Abstract
The overproduction and extracellular buildup of amyloid-β peptide (Aβ) is a critical step in the etiology of Alzheimer’s disease. Recent data suggest that intracellular trafficking is of central importance in the production of Aβ. Here we use a neuronal cell line to examine two structurally similar clathrin assembly proteins, AP180 and CALM. We show that RNA interference-mediated knockdown of AP180 reduces the generation of Aβ 1-40 and Aβ 1-42, whereas CALM knockdown has no effect on Aβ generation. Thus AP180 is among the traffic controllers that oversee and regulate amyloid precursor protein processing pathways. Our results also suggest that AP180 and CALM, while similar in their domain structures and biochemical properties, are in fact dedicated to separate trafficking pathways in neurons.
Keywords: amyloid-β peptide, APP, AP180, CALM, clathrin assembly protein, neuron, Alzheimer’s disease
Introduction
Extracellular accumulation and aggregation of amyloid-β peptide (Aβ) plays a pivotal in the pathogenesis of Alzheimer’s disease (AD) [1]. Within the brain, Aβ is mostly produced by neurons and is derived from the amyloid precursor protein (APP). APP can undergo two different cleavage processes, non-amyloidogenic processing and amyloidogenic processing; the latter route leads to overproduction and extracellular accumulation of Aβ [3,4]. Recent advances in understanding APP processing have led to the realization that many of the molecules that play fundamental roles in intracellular trafficking also function to direct APP away or towards the amyloidogenic processing pathway [5–10]. We therefore examined the possible roles of AP180 and CALM (clathrin assembly lymphoid myeloid protein) – two regulators of clathrin-mediated trafficking – in Aβ production.
AP180 and CALM belong to clathrin assembly proteins because of their widely recognized role in promoting the assembly of clathrin-coated vesicles [11–16]. AP180 and CALM share a remarkably similar domain structure [14] and both are present in neurons [13, 17, 18]. However, we previously found that AP180 and CALM carry out different functions in neurons [19], raising the possibility that AP180 and CALM might be involved in different populations of clathrin-coated vesicles and thus regulate different clathrin-mediated pathways. Consistent with this idea, we have found in this study that AP180 but not CALM influences the level of extracellular Aβ.
Materials and Methods
Cells, shRNAs, and transfection
Human neuroblastoma SH-SY5Y cells expressing human Swedish mutant APP695 cDNA were obtained from Dr. Wataru Araki (National Institute for Longevity Sciences) [20]. The pSuper-AP180shRNA has been described in detail in a previous study [19]. The pSuper-CALMshRNA has also been previously characterized and described [19, 21]. Cells were transfected using Lipofectamine following the manufacturer’s (Invitrogen) protocol and analyzed 3–4 days after transfection.
ELISA
The Aβ 1-40 and 1-42 in the media of transfected cells were measured using enzyme-linked immunosorbent assay (ELISA) kits (Biosource International Inc.). For each experiment, samples were assayed in triplicate and all experiments were repeated at least three times. To confirm changes in Aβ 1-40, we also used a non-commercial ELISA protocol [22]. The data from both ELISA protocols were similar.
Antibodies, immunoblotting, and immunolabeling
The following antibodies were used: mouse monoclonal anti-AP180 (clone AP180-I; Sigma), goat polyclonal anti-CALM (sc5395 and sc6433; Santa Cruz Biotechnology), mouse monoclonal anti-APP N-terminus (clone 22C11; Chemicon/Millipore), and rabbit polyclonal anti-APP C-terminus (IBL Co., LTD, Japan). For immunoblotting, cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blots were incubated with primary antibodies followed by appropriate HRP-conjugated secondary antibodies, and visualized using ECL chemiluminescence. For immunolabeling, cells were fixed with 4% paraformaldehyde and 4% sucrose, and permeabilized with 0.1% Triton X-100. Cells were then incubated with primary antibodies followed by appropriate fluorescently tagged secondary antibodies.
Results and Discussion
The goal of this study was to determine whether AP180 and CALM have effects on Aβ production. We chose to use the neuroblastoma SH-SY5Y cells expressing the AD-associated Swedish mutant APP [20] for several reasons. First, these cells have been used as a simple system for studying APP processing and Aβ production [8, 20]. Second, because SH-SY5Y cells are neural, we were able to compare the neuron-specific AP180 and the ubiquitously expressed CALM. Third, higher transfection efficiency in cell lines compared with primary cultured neurons provides a practical means for biochemical assays.
To suppress the expression of AP180 or CALM, we transfected the SH-SY5Y cells with AP180 shRNA or CALM shRNA. The specificity and efficacy of these shRNAs in reducing the level of AP180 or CALM in the SH-SY5Y cells were analyzed by immunoblotting and immunolabeling. AP180 shRNA was originally designed to silence the rat AP180 gene and has proven to be highly effective in the knockdown of AP180 in rat neurons [19]. However, the shRNA targeting region of rat and human AP180 differs in two nucleotides (Figure 1A). The difference could potentially render AP180 shRNA ineffective in human SH-SY5Y cells, as gene silencing by RNAi is known to be specific [23, 24]. To address this question, we examined the cells after they had been transfected with AP180 shRNA for 3–4 days. Immunoblotting of the cell lysates showed that the level of AP180 in the AP180 shRNA-transfected cells was significantly lower (<50%) compared to those transfected with the control vector (Figure 1B, upper panel). The level of AP180 in the CALM shRNA-transfected cells, however, was not reduced (Figure 1B, also upper panel), suggesting the specificity of the AP180 shRNA. To confirm the immunoblotting observation and to determine if the residual AP180 was derived from non-transfected cells, we co-transfected the cells with EGFP to mark transfected cells and carried out immunofluoresence labeling. While not all cells were transfected, those EGFP-expressing transfected cells were devoid of AP180 labeling (Figure 1C).
Figure 1.
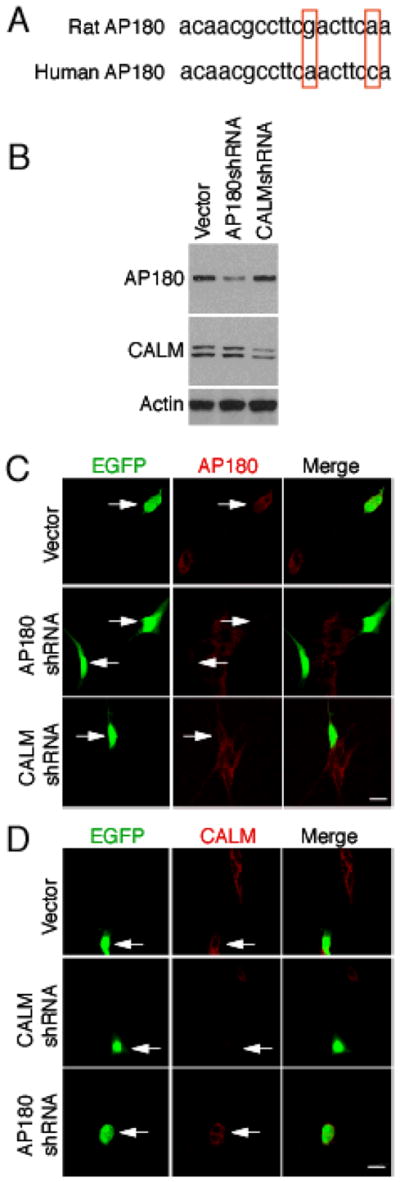
Characterization of the AP180 shRNA and CALM shRNA in SH-SY5Y cells. (A) Comparison of the shRNA-targeting sequences between rat and human AP180. The two nucleotides that are different between the rat AP180 and the human AP180 are indicated (the rat AP180 shRNA-targeting sequence was nt2157-nt2175, accession number X68877; the human AP180 shRNA-targeting sequence was nt2424-nt2442, accession number NM_014841). (B) Immunoblots of the cells transfected with the indicated shRNA showed that AP180 shRNA and the CALM shRNA respectively suppressed the expression of AP180 and CALM. (C)(D) Immunolabeling of the cells co-transfected with EGFP and the indicated shRNA confirmed that the AP180 shRNA suppressed AP180 expression but not CALM, whereas the CALM shRNA suppressed CALM expression but not AP180. Bars = 10 μm.
CALM shRNA was also designed to silence rat CALM [21]. Unlike AP180 shRNA, the sequence within the CALM shRNA-targeting region is identical between rats and human, as well as between the long- and short-splice variants (see Materials and Methods). This CALM shRNA has been characterized in rat neurons [19] and in human embryonic kidney (HEK) 293 cells [21]. As expected, the shRNA suppressed the expression of CALM, as determined by immunoblotting (Figure 1B, middle panel) and immunolabeling (Figure 1D). Thus, AP180 shRNA and CALM shRNA can specifically and effectively suppress the expression of AP180 and CALM in the SH-ST5Y cells.
To determine if suppressing AP180 or CALM would influence Aβ production, we measured Aβ1-40 and Aβ1-42 levels in the media of the shRNA transfected cells using ELISA. We observed that the AP180-knockdown cells produced significantly less Aβ1-40 (63% ± 10.8) compared to the control cells (Figure 2A). The level of Aβ1-40 from the CALM-knockdown cells was slightly reduced (88.8% ± 6.2), but the reduction was not statistically significant (Figure 2A). The AP180-knockdown cells also produced less Aβ1-42 (78.2% ± 1.3) (Figure 2A). In contrast, the level of Aβ1-42 in the CALM-knockdown cells remained unchanged (100% ± 2.2) (Figure 2B). Therefore, suppressing AP180 reduces the production of both Aβ1-40 and Aβ1-42. Suppressing CALM, on the other hand, does not alter Aβ levels.
Figure 2.
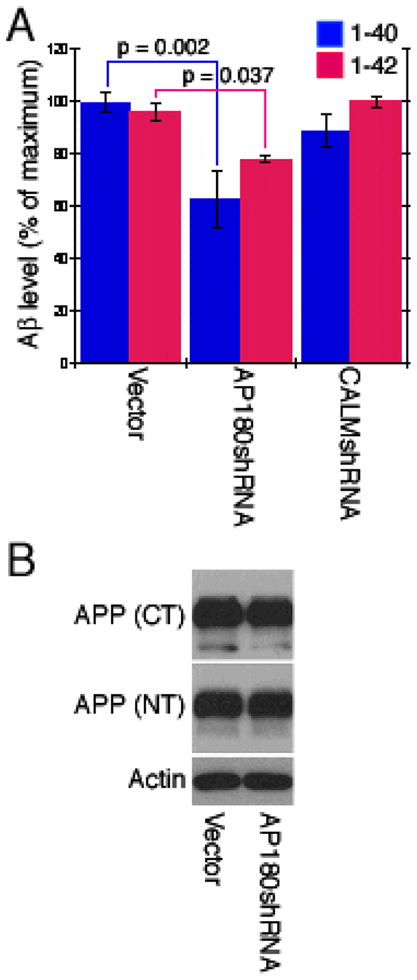
Knockdown of AP180 reduces the levels of Aβ but not APP. (A) Cells were transfected with the indicated shRNAs for 3 to 4 days and the amount of Aβ 1-40 and 1-42 in the media were measured (n ≥ 3 experiments, triplicate samples per experiment). Data represent means ± SEM. (B) Cell lysates from AP180 shRNA-transfected cells used in (A) were analyzed by immunoblotting. The same major band revealed by two different APP antibodies migrated at ~120 kDa.
To assess whether the reduction of Aβ resulted from a decrease of total APP, we next measured APP level in the lysates of the AP180 knockdown cells using immunoblotting. We used two different APP antibodies: one reacts against APP’s N-terminus [25] and the other against APP’s C-terminus. We found that both antibodies revealed a major protein band at ~120 kDa and that the intensity of this band was not different between the AP180 knockdown cells and the control cells (Figure 2B). This result suggests that AP180 knockdown has no effect on the level of APP.
Our results add AP180 to the growing list of endocytic proteins that are actively involved in Aβ production by neurons. Endocytosis and subsequent intracellular sorting direct APP to subcellular compartments where the amylidogenic cleavages of APP that generate Aβ take place [2, 3]. Therefore, the production of Aβ is not only controlled by the enzymes that carry out the cleavage, but also by cell’s ability to control the traffic and the destination of APP.
Our finding also raises several questions about AP180. First, does AP180 exert the same effects on mutant APP and on the wild-type APP? In the initial course of this study, we planned to analyze two different SH-SY5Y cells, expressing either the AD-associated Swedish mutant APP or the wild-type APP. However, we found that the level of AP180 in the wild-type APP expressing cells was nearly undetectable (data not shown), precluding us from carrying out analysis on AP180 in those cells. The disappearance of AP180 could directly result from the overexpression of wild-type APP. It is, then, tempting to ask if a two-way regulatory relationship exists between APP and AP180 and if the mutation in APP alters this relationship.
Second, how can we consolidate the data described here and the previous observations from tissues of AD patients? We previously found that AP180 was significantly and consistently reduced in the postmortem brain tissues of patients suffering late-stage AD [26, 27]. The in vitro finding that AP180 suppression leads to Aβ reduction seems to conflict with the observation of low AP180 but high Aβ in vivo [26, 27]. The seeming contradiction, however, can be ascribed to the complexity of the disease. Important issues to address in future studies include at what disease stage the loss of AP180 occurs, whether its loss simply reflects the disease or contributes to the disease pathology, and how AP180 cooperates with other endocytic proteins in regulating APP trafficking and Aβ production.
Third, how are AP180 and CALM related to each other? AP180 and CALM have been regarded as homologues because of their overlapping amino acid sequences, similar domain structures, and ability to promote the assembly of clathrin-coated vesicles [11–16]. In neurons, however, AP180 and CALM do not co-localize [18, 28], and they play different roles in the growth of neurons [19]. Our finding that AP180, but not CALM, affects Aβ production supports the notion put forward previously: that AP180 and CALM are two functionally separate clathrin assembly proteins.
Acknowledgments
This research was supported by the Intramural Research Program of the Natinal Institute on Aging of the NIH, and extramural grants (R01AG026478 and K01AG022455 to Y.M.). We also thank Ms. Chiho Hirata-Fukae for her technical assistance.
References
- 1.Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Small SA, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song YQ, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider A, Rajendran L, Honsho M, Gralle M, Donnert G, Wouters F, Hell SW, Simons M. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28:2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schöbel S, Neumann S, Hertweck M, Dislich B, Kuhn PH, Kremmer E, Seed B, Baumeister R, Haass C, Lichtenthaler SF. A novel sorting nexin modulates endocytic trafficking and alpha-secretase cleavage of the amyloid precursor protein. J Biol Chem. 2008;283:14257–14268. doi: 10.1074/jbc.M801531200. [DOI] [PubMed] [Google Scholar]
- 8.Kyriazis GA, Wei Z, Vandermey M, Jo DG, Xin O, Mattson MP, Chan SL. Numb endocytic adapter proteins regulate the transport and processing of the amyloid precursor protein in an isoform-dependent manner: implications for Alzheimer disease pathogenesis. J Biol Chem. 2008;283:25492–25502. doi: 10.1074/jbc.M802072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F, Yao PJ. Clathrin-mediated endocytosis and Alzheimer’s disease: An update. Ageing Res Rev. 2009 doi: 10.1016/j.arr.2009.03.002. in press. [DOI] [PubMed] [Google Scholar]
- 11.Ahle S, Ungewickell E. Purification and properties of a new clathrin assembly protein. EMBO J. 1986;5:3143–3149. doi: 10.1002/j.1460-2075.1986.tb04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye W, Lafer EM. Bacterially expressed F1-20/AP-3 assembles clathrin into cages with a narrow size distribution: implications for the regulation of quantal size during neurotransmission. J Neurosci. 1995;41:15–26. doi: 10.1002/jnr.490410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreyling MH, Martinez-Climent JA, Zheng M, Mao J, Rowley JD, Bohlander SK. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc Natl Acad Sci. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, Lafer EM. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J Neurosci. 1999;19:10201–10212. doi: 10.1523/JNEUROSCI.19-23-10201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol Biol Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyerholz A, Hinricchsen L, Groos S, Esk PC, Brandes G, Ungewickell EJ. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 17.Sousa R, Tannery NH, Zhou S, Lafer LM. Characterization of a novel synapse specific protein. I. Developmental expression and cellular localization of the F1-20 protein and mRNA. J Neurosci. 1992;12:2130–2143. doi: 10.1523/JNEUROSCI.12-06-02130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao PJ, Petralia RS, Bushlin I, Wang Y, Furukawa K. Synaptic distribution of the endocytic accessory proteins AP180 and CALM. J Comp Neurol. 2005;481:58–69. doi: 10.1002/cne.20362. [DOI] [PubMed] [Google Scholar]
- 19.Bushlin I, Petralia RS, Wu F, Harel A, Mughal MR, Mattson MP, Yao PJ. Clathrin assembly protein AP180 and CALM differentially control axogenesis and dendrite outgrowth in embryonic hippocampal neurons. J Neurosci. 2008;28:10257–10271. doi: 10.1523/JNEUROSCI.2471-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda K, Araki W, Tabira T. Enhanced generation of intracellular Abeta42 amyloid peptide by mutation of presenilins PS1 and PS2. Eur J Neurosci. 2004;19:258–264. doi: 10.1111/j.0953-816x.2003.03135.x. [DOI] [PubMed] [Google Scholar]
- 21.Harel A, Wu F, Mattson MP, Morris CM, Yao PJ. Evidence for CALM in directing VAMP2 trafficking. Traffic. 2008;9:417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 22.Hirata-Fukae C, Sidahmed EH, Gooskens TP, Aisen PS, Dewachter I, Devijver H, Van Leuven F, Matsuoka Y. Beta-site amyloid precursor protein-cleaving enzyme-1 (BACE1)-mediated changes of endogenous amyloid beta in wild-type and transgenic mice in vivo. Neurosci Lett. 2008;435:186–189. doi: 10.1016/j.neulet.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharp PA. RNA interference—2001. Genes Dev. 2001;15:485–490. doi: 10.1101/gad.880001. [DOI] [PubMed] [Google Scholar]
- 24.Birmingham A, Anderson E, Sullivan K, Reynolds A, Boese Q, Leake D, Karpilow J, Khvorova A. A protocol for designing siRNAs with high functionality and specificity. Nat Protoc. 2007;2:2068–2078. doi: 10.1038/nprot.2007.278. [DOI] [PubMed] [Google Scholar]
- 25.Hilbich C, Mönning U, Grund C, Masters CL, Beyreuther K. Amyloid-like properties of peptides flanking the epitope of amyloid precursor protein-specific monoclonal antibody 22C11. J Biol Chem. 1993;268:26571–26577. [PubMed] [Google Scholar]
- 26.Yao PJ, Coleman PD. Reduction of O-linked N-acetylglucosamine-modified assembly protein-3 in Alzheimer’s disease. J Neurosci. 1998;18:2399–2411. doi: 10.1523/JNEUROSCI.18-07-02399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao PJ, Morsch R, Callahan LM, Coleman PD. Changes in synaptic expression of clathrin assembly protein AP180 in Alzheimer’s disease analysed by immunohistochemistry. Neuroscience. 1999;94:389–394. doi: 10.1016/s0306-4522(99)00360-7. [DOI] [PubMed] [Google Scholar]
- 28.Petralia RS, Yao PJ. AP180 and CALM in the developing hippocampus: expression at the nascent synapse and localization to trafficking organelles. J Comp Neurol. 2007;504:314–327. doi: 10.1002/cne.21454. [DOI] [PubMed] [Google Scholar]


