 1.
Schematic representation of events occurring within 365 days of activation on the liver transplant waiting list.
Posttransplantation Survival
In addition, postoperative survival was determined for 691 consecutive patients who had primary liver transplantation from July 1, 1989, to December 31, 1990, the last 18 mo of the candidacy study. These recipients were not all derivative from the candidates under study because about 10% of them were already on the waiting list before January 1, 1989. The profiles of the transplant recipients, including their final UNOS scores are summarized in Table 2.
Table 2.
Demographic information on 691 consecutive primary adult liver transplants, 7/1/89-12/31/90
| Median follow-up | 22.4 mo |
| Range | 1.0–39.1 mo |
| Primary immunosuppression | |
| Cyclosporinea | 314 (45.4%) |
| FK506 | 377 (54.6%) |
| M/F ratio | 405:286 |
| Age (yr)b | 47.7 ± 12.3 |
| UNOS classification at time of transplant | |
| 1,2 | 85 (12.4%) |
| 3 | 260 (37.6%) |
| 4 | 174 (25.2%) |
| 5 | 172 (24.9%) |
FK 506 was available if necessary.
Data expressed as mean ± S.D.
This separate study was undertaken so that we might have a better assessment of survival expectation at the various risk levels than was available, even in the current literature. Just before this series began, there had been two significant improvements with a potential impact on high-risk (as well as all other) patients: University of Wisconsin solution in 1988 (2, 3), and, perhaps more important, FK 506, which after July 1989 could be used for primary immunosuppression or for the rescue of patients whose initial baseline drug was cyclosporine (4, 5).
By cross analysis of those results and those from the candidacy study, it was possible to compare the risk of mortality after liver transplantation (treatment group) with the risk of mortality on the waiting list (no treatment group). This was expressed as the relative risk (RR) score.
Statistical Analysis
The Kruskal-Wallis test, a nonparametric test equivalent to one-way ANOVA, was used to compare the median times to exit, transplantation or death while waiting with each UNOS entry category. The χ2 test of association was used to compare percent mortality across the different UNOS risk categories.
Patient survival after transplantation during the study period was calculated from the date of liver transplantation until patient death. Survival curves were generated with the Kaplan-Meier (product-limit) method and were compared by means of the generalized Wilcoxon (Breslow) test. A p value less than 0.05 on comparison of UNOS categories was considered statistically significant.
The methods of Crowley and Hu (6) and Kalbfleisch and Prentice (7) were adapted to investigate whether liver transplantation is life extending at different levels of pretransplant risk. A proportional-hazards model was used to assess the effect of liver transplantation, allowing us to use the partial-likelihood approach of Cox (8) and to avoid specification of a model for the underlying baseline hazard function (or force of mortality) assumed conventionally to be common to all individuals. Because the candidacy list was a mixed population consisting of patients with high forces of mortality (UNOStat) and patients with low forces of mortality (UNOS 1), the assumption of a common hazard was invalid, necessitating stratification into the entry subgroups defined according to UNOS criteria. In the Cox proportional hazards model, liver transplantation was incorporated as a time-varying covariate. Patients who were alive at 1 yr, lost to follow-up, given transplants elsewhere or inactivated (these were considered lost to follow-up) were right censored (i.e., exact survival time for each patient is unknown but failure or death is assumed to occur sometime in the future).
Relative risk (RR) was computed by means of Cox regression analysis as a measure of the associated risk of mortality due to liver transplantation. The analysis excluded patients whose medical urgency status changed as a result of disease progression on the waiting list. Therefore estimates of RR were based on a homogeneous subgroup of patients whose pretransplant risk was similar to their risk at activation. This was done so that we might avoid any bias in the RR attributable to disease progression.
An RR greater than 1.0 during the year’s observation indicated an increased risk of mortality after transplantation relative to that in the steadily declining population of candidates who did not have this intervention. An RR less than 1.0 indicated a reduction in mortality attributable to transplantation. Approximate 95% confidence intervals were generated according to Woolf’s method (9). Statistical analyses were performed with statistical software package EGRET (10).
RESULTS
Dynamics on the Waiting List
Waiting-list dynamics are summarized for all risk categories in Table 3. Most patients (56%) entered as UNOS 1 passed through the year without transplantation, excluding 5% who were lost to follow-up. Most of the 5% had improved under medical management or had been treated with conservative surgical procedures (most commonly, distal splenorenal shunt) with discharge from the candidacy clinic to distant referring physicians. The median waiting time for UNOS 1 patients was 365 days (range, 2 to 365 days). Four patients (3%) exited by dying after a wait of 114 to 345 days (median, 229.5 days). Of the 36 who exited by transplantation, almost three fourths had graduated to a higher risk category (discussed in the next section), including three recipients with calamitous complications that vaulted them to UNOStat.
Table 3.
Median time to exit from the waiting list stratified by UNOS score at enrollment
| Features | UNOS Status at Time of Activation |
P Valuea | ||||
|---|---|---|---|---|---|---|
| 1 (n = 129) | 2 (n = 203) | 3 (n = 408) | 4 (n = 228) | 5 (n = 160) | ||
| Median time to exit (days) | 365 | 80 | 34 | 16 | 5 | <0.00001 |
| Range (days) | 2–365 | 1–365 | 0–365 | 0–365 | 0–365 | |
| Patients given transplants (%) | 36 | 72 | 80 | 73 | 67 | <0.00001 |
| Median time to transplant (days) | 97 | 58 | 30 | 14 | 5 | <0.00001 |
| Range (days) | 2–360 | 1–332 | 0–322 | 0–123 | 0–73 | |
| Died while waiting (%) | 3 | 4 | 10 | 18 | 28 | <0.00001 |
| Median time to death (days) | 229.5 | 30 | 33.5 | 13 | 5.5 | <0.00001 |
| Range (days) | 114–345 | 4–325 | 4–325 | 2–30 | 0–64 | |
| Alive without transplant (%) | 56 | 15 | 6 | 4 | 3 | <0.00001 |
| Lost to follow-up (%) | 5 | 9 | 4 | 6 | 2 | 0.0242 |
Based on comparisons across all UNOS status at activation.
The median time of waiting before exit decreased with each successive increase in entry risk category: from 365 days in UNOS 1 to 80 days in UNOS 2 and only 5 days in UNOS 5. Exit by death while waiting occurred with the same kind of sliding scale, at a median time of 229.5 days in UNOS 1, 1 mo in UNOS 2, 13 days in UNOS 4 and 5 days in UNOS 5 (Table 3). The frequency of this distressing outcome rose from 3% to 28% from UNOS 1 to UNOS 5; this was reflected in the decline of exit by transplantation. Most of the small number of patients in UNOS classes 3 to 5 who were alive at the year’s end without transplantation had entered with the diagnosis of fulminant or subfulminant liver failure.
Survival Studies
The variable stability of consecutive patients coming to transplantation over an 18-mo period can be seen in Table 4, which relates the entry UNOS score to the score on the day of transplantation. Of those entered as UNOS 1, only 28.3% were still in this category at the time of liver replacement. The other 71.7% had UNOS grade slippage to a higher risk category, including UNOS 4 and 5.
Table 4.
UNOS score at time of candidacy activation vs. time of transplantation
| UNOS score at enrollment | UNOS score at transplantation |
TOTAL | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | 28.3% | 15.2% | 34.8% | 15.2% | 6.5% | 100% |
| 2 | 0% | 38.4% | 34.2% | 17.1% | 10.3% | 100% |
| 3 | 0.3% | 2.7% | 56% | 26% | 15% | 100% |
| 4 | 0% | 3.6% | 26.2% | 47% | 23.2% | 100% |
| 5 | 0% | 0% | 9.3% | 18.5% | 72.2% | 100% |
The same trend was seen in all risk categories. Of interest, however, was the fact that classification to a lower risk status was not uncommon during waiting in the dangerous UNOS 4 and 5 classes. This reflected benefit of supportive care allowing the patients to be removed from the intensive care unit or occasionally even discharged home under close surveillance.
Six-month and 1-yr survival rates were equivalent in patients whose UNOS status was class 1 to class 3 at the time of transplantation. It was slightly but not significantly less in the UNOS 4 group and significantly decreased in UNOS 5 (p = 0.002). Between 1 and 2 yr, the percentage of patient losses was least (3.5%) in the originally highest risk UNOS 5 group (Table 5).
Table 5.
Actuarial percent patient survival
| Time | Overall | UNOS classification |
|||
|---|---|---|---|---|---|
| 1, 2 | 3 | 4 | 5 | ||
| 6 mo | 84.9 ± 1.4a | 88.2 ± 3.5 | 89.2 ± 1.9 | 85.6 ± 2.7 | 76.0 ± 3.3 |
| 1 yr | 82.0 ± 1.5 | 88.2 ± 3.5 | 87.3 ± 2.1 | 81.6 ± 2.9 | 71.4 ± 3.5 |
| 2 yr | 76.8 ± 1.6 | 80.7 ± 4.3 | 82.1 ± 2.4 | 76.4 ± 3.3 | 67.9 ± 3.6 |
p = 0.0017, UNOS 5 vs. combined UNOS 1–4.
Data expressed as survival ± S.E.
Graft survival curves were similar to those of patient survival but about 10% lower at 6, 12 and 24 mo (Table 6). The difference in patient (Table 5) and graft survival rates (Table 6) reflected the benefit of repeat transplantation.
Table 6.
Actuarial percent graft survival
| Time | Overall | UNOS classification |
|||
|---|---|---|---|---|---|
| 1, 2 | 3 | 4 | 5 | ||
| 6 mo | 75.8 ± 1.6a | 78.8 ± 4.4 | 80.8 ± 2.4 | 75.9 ± 3.2 | 66.7 ± 3.6 |
| 1 yr | 72.2 ± 1.7 | 76.9 ± 2.6 | 71.8 ± 3.4 | 68.7 ± 3.5 | 63.2 ± 3.7 |
| 2 yr | 68.3 ± 1.8 | 72.7 ± 4.9 | 68.7 ± 3.5 | 68.7 ± 3.5 | 60.7 ± 3.8 |
p = 0.0175, UNOS 5 vs. combined UNOS 1–4.
Data expressed as survival ± S.E.
The overall rate of repeat transplantation at any time during the study was 17.1% (Table 7). The rate of repeat transplantation was incongruously highest in the UNOS 1 and 2 categories and lowest in the UNOS 4 group (NS).
Table 7.
UNOS classification and need for repeat transplantation
| No. of grafts | UNOS classification at time of initial transplant |
||||
|---|---|---|---|---|---|
| 1, 2 (n = 85) | 3 (n = 260) | 4 (n = 174) | 5 (n = 172) | TOTAL (n = 691) | |
| One | 67 (78.8%) | 217 (83.5%) | 149 (85.6%) | 140 (81.4%) | 573 (82.9%) |
| Multiple | 18 (21.2%) | 43 (16.5%) | 25 (14.4%) | 32 (18.6%) | 118 (17.1%) |
RR Score
Although the failing and unstable patients were systematically pruned from the candidacy list by transplantation, creating a dwindling pool of “no-treatment” survivors, the highly elective UNOS 1 group was particularly interesting because about 54% went through the 1-yr period of observation in the same risk category without transplantation. In this subgroup and in the smaller, similar one of UNOS 2, those who remained had a higher 1-yr cumulative survival than did patients who underwent transplantation at their original UNOS entry level. This was in contrast to the lethal outcome of those who did not have transplantation after entry with UNOS classifications of 3, 4 and 5.
Statistically expressed (Table 8), the RR score from transplantation in the UNOS 1 and 2 cohorts exceeded 1.0, connoting a potential negative benefit of trans plantation in spite of the high rate (88%) of 1-yr posttransplant survival. In contrast, patients entered and kept at UNOS classifications of 3, 4 and 5 had RR scores of less than 1.0, showing treatment value. The most favorable RR outcome was found in UNOS 3 patients.
Table 8.
RR of mortality with liver transplantation stratified by UNOS classification at time of enrollment using Cox’s proportional-hazards model
| UNOS score at enrollment and exit | RR | 95% Confidence limits |
||
|---|---|---|---|---|
| Lower | Upper | p Value | ||
| 1 (n = 78) | 3.375 | 0.342 | 33.33 | 0.298 |
| 2 (n = 71) | 2.098 | 0.608 | 7.243 | 0.241 |
| 3 (n = 162) | 0.273 | 0.126 | 0.594 | 0.001 |
| 4 (n = 77) | 0.352 | 0.141 | 0.881 | 0.026 |
| 5 (n = 81) | 0.528 | 0.206 | 1.355 | 0.184 |
DISCUSSION
Although transplantation has become a dominant factor in hepatology, the role of this procedure and the appropriate time for its application have yet to be fully defined (11–15). We have presented a one-dimensional examination in which extension of life was the focus, excluding the improved quality of life that must be a pervasive consideration. This monolithic approach is dismissive of quality-of-life considerations and ignores extremely frail candidates with severe disease or other factors not reflected in the UNOS score. These latter patients are in a narrow window of opportunity that is closed with any significant pretransplant complication. Their identification (epitomized by the older patient with PBC) requires sophisticated clinical judgment at a hands-on level.
However, a surprising number of elective UNOS 1 and 2 patients are physically functional but disabled in part because they perceive their disease as inexorable. For such transplant candidates, of whom some have adequate or restorable liver function, there may be other, and often safer, treatments. This is exemplified medically by autoimmune hepatitis, the progression of which can be delayed with the same drugs used to prevent rejection, putting off the need for transplantation for years, or perhaps permanently in some cases (16).
Surgeons also can often offer treatment short of transplantation to patients who are still in good condition. Henderson et al. (17) emphasized the neglected role of distal splenorenal shunt for good-risk patients whose principal complication was variceal hemorrhage, pointing out that the 3- and 5-yr survival rates after this procedure were superior to those of the admittedly sicker transplant recipients at the same institution (Emory University). In their study, the quality-of-life score was essentially the same in both groups 1 yr after either kind of operation, but the cost was less than a quarter that for those treated with Warren shunts.
To have access to these and other therapeutic alternatives, the patient with liver disease must be treated at a hepatology center where all of the medical tools and surgical procedures that have been acquired over the years are available. Otherwise, the introduction of liver transplantation, detached from its historical roots in hepatology and general surgery, will degrade instead of advance the care of the patient with liver disease.
Our investigation provided an assessment of the risk of attempts at conservative care by quantitating the rate of slippage of patients from one UNOS risk category to another while waiting for transplantation, as well as the mortality during this period in candidates who were prospectively determined to be at low, medium or high risk. The results were remarkably similar to those of a similar study by Gordon et al. (15) of the candidate population of the mid-1980s, a time when far fewer liver centers offered transplant services. Although few in this analysis, those in our analysis who underwent transplantation after entry with good UNOS risk factors had a very high survival rate at 1 yr (88%). Yet their survival was not as high as actually realized by the nontransplant good-risk residual patients who did not undergo this procedure. Only when the urgency status reached UNOS 3 was there an obvious gain in survival.
This was not surprising in view of the results in historical series of patients who came to transplantation with generally more advanced disease than the cross-section of potential recipients who are admitted to candidacy today. One such study was of patients with PBC treated by transplantation from March 1980 through June 1987 at the universities of Colorado and Pittsburgh (18). The cases were studied retrospectively by physicians at the Mayo Clinic who independently stratified the patients into three categories–low, midrange and high. The sickest patients had the worst results after transplantation. Only 58% of those at highest preoperative risk survived for 1 yr, compared with 74% with an intermediate degree of illness and 83% for those with the most favorable preoperative scores.
Using the Mayo prognostic model for PBC (19) which combined five factors at the time of transplantation (age, serum bilirubin, serum albumin, prothrombin time and edema index), we compared with the posttransplant results those predicted by this model without transplant intervention. This analysis showed a 58% gain in survival at 1 yr in the highest-risk patients (average preoperative bilirubin, 28 mg/dl), 55% in the intermediate group (bilirubin, 24 mg/dl) but only 14% in the best-risk cohort (bilirubin, 12 mg/dl), whose predicted 1-yr survival without transplantation had been 69%. The degree of rehabilitation of the survivors and the death rate after 1 yr were the same no matter how sick the patients were at surgery (18).
Similar trends were reported in good-, intermediate-and high-risk patients with the diagnosis of sclerosing cholangitis (20) whose actual posttransplant outcomes were compared with those predicted with a second Mayo prediction model (21) derived from age, bilirubin, splenomegaly and histopathological stage. The percent gain in survival of best-risk patients in the first year compared with that in their surrogate controls was a modest 7%; even after 7 yr, the percentage difference was still only 7% (60% vs. 53%). In contrast, the highest-risk recipients achieved a 40% gain in survival by 12 mo, an improvement that had grown to nearly 80% after 4 yr, when all were predicted to be dead by the Mayo model. By 7 yr, the best absolute results (80%) were from the patients who had been the most ill at the outset because there had been no deaths after 18 mo in this cohort.
With the five-tier UNOS score, a study of adults and children with heterogeneous diagnoses at the New England Medical Center (1984–1992) showed the same effect of disease severity on survival and correlated in addition the preoperative status with costs, including those incurred before transplantation (13), which in our own experience may exceed those after transplantation. Each higher level of risk added to the expenditures in this study. The elective UNOS 1 and 2 recipients lived 35% more frequently than did those in the UNOStat stage 5 (88% vs. 53%), with UNOS 3 and 4 recipients in between. Yet, in those who survived to undergo quality-of-life (Karnofsky) testing 1 yr later (the majority in every subgroup), the scores were the same no matter what the preoperative risk score.
In all such analyses, the significant salvage of doomed UNOS 4 and 5 recipients with catastrophic disease has been at least as noteworthy as the fact that the survival curve was degraded by their admission into candidacy and that their care was costly. Such complete and repeated reversal of fortune of patients with liver disease was without precedent before the availability of transplantation. The results in our survival study were congruent with the earlier ones, but the gap between urgency classes had narrowed to the point of nonsignificance except for the UNOS 5 (UNOStat) group which trailed the other four groups by 10% at 6, 12 and 24 mo. Still, even in this highest-risk cohort, 71% and 68% of the patients were alive at 1 and 2 yr. After this time, further decay in survival had no relation to preexisting illness.
Although there was a high proportion of profoundly ill patients on the candidacy list during the study period, care was provided with reasonable efficiency because of the two key features of the organ allocation system then operational: emphasis on urgency of patient need and an interlocking national donor reservoir designed to meet this need as mandated by the Gore-Waxman-Kennedy law (1985). The national character of the system was changed on January 1, 1991, by a directive from UNOS that created a confederacy of regions from which the free national movement of organs was discouraged in favor of elective regional use.
The directive secondarily removed urgency of need from the American organ allocation framework as its most pervasive national objective because it permitted or even encouraged the elective use of organs in some parts of the country while candidates languished elsewhere in the progressively more lethal UNOS stages 3, 4 and 5. This has been justified increasingly by the argument that high-risk recipients survive less frequently after transplantation than those with lesser need. Our study has verified this conclusion, but our RR analysis has added the disturbing possibility that the elective use of livers for low-risk recipients could result in their net loss of life in at least a 1-yr framework while retarding the use of these organs for patients who otherwise have little hope of survival. Similar observations and questions have been raised by critical appraisals of heart transplantation (22).
The selective exclusion of seriously ill patients from candidacy is an administrative syndrome of subtle triage that has been encouraged with the establishment of minimum life survival standards by government agencies or insurance carriers as a measure of transplant team competence, without stratification of disease severity (23). The incentive that this will create to divert transplant services from those who could benefit most has been noted by Kilpe, Krakauer and Wren (24) of the United States Bureau of Policy Development, Office of Coverage and Eligibility Policy, Health Care Financing Administration. An even more powerful disincentive to treat ill patients could be the growing tendency of insurance carriers to pay a fixed fee to hospitals per liver transplant case. The predictably higher cost of providing life-saving vs. elective liver transplantation has been discussed.
These may be uniquely our problems in the United States, but this seems doubtful. The best-known European system of allocation is probably more patient-driven than the current American one, but only indirectly. It regulates organ distribution on the basis of case production by a given team during a preceding 6-mo interval. A stipulated level of life survival is required, but without a means of case stratification. This would seem to present a temptation to anyone lacking a strong character to churn out easy cases with an eye to the organ supply 6 mo down the line, skipping over those whose less certain salvation is more apt to cause monumental headaches in the operating room and afterward.
Rather than having center-driven organ allocation, autoregulation of the supply-and-demand balance would be accomplished with less micromanagement by having organ distribution reflect patient need. This could actually ameliorate the organ shortage by encouraging the use of alternative therapies for patients with minimal risk factors, particularly if the possibility identified in our RR analysis is confirmed that there is a potential negative survival effect from premature transplantation. At the other extreme, the wastage of organs by teams with the compulsion to treat inappropriate diseases or hopelessly ill patients could be an undesirable consequence of a system driven by urgency. However, monitoring to curb such practices would be easy because this kind of activity is so readily detected. Equally detectable is the “cheating” by misclassification of which each center is inclined to accuse all others. Although this may occur in isolated cases, the analyses herein presented showed that the stability (or instability) while waiting and death rate as well as time to death during this interval were in strict conformity to what was expected from the UNOS entry score.
Finally, it is important to consider the ripple effect of allocation policy on organ supply. If lifesaving need does not translate into the kind of prompt response that requires a large donor pool, a sobering effect will be shrinkage of the organ availability by more than the loss of public confidence. Livers with minor functional or anatomic imperfections, or from older donors, will be systematically discarded in regions with a superfluity of organs. Even if they were procured, such donor organs would not be accepted elsewhere if the perception or the reality exist that prompt rescue will be unavailable or difficult in the event that such a “marginal” organ fails. It would seem that an organ pool for the united countries of Europe would be just as important as for the United States of America.
Acknowledgments
This work was aided by project grant DK 29961 from the National Institutes of Health (Bethesda, MD).
References
- 1.Bronsther O, Fung JJ, Tzakis A, Van Thiel D, Starzl TE. Prioritization and organ distribution for liver transplantation. JAMA. 1994;271:140–143. [PMC free article] [PubMed] [Google Scholar]
- 2.Kalayoglu M, Sollinger HW, Stratta RJ, D’Alessandro AM, Hoffman RM, Pirsch JD, Belzer FO. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617–619. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 3.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todo S, Fung JJ, Starzl TE, Tzakis A, Demetris AJ, Kormos R, Jain A, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowley J, Hu M. Covariance analysis of heart transplant survival data. J Am Stat Assoc. 1977;72:27–36. [Google Scholar]
- 7.Kalbfleisch JD, Prantice RL. In: The statistical analysis of failure time data. Wiley J, editor. New York: John Wiley and Sons; 1980. pp. 135–142. [Google Scholar]
- 8.Cox DR. Regression models with life tables (with discussion) J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 9.Kahn H, Sempos C. Statistical methods in epidemiology. New York: Oxford University Press; 1989. pp. 56–58. [Google Scholar]
- 10.EGRET Version 1.0. Statistics and Epidemiology Research Corp. and Cytel Software Corp; 1985. [Google Scholar]
- 11.Malatack JJ, Schaid DJ, Urbach AH, Zitelli BJ, Gartner JC, Rockette PHD, Fischer J, et al. Choosing a pediatric recipient for orthotopic liver transplantation. J Pediatr. 1987;111:479–489. doi: 10.1016/s0022-3476(87)80105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw BW, Jr, Wood RP, Gordon RD, Iwatsuki S, Gillquist WP, Starzl TE. Influence of selected patient variables and operative blood loss on six-month survival following liver transplantation. Semin Liver Dis. 1985;5:385–393. doi: 10.1055/s-2008-1040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muto P, Freeman RB, Haug CE, Lu A, Rohrer RJ. Liver transplant candidate stratification systems: implications for third-party payors and organ allocation. Transplantation. 1994;57:306–308. [PubMed] [Google Scholar]
- 14.Delmonico RL, Jenkins RL, Freeman R, Vacanti J, Bradley J, Dienstag JL, Trey C, et al. The high-risk liver allograft recipient: should allocation policy consider outcome? Arch Surg. 1992;127:579–584. doi: 10.1001/archsurg.1992.01420050103013. [DOI] [PubMed] [Google Scholar]
- 15.Gordon RD, Hartner CM, Casavilla A, Selby RR, Bronsther O, Mieles L, Martin M, et al. The liver transplant waiting list: a single-center analysis. Transplantation. 1991;51:128–134. doi: 10.1097/00007890-199101000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Thiel D, Wright H, Carroll P, Abu-Elmagd K, Rodriguez-Rilo H, McMichael J, Irish W, et al. FK 506: a treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol. in press. [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson JM, Gilmore GT, Hooks MA, Galloway JR, Dodson TF, Hood MM, Kutner MH, et al. Selective shunt in the management of variceal bleeding in the era of liver transplantation. Ann Surg. 1992;216:248–255. doi: 10.1097/00000658-199209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markus B, Dickson ER, Grambsch P, Fleming T, Mazzaferro V, Klintmalm G, Weisner RH, et al. Efficacy of liver transplantation in patients with primary biliary cirrhosis. N Engl J Med. 1989;320:1709–1713. doi: 10.1056/NEJM198906293202602. [DOI] [PubMed] [Google Scholar]
- 19.Dickson ER, Grambsch PM, Flening TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Elmagd K, Malinchoc M, Dickson ER, Fung JJ, Murtaugh PA, Langworthy AL, Demetria AJ, et al. Efficacy of liver transplantation in patients with primary sclerosing cholangitis. Surg Gynecol Obstet. 1993;177:335–344. [PMC free article] [PubMed] [Google Scholar]
- 21.Dickerson ER, Murtaugh PA, Weisner RH, Grambsch PM, Fleming TR, Ludwig J, LaRusso NF, Malinchoc M, Chapman RW, Kaplan MM, Maddrey WC, Williams R, Farrant M, Langworthy A. Primary sclerosing cholangitis: refinement and validation of survival model. Gastroenterology. 1992;103:1893–1901. doi: 10.1016/0016-5085(92)91449-e. [DOI] [PubMed] [Google Scholar]
- 22.Murray A, Laird N, Francis B. A re-analysis of the Stanford heart transplant data. J Am Stat Assoc. 1983;78:264–274. [Google Scholar]
- 23.Burk R, Sheldon M, Burton LA, Williams J, Foster P, Bone R, Jensen D, et al. Limiting access and patient selection in liver transplantation [Letter] N Engl J Med. 1992;326:413. [PubMed] [Google Scholar]
- 24.Kilpe VE, Krakauer H, Wren RE. An analysis of liver transplant experience from 37 transplant centers as reported to medicare. Transplantation. 1993;56:554–561. doi: 10.1097/00007890-199309000-00012. [DOI] [PubMed] [Google Scholar]