Abstract
The retina is an integral part of the central nervous system and retinal cells are known to express insulin receptors (IR), although their function is not known. This article describes recent studies that link the photoactivation of rhodopsin to tyrosine phosphorylation of the IR and subsequent activation of phosphoinositide 3-kinase (PI3K), a neuron survival factor. Our studies suggest that the physiological role of this process is to provide neuroprotection of the retina against light-damage by activating proteins that protect against stress-induced apoptosis. We focus mainly on our recently identified regulation of the IR pathway through the G-protein-coupled receptor rhodopsin. Various mutant and knockout proteins of phototransduction cascade have been used to study the light-induced activation of the retinal IR. Our studies suggest that rhodopsin may have additional previously uncharacterized signaling functions in photoreceptors.
Keywords: Insulin receptor, photobleaching, rhodopsin, phosphoinositdie 3-kinase, retina, rod outer segments
INTRODUCTION
Insulin receptors (IR) and insulin signaling proteins are widely distributed throughout the central nervous system (CNS) (1). Previous studies have suggested a role for insulin signaling in the regulation of food intake (2,3) and neuronal growth and differentiation (4,5). Dysregulation of insulin signaling in the CNS has been linked to the pathogenesis of neurodegenerative disorders such as Alzheimer's and Parkinson's disease (6,7). Insulin and insulin growth factor-1 receptors are expressed at high levels in many brain areas and different cell types, including glial and neuronal cells (1). Because neurons metabolize glucose in an insulin-independent manner and ablation of the IR in brain results in increased food intake, moderate diet-dependent obesity, and hypergonadotropic hypogonadism; the latter is associated with impaired maturation of ovarian follicles in females and reduced spermatogenesis in males and leads to reduced fertility (8). These studies indicate that IRs play a role in the control of appetite suppression and reproduction.
Cells of bovine and rat retina contain high affinity receptors for insulin (1). IR signaling provides a trophic signal for transformed retinal neurons in culture (9), but the role of the IR in vivo is unknown. We have used a targeted deletion strategy to specifically inactivate the IR gene in rod photoreceptors (10). Reduced IR expression in rod photoreceptors significantly decreased retinal function and caused the loss of photoreceptors in mice exposed to bright light stress (10).
IR activation has been shown to rescue retinal neurons from apoptosis through the phosphoinositide 3-kinase (PI3K) cascade (9). We previously reported that light induces tyrosine phosphorylation of the retinal IR and that this activation leads to the binding of PI3K to rod outer segment (ROS) membranes (11). More recently, we demonstrated that light-dependent IR activation is mediated through the G-protein-coupled receptor rhodopsin (12), the major protein in ROS. IR signaling is also involved in 17ß-estradiol-mediated neuroprotection in the retina (13). Recent evidence suggests a down-regulation of IR kinase activity in diabetic retinopathy that is associated with the deregulation of downstream signaling molecules (14). Our laboratory has shown that light-induced activation of the IR leads to the activation of downstream effectors, PI3K and Akt (12,15). Activated Akt phosphorylates and inactivates components of the apoptotic machinery (16-19). There are three isoforms of Akt (20-25) and we found that all three isoforms are expressed in rod photoreceptor cells (26). We also found that physiological light-activated IR results in the activation of Akt1 and Akt3 but not in the activation of the Akt2 isoform (15). Deletion of several downstream effector molecules of the IR signaling pathway, such as IRS-2 (27), Akt2 (26), and bcl-xl (28), in the retina resulted in photoreceptor degeneration. These studies clearly indicate the importance of the IR signaling pathway in the retina.
The IR is highly conserved with a high degree of IR signaling homology between C. elegans, Drosophila and humans suggests functional conservation in the mammalian retina. The IR regulates neuronal survival in C. elegans (29). In Drosophila, the IR serves an important function to guide retinal photoreceptor axons from the retina to the brain during development (30) and the IR influences the size and number of photoreceptors (31). Mutation in either IR autophosphorylation sites (30) or its binding partner Dock (32) in Drosophila results in a severe photoreceptor axonal misguidance phenotype. In humans, defects in IR signaling in the central nervous system are associated with Alzheimer's disease (33-35). The lack of IR activation leads to neurodegeneration in brain/neuron-specific IR knock-out mice (36). These studies clearly suggest that the IR pathway is important for neuronal survival and maintenance.
This article focuses on our recently identified regulation of the IR pathway through the G-protein-coupled receptor rhodopsin. Our studies suggest that rhodopsin photoexcitation may trigger signaling events alternative to the classical transducin activation.
Increased IR Phosphorylation and PI3K Enzyme Activity Associated with IRs of Light-adapted Rat Retinas
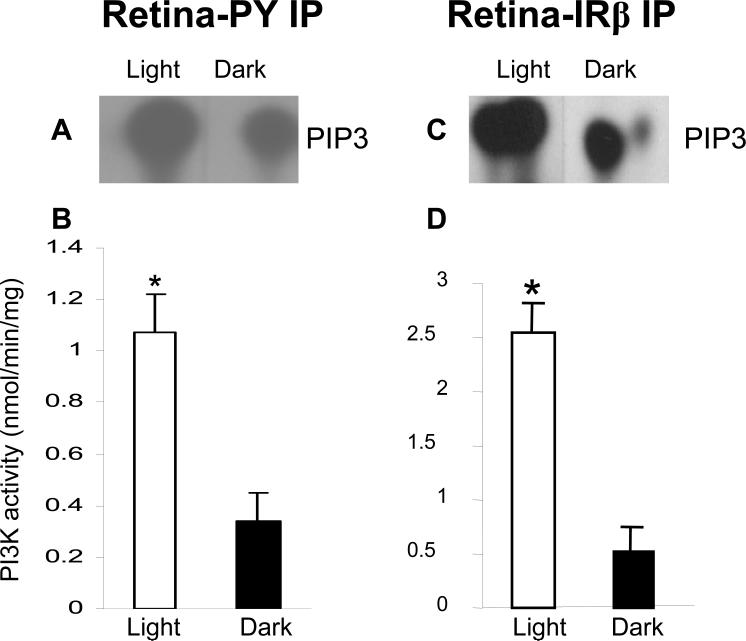
Ghalayini et al (37) previously reported that light stimulates tyrosine phosphorylation of multiple proteins in ROS in vivo. To determine whether light has an effect on PI3K activity and phosphorylation of the IR, rats were dark-adapted overnight, and one-half were subjected to normal room light for 30 min (11). Retinal lysates were immunoprecipitated with anti-PY-99 and anti-IRβ antibodies. The PI3K activity was higher in retinas from light-adapted rats, compared to those from dark-adapted animals (Fig. 1). These experiments suggested the light-induced activation of PI3K through light-induced tyrosine phosphorylation of IR (11).
Figure 1.
PI3K enzyme activity in anti-PY-99 and anti-IRβ immunoprecipitates from dark- and light-adapted rat retina homogenates. PI3K activity was measured from anti-IRβ immunoprecipitates of lysates from light- and dark-adapted retinas. PI3K activity was measured using PI-4,5-P2 and [γ32P]ATP as substrate. The radioactive spots of PI-3,4,5-P3 (A, C) were scraped from TLC plates and counted (B,D). Data are mean ± SD, n=6, *p<0.05. Reprinted with permission from (11).
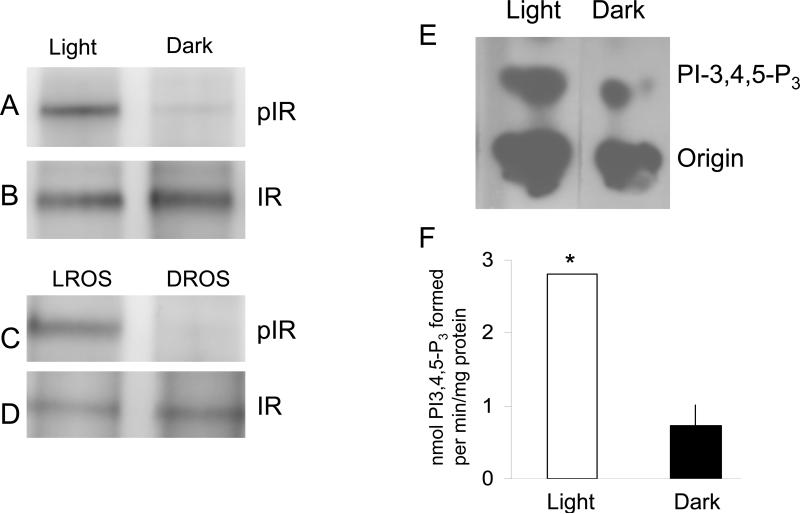
The catalytic loops within the tyrosine kinase domain of the IR contain three (Y1158, Y1162 and Y1163) tyrosine motifs (38,39). It is generally believed that autophosphorylation within the activation loop proceeds in a processive manner initiated at the second tyrosine (1162), followed by phosphorylation at the first tyrosine (1158) and finally the last (1163), upon which the IR becomes fully active (38,39). To determine whether light activates the IR in the same manner as insulin, we immunoprecipitated the IR (Fig. 2B) from retinal lysates that were prepared from light- and dark-adapted mice followed by Western blot analysis with phosphospecific anti-IR (pYpYpY1158/1162/1163) antibody. The results indicated increased phosphorylation in light-adapted compared to dark-adapted retinas (Fig. 2A). Light-adapted rod outer segments (LROS) and dark-adapted ROS (DROS) were solubilized with 1% NP-40 and the IR was immunoprecipitated followed by Western blot analysis with anti-IR (pYpYpY1158/1162/1163) antibody. The results indicated increased tyrosine phosphorylation of the IR in LROS (Fig. 2C). The blot was stripped and reprobed with anti-IRß antibody to ensure equal amount of protein in each line (Fig. 2D). These results suggest that light activates IR phosphorylation in the catalytic loop within the tyrosine kinase domain. When retinal homogenates were immunoprecipitated with anti-IRβ antibodies, PI3K activity was more than 3-fold higher in IPs from light-adapted rats compared to those from dark-adapted animals (Fig. 2E and F). These results demonstrate light-induced phosphorylation of the IRβ, as well as light-induced binding of p85 to the insulin receptor.
Figure 2.
Light activation of tyrosine phosphorylation in the catalytic loop of the IR. Two hundred micrograms of protein were immunoprecipitated with anti-IRβ antibody from either mouse retina lysates (A) or ROS (C) prepared from light- and dark-adapted rats. The immunoprecipitates were subjected to Western blot analysis with anti-IR (pYpYpY1158/1162/1163) antibody. The blots were stripped and reprobed with anti-IRβ antibody to ensure equal amount of protein in each lane (B and D). PI3K activity was measured from anti-IRβ immunoprecipitates of lysates from light- and dark-adapted retinas (E). PI3K activity was measured using PI-4,5-P2 and [γ32P]ATP as substrate. The radioactive spots of PI-3,4,5-P3 were scraped from TLC plates and counted (F). Data are mean ± SD, n=6, *p<0.05. Reprinted with permission from (12).
Location of Insulin Receptors in the Rod Photoreceptors
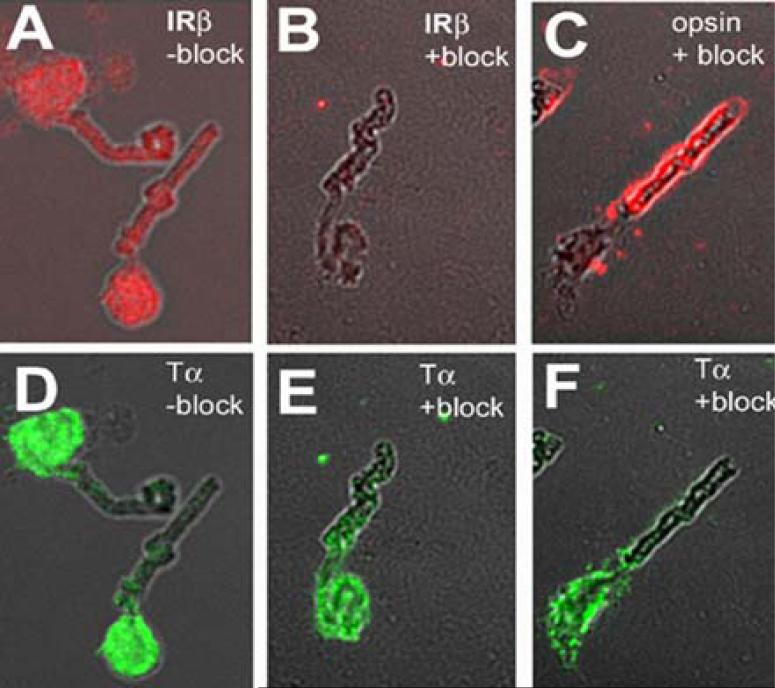
Rodrigues et al (40) found the insulin receptor was localized in photoreceptor and neuronal cell bodies, with lower immunoreactivity in ROS. To further demonstrate that IRs are present in photoreceptor outer segments, we immunolabeled bovine ROS (containing some attached inner segment) with anti-IRβ antibody. Immunostaining was found in both outer and inner segments (Fig. 3A). Inclusion of the IRβ-blocking peptide inhibited the immunoreactivity of IRβ (Fig. 3B). For positive controls, anti-opsin antibody showed the localization of opsin only in the ROS (Fig. 3C), and transducin immunoreactivity was observed primarily in the inner segment of this light-adapted preparation (Fig. 3D and E). The IRβ blocking peptide did not block the binding of the anti-opsin (Fig. 3C) or transducin alpha antibodies (Fig. 3F). These results provide strong evidence that the IR is present in ROS membranes.
Figure 3.
Immunolocalization of IRβ in methanol fixed ROS. Bovine ROS were prepared on glass slides as described (62). Slides were co-immunolabeled with opsin (C) and transducin alpha (D), or with IRβ (A) and transducin alpha (E). The immuno-staining of IRβ was significantly blocked using the peptide from which the antibody was generated (B). The same peptide did not block opsin (C) or transducin alpha staining (D and F). Reprinted with permission from (11).
Insulin Receptors are localized to Plasma membrane
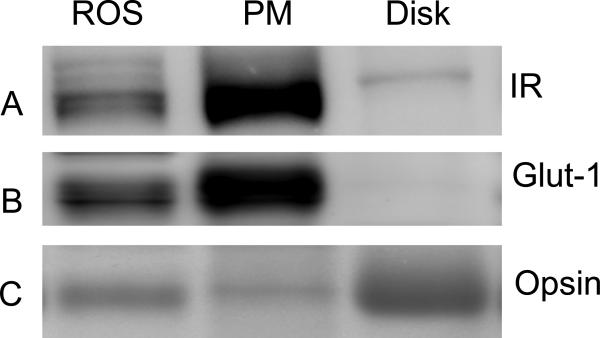
Crude bovine rod outer segments were subjected to FICOLL gradient centrifugation to isolate ROS disks (41). ROS, plasma membrane, and isolated disks were subjected to Western blot analysis with anti-IRβ, anti-Glut1, and anti-opsin antibodies. The results indicate that IR, Glut1, and opsin immunoreactivity was present in ROS (Fig. 4). IR and the plasma membrane marker Glut1 were enriched in the plasma membrane fraction of the ROS (Fig. 4A and B). Opsin blots show the enrichment of opsin in the disk membranes (Fig. 4C). These results suggest that IRs are localized to the plasma membrane.
Figure 4.
IR localization to the plasma membrane. Bovine ROS, plasma membrane and disk proteins were subjected to Western blot analysis with anti-IRβ (A), anti-Glut1 (B), and anti-opsin antibodies (C). Reprinted with permission from (12).
Light Activation of IR is localized to Photoreceptor Neurons
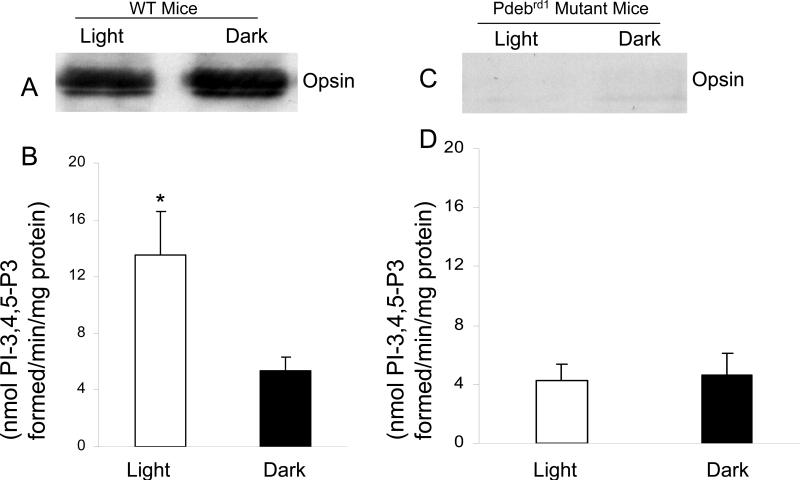
Light absorption by rhodopsin activates transducin, a G-protein, which in turn promotes cGMP hydrolysis by cGMP-phosphodiesterase, leading to hyperpolarization of rod photoreceptor cells (42,43). FVB/N mice are homozygous for the Pdebrd1 mutation (formally known as rd1) in the cGMP-phosphodiesterase β-subunit. As a result, these mice undergo rapid photoreceptor degeneration beginning at postnatal day 9 (44-47). The retinas from adult FVB/N mice completely lack photoreceptors. To determine whether the light-induced tyrosine phosphorylation is mediated through photoreceptor neurons, we conducted experiments on 2-month old wild type and mutant FVB/N mice to investigate the involvement of photobleachable visual pigments in the regulation of PI3K activity. Also as shown in Figure 1 PI3K activity was significantly higher in anti-IRβ IPs of light-adapted wild type mouse retinas compared to dark-adapted mouse retinas (Fig. 5B). However, there was no difference in PI3K activity in light- and dark-adapted Pdebrd1 mutant mice, which lack photoreceptors (Fig. 5D). Lack of photoreceptors was confirmed by probing the retina lysates with anti-opsin antibody, where no detectable opsin was observed in FVB/N mutant (Fig. 5C) compared to wild type retinas (Fig. 5A). The results suggest that the observed light/dark differences in IRβ phosphorylation and subsequent binding of PI3K are photoreceptor-specific phenomena that are mediated by photon capture in the ROS. These studies also suggest that the phototransduction cascade is coupled to the activation of IR phosphorylation.
Figure 5.
PI3K activity and opsin in retinas of wild type and FVB mice. PI3K activity was measured in the immunoprecipitates of IRβ from (B) wild type (n=9) and (4) FVB mouse retinas. Data are mean ± SE. *P<0.05. Opsin (A, C) expression was examined with 10 μg of protein from light and dark-adapted wild type and FVB mice. Reprinted with from (11).
The visual transduction cascade and IR phosphorylation
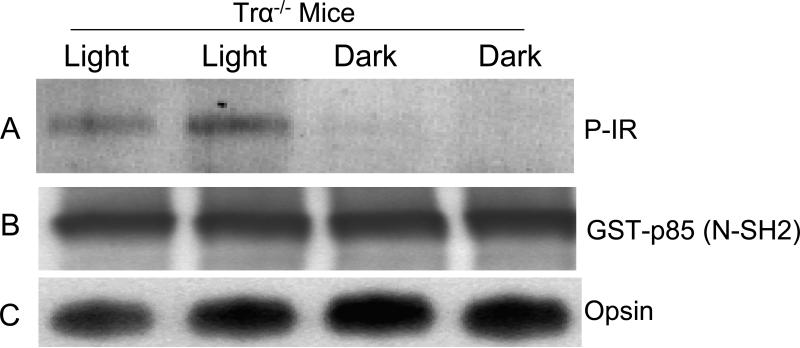
To determine if phototransduction events are required for IR phosphorylation, we examined IR phosphorylation in mice lacking transducin (Trα-/-). These mice contain normal amounts of rhodopsin, but its photoexcitation does not initiate phototransduction due to the lack of transducin. Trα-/- mice were dark-adapted overnight and half were exposed to normal room light for 30 min. Retinal lysates from light- and dark-adapted Trα-/- mice were subjected to GST pull-down assays using the p85(N-SH2) domain (which specifically binds to the phosphorylated form of the IR) followed by Western blot analysis with anti-IRß antibody. The blot shows that Trα-/- mice still exhibit a light-dependent phosphorylation of IR (Fig. 6A). These results suggest that the visual transduction cascade, downstream of rhodopsin, is not necessary for light-dependent IR phosphorylation.
Figure 6.
Light-dependent phosphorylation of IR in Trα-/- mice. Lysates of retina from dark- and light-adapted Trα-/- mice were subjected to GST pull-down assays with GST-p85 (N-SH2) domain. Bound proteins were probed with anti-IRβ antibody (A). To ensure equal protein in both light and dark conditions, the original lysates were probed with anti-GST antibodies (B) and anti-opsin (C) antibodies. P-IR, phosphorylated IR. Reprinted with permission from (12).
Photobleaching of rhodopsin and IR phosphorylation
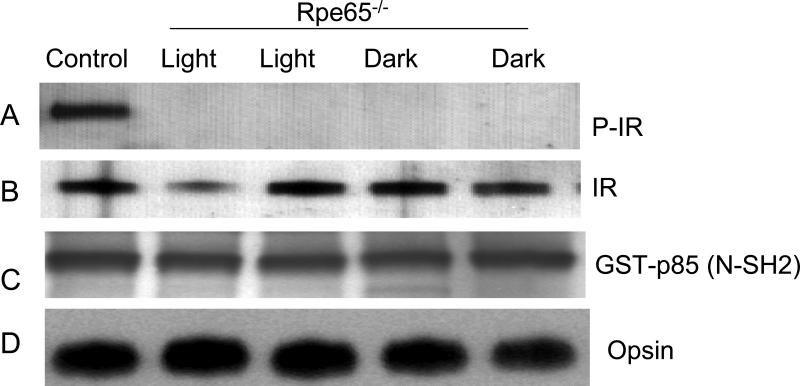
To confirm that light-induced IR phosphorylation is signaled through rhodopsin, we examined the phosphorylation of the IR in retinas from retinal pigment epithelium protein (Rpe65) knockout mice that are deficient in 11-cis-retinal, the chromophore for rhodopsin (48). These animals have the opsin apoprotein in their ROS, but do not have a bleachable rhodopsin due to the absence of the chromophore. Rpe65-/- mice were dark-adapted overnight and half were exposed to normal room light for 30 min. Retinal lysates from light- and dark-adapted Rpe65-/- mice were subjected to GST pull-down assays using the p85(N-SH2) domain, followed by Western blot analysis with anti-IRß antibody. The blot shows the absence of light-induced IR phosphorylation in Rpe65-/- mice (Fig. 7A). These results suggest that photobleaching of rhodopsin is necessary for IR phosphorylation.
Figure 7.
Absence of IR phosphorylation in Rpe65-/- mice. Dark- and light-adapted Rpe65-/- mouse retinas were lysed and subjected to GST pull-down assays with GST-p85 (N-SH2) domain. The bound proteins were subjected to Western blot analysis with anti-IRβ antibody (A). The blot was probed with anti-IRβ (B), anti-GST (C) and anti-opsin (D) antibodies to demonstrated equal protein in each lysate. Control, Wild-type retina stimulated with insulin was used as positive control (A). P-IR, phosphorylated IR. Reprinted with permission from (12).
Insulin activation of IR phosphorylation in Rpe65-/- mice
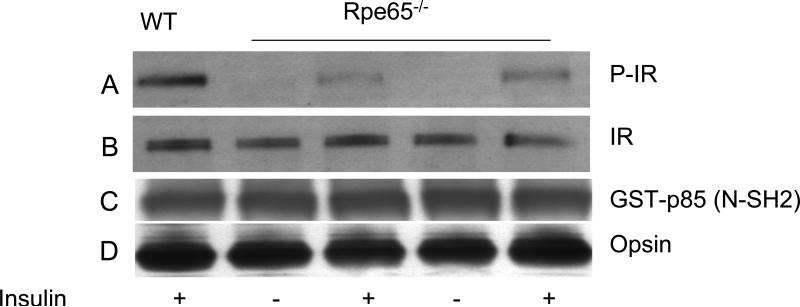
Rpe65-/- mouse retinas were stimulated with 1 μM insulin in organ cultures, after which the retinas were lysed and subjected to GST pull-down assays using the p85 (N-SH2) domain followed by Western blot analysis with anti-IRß antibody. The results indicate that the IR can be phosphorylated in response to insulin stimulation in Rpe65-/- retinas (Fig. 8A) and suggest the existence of a light-mediated IR pathway in the retina that is different from the known insulin-mediated pathway in non-neuronal tissues. Collectively, these experiments show that photobleaching of rhodopsin is necessary for light-mediated phosphorylation of the IR.
Figure 8.
Insulin-induced activation of the IR in Rpe65-/- mouse retinas. Rpe65-/- mouse retinas were stimulated with 1 μM insulin in organ cultures, lysed and subjected to GST pull-down assay with GST-p85 (N-SH2) domain. The bound proteins were subjected to Western blot analysis with anti-IRβ antibody (A). The blot was probed with anti-IRβ (B), anti-GST (C) and anti-opsin (D) antibodies to demonstrated equal protein in each lysate. Wild type (WT) retinas stimulated with insulin were used as a positive control (A). P-IR, phosphorylated IR. Reprinted with permission from (12).
Activation of IR signaling through light stimulation of rods
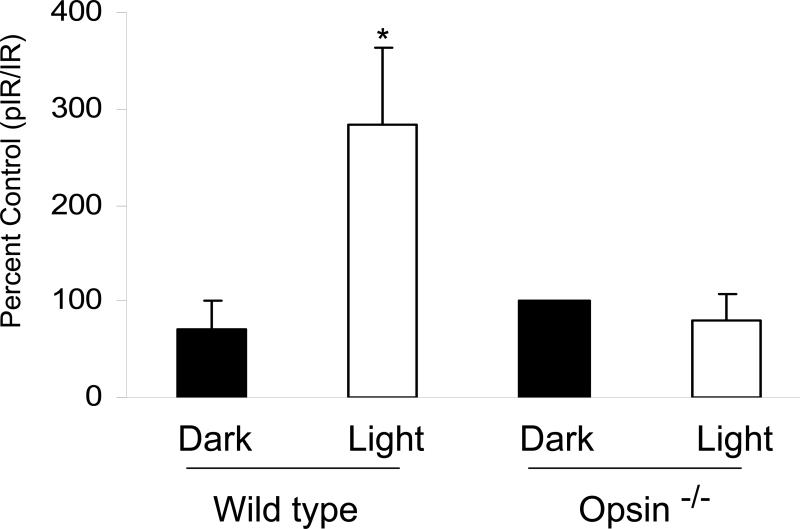
To further explore whether the activation of the IR is signaled through light stimulation of rods, we examined the phosphorylation of the IR in retina lysates from opsin-/- mice. Although these mice have the full complement of rod photoreceptor cells, they lack rod outer segments and scotopic electroretinographic (ERG) signal (49). However, retinas of opsin-/- mice exhibit normal photopic ERG responses, reflecting the existence of a functional cone transduction pathways (49,50). Therefore, these mice afforded the ideal system to test the activation of IR signaling through light stimulation of rods and to determine if any contributions are made by cones. To that end, C57Bl/6 control, and homozygous opsin-/- mice were dark-adapted overnight and half were exposed to normal room light for 30 min and the rest were kept in the dark. Retinal lysates from light- and dark-adapted mice from the two groups were subjected to immunoprecipitation with anti-IRß antibody followed by Western blot analysis with anti-IR (pYpYpY1158/1162/1163) antibody. The blots were stripped and reprobed with anti-IRß antibody. Densities were calculated from the respective immunoblots and the results are expressed as phospho-IR/total IR. In control mice, we observed a significant increase in IR phosphorylation from light-adapted retinas compared to dark-adapted retinas (Fig. 9). In homozygous opsin-/- mice, the light-dependent activation of the IR was lost (Fig. 9). These results suggest that the light-dependent activation of the IR may be regulated through rod transduction pathway.
Figure 9.
Activation of the IR through light stimulation of rods. Two hundred micrograms of protein were immunoprecipitated with anti-IRβ antibody from retina lysates from light- and dark-adapted wild type and homozygous opsin-/- mice. The immunoprecipitates were subjected to Western blot analysis with anti-IR (pYpYpY1158/1162/1163) antibody. The blots were stripped and reprobed with anti-IRβ antibody to ensure equal amount of protein in each lane. Densities were calculated from the immunoblots and the results are expressed as phospho-IR/total IR. Data mean ± SD, n=6, *p<0.05. Reprinted with permission from (11).
Our results also suggest that cross-talk exists between phototransduction and other signal transduction pathways. This cross-talk phenomenon has been shown for other G-protein-coupled receptors (GPCRs), and many tyrosine kinase cascades are regulated by GPCRs (51,52). Examples include mitogen-activated protein kinase cascade, extracellular-regulated kinases, and stress-activated protein kinases (52). Further, the binding of PYK2, a non-receptor protein tyrosine kinase, to N-terminal domain-interacting receptors (Nir) is activated by GPCRs (53). The Nir proteins are the human homologs of the Drosophila retinal degeneration B protein (rdgB), a protein implicated in the visual transduction pathway in flies (53).
Several studies have shown that the retinal ROS contains intrinsic tyrosine kinase(s) that can be activated by light (37) to phosphorylate several ROS proteins (54,55). It has been shown that light exposure in vivo activates Src and promotes its association with a complex containing bleached rhodopsin and arrestin (56). Retinal BIT (brain immunoglobulin-like molecules with a tyrosine-based activation motif) protein has also been shown to be tyrosine phosphorylated in vitro in a light-dependent manner (57). Evidence also indicates that the small G-protein Rac-1 may be regulated by rhodopsin in both Drosophila (58) and vertebrates (59). The α-subunit of heterotrimeric G-protein, G11α, does not participate in visual transduction, but the opsin-G11-mediated signaling pathway is important for photic entrainment of the chicken pineal circadian clock (60). These earlier studies along with the present study clearly suggest that photobleaching of rhodopsin may activate more than one signaling pathway.
Conclusion
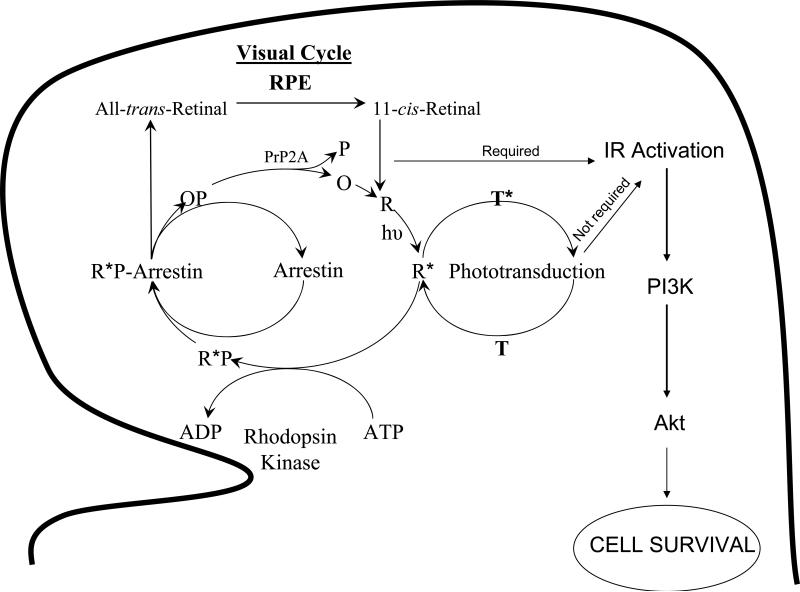
The retina expresses PI3K, which is regulated through the light-induced tyrosine phosphorylation of the IR in vivo (11,61). Light-induced activation of the retinal IR is independent of insulin secretion, and the light effect is localized to photoreceptor neurons (11). These studies suggest that there exists a cross talk between phototransduction and other signal transduction pathways. Our studies also suggest that light-induced tyrosine phosphorylation of the IR requires the photobleaching of rhodopsin, but not transducin signaling (Fig. 10). Collectively these studies suggest that rhodopsin can cross-talk with other signaling pathways in addition to the classical phototransduction cascade.
Figure 10.
Working model of IR activation in rod photoreceptor cells. The IR activation in photoreceptor cells requires the photobleaching of rhodopsin but not transducin signaling. Light-activated IR is subsequently associated with PI3K, a cell survival factor and thus regulates the downstream survival pathway. R*, photoactivated rhodopsin.
ACKNOWLEDGMENTS
This work was supported by grants from National Institutes of Health (EY016507, EY00871, EY04149, EY012190, and RR17703) and an unrestricted grant from Research to Prevent Blindness, Inc.
REFERECES
- 1.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 2.Baskin DG, Figlewicz LD, Seeley RJ, Woods SC, Porte D, Jr., Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology. 1992;130:3608–3616. doi: 10.1210/endo.130.6.1597158. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich KA. Insulin and IGF-I receptor signaling in cultured neurons. Ann. N. Y. Acad. Sci. 1993;692:72–88. doi: 10.1111/j.1749-6632.1993.tb26207.x. [DOI] [PubMed] [Google Scholar]
- 5.Robinson LJ, Leitner W, Draznin B, Heidenreich KA. Evidence that p21ras mediates the neurotrophic effects of insulin and insulin-like growth factor I in chick forebrain neurons. Endocrinology. 1994;135:2568–2573. doi: 10.1210/endo.135.6.7988444. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M, Yamada T, Tooyama I, Moroo I, Kimura H, Yamamoto T, Okada H. Insulin receptor mRNA in the substantia nigra in Parkinson's disease. Neurosci. Lett. 1996;204:201–204. doi: 10.1016/0304-3940(96)12357-0. [DOI] [PubMed] [Google Scholar]
- 7.Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J. Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 8.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 9.Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- 10.Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J. Biol. Chem. 2008;283:19781–19792. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajala RV, McClellan ME, Ash JD, Anderson RE. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J. Biol. Chem. 2002;277:43319–43326. doi: 10.1074/jbc.M206355200. [DOI] [PubMed] [Google Scholar]
- 12.Rajala A, Anderson RE, Ma JX, Lem J, Al Ubaidi MR, Rajala RV. G-protein-coupled Receptor Rhodopsin Regulates the Phosphorylation of Retinal Insulin Receptor. J. Biol. Chem. 2007;282:9865–9873. doi: 10.1074/jbc.M608845200. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Rajala RV, McGinnis JF, Li F, Anderson RE, Yan X, Li S, Elias RV, Knapp RR, Zhou X, Cao W. Involvement of insulin/phosphoinositide 3-kinase/Akt signal pathway in 17 beta-estradiol-mediated neuroprotection. J. Biol. Chem. 2004;279:13086–13094. doi: 10.1074/jbc.M313283200. [DOI] [PubMed] [Google Scholar]
- 14.Reiter CE, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RS, Fort PE, Antonetti DA, Gardner TW. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Rajala A, Wiechmann AF, Anderson RE, Rajala RV. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J. Neurochem. 2008;107:1382–1397. doi: 10.1111/j.1471-4159.2008.05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khwaja A. Akt is more than just a Bad kinase. Nature. 1999;401:33–34. doi: 10.1038/43354. [DOI] [PubMed] [Google Scholar]
- 17.Mora A, Sabio G, Gonzalez-Polo RA, Cuenda A, Alessi DR, Alonso JC, Fuentes JM, Soler G, Centeno F. Lithium inhibits caspase 3 activation and dephosphorylation of PKB and GSK3 induced by K+ deprivation in cerebellar granule cells. J. Neurochem. 2001;78:199–206. doi: 10.1046/j.1471-4159.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 18.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 19.Sanvicens N, Gomez-Vicente V, Masip I, Messeguer A, Cotter TG. Oxidative stress-induced apoptosis in retinal photoreceptor cells is mediated by calpains and caspases and blocked by the oxygen radical scavenger CR-6. J. Biol. Chem. 2004;279:39268–39278. doi: 10.1074/jbc.M402202200. [DOI] [PubMed] [Google Scholar]
- 20.Jones PF, Jakubowicz T, Pitossi FJ, Maurer F, Hemmings BA. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc. Natl. Acad. Sci U. S. A. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones PF, Jakubowicz T, Hemmings BA. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991;2:1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl. Acad. Sci U. S. A. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodbeck D, Cron P, Hemmings BA. A human protein kinase Bgamma with regulatory phosphorylation sites in the activation loop and in the C-terminal hydrophobic domain. J. Biol. Chem. 1999;274:9133–9136. doi: 10.1074/jbc.274.14.9133. [DOI] [PubMed] [Google Scholar]
- 24.Masure S, Haefner B, Wesselink JJ, Hoefnagel E, Mortier E, Verhasselt P, Tuytelaars A, Gordon R, Richardson A. Molecular cloning, expression and characterization of the human serine/threonine kinase Akt-3. Eur. J. Biochem. 1999;265:353–360. doi: 10.1046/j.1432-1327.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani K, Thompson DA, Barthel A, Sakaue H, Liu W, Weigel RJ, Roth RA. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J. Biol. Chem. 1999;274:21528–21532. doi: 10.1074/jbc.274.31.21528. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Anderson RE, Tomita H, Adler R, Liu X, Zack DJ, Rajala RV. Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J. Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi X, Schubert M, Peachey NS, Suzuma K, Burks DJ, Kushner JA, Suzuma I, Cahill C, Flint CL, Dow MA, Leshan RL, King GL, et al. Insulin receptor substrate 2 is essential for maturation and survival of photoreceptor cells. J. Neurosci. 2005;25:1240–1248. doi: 10.1523/JNEUROSCI.3664-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng L, Anderson RE, Agbaga MP, Rucker IEB, Le YZ. Loss of Bcl-XL causes increased rod photoreceptor susceptibility to bright light damage. Invest. Ophthalmol. Vis. Sci. 2006;47:5583–5589. doi: 10.1167/iovs.06-0163. [DOI] [PubMed] [Google Scholar]
- 29.Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 30.Song J, Wu L, Chen Z, Kohanski RA, Pick L. Axons guided by insulin receptor in Drosophila visual system. Science. 2003;300:502–505. doi: 10.1126/science.1081203. [DOI] [PubMed] [Google Scholar]
- 31.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 32.Garrity PA, Rao Y, Salecker I, McGlade J, Pawson T, Zipursky SL. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell. 1996;85:639–650. doi: 10.1016/s0092-8674(00)81231-3. [DOI] [PubMed] [Google Scholar]
- 33.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J. Alzheimers. Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 34.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J. Alzheimers. Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 35.de la Monte SM, Wands JR. Alzheimer-associated neuronal thread protein mediated cell death is linked to impaired insulin signaling. J. Alzheimers. Dis. 2004;6:231–242. doi: 10.3233/jad-2004-6304. [DOI] [PubMed] [Google Scholar]
- 36.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci U. S. A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghalayini AJ, Guo XX, Koutz CA, Anderson RE. Light stimulates tyrosine phosphorylation of rat rod outer segments In vivo. Exp. Eye Res. 1998;66:817–821. doi: 10.1006/exer.1998.0498. [DOI] [PubMed] [Google Scholar]
- 38.Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, Ou JH, Masiarz F, Kan YW, Goldfine ID. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985;40:747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- 39.Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, Gray A, Coussens L, Liao YC, Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues M, Waldbillig RJ, Rajagopalan S, Hackett J, LeRoith D, Chader GJ. Retinal insulin receptors: localization using a polyclonal anti-insulin receptor antibody. Brain Res. 1988;443:389–394. doi: 10.1016/0006-8993(88)91639-3. [DOI] [PubMed] [Google Scholar]
- 41.Smith HG, Jr., Litman BJ. Preparation of osmotically intact rod outer segment disks by Ficoll flotation. Methods Enzymol. 1982;81:57–61. doi: 10.1016/s0076-6879(82)81012-4. [DOI] [PubMed] [Google Scholar]
- 42.Roof DJ, Heuser JE. Surfaces of rod photoreceptor disk membranes: integral membrane components. J. Cell Biol. 1982;95:487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roof DJ, Korenbrot JI, Heuser JE. Surfaces of rod photoreceptor disk membranes: light-activated enzymes. J. Cell Biol. 1982;95:501–509. doi: 10.1083/jcb.95.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidman RL, Green MC. Retinal degeneration in the mosue: Location of the RD locus in linkage group XVIII. J. Hered. 1965;56:23–29. doi: 10.1093/oxfordjournals.jhered.a107364. [DOI] [PubMed] [Google Scholar]
- 45.Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 46.Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc. Natl. Acad. Sci. U. S. A. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lem J, Flannery JG, Li T, Applebury ML, Farber DB, Simon MI. Retinal degeneration is rescued in transgenic rd mice by expression of the cGMP phosphodiesterase beta subunit. Proc. Natl. Acad. Sci. U. S. A. 1992;89:4422–4426. doi: 10.1073/pnas.89.10.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 49.Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makino CL, Sidman RL. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc. Natl. Acad. Sci U. S. A. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaissle GB, May CA, Reinhard J, Kohler K, Fauser S, Lutjen-Drecoll E, Zrenner E, Seeliger MW. Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest Ophthalmol. Vis. Sci. 2001;42:506–513. [PubMed] [Google Scholar]
- 51.Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Krueger KM, Touhara K, Lefkowitz RJ. G-protein-coupled receptors and their regulation: activation of the MAP kinase signaling pathway by G-protein-coupled receptors. Adv. Second Messenger Phosphoprotein Res. 1997;31:263–277. [PubMed] [Google Scholar]
- 52.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 53.Lev S, Hernandez J, Martinez R, Chen A, Plowman G, Schlessinger J. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol. Cell Biol. 1999;19:2278–2288. doi: 10.1128/mcb.19.3.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell MW, Desai N, Guo XX, Ghalayini AJ. Tyrosine phosphorylation of the alpha subunit of transducin and its association with Src in photoreceptor rod outer segments. J. Neurochem. 2000;75:2006–2019. doi: 10.1046/j.1471-4159.2000.0752006.x. [DOI] [PubMed] [Google Scholar]
- 55.Bell MW, Alvarez K, Ghalayini AJ. Association of the tyrosine phosphatase SHP-2 with transducin-alpha and a 97-kDa tyrosine-phosphorylated protein in photoreceptor rod outer segments. J. Neurochem. 1999;73:2331–2340. doi: 10.1046/j.1471-4159.1999.0732331.x. [DOI] [PubMed] [Google Scholar]
- 56.Ghalayini AJ, Desai N, Smith KR, Holbrook RM, Elliott MH, Kawakatsu H. Light-dependent association of Src with photoreceptor rod outer segment membrane proteins in vivo. J. Biol. Chem. 2002;277:1469–1476. doi: 10.1074/jbc.M011432200. [DOI] [PubMed] [Google Scholar]
- 57.Hamada J, Okumura N, Inagaki M, Taniguchi H, Nakahata Y, Sano S, Nagai K. Tyrosine phosphorylation of BIT on photic stimulation in the rat retina. FEBS Lett. 2004;557:204–208. doi: 10.1016/s0014-5793(03)01493-5. [DOI] [PubMed] [Google Scholar]
- 58.Chang HY, Ready DF. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science. 2000;290:1978–1980. doi: 10.1126/science.290.5498.1978. [DOI] [PubMed] [Google Scholar]
- 59.Balasubramanian N, Slepak VZ. Light-mediated activation of Rac-1 in photoreceptor outer segments. Curr. Biol. 2003;13:1306–1310. doi: 10.1016/s0960-9822(03)00511-6. [DOI] [PubMed] [Google Scholar]
- 60.Kasahara T, Okano T, Haga T, Fukada Y. Opsin-G11-mediated signaling pathway for photic entrainment of the chicken pineal circadian clock. J. Neurosci. 2002;22:7321–7325. doi: 10.1523/JNEUROSCI.22-17-07321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajala RV, Anderson RE. Light regulation of the insulin receptor in the retina. Mol. Neurobiol. 2003;28:123–138. doi: 10.1385/MN:28:2:123. [DOI] [PubMed] [Google Scholar]
- 62.Muresan V, Joshi HC, Besharse JC. Gamma-tubulin in differentiated cell types: localization in the vicinity of basal bodies in retinal photoreceptors and ciliated epithelia. J. Cell Sci. 1993;104(Pt 4):1229–1237. doi: 10.1242/jcs.104.4.1229. [DOI] [PubMed] [Google Scholar]