Abstract
Quorum sensing (QS)-based transcriptional responses in Pseudomonas aeruginosa have been defined on the basis of increases in transcript levels of QS-controlled genes such as lasB and aprA following the hierarchical transcriptional increases of central controllers such as the lasR gene. These increases occur at high bacterial concentrations such as early-stationary-phase growth in vitro. However, the extent to which the increases occur in a variety of clinical and environmental isolates has not been determined nor is there extensive information on allelic variation in lasR genes. An analysis of the sequences of the lasR gene among 66 clinical and environmental isolates showed that 81% have a sequence either identical to that of strain PAO1 or with a silent mutation, 15% have nucleotide changes resulting in amino acid changes, and 5% have an insertion sequence in the lasR gene. Using real-time PCR to quantify transcript levels of lasR, lasB, and aprA in the early log and early stationary phases among 35 isolates from bacteremia and pneumonia cases and the environment, we found most (33 of 35) strains had increases in lasR transcripts in early stationary phase but with a very wide range of final transcript levels per cell. There was a strong correlation (r2 = 0.84) between early-log- and early-stationary-phase transcript levels in all strains, but this finding remained true only for the 50% of strains above the median level of lasR found in early log phase. There were significant (P < 0.05) but weak-to-modest correlations of lasR transcript levels with aprA (r2 = 0.2) and lasB (r2 = 0.5) transcript levels, but again this correlation occurred only in the 50% of P. aeruginosa strains with the highest levels of lasR transcripts in early stationary phase. There were no differences in distribution of lasR alleles among the bacteremia, pneumonia, or environmental isolates. Overall, only about 50% of P. aeruginosa strains from clinical and environmental sources show a lasR-dependent increase in the transcription of aprA and lasB genes, indicating that for about 50% of clinical isolates this regulatory system may not play a significant role in pathogenesis.
Pseudomonas aeruginosa is a ubiquitous environmental organism able to colonize and infect humans with underlying genetic susceptibilities (14) or under opportunistic conditions. Ventilated patients hospitalized in intensive care units are often highly susceptible to P. aeruginosa colonization in the upper respiratory tract (throat and/or trachea) (1, 8). The transition from colonization to infection by P. aeruginosa is often seen in the setting of immunosuppression of host defenses such as occurs in cancer patients undergoing chemotherapy, when the blood levels of polymorphonuclear neutrophils are below 100/mm3 (26). In addition, controlled transcription and synthesis of genes and proteins involved in virulence are thought to be instrumental in sensing the host environment and producing appropriate bacterial responses.
The transcription of genes encoding several virulence factors of P. aeruginosa is controlled by the two quorum-sensing (QS) systems, las and rhl (19), which are regulated by autoinducers (30). Our knowledge of these two QS systems in P. aeruginosa has rapidly progressed in the last decade (for a recent review, see reference 4). The las system controls the expression of virulence genes such as lasA, lasB, aprA, toxA, and lasI (6, 7, 17, 23, 28). The rhl system controls, for example, the expression of lasA, lasB, and rhlAB (2, 3, 11, 12, 15, 18, 30). The las and rhl QS systems are hierarchically linked. The las system positively regulates the expression of both rhlR and rhlI (11, 19).
In vitro studies with primarily laboratory strains and virulence studies in animals with these same strains have suggested a role for QS systems in pathogenesis. However, the importance of QS in clinical isolates from typical human P. aeruginosa infections is not clearly known (21). A recent study (25) showed a positive correlation between the accumulation of lasR transcripts and those of lasA, lasB, and toxA in sputum samples from cystic fibrosis (CF) patients infected with P. aeruginosa, suggesting that the las system controls virulence gene expression during the course of this specific infection. The autoinducers have also been detected in sputum from patients with CF that also had high levels of lasI and lasR transcripts (5, 24, 25). However, detection of the autoinducers and lasI and lasR transcripts remained low for several CF patients infected by P. aeruginosa (5, 25), and due to the high immune responses of CF patients to virulence factors, it is unclear in this setting of chronic infection whether QS-controlled proteins remain effective virulence factors. As there has been little investigation into the role of QS in P. aeruginosa strains outside of CF, it may be that some isolates do not possess or do not express the QS systems yet remain capable of causing serious human infections.
In order to determine the presence and function of the QS gene lasR among non-CF clinical isolates, we analyzed the sequence of most of the lasR gene from a collection of 66 P. aeruginosa strains isolated from cases of nosocomial pneumonia in ventilated patients hospitalized in an intensive care unit, from cases of bacteremia in cancer patients with neutropenia, and from water from swimming pools and rivers (20). A subset of these strains selected on the basis of genetic diversity of the lasR gene sequence and of the origin of strains was further used to quantify the amount of mRNA transcribed by lasR, lasB, and aprA genes during the growth of these strains by using real-time reverse transcription-PCR.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
We studied 68 strains of P. aeruginosa, including PAO1, a wild-type prototroph (9) sensitive to chloramphenicol and thus not overexpressing the MexEF-oprN multidrug efflux pump (10); PAOR, a lasR mutant derived from PAO1 (11); 30 strains isolated from patients with nosocomial pneumonia; 20 strains isolated from patients with bacteremia; and 16 strains isolated from water in swimming pools and rivers. The strains were stored frozen at −80°C in individual aliquots in peptone with 10% glycerol. The clinical and environmental strains were part of a collection of strains previously studied for their genetic diversity (20). Some strains were retrieved from six closely related clusters of infection, and we did not use all of the strains likely to be closely related in this analysis. Criteria for inclusion of clinical strains were clearly identified (20), and details of the properties of the clinical and environmental strains are presented at the website http://www.chez.com/rruimy. On the basis of the results of lasR gene sequencing and the origin of strains, we selected 13 strains from the two clinical groups and 9 strains from the environmental group to quantify the amount of mRNA of lasR, lasBI, and aprA genes in early log phase after 120 min of growth in broth and in early stationary phase after 300 min of growth in broth.
DNA extraction, amplification, and sequencing of lasR, lasB, and aprA.
DNA extraction was performed by using MagnaPure LC (Roche, Mannheim, Germany) according to the manufacturer's recommendations with slight modifications. Bacteria from the frozen stocks were inoculated onto Mueller-Hinton agar (Sanofi-Pasteur, Marnes-la-coquette, France). After overnight growth at 37°C, one colony was suspended in 60 μl of DNase-RNase-free water (Sigma-Aldrich, Saint Quentin Fallavier, France) with 2 μl of lysozyme at 20 mg/ml (Sigma-Aldrich) and incubated for 5 min at 37°C. One hundred thirty microliters of bacterial lysis buffer supplied in isolation kit III (Roche) was added to the mixture, and the mixture was incubated for 10 min at 65°C. Twenty microliters of proteinase K was also added to the mixture. After the mixture was incubated for 10 min at 95°C, DNA was extracted by using the MagNA Pure LC and the other products of kit III (Roche). DNA was quantified at an optical density of 260 nm with a Gene Quant II spectrophotometer (Amersham-Pharmacia Biotech, Orsay, France).
Internal fragments of 665 bp of the lasR gene, 441 bp of the lasB gene, and 728 bp of the aprA gene were amplified in an I-cycler (Bio-Rad, Marnes-la-coquette, France). An internal fragment of the lasR gene from each of the 66 strains was amplified and sequenced by using primers that encompassed the 5′ and 3′ ends of the gene to ensure that most of the gene from each strain would be amplified. We did not use primers outside of the coding sequence for lasR because the variability of the nucleotide sequence around the lasR gene was unknown. The nucleotide sequence recovered 92.4% of the lasR gene, which was a good reflection of the overall variation in the lasR gene; the remaining 7.6% of the lasR sequence was from the primers used for PCR amplification. Internal fragments of lasB and aprA genes from the 35 strains selected for real-time PCR were amplified and sequenced in order to choose pairs of primers for amplification and fluorogenic probes that encompassed the region of the genes with the fewest nucleotide changes among all 35 strains. The primers used for amplification are listed in Table 1.
TABLE 1.
Primers used for amplification and sequencing and fluorogenic probes used for real-time PCR
| Gene target | GenBank no. | Forward and reverse primersa
|
Fluorogenic probe
|
Internal primera
|
|||
|---|---|---|---|---|---|---|---|
| Positionb | Sequence | Positionb | Sequence | Positionb | Sequence | ||
| lasR | D30813 | 4 (F) | 5′-GCCTTGGTTGACGGTTTTCTTG-3′ | 473 | 5′-GCGTAGTCCTTGAGCATCCA-3′ | ||
| 713 (R) | 5′-GTAATAAGACCCAAATTAACGGC-3′ | 454 | 5′-TGGATGCTCAAGGACTACGC-3′ | ||||
| 271 | 5′-TCCATCTACCAGACGCGAAAG-3′ | ||||||
| 532 (F) | 5′-ACCAGCTGGGAGAAGGAAG-3′ | 572 | 5′-CCGATGGCGCACCAC-3′ | ||||
| 595 (R) | 5′-CCGATATCTCCCAACTGGTCTTG-3′ | ||||||
| lasBc | M19472 | 363 (F) | 5′-TCGTTGCGATCATGGGTGT-3′ | ||||
| 842 (R) | 5′-CGGGAATCAGGTAGGAGACG-3′ | ||||||
| 588 (F) | 5′-GCGAAGCCATCACCGAAGT-3′ | 646 | 5′-CCATTTCGTCGCCAACA-3′ | ||||
| 713 (R) | 5′-CCTGCTCGGCGGATACC-3′ | ||||||
| 588 (F) | 5′-CGAGGCCATCACCGAAGT-3′ | 646 | 5′-CCATTTCGTCGCCAACA-3′ | ||||
| 713 (R) | 5′-ACCTGCTCGGCGGAGAC-3′ | ||||||
| aprAc | X64558 | 5042 (F) | 5′-TTGCATTGAAAGGTCGTAGCGATG-3′ | 5270 | |||
| 5813 (R) | 5′-GTGCGAGTGGTCAGGTTGGC-3′ | 5373 | 5′-GGTGACGTCCGACCAGGAT-3′ | ||||
| 5495 (F) | 5′-GGCAATCCTGGTACCTGATCAA-3′ | 5526 | 5′-CAGCGCCAACGTCAA-3′ | ||||
| 5554 (R) | 5′-AGGGTCTGGCGTCCGTAGTT-3′ | ||||||
| 5495 (F) | 5′-GCCAGTCCTGGTACCTGATCAA-3′ | 5526 | 5′-CAGCGCCAACGTCAA-3′ | ||||
| 5554 (R) | 5′-AGCGTCTGGCGTCCGTAGTT-3′ | ||||||
| 5495 (F) | 5′-GGCAATCCTGGTACCTGATCAA-3′ | 5526 | 5′-CAGCGCCAACGTCAA-3′ | ||||
| 5554 (R) | 5′-AGCGTCTGGCGCCCGTAGTT-3′ | ||||||
| Small-subunit rDNA | X06684 | 485 (F) | 5′-CAACAGAATAAGCACCGGCTAA-3′ | 525 | 5′-CGCGGCTGCTGGCACGAA-3′ | ||
| 547 (R) | 5′-ACGCTTGCACCCTTCGTATTA-3′ | ||||||
For each gene target, two primers, forward (F) and reverse (R), were used for amplification and sequencing, while the internal primer was used only for sequencing.
The numbers indicate the position in the gene sequence, as published in GenBank (see the accession numbers in the GenBank column), corresponding to the nucleotide at the 5′ end of each forward, reverse, and internal primer and of each fluorogenic probe.
For the lasB and aprA genes, different primers were chosen according to the strain to be amplified by real-time PCR.
The amplification mixture for each gene fragment contained 100 ng of bacterial DNA, two primers at 400 nM each, 250 μM (each) deoxynucleoside triphosphate (Boehringer GmbH, Mannheim, Germany), and 1× reaction buffer supplied by the manufacturer with 1.5 mM MgCl2 and 1 U of AmpliTaq DNA polymerase (Applera, Courtaboeuf, France) in a final volume of 50 μl. DNA was amplified by using the following protocol: 94°C for 4.5 min, then 27 cycles of 94°C for 30 s, 57°C for 1 min, and 72°C for 1 min, followed by 72°C for 10 min. The PCR products were electrophoresed through agarose gels (2%, wt/vol) containing ethidium bromide (0.5 μg ml−1) and visualized under UV irradiation.
The PCR products were purified by using the PCR purification kit Qiaquick (Qiagen, Courtaboeuf, France) and then quantified following electrophoresis on agarose gels by using visual comparisons with markers of known quantity (Low DNA Mass Ladder; Boehringer GmbH). The PCR products were sequenced according to the manufacturer's recommendations for the ABI Prism sequencing kit (Applera) by using the same two primers that were used in amplification (Table 1). The non-primer-derived sequences were aligned and compared to those of the published PAO1 strain by using the Sequence Navigator program (Applera).
RNA extraction and purification.
RNA was extracted from 35 of the strains, which were chosen based on their origin and their lasR gene sequences, which are representative of each category of clinical isolate, and from PAO1 and its mutant in the lasR gene, PAOR. The 35 strains included the following: (i) 11 strains with lasR gene sequences identical to that of PAO1 and 11 strains with silent lasR mutations, both groups having a similar distribution in terms of clinical source and comprising 4 strains isolated from patients with pneumonia, 4 strains from patients with bacteremia, and 3 strains from environmental water isolates; (ii) 10 strains with mutations in the lasR gene compared with the PAO1 lasR sequence and with a similar distribution in regard to clinical source; and (iii) three strains with an inserted sequence in lasR.
One colony of each strain was inoculated into 5 ml of Luria-Bertani broth and grown overnight at 37°C with shaking. Five hundred microliters of this overnight culture was inoculated into 200 ml of fresh broth and incubated under the same growth conditions. For PAOR cultures, 300 μg of carbenicillin (Sigma-Aldrich, Saint Quentin Fallavier, France) per ml was added. After 120 min of growth, the optical density of the culture at 600 nm was measured with a Spectronic 301 spectrophotometer (Bioblock Scientific, Paris, France). After 120 and 300 min of growth, a volume of culture corresponding to 2 × 108 bacteria was centrifuged for 5 min at 6,000 × g at 4°C. The bacterial pellet was immediately suspended in 100 μl of Tris-EDTA lysis buffer (10 mM Tris-Cl, 1 mM EDTA, pH 8.0) containing 400 μg of lysozyme (Sigma-Aldrich) per ml and incubated for 3 min at room temperature. Three hundred fifty microliters of RLT buffer (Qiagen) containing 0.145 μM β-mercaptoethanol was added, and the mixture was vigorously shaken, immediately frozen in liquid nitrogen, and stored at −80°C until RNA extraction.
The bacterial lysates were defrosted and centrifuged for 2 min at 6,000 × g at 4°C. Supernatant samples of 450 μl were transferred to microcentrifuge tubes, and 250 μl of absolute ethanol was added to each tube to precipitate nucleic acids. The samples were loaded onto RNeasy columns (Qiagen) and centrifuged for 15 s at 6,000 × g, washed with 700 μl of RW1 buffer, and centrifuged for 15 s at 20,200 × g. Eighty microliters of RD buffer per sample containing 0.34 U of RNase-free DNase (Qiagen) per μl was added, the samples were left to stand for 20 min at 37°C, and the column was then washed twice with 500 μl of RPE buffer and centrifuged for 15 s at 6,000 × g for the first wash and 2 min for the second. RNA was then eluted twice in 50 μl of RNase-DNase-free water (Sigma-Aldrich) by centrifugation for 1 min at 6,000 × g and quantified at an optical density of 260 nm with a Gene Quant II spectrophotometer (Amersham-Pharmacia Biotech, Orsay, France). Any RNA breakdown was detected by ethidium bromide staining after electrophoresis (0.5× Tris-acetate-EDTA) on agarose gel (1.4%, wt/vol). RNAs were judged undamaged when only two sharp bands corresponding to the large and small subunits of rRNA were visible.
Reverse transcription.
Immediately after extraction, reverse transcription was performed on 500 ng of RNA by using the Taqman RT reagents kit (Applera) according to the manufacturer's recommendations.
Real-time PCR.
The amounts of cDNA obtained by reverse transcription were quantified with the real-time fluorogenic 5′ nuclease assay by using an ABI Prism 7000 sequence detector (Applera). We determined the level of transcripts of the lasR gene, which controls both of the QS systems, of the lasB and aprA genes, which are regulated by these QS systems, and of the small-subunit rRNA gene. The probes and primers (Table 1) used to quantify their expression were designed by using the Primer Express ABI Prism program (Applera) and considering the published sequences of lasR, lasB, and aprA. The probes were obtained from Applera and labeled 5′ with the 6-carboxyfluorescein fluorescent dye as reporter and 3′ with the 6-carboxytetramethylrhodamine as quencher. Amplification was performed in a final volume of 20 μl in MicroAmp Optical plates (Applera). The 20-μl reaction mixture contained 10 μl of cDNA sample diluted 1:20, 1× Taqman buffer A, 5.5 mM MgCl2, 200 μM (each) dATP, dCTP, and dGTP, 400 μM dUTP, 200 nM forward primer, 200 nM reverse primer, 100 nM probe, 0.01 U of uracil-N-glycosylase (AmpErase UNG) per μl, and 0.025 U of AmpliTaq Gold DNA polymerase per ml. Before amplification, the PCR mixtures were heated at 50°C for 2 min to prevent carryover of PCR products and then to 95°C for 10 min to denature nucleic acids. All PCRs were run in duplicate and included 40 cycles (95°C for 15 s and 60°C for 1 min). The evolution of the fluorescent intensity of each dye was recorded continuously by using the ABI Prism 7000 sequence detection system (PE Applied Biosystems). The amount of DNA in the sample was calculated by comparing it with the values obtained with standards comprising 10-fold dilutions of P. aeruginosa DNA ranging from 25 to 0.025 ng/ml (corresponding approximately to 3.8 × 106 to 3.8 × 101 copies per ml of each amplified target). Negative controls consisting of distilled water or total RNA were included in each test to detect DNA contamination. Data were analyzed with Sequence Detector version 16 application software (Applera) on a personal computer linked directly to the ABI Prism 7000 sequence detection system, as recommended by the manufacturer. The mRNA of lasB, aprA, and lasR genes extracted from each strain was normalized on the basis of the small-subunit rRNA levels, which were determined in each of the real-time PCR experiments. Normalization consisted of dividing the number of copies of the larR, aprA, or lasB transcripts by the number for the small-subunit rRNA and multiplying by 1,000. The PCRs for each strain were repeated three times. Analysis of our data indicated that small-subunit rRNA levels were essentially identical at 120 and 300 min of growth for every strain.
Statistical analysis.
All analyses were carried out by using the Prism software package (GraphPad Prism software).
RESULTS
Sequences of the internal fragments of lasR genes.
An internal fragment (665 bp) of the 720-bp lasR gene (92.4% of the sequence) was detected in all 66 strains isolated from pneumonia, bacteremia, and environmental sources. The distribution of strains, based on the change in the lasR sequences of the 66 strains, is given in Table 2. Nucleotide changes resulting in amino acid changes were found in nine strains in various regions of the lasR gene. Details of the amino acid changes resulting from the nucleotide substitutions in the lasR protein from these nine strains are given in Table 3. Finally, one strain in each group had an inserted sequence of roughly 1,300 bp that was always in the same region. These sequences were compared with insertion sequence elements from the database available at http://www-is.biotoul.fr/page-is.html and were found to be members of the IS5 family group of insertion sequences, IS427, IS5, and IS6 for the pneumonia, bacteremia, and environmental strains, respectively. Characteristics of the three insertion sequences can be found at http://www.chez.com/rruimy. By chi-square analysis there were no significant associations between changes in the lasR gene and the source of isolation of the strain.
TABLE 2.
Comparison of the lasR sequence of 66 P. aeruginosa strains and a subset of 35 strains with the internal fragment of the lasR gene of PAO1b
| Identity or difference | Origin of strain
|
||
|---|---|---|---|
| Pneumoniaa (n = 30) | Bacteremiaa (n = 20) | Environmenta (n = 16) | |
| Full identity | |||
| All strains | 15 (50) | 8 (40) | 7 (44) |
| RT-PCR strains | 4 (13) | 4 (20) | 3 (19) |
| Silent mutations | |||
| All strains | 11 (37) | 7 (35) | 6 (38) |
| RT-PCR strains | 4 (13) | 4 (20) | 3 (19) |
| Nucleotide changes resulting in amino acid changes | |||
| All strains | 3 (10) | 4 (20) | 2 (12) |
| RT-PCR strains | 4 (13) | 4 (20) | 2 (12) |
| Insertions | |||
| All strains | 1 (3) | 1 (5) | 1 (6) |
| RT-PCR strains | 1 (3) | 1 (5) | 1 (6) |
The numbers in parentheses indicate the percentages of lasR sequence retrieved from all strains from each origin.
The strains were studied by using real-time PCR (RT-PCR). The internal fragment used for comparison was 665 bp; the lasR gene of PAO1 is 720 bp.
TABLE 3.
Comparisons of lasB, aprA and lasR transcript levels in early log phase (120 min) and stationary phase (300 min) for P. aeruginosa strains
| Strain no. and origina | Amt of transcript at indicated culture timeb
|
Comparison with lasR gene of PAO1 | |||||
|---|---|---|---|---|---|---|---|
|
lasR transcript
|
lasB transcript
|
aprA transcript
|
|||||
| 120 min | 300 min | 120 min | 300 min | 120 min | 300 min | ||
| 47, B | 0.24 | 1.94 | 0.04 | 3.46 | 1.7 | 197.93 | Full identity |
| 63, E | 0.24 | 4.09 | 0.6 | 1.74 | 0.09 | 3.23 | C201 stopc |
| 41, B | 0.45 | 0.66 | 0.04 | 0.01 | 1.03 | 6.93 | Full identity |
| 45, B | 0.59 | 4.14 | 1.51 | 13.41 | 0.17 | 0.57 | Silent mutations |
| 53, B | 0.88 | 10.36 | 1.43 | 1.44 | 4.5 | 12.91 | Insert |
| 42, B | 0.92 | 1.67 | 2.58 | 4.38 | 0.13 | 1 | Q186P |
| 58, E | 1.09 | 2.59 | 0.27 | 18.94 | 0.1 | 11.78 | Silent mutations |
| 55, B | 1.36 | 17.66 | 0.83 | 0.46 | 1.83 | 3.11 | Full identity |
| 43, B | 1.64 | 7.77 | 0.16 | 0.17 | 0.23 | 1.81 | A70E |
| 37, B | 2.26 | 8.09 | 1.59 | 20.92 | 2.85 | 64.95 | Silent mutations |
| 30, P | 2.42 | 9.11 | 0.46 | 12.14 | 0.29 | 29.62 | Silent mutations |
| 67, E | 2.76 | 3.21 | 1.27 | 0.58 | 1.08 | 3.64 | Full identity |
| 15, P | 2.96 | 5.58 | 0.6 | 35.98 | 1.42 | 309.28 | Silent mutations |
| 51, B | 3.19 | 7.38 | 2.47 | 3.35 | 15.59 | 17.21 | Full identity |
| 66, E | 3.62 | 6.49 | 3.02 | 2.63 | 5.09 | 9.56 | Insertions |
| 24, P | 3.93 | 44.01 | 0.87 | 27.61 | 0.88 | 111.67 | Silent mutations |
| 48, B | 4.02 | 5.16 | 15.05 | 9.86 | 2.14 | 1.72 | F210L |
| 16, P | 4.83 | 12.93 | 0.25 | 4.74 | 0.86 | 44.89 | Full identity |
| 39, B | 5.15 | 27.22 | 0.96 | 7.73 | 0.88 | 21.5 | Silent mutations |
| 11, P | 6.86 | 27.99 | 0.49 | 5.07 | 0.29 | 33.74 | Multiple substitutionsd |
| 64, E | 7.45 | 28.91 | 1.87 | 201.68 | 4.51 | 144.01 | Silent mutations |
| 23, P | 9.54 | 88.21 | 3.01 | 87.95 | 4.25 | 141.91 | Silent mutations |
| 71, E | 11.35 | 12.94 | 0.5 | 6.36 | 0.34 | 35.45 | Multiple substitutions |
| 20, P | 11.55 | 6.2 | 2.59 | 0.61 | 6.95 | 2.01 | Full identity |
| 50, B | 12.67 | 26.98 | 2.27 | 42.86 | 0.41 | 134.41 | K25M |
| 65, E | 14.7 | 70.88 | 1.7 | 121.08 | 3.38 | 361.46 | Full identity |
| 69, E | 15.24 | 19.47 | 7.51 | 119.05 | 7.69 | 197.64 | Silent mutations |
| 36, P | 15.84 | 33.05 | 3.06 | 0.44 | 0.45 | 4.78 | Insertions |
| 19, P | 18.57 | 44.88 | 10.01 | 8.84 | 3.31 | 31.67 | A50G |
| 38, B | 21.24 | 35 | 1.73 | 155.55 | 2.12 | 203.25 | Silent mutations |
| 9, P | 22.15 | 51.88 | 2.32 | 73.68 | 1.22 | 3.24 | Full identity |
| 14, P | 34.27 | 37.36 | 1.53 | 3.14 | 0.94 | 9.12 | Full identity |
| 13, P | 37.21 | 25.97 | 2.49 | 77.86 | 0.3 | 59.51 | V226I |
| 61, E | 42.1 | 137.97 | 4.57 | 58.77 | 6.48 | 133.12 | Full identity |
| 27, P | 106.29 | 414.39 | 5.02 | 258.05 | 16.84 | 269.79 | Silent mutations |
| PAO1 | 8.39 | 12.23 | 8.22 | 129.07 | 5.12 | 210.15 | Full identity |
B, strain isolated from patient with bacteremia; P, strain isolated from patient with pneumonia; E, strain isolated from the environment.
lasB, aprA, and lasR transcript levels were normalized by using the ratio of the amount of these transcripts to the small-subunit rRNA level and multiplying by 1,000.
Amino acid substitution.
Strains 11 and 71 had the following amino acid substitutions: R66K, N136S, A137N, and G172N.
Transcriptional analysis of lasR genes in early log and stationary phase.
To explore the transcriptional manifestations of QS activity in each strain, the mRNA levels of lasR, lasB, and aprA genes were assayed by real-time PCR at 120 and 300 min of growth, corresponding to the beginning of early exponential phase and entry into stationary phase, respectively. The small-subunit rRNA gene was assayed in parallel to normalize the transcript levels of lasR, lasB, and aprA genes. The results for each strain are in Table 3. The resultant amounts of mRNA transcripts were then compared by paired t tests, analysis of variance, correlation analysis, and linear regression.
Overall, there were increases in the levels of lasR transcripts in stationary phase compared with those in early log phase in 33 of the 35 strains (P = 0.01, paired t test), indicating that for most clinical isolates some type of QS response occurred, as reflected by an increase in the lasR transcript level. There was no correlation in the changes in lasR transcript levels based on the genotypic relationship of the lasR gene to the lasR gene of strain PAO1. Two strains isolated from patients with pneumonia had no increase in lasR transcript level. The geometric mean increase in the level of lasR transcripts between 120 and 300 min of growth was 7.1 transcripts per cell, but there was a wide range between a 0.54-fold geometric change (i.e., decrease) and a 17-fold increase. Thus, almost all of the strains showed an increase in lasR transcript levels going from early-log- to stationary-phase growth in vitro.
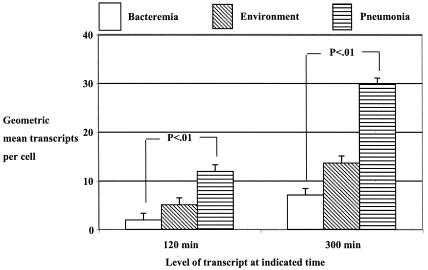
Interestingly, there were significant differences noted in the levels of lasR transcripts in isolates from different sources (Fig. 1). Overall, the pneumonia isolates had significantly higher levels (P < 0.01) of lasR transcripts at both 120 and 300 min of growth than those of the bacteremia strains. Isolates from the environment, which likely represents the source of most clinical P. aeruginosa isolates, were intermediate in their lasR transcript levels between the other two clinical sources and not significantly different from the strains isolated from these other two sources. It is unclear if this association of clinical source and lasR transcript level is of significance in regard to virulence, as there were no significant differences (P > 0.5) by clinical source in the levels of the LasR-regulated transcripts for lasB or aprA at either 120 or 300 min of growth.
FIG. 1.
Comparison by source of isolate of lasR transcript levels in the early log (120 min) and stationary (300 min) phases of growth. The bars represent the geometric means for the strains, and the error bars represent the standard errors of the means. There were 13 isolates from patients with bacteremia, 9 from the environment, and 13 from patients with pneumonia. The P values represent Fisher's probable least square differences for pair-wise comparisons. Values for the overall analysis of variance were as follows: at 120 min, F = 6.317, P = 0.005; at 300 min, F = 4.44, P = 0.02.
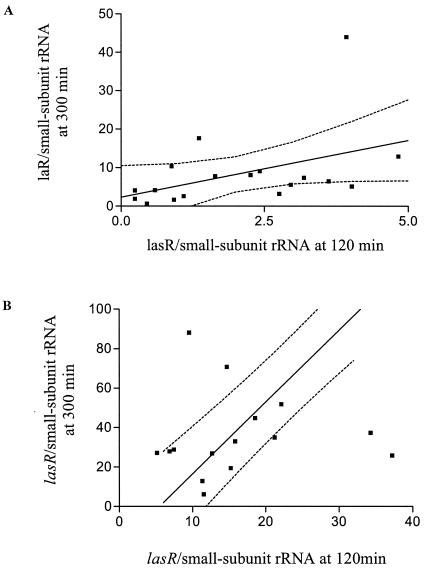
To determine if there was any relationship between the levels of lasR transcripts in the early log and stationary phases, we carried out a correlation analysis of lasR transcript levels at 120 and 300 min of growth. There was a strong correlation between the level of lasR transcripts at 120 min and 300 min of growth (r2 = 0.84, P < 0.001). However, for 18 strains below the median level of all lasR transcripts at 120 min (≤4.8 transcripts per cell), there was no real correlation with the transcript levels present in stationary phase (r2 = 0.2, P > 0.05), as shown in Fig. 2A. In contrast, almost all of the correlation between the levels of lasR transcripts at 120 min and 300 min of growth occurred with the 17 strains above the initial median level at early log phase (r2 = 0.83, P < 0.001), as shown in Fig. 2B. The geometric mean level of lasR transcripts at 300 min of growth for the strains with ≤4.8 transcripts per cell at 120 min of growth was 5.5 (lower and upper standard error of the mean, 4.1, 7.1) (P < 0.001 compared with the level at 120 min, as determined by the paired t test), whereas for strains with >4.8 lasR transcripts per cell at 120 min of growth the geometric mean at 300 min of growth was over nine times higher, 38.4 (35.3, 41.6) (P < 0.001 compared with the level at 120 min, as determined by the paired t test; P < 0.001 for comparison of the geometric means at 300 min of both the initial 50% lowest and 50% highest levels of lasR transcripts). This finding indicates that the initial level of lasR transcripts in those strains with high constitutive levels in early log phase strongly influences the transcript level in stationary phase, whereas for strains with low constitutive levels the amount of lasR transcript produced in stationary phase was still low and not correlated with the initial transcript level.
FIG. 2.
Correlation between the level of lasR/small-subunit rRNA transcripts at 120 min and 300 min of growth for 18 strains below the median level of all lasR/small-subunit rRNA transcripts at early log phase (r2 = 0.2, P > 0.05) (A) and for the other 17 strains above the median level of all lasR/small-subunit rRNA transcripts at early log phase (r2 = 0.83, P < 0.0001) (B).
Transcriptional analysis of lasB and aprA genes in early log and stationary phase.
A similar analysis of the changes in transcript levels of the LasR-regulated lasB and aprA genes showed significant increases (P ≤ 0.001, as determined by paired t tests) in transcript levels for both lasB and aprA genes among the 35 strains, but there was no correlation between levels at 120 and 300 min of growth. This finding may indicate that factors other than the initial level of lasB and aprA determined the level of transcripts in stationary phase.
Analysis of the correlation of lasR transcripts with lasB and aprA transcripts in early log and stationary phases.
To determine if lasR transcription was one of the factors influencing levels of lasB and aprA transcripts in both the early log and stationary phases, we performed a variety of linear regression analyses. The level of lasR transcripts at 120 min in all 35 strains analyzed correlated significantly but very modestly with that of aprA in early log phase (Table 4, group A). At 300 min of growth, the level of lasR transcripts for all 35 strains actually correlated less well, but still significantly, with that of aprA (Table 4, group A). There was no correlation between the transcript levels of lasR and lasB at early log phase for any of the 35 strains (Table 4, group A), but in stationary phase there was a reasonably good and significant correlation between these transcript levels (Table 4, group A). Thus, lasB transcript levels at 300 min seemed to be much more strongly influenced by lasR than were those of aprA.
TABLE 4.
Correlation analysis of lasR, lasB and aprA transcript levels in early log phase and stationary phase for 35 P. aeruginosa strains and for strains below the median level of lasR at 300 min or above the median level of lasR at 300 min
| Groupa | 120 min
|
300 min
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
lasR
|
aprA
|
lasR
|
aprA
|
|||||||||
|
lasB
|
aprA
|
lasB
|
lasB
|
aprA
|
lasB
|
|||||||
| r2 | P | r2 | P | r2 | P | r2 | P | r2 | P | r2 | P | |
| A | 0.06 | NSb | 0.3 | <0.001 | 0.1 | NSb | 0.5 | <0.001 | 0.2 | <0.01 | 0.5 | <0.001 |
| B | 0.06 | NSb | 0.2 | NSb | 0.02 | NSb | 0.0006 | NSb | 0.002 | NSb | 0.4 | <0.01 |
| C | 0.1 | NSb | 0.6 | <0.001 | 0.3 | <0.05 | 0.4 | <0.01 | 0.2 | <0.05 | 0.6 | <0.001 |
A, 35 P. aeruginosa strains; B, strains below the median level for lasR transcripts at 300 min; C, strains above the median level for lasR at 300 min.
NS, not significant.
Given that the correlation in early stationary phase between lasR and lasB transcript levels was only 0.5 and that the correlation in transcript levels for lasR and aprA was 0.2, we sought to determine if among the 35 strains there were those with a greater or lesser correlation between lasR and lasB or aprA transcript levels. For 17 strains below the median level for lasR transcripts at 300 min (12.3 transcripts per cell), there was no correlation with transcript levels for lasB or aprA (Table 4, group B) at either 120 min or 300 min of growth. For the 18 strains above the median level for lasR transcripts measured at 300 min of growth, the correlation with transcript levels for lasB were comparable to that for the entire population of strains, as was the correlation with transcript levels for aprA (Table 4, group C). This finding indicates that in only about one-half of P. aeruginosa strains that produce high levels of lasR transcripts does this transcript level correlate with that for lasB or aprA in stationary phase, suggesting that about one-half of strains do not have a LasR-dependent, QS-based regulation of lasB and aprA transcription.
Analysis of the correlation of lasB and aprA transcripts in early log and stationary phases.
Since LasR regulation of the transcription of lasB and aprA in stationary phase was limited to about one-half of the strains as determined by correlation analysis, we sought to determine if there were other factors regulating transcription of lasB or aprA by analyzing the correlation between lasB and aprA transcripts. An analysis of the entire group of 35 isolates revealed a reasonable and significant correlation between the transcript levels of lasB and aprA in stationary phase (Table 4, group A). The correlation at 300 min of growth was present both for strains with lasR transcript levels below the median for the entire population in stationary phase (Table 4, group B) and for strains with lasR transcript levels above the population median at stationary phase (Table 4, group C). Also, there was a modest correlation (r2 = 0.3, P < 0.05) between aprA and lasB transcript levels at 120 min in those strains with lasR transcript levels above the median measured at 300 min. This correlation of lasB and aprA transcript levels with each other for all strains regardless of the lasR level indicates that LasR does not always play a major role in regulating transcription of lasB and aprA.
Other analyses indicated no difference in transcript levels or in correlations for lasR, lasB, or aprA among bacteremia, pneumonia, or environmental isolates. These individual analyses gave essentially the same results as did the analysis done on the entire group of 35 strains.
DISCUSSION
QS responses in P. aeruginosa have been principally defined by using transcriptional analysis of the las and rhl regulators and the regulated genes, such as lasA, lasB, aprA, and toxA (for a recent review, see reference 4). However, almost all of these measurements have been carried out with a limited number of mostly laboratory strains, with little data available for clinical isolates from different infections (16, 22, 29). Although animal studies in models of burn wound infection and pneumonia (13, 21, 27, 31) indicate a role for QS-regulated genes in pathogenesis, these studies were also carried out by using single laboratory strains. Here, we analyzed the relationship between transcription of one of the key QS regulators, lasR, and two important virulence factors whose transcription is controlled by LasR, lasB, and aprA. We found overall modest correlations in transcript levels between lasR and the other two genes, but this correlation was found for only one-half of the clinical and environmental isolates studied. For the other half, those with low, constitutive levels of lasR, there was no correlation of transcript levels for this regulator with those of the target genes. Thus, only about one-half of P. aeruginosa strains seem to depend upon lasR transcription for regulating transcription of other virulence factors.
Even among comparable laboratory strains of P. aeruginosa there are differences in QS-regulated transcriptional responses. When mutant strains of PAO1 unable to produce endogenous homoserine lactone (HSL) due to deletion of both the lasI and rhlI genes (29) or rhlR and lasR (22) were given exogenous HSL, both studies reported increased transcription of lasB but with a large difference in the magnitude of the transcriptional response. Shuster et al. (22) reported a 110-fold increase in lasB transcription in response to 2 μM N-3-oxododecanoyl-HSL (3O-C12-HSL) and a 180-fold increase in response to a combination of 2 μM 3O-C12-HSL and 10 μM N-butanoyl-HSL (C4-HSL). Wagner et al. (29) reported only a 39-fold increase in lasB transcription in response to 1 μM 3O-C12-HSL plus 2 μM C4-HSL. Both studies also reported increases in aprA transcript levels, with Wagner et al. (29) reporting a 6.9-fold change in response to 1 μM 3O-C12-HSL plus 2 μM C4-HSL, while Shuster et al. (22) found a 25-fold increase in the response of their mutant strain to 2 μM 3O-C12-HSL and a 27-fold increase in response to a combination of 2 μM 3O-C12-HSL and 10 μM C4-HSL. Note that another important difference between these two PAO1 variants is that the strain used by Wagner et al. (29) has also been found to harbor a mutation in the nfxC gene, leading to overexpression of the MexEF-OprN efflux pump (10), which increases antibiotic resistance, decreases virulence, alters cell-to-cell signaling, and decreases transcription of lasB, the latter result being apparent when comparing the transcriptional responses reported by Wagner et al. (29) and Shuster et al. (22).
Since the differences in transcript levels between two otherwise closely related strains with a difference in a gene outside of the QS system were large, it is clear that even small genetic differences that likely occur among the wide variety of clinical and environmental strains of P. aeruginosa can also impact the QS response in many ways. Therefore, it is not surprising that among about one-half of the strains we studied there was very little impact of the QS response that occurs in early stationary phase on the transcription of lasB and aprA.
A functional link between lasR gene transcription and the consequent transcription of the QS-activated genes has been suggested in lung infections associated with CF (5, 25). This link was made by using an approach based on the detection and correlation of gene transcripts in samples of CF sputa. The difficulty with analysis of transcript levels in sputum is that different strains of bacteria in the sputum may be responding to different concentrations of QS signals and thus transcribing different sets of genes at the same time (5, 25). Also, the rapid degradation of mRNA resulting from the interactions between bacterial mRNA and human products present in infected sputa was problematic for quantifying the transcript levels. Thus, it is difficult to use such results for accurate analysis of QS responses during human infection.
Further support for the idea that a QS-based transcriptional response of virulence factor genes modulated by LasR may be present in only about half of P. aeruginosa clinical and environmental isolates came from the analysis of the correlation of the levels of lasB and aprA transcripts. Here, we found that the levels of these transcripts in early stationary phase correlated significantly with each other regardless of the strain's initial or final level of lasR transcripts. Thus, factors other than the lasR transcript level affected transcription of lasB and aprA, and these other factors appeared to have a more common effect among the isolates than did the lasR transcript level. While we cannot fully exclude the possibility that some of these factors may be related to the levels or functions of LasR that are independent of the lasR transcript level, these findings do point out that for about 50% of P. aeruginosa isolates conclusions regarding the role of LasR-dependent QS responses cannot be inferred from lasR transcript levels.
Overall, an extensive analysis of genetic sequences and transcriptional responses of a large set of P. aeruginosa clinical and environmental isolates indicates that the lasR-dependent QS transcriptional response of the lasB and aprA genes may be present in about one-half of the strains. There was no indication of a large amount of allelic variation among the lasR genes, with the vast majority of strains having a nucleotide sequence very close or identical to that of PAO1. Differences were noted principally between the strains with low and high constitutive levels of lasR transcripts in early log phase. Strains with levels above the median for 35 isolates tended to produce more lasR transcripts in stationary phase, with a strong correlation between the initial and final levels. These strains also had the better correlation of lasR transcripts with lasB and aprA, although for the latter, the correlation, while significant, was weak. For strains with lasR transcript levels below the median for the population, there was little increase in lasR transcripts in stationary phase and no correlation with the transcription of lasB or aprA genes. Given that many of these strains were, nonetheless, clinical isolates, it appears that among non-CF isolates of P. aeruginosa the lasR-dependent transcriptional activation of lasB and aprA is present in only one-half of strains and thus unlikely to contribute significantly to virulence in these strains. Finally, as we did not analyze protein levels, we cannot exclude the possibility that there was, nonetheless, production of LasB and AprA among the strains with low transcript levels that was comparable to that of strains with high transcript levels. But as the molecular aspects of the LasR-QS system have been defined by microarray analysis (22, 29) and correlative analysis of transcript levels, particularly in CF (5, 25), this study shows that correlations among the lasR, lasB, and aprA transcripts may be found in only about 50% of clinical strains of P. aeruginosa.
Acknowledgments
We thank A. Ladzunski and M. Foglino for providing PAO1 and PAOR strains and for helpful discussions. We thank P. Siguier from C.N.R.S. (UPR9007), Toulouse, France, for technical assistance with analysis of IS elements.
This work was supported in part by a grant from the Ministère de l'Education Nationale de la Recherche et de la Technologie: réseau infections nosocomiales à Pseudomonas aeruginosa and by a grant from Université Paris VII, Faculté X. Bichat: BQR.
REFERENCES
- 1.Bonten, M. J., D. C. Bergmans, A. W. Ambergen, P. W. de Leeuw, S. van der Geest, E. E. Stobberingh, and C. A. Gaillard. 1996. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am. J. Respir. Crit. Care Med. 154:1339-1346. [DOI] [PubMed] [Google Scholar]
- 2.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapon-Herve, V., M. Akrim, A. Latifi, P. Williams, A. Lazdunski, and M. Bally. 1997. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol. Microbiol. 24:1169-1178. [DOI] [PubMed] [Google Scholar]
- 4.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson, D. L., R. Endersby, A. Kirkham, K. Stuber, D. D. Vollman, H. R. Rabin, I. Mitchell, and D. G. Storey. 2002. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 70:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gambello, M. J., S. Kaye, and B. H. Iglewski. 1993. LasR of Pseudomonas aeruginosa is a transcriptional activator of the alkaline protease gene (apr) and an enhancer of exotoxin A expression. Infect. Immun. 61:1180-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrouste-Orgeas, M., S. Chevret, G. Arlet, O. Marie, M. Rouveau, N. Popoff, and B. Schlemmer. 1997. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients. A prospective study based on genomic DNA analysis. Am. J. Respir. Crit. Care Med. 156:1647-1655. [DOI] [PubMed] [Google Scholar]
- 9.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler, T., C. van Delden, L. K. Curty, M. M. Hamzehpour, and J. C. Pechere. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 183:5213-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 12.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 13.Lesprit, P., F. Faurisson, O. Join-Lambert, F. Roudot-Thoraval, M. Foglino, C. Vissuzaine, and C. Carbon. 2003. Role of the quorum-sensing system in experimental pneumonia due to Pseudomonas aeruginosa in rats. Am. J. Respir. Crit. Care Med. 167:1478-1482. [DOI] [PubMed] [Google Scholar]
- 14.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 15.Ochsner, U. A., A. K. Koch, A. Fiechter, and J. Reiser. 1994. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176:2044-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 17.Pearson, J. P., K. M. Gray, L. Passador, K. D. Tucker, A. Eberhard, B. H. Iglewski, and E. P. Greenberg. 1994. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA 91:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruimy, R., E. Genauzeau, C. Barnabe, A. Beaulieu, M. Tibayrenc, and A. Andremont. 2001. Genetic diversity of Pseudomonas aeruginosa strains isolated from ventilated patients with nosocomial pneumonia, cancer patients with bacteremia, and environmental water. Infect. Immun. 69:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rumbaugh, K. P., J. A. Griswold, B. H. Iglewski, and A. N. Hamood. 1999. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 67:5854-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seed, P. C., L. Passador, and B. H. Iglewski. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 25.Storey, D. G., E. E. Ujack, H. R. Rabin, and I. Mitchell. 1998. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 66:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tancrede, C. H., and A. O. Andremont. 1985. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J. Infect. Dis. 152:99-103. [DOI] [PubMed] [Google Scholar]
- 27.Tang, H. B., E. DiMango, R. Bryan, M. Gambello, B. H. Iglewski, J. B. Goldberg, and A. Prince. 1996. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect. Immun. 64:37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toder, D. S., M. J. Gambello, and B. H. Iglewski. 1991. Pseudomonas aeruginosa LasA: a second elastase under the transcriptional control of lasR. Mol. Microbiol. 5:2003-2010. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winson, M. K., M. Camara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. Salmond, B. W. Bycroft, A. Lazdunski, G. S. A. B. Stewart, and P. Williams. 1995. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu, H., Z. Song, M. Givskov, G. Doring, D. Worlitzsch, K. Mathee, J. Rygaard, and N. Hoiby. 2001. Pseudomonas aeruginosa mutations in lasI and rhlI quorum sensing systems result in milder chronic lung infection. Microbiology 147:1105-1113. [DOI] [PubMed] [Google Scholar]