Abstract
To determine the function of the wabG gene in the biosynthesis of the core lipopolysaccharide (LPS) of Klebsiella pneumoniae, we constructed wabG nonpolar mutants. Data obtained from the comparative chemical and structural analysis of LPS samples obtained from the wild type, the mutant strain, and the complemented mutant demonstrated that the wabG gene is involved in attachment to α-l-glycero-d-manno-heptopyranose II (l,d-HeppII) at the O-3 position of an α-d-galactopyranosyluronic acid (α-d-GalAp) residue. K. pneumoniae nonpolar wabG mutants were devoid of the cell-attached capsular polysaccharide but were still able to produce capsular polysaccharide. Similar results were obtained with K. pneumoniae nonpolar waaC and waaF mutants, which produce shorter LPS core molecules than do wabG mutants. Other outer core K. pneumoniae nonpolar mutants in the waa gene cluster were encapsulated. K. pneumoniae waaC, waaF, and wabG mutants were avirulent when tested in different animal models. Furthermore, these mutants were more sensitive to some hydrophobic compounds than the wild-type strains. All these characteristics were rescued by reintroduction of the waaC, waaF, and wabG genes from K. pneumoniae.
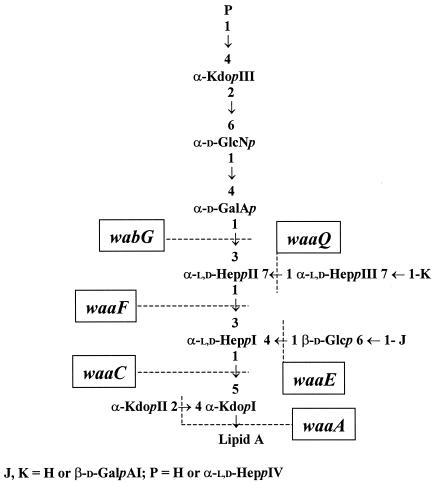
In gram-negative bacteria the lipopolysaccharide (LPS) is one of the major structural and immunodominant molecules of the outer membrane. LPS consists of three domains: lipid A, core oligosaccharide, and O-specific antigen or O side chain. In smooth LPS, the core region is conceptually divided into two regions: a lipid A proximal inner core and an outer core that provides the attachment site for the O antigen (21). Comparison of the known core LPS structures from Enterobacteriaceae organisms reveals that the first outer core residue might be either glucose (Glc) or a galacturonic acid (GalA) residue. In the four known Escherichia coli core types and in Salmonella enterica, a substitution of the l-glycero-d-manno-heptopyranose II (l,d-HeppII) at the O-3 position for a Glcp residue was found (12). For Klebsiella pneumoniae, Proteus mirabilis, and Yersinia enterocolitica, a substitution of the l,d-HeppII at the O-3 position for an α-d-galacturonic acid residue (α-d-GalpA) residue has been described (20, 29, 30). On the other hand, in most of the Enterobacteriaceae studied, the core LPS contains inner core phosphoryl modifications (21), but K. pneumoniae core LPS is devoid of such modifications (29) (Fig. 1).
FIG. 1.
Conserved region in the core LPS structure of K. pneumoniae (29) and genes involved in inner core biosynthesis (24, 14). Kdop, 3-deoxy-d-manno-octulopyranosonic acid; Glcp, d-glucopyranose; GlcNp, glucosamine; GalAp, galacturonic acid. Depending on the K. pneumoniae strain, residues J and K could be H or GalA, and residue P could be H or Hep (29).
Important roles in outer membrane permeability and in pathogenesis have been shown for the outer core and for the negative charges contributed by phosphoryl inner core modification in E. coli and/or S. enterica serovar Typhimurium (32, 33, 34). In view of the peculiarities of the K. pneumoniae core LPS, we sought in this work to determine the importance of the outer core LPS in K. pneumoniae outer membrane permeability and in pathogenesis. The previous knowledge of the K. pneumoniae waa gene cluster (the nomenclature proposed by Reeves et al. [23] for proteins and genes involved in core LPS biosynthesis is used in this work) and the elucidation of the genes involved in its inner core biosynthesis (14, 24) (Fig. 1) facilitated the identification of the gene involved in the transfer of the first outer core residue to construct and characterize K. pneumoniae mutants devoid of the outer core LPS.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
K. pneumoniae strains 889 (serovar O8:K69) (11), 52145 (O1:K2) (17), NC20 (waaL mutant) (24), and Serratia marcescens N28b (10) were used in this study. Bacterial strains were grown in Luria-Bertani (LB)broth and LB agar (16). LB media were supplemented with kanamycin (50 μg ml−1), ampicillin (100 μg ml−1), chloramphenicol (20 μg ml−1), and tetracycline (25 μg ml−1) when needed. The plasmids used in this study and their characteristics are shown in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristica | Source or reference |
|---|---|---|
| pKO3 | CmrsacB temperature-sensitive replication | 15 |
| pKO3ΔwaaC | Contains the K. pneumoniae engineered waaC deletion | This work |
| pKO3ΔwaaF | Contains the K. pneumoniae engineered waaF deletion | This work |
| pKO3ΔwaaL | Contains the K. pneumoniae engineered waaL deletion | This work |
| pKO3ΔwabG | Contains the K. pneumoniae engineered waabG deletion | This work |
| pGEMT | Apr plasmid vector | Promega |
| pGEMT-WaaC | waaC gene from K. pneumoniae in pGEMT | This work |
| pGEMT-WaaF | waaE gene from K. pneumoniae in pGEMT | This work |
| pGEMT-WaaL | waaL gene from K. pneumoniae in pGEMT | This work |
| pGEMT-WabG | wabG gene from K. pneumoniae in pGEMT | This work |
| pGEMT-Orf9Sm | orf9 gene from S. marcescens in pGEMT | This work |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance.
General DNA methods.
General DNA manipulations were done essentially as described previously (26). DNA restriction endonucleases, T4 DNA ligase, E. coli DNA polymerase (Klenow fragment), and alkaline phosphatase were used as recommended by the respective manufacturers.
LPS isolation and electrophoresis.
Cultures for analysis of LPS were grown in tryptic soy broth at 37°C. LPS was purified by the Pneumocystis carinii pneumonia method (7), resulting in a yield of 2.3%. For screening purposes, LPS was obtained after proteinase K digestion of whole cells (13). LPS samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or SDS-tricine-PAGE and visualized by silver staining as previously described (19, 28).
Isolation of oligosaccharides.
LPS (20 mg) was hydrolyzed with 1% AcOH (100°C for 1 h). The resulting precipitate (8 mg) was removed by centrifugation, and the supernatant (10 mg) was analyzed by mass spectrometry. Another sample of LPS (40 mg) was deacylated and purified as described previously (3), yielding 6 mg of alditol oligosaccharide mixture.
LPS chemical analysis.
For chemical analysis, either purified LPS or core LPS oligosaccharide samples were hydrolyzed with 1 N trifluoroacetic acid for 4 h at 100°C. Alditol acetates and methyl glycoside acetates were analyzed on an Agilent Technologies model 5973N mass spectrometry (MS) instrument equipped with a model 6850A gas chromatography and an RTX-5 capillary column (Restek; 30 m × 0.25 mm inside diameter, flow rate 1 ml min−1, He used as carrier gas). Acetylated methyl glycoside analysis was performed with the following temperature program: 150°C for 5 min, 150 to 250°C at 3°C min−1, and 250°C for 10 min. Acetylated methyl ester lipid analysis was performed as follows: 150°C for 3 min, 150 to 280°C at 10°C min−1, and 280°C for 15 min. The alditol acetate mixture was analyzed with the following temperature program: 150°C for 5 min and 150 to 300°C at 3°C min−1. For partially methylated alditol acetates, the temperature program was 90°C for 1 min, 90 to 140°C at 25°C min−1, 140 to 200°C at 5°C min−1, 200 to 280°C at 10°C min−1, and 280°C for 10 min.
Glycosyl and lipid analysis.
A sample (1 mg) of LPS was dried over P2O5 overnight and was then treated with 1 M HCl-CH3OH (1 ml) at 80°C for 20 h to analyze both glycosyl and fatty acid composition. The crude reaction was extracted twice with hexane, and the two extracts were pooled, dried under a stream of air, and treated with acetic anhydride (100 μl) at 100°C for 15 min. The methanol layer was neutralized with Ag2CO3, dried, and acetylated. Both samples were subjected to gas chromatography-MS. Another sample of LPS (1 mg) was hydrolyzed with 4 M trifluoroacetic acid for 1 h at 100°C, reduced with deuterated sodium tetrahydridoborate (NaBD4), acetylated, and analyzed by gas chromatography-MS.
MS studies.
Electrospray MS was performed on a Micromass ZQ instrument (Waters). The sample (100 pmol) was deionized on Dowex H+ resin (Fluka) and dissolved in 2% triethylamine in 50% acetonitrile and injected into the ion source at a flow rate of 5 μl min−1. The spectrum was acquired in negative mode. Positive-ion reflectron matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectra were acquired on a Voyager DE-PROinstrument (Applied Biosystems) equipped with a delayed-extraction ion source. Ion acceleration voltage was 20 kV, grid voltage was 14 kV, mirror/voltage ratio was 1/12, and the delay time was 100 ns. Samples were irradiated at a frequency of 5 Hz by 337-nm photons from a pulsed-nitrogen laser. Postsource decay (PSD) was performed using an acceleration voltage of 20 kV (27). The reflectron voltage was decreased in 10 successive 25% steps. Mass calibration was obtained with a malto-oligosaccharide mixture from corn syrup (Sigma). A solution of 2,5-dihydroxybenzoic acid in 20% CH3CN in water at a concentration of 25 mg/ml was used as the MALDI matrix. One microliter of matrix solution and 1 μl of the sample were premixed and then deposited on the target. The droplet was allowed to dry at ambient temperature. Spectra were calibrated and processed under computer control by using the Applied Biosystems Data Explorer software.
Methylation analysis.
The alditol oligosaccharide mixture was N-acetylated by dissolving a sample (2 mg) in dry methanol and treating it with 50 μl of acetic anhydride for 16 h. After evaporation of the solvents, the sample was methylated as previously reported (2). Linkage analysis was performed as follows: the methylated sample was carboxymethyl reduced with lithium triethylborohydride (Aldrich), mildly hydrolyzed to cleave ketosidic linkage, reduced by means of NaBD4, and was then totally hydrolyzed, reduced with NaBD4, and finally acetylated as described previously (6).
K. pneumoniae waaC, waaF, waaL, and wabG mutant construction.
To obtain K. pneumoniae mutant strains, a method was used to create chromosomal in-frame waa deletions (15). Primers for mutant construction were designed from the known K. pneumoniae waa gene cluster sequence. Primer pairs Fa (5′-CGCGGATCCAAATCCCGTTCCTGTACGCC-3′) and Fb (5′-CCCATCCACTAAACT TAAACACATCATCATGTCGCCCACC-3′) and Fc (5′-TGTTTAAGTTTAGTG GATGGGTTAGCGGAAAAACCGAGCAC-3′) and Fd (5′-CGCGGATCCGCAGAAACACCAGATAGGGC-3′) were used in two sets of asymmetric PCRs to amplify DNA fragments of 697 (AB) and 618 (CD) bp, respectively. DNA fragment Fa-Fb encompasses nucleotide 470, inside gmhD, to nucleotide 1137, corresponding to the first base of codon 16 of waaF. DNA fragment Fc-Fd encompasses nucleotide 2116, corresponding to the first base of the 393rd codon of waaF, to nucleotide 2704, which lies within the waaC gene. DNA fragments Fa-Fb and Fc-Fd were annealed at their overlapping region (the underlined letters in primers Fb and Fc) and amplified by PCR as a single fragment, using primers Fa and Fd. The fusion product was purified, digested with BamHI (the BamHI site is shown as the double-underlined letters in primers Fa and Fd), ligated into BamHI-digested and phosphatase-treated pKO3 vector (15), electroporated into E. coli DH5α, and plated on chloramphenicol-kanamycin plates at 30°C to obtain plasmid pKO3ΔwaaF. Primer pairs Ca (5′-CGCGGATCCGCGCTTTTAACC TGTCCTAC-3′) and Cb (5′-CCCATCCACTAAACTTAAACAAACGATCAAT ACCCGCATCC-3′) and Cc (5′-TGTTTAAGTTTAGTGGATGGGCA CACTCTAATATCTCCGACC-3′) and Cd (5′-CGCGGATCCGCTCCATGACCCTTTTTGAC-3′) were used to obtain plasmid pKO3ΔwaaC, containing an internally deleted waaC gene (the first 6 codons, a 7-codon tag, and the last 26 codons). Primer pairs La (5′-CGCGCGGCCGCGGATATTGCAG GACAAAGGGC-3′) and Lb (5′-CCCATCCACTAAACTTAAACAAAGCAAACC GGCAAGGTTAAG-3′) and Lc (5′-TGTTTAAGTTTAGTGGATGGGGAT GAGAACCATGAGTGACAAG-3′) and Ld (5′-CGCGCGGCCGCATATGCCAGTGG GAACGAC-3′) were used to obtain plasmid pKO3ΔwaaL, containing an internally deleted waaL gene (the first 22 codons, a 7-codon tag, and the last 5 codons of waaL). Primer pairs Ga (5′-CGCGGATCCCCACCCAACAGCACAACC-3′) and Gb (5′-CCCATCCACTAAACT TAAACAGACAAACCGTTCTGCGCC-3′) and Gc (5′-TGTTTAAGTTTAGTGGATGGGAGCGAGCGACTCTCAACC-3′) and Gd (5′-CGCGGATCCGCGACCGACGTGAATCAG-3′) were used to obtain plasmid pKO3ΔwabG, containing an internally deleted wabG gene (the first 23 codons, a 7-codon tag, and the last 17 codons of wabG). Plasmids pKO3ΔwaaC, pKO3ΔwaaF, pKO3ΔwaaL, and pKO3ΔwabGwere used to construct nonpolar mutations in the waaC, waaF, waaL, and wabG genes, respectively.
Plasmid construction.
To complement the constructed mutants, the waaC, waaF, waaL, and wabGKp genes from K. pneumoniae and the S. marcescens wabGSm homologue were PCR amplified and ligated to the vector pGEMT as follows: pGEMT-WaaC (5′-GTTTAAATCGGCATTAGTCC-3′ and 5′-AAGCAAACCGGCAAGGTTAAG-3′), pGEMTWaaF (5′-TCAGCCCAGCACCTTATTC-3′ and 5′-TTTTACCGTATCCGCCAATC-3′), pGEMTWaaL (5′-TACAGGGAACGTCAGAAGC-3′ and 5′-ATGCCTTGCATCACATTAC-3′), pGEMT-WabGKp (5′-CAATGGCAGCTCATTCAGAC-3′ and 5′-TGAAAGCCTTTGAACCACAC-3′), and pGEMT-Orf9Sm (5′-TCAAATGCTGGAGCGAAGAG-3′ and 5′-CCTGATAATCAATGCCTGAC-3′).
Urinary tract infections (UTIs) in rats.
The bacterial strains used to establish infection were grown overnight in LB agar supplemented with antibiotics when needed and gently suspended in phosphate-buffered saline to the appropriate concentration. In each experiment, 12 female Wistar rats (weight, 200 to 250 g) of strain CFHB (Interfauna UK, Hungtinton, United Kingdom) were used. Ten animals were infected and two were used as controls. The infections were established and quantified as previously described (1).
Murine pneumonia model.
The experiments were performed as previously described (4). Briefly, ICR-CDI mice (Harlan Ibérica, S.L.) were anesthetized and intubated intratracheally with a blunt-end needle. Approximately 107 CFU of exponential K. pneumoniae cells was suspended in 50 μl of phosphate-buffered saline and inoculated through the blunt-end needle. The mice were observed daily, and bacteremia was assessed at days 2, 4, and 6 by culturing blood obtained from the tail vein (approximately 20 μl) on LB agar plates. Lung and spleen tissues from surviving or dead animals were aseptically removed, homogenized, and plated for growth of quantitative bacterial cultures. Each experiment was performed with nine animals.
LD50.
Albino Swiss female mice (5 to 7 weeks old; Harlan Ibérica, S.L.) were injected intraperitoneally with 0.2 ml of the test samples. Mortality was recorded up to 7 days postinjection, and all deaths occurred within 1 to 5 days. The 50% lethal dose (LD50) was calculated as previously described (22).
RESULTS
Construction of K. pneumoniae wabG mutants.
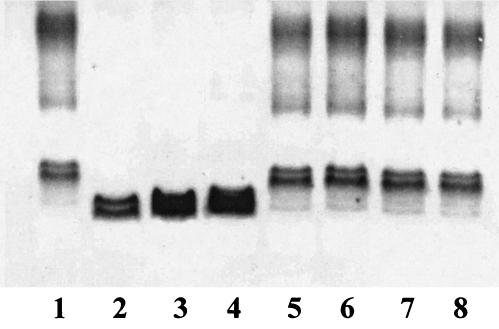
We have previously reported the nucleotide sequence of the K. pneumoniae waa gene cluster and have identified the functions of the genes involved in the biosynthesis of its inner core LPS (Kdo, l,d-HeppI, l,d-HeppII, l,d-HeppIII, and branched d-Glcp transferases) (14, 24). Comparison of the known outer core LPS structures among Enterobacteriaceae revealed that the first outer core residue is either d-Glc or d-GalA, linked to the l,d-HeppII residue by an α1,3 bond. Thus, it could be expected that the enzymes involved in the transfer of the first outer core residue would share some similarity. The WaaG protein has been identified as the glucosyltransferase involved in the transfer of the first outer core LPS residue in E. coli and S. enterica serovar Typhimurium. Only the K. pneumoniae orf8-encoded protein showed significant albeit low levels of identity (26%) and similarity (44%) to the WaaG protein from E. coli; therefore, this K. pneumoniae gene was named wabG. To determine the wabG function, nonpolar mutants were constructed in K. pneumoniae 889 and 52145. K. pneumoniae 889 (08:K69) (11) was used because its core LPS structure has been recently updated using the nonencapsulated mutant NRC6121 (Fig. 1) (29). Strain 52145 was used because it shows high virulence in different experimental animal models (17). To construct the K. pneumoniae wabG nonpolar mutants, an in-frame tagged deletion approach was used. Plasmid pKO3ΔwabG, containing the engineered deletion, was used to introduce the wabG deletion into K. pneumoniae 889 and 52145 by double recombination as previously described (15, 24). Candidate mutants were screened by PCR, and two of them, strains 889ΔwabG and 52145ΔwabG, were proved to contain the desired mutation by DNA nucleotide sequence determination. LPS from strains 889 and 52145 (wild type) and 889ΔwabG and 52145ΔwabG was extracted and analyzed by SDS-tricine-PAGE. The core LPS from the mutant strains migrated faster than that from the wild-type strains, suggesting that the wabG mutants contain a truncated-core LPS (Fig. 2, lane 4).
FIG. 2.
SDS-tricine-PAGE analysis of LPS samples from K. pneumoniae 52145 (lane 1), 52145ΔwaaC (lane 2), 52145ΔwaaF (lane 3), 52145ΔwabG (lane 4), 52145ΔwaaC plus pGEMT-WaaC (lane 5), 52145ΔwaaF plus pGEMT-WaaF (lane 6), 52145ΔwabG plus pGEMT-WabGKp (lane 7), and 52145ΔwabG plus pGEMT-ORF9Sm (lane 8).
To test whether the WabG and the S. enterica serovar Typhimurium WaaG proteins perform the same function, two complementation assays were performed. Plasmids pGEMT-WabG and pGEMT-WaaG were unable to complement S. enterica serovar Typhimurium SL3768 waaG (25) and K. pneumoniae wabG mutants, respectively, as determined by SDS-tricine-PAGE of LPS.
K. pneumoniae wabG LPS analysis.
To determine the core LPS changes produced by the mutation of wabG, LPS was obtained from strains 889ΔwabG, 52145ΔwabG, 889, and 52145. Comparative monosaccharide composition analysis of these LPS samples revealed major changes in LPS composition, with a complete loss of GalA and about a 30% reduction in glucosamine (GlcN) in the mutant wabG strains. The putative GalA residues (J and K in Fig. 1) are not present in all the K. pneumoniae strains studied (29). Our data suggest that the strains used in this study lack both GalA residues (J and K in Fig. 1).
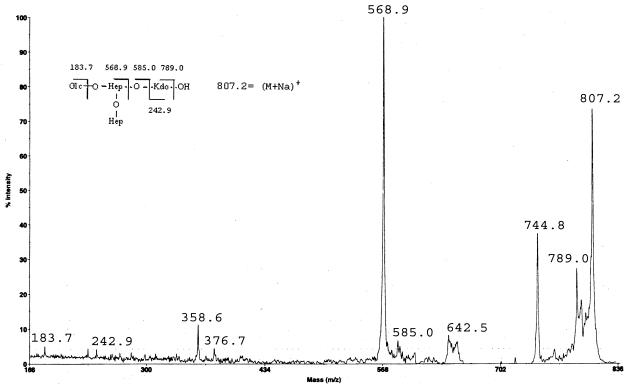
To elucidate the chemical structure of the LPS core region of the wabG mutant (889ΔwabG) in more detail, LPS was hydrolyzed with 1% acetic acid, which cleaves the acid-labile ketosidic linkages between KdoI and lipid A, KdoI and KdoII, and KdoIII-GlcN as reported (29). The negative ions' ElectroSpray ionization mass spectra of core oligosaccharide (data not shown) showed several signals. One of these signals indicated the presence of a pseudomolecular ion (M-H)− at m/z 783.37, a value which was in agreement with the calculated average molecular weight (783.67) of the expected molecular structure, with one hexose, two heptose, and one Kdo units. The presence of a −18 signal at m/z 765.38 is attributable to the anhydrous form of the reducing Kdo end, and it is well documented for LPS samples that are hydrolyzed in presence of acetic acid (18).
In order to determine the oligosaccharide sequence, we analyzed the sample with the MALDI-PSD technique, which enabled us to obtain a total fragment spectrum in a single experiment. The positive ions' PSD spectra of the acetic acid product (Fig. 3) contains many fragment ions, most of them attributable to B-type ions (5). Either molecular ions or fragment ions contain sodium, as has been reported for MALDI spectra. It is also known that interpretation of a PSD spectrum of unknown sample is potentially difficult, but in this case we were able to assign almost all of the signals. Actually, other than the signals reported in the fragmentation scheme, the fragment ion at m/z 744.8 can be attributed to the decarboxylated anhydrous core structure (M-18-44) (9). Particularly important to define the ramified nature of the core oligosaccharide are the two signals at m/z 376.7 and 358.6. These signals are attributable to internal fragmentation (8), as they might arise from a loss of the terminal heptose residue from the signal at 568.9, leaving a hydroxyl group (m/z 376.7) or a double bond (m/z 358.6). In agreement with the proposed structure was the 1H nuclear magnetic resonance spectrum of this sample, which mainly showed three anomeric signals at δ 5.31 and 5.09 (bs) and at δ 4.54 (doublet, 3JH,H 7.8 Hz) (data not shown), according to the presence of two heptose units and one Glc unit, respectively.
FIG. 3.
PSD spectrum of m/z 807.2 of K. pneumoniae 889ΔwabG core oligosaccharide after acidic release of Lipid A, in the positive-ion mode. Insert shows the proposed structure and fragmentation pattern.
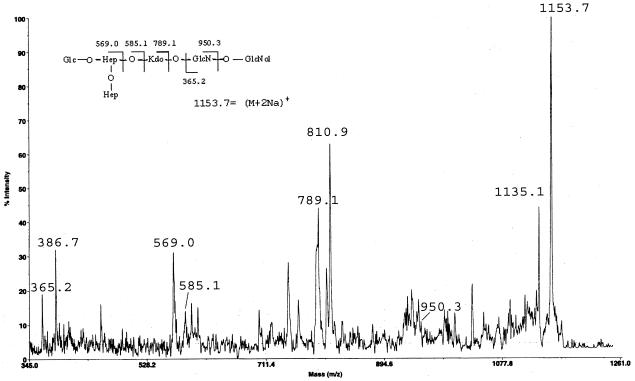
Similar results were obtained from the complete O,N-deacylated LPS from the K. pneumoniae wabG mutant. This sample was obtained after hydrazinolysis, HF treatment, NaBH4 reduction,and KOH hydrolysis as already reported (3). The reflectron-positive ions' MALDI-TOF spectra of this fraction showed the presence of three clusters of signals: the first at m/z 1,153.87 (M1 plus 2Na)+ (calculated to be 1,154.95), corresponding to one hexose, two heptose, one Kdo, one hexosamine and one hexosaminitol; the second at m/z 1,374.16 (M1 plus 2Na)+ (calculated to be 1,375.08), corresponding to the same composition plus one Kdo unit; and the last at m/z 1,296.2 (M2)+ (calculated to be 1,291.1), corresponding to one hexose, three heptose, one Kdo, one hexosamine and one hexosaminitol. Other than the two signals already found in the acetic acid hydrolysis product (m/z 569.0 and 789.1) in the PSD spectrum of the signal at m/z 1153.7 (Fig. 4), there are signals at m/z 365.2 and 386.7 (365.2 plus Na) which clearly indicate the presence of the two GlcN residues of the lipid A. Moreover, the intense signal at m/z 810.9 can be attributed to the C fragment (5), which contains one hexose residue, two heptose residues, and one Kdo residue (789.1) (26). These results strongly suggest the presence of the oligosaccharide reported in the fragmentation scheme (Fig. 4).
FIG. 4.
PSD spectrum of m/z 1153.7 of O,N-deacylatedand dephosphorylated LPS from K. pneumoniae 889ΔwabG, in the positive-ion mode. Insert shows the proposed structure and fragmentation pattern.
In order to confirm the proposed structure, a methylation analysis on the N-acetylated oligosaccharide alditol mixture was performed. The results obtained confirm the presence of three terminal residues (Glcp, Hepp, and Kdop). The presence of a 3,4-linked Hep confirmed the branching point in the oligosaccharide structure. The detection of terminal Kdo and 5,4-linked Kdo units can be attributed to the oligosaccharide alditol having one additional Kdo (KdoII) unit linked at the O4 position of the KdoI residue. The above results strongly suggest that the WabG protein is involved in attachment of the first outer core residue (GalAp) to the l,d-HeppII by an α1,3 linkage.
Phenotypic characterization of the mutant strains.
The chemical characterization of the wabG mutants revealed that its core LPS is devoid of the outer core region. Furthermore, the nonmucoid colony morphology of the wabG mutants suggests that they are unencapsulated. Since it is well known that the K. pneumoniae capsule plays an important role in pathogenesis (31), we decided to determine if the K. pneumoniae capsule is linked to the core LPS. The approach was based on the analysis of several waa nonpolar deletion mutants, i.e., 52145ΔwaaC, 52145ΔwaaF, 52145ΔwabG, and 52145ΔwaaL, derived from wild-type strain 52145 (O1:K2). These strains and a previously constructed waaL mutant (NC20) derived from wild-type strain C3 (O1:K66) (24) were analyzed for the presence of capsule by sensitivity to capsule-specific bacteriophages, by electron microscopy (EM) studies, and by enzyme immunoassay (EIA) with capsular-specific serum. The NC20 and 52145ΔwaaL strains contained K66 and K2 capsule, respectively, as can be deduced from their sensitivity to capsule-specific bacteriophages, EM studies, and reactivity against K66- and K2-specific antibodies in EIA. In contrast, no K2 capsule was detected in the 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwabG whole-cell mutants. Culture supernatants of strains 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwabG reacted by EIA with K2-specific antibodies. Neither whole cells nor culture supernatants from a 52145 K2− mutant (with a mini-Tn5 inserted in the known K2 capsular biosynthetic cluster) were unable to react by EIA with K2-specific serum. These results clearly show that in K. pneumoniae, the outer core LPS is somehow involved in K2 capsule's, and probably other capsular types', attachment to the cell surface.
The truncation of the core LPS in other Enterobacteriaceae results in profound changes in the bacterial cell behavior and permeability (reviewed in reference 21). Since these assays have been used with Enterobacteriaceae strains containing phosphoryl modifications in their inner core LPS (32, 33, 34), we decided to determine the behavior of LPS core-truncated mutants from K. pneumoniae, an Enterobacteriaceae organism that is naturally devoid of such inner core phosphoryl modifications. The sensitivity to hydrophobic compounds of the 52145 mutants was compared to that of the wild-type strain. For the 52145ΔwabG mutant, MICs of SDS, deoxycholate, and polymyxin B were found to be 0.5 mg ml−1 (a greater than 20-fold reduction), 10 mg ml−1 (a 50% reduction), and 2 μg ml−1 (a 60% reduction), respectively. The MICs obtained for mutants devoid of the inner core Hep region (52145ΔwaaC) or containing only the first l,d-HeppI (52145ΔwaaF) residue were essentially similar to those for the mutants lacking the outer core region (52145ΔwabG). For the 52145ΔwabG mutant, MICs of antibiotics (nalidixic acid, erythromycin, novobiocin, and rifampin) showed about 50% reduction in comparison to those for the wild-type strain, while the MICs for the 52145ΔwaaC and 52145ΔwaaF mutants showed about 80 to 90% reduction when these same antibiotics were used. Similar MICs were obtained for strains 889ΔwaaC, 889ΔwaF, and 889ΔwabG.
Complementation studies.
The wild-type pattern of electrophoretic banding (Fig. 2, lanes 1, 5, 6, and 7), the presence of K2 capsule (as determined by sensitivity to specific capsule bacteriophage, EM studies, and reactivity against K2-specific antibodies in EIA studies), SDS, deoxycholate, and polymyxin B sensitivity were demonstrated by the mutant strains 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwabG upon complementation with plasmids pGEMT-WaaC, pGEMT-WaaF, and pGEMT-WabG, respectively. The phenotypic characteristics of the waaC, waaF, and wabG mutants were not changed when the plasmid vector alone (pGEMT) was introduced by transformation.
The S. marcescens waa gene cluster has been sequenced (GenBank accession number U52844). An open reading frame (ORF), orf9, has been identified as encoding a putative protein highly similar (69% identity and 81% similarity) to the K. pneumoniae WabG protein, suggesting that the orf9Sm could be a wabG homologue. To test this possibility, the mutant 52145ΔwabG was transformed with plasmid pGEMT-Orf9Sm. The transformed strain showed full-length LPS (Fig. 2, lane 8), produced K2 capsule, and exhibited wild-type levels of sensitivity to hydrophobic compounds. This result strongly suggests that the first residue in the S. marcescens N28b core LPS would be an α-d-GalpA residue linked to the l,d-HeppII by an α1,3 linkage.
Colonization and virulence studies.
As a colonization model, we used experimental UTI in rats. As shown in Table 2, 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwabG mutants are unable to induce experimental UTIs in rats (unable to colonize the rat UT). However, the 52145ΔwaaL mutant showed a reduced ability to colonize the rat UT in comparison with that of the wild-type strain, but the mutant was still able to perform some colonization. The plasmid vector harboring the corresponding wild-type gene(s) introduced by transformation restored (to the level of the wild-type strain) the ability of all the mutants to induce experimental UTIs in rats. The plasmid vector alone was unable to restore this ability when introduced in the mutant strains.
TABLE 2.
Experimental UTI in rats by different K. pneumoniae strains
| Strain (infection dose CFU/rat) | % Of infected rats and viable countsa
|
||
|---|---|---|---|
| Sample | % Infectedb | Mean ± SDc | |
| K. pneumoniae 51245 (O1:K2, wild type) (0.8 × 109) | Kidney | 100 | 6.9 ± 0.6 |
| Bladder | 100 | 6.7 ± 0.5 | |
| Urine | 100 | 8.4 ± 0.4 (6) | |
| 52145ΔwaaC (1.1 × 109) | Kidney | 10 | 4.3 |
| Bladder | 10 | 3.8 | |
| Urine | 0 | 0 | |
| 52145ΔwaaC + pGEMT (1.0 × 109) | Kidney | 10 | 4.8 |
| Bladder | 0 | 0 | |
| Urine | 10 | 4.6 | |
| 52145ΔwaaC + pGEMT-WaaC (0.9 × 109) | Kidney | 100 | 6.4 ± 0.6 |
| Bladder | 100 | 6.1 ± 0.4 | |
| Urine | 100 | 8.1 ± 0.4 (6) | |
| 52145ΔwaaF (1.1 × 109) | Kidney | 10 | 4.8 |
| Bladder | 10 | 3.5 | |
| Urine | 0 | 0 | |
| 52145ΔwaaF + pGEMT (0.9 × 109) | Kidney | 10 | 4.3 |
| Bladder | 0 | 0 | |
| Urine | 0 | 0 | |
| 52145ΔwaaF + pGEMT-WaaF (1.2 × 109) | Kidney | 100 | 6.5 ± 0.7 |
| Bladder | 100 | 6.4 ± 0.6 | |
| Urine | 100 | 8.2 ± 0.3 (6) | |
| 52145ΔwabG (1.2 × 109) | Kidney | 10 | 4.2 |
| Bladder | 0 | 0 | |
| Urine | 0 | 0 | |
| 52145ΔwabG + pGEMT (1.2 × 109) | Kidney | 10 | 4.6 |
| Bladder | 10 | 3.7 | |
| Urine | 0 | 0 | |
| 52145ΔwabG + pGEMT-WabG (1.1 × 109) | Kidney | 100 | 6.2 ± 0.7 |
| Bladder | 100 | 6.0 ± 0.3 | |
| Urine | 100 | 8.3 ± 0.8 (6) | |
| 52145ΔwaaL (1.2 × 109) | Kidney | 40 | 4.7 ± 0.8 |
| Bladder | 40 | 4.3 ± 0.9 | |
| Urine | 30 | 7.2 ± 0.6 | |
| 52145ΔwaaL + pGEMT (1.3 × 109) | Kidney | 40 | 4.9 ± 0.5 |
| Bladder | 30 | 4.0 ± 0.7 | |
| Urine | 30 | 5.1 ± 0.3 | |
| 52145ΔwaaL + pGEMT-WaaL (1.4 × 109) | Kidney | 100 | 6.8 ± 0.4 |
| Bladder | 100 | 6.0 ± 0.3 | |
| Urine | 100 | 7.9 ± 0.6 (6) | |
A total of 20 kidneys and 10 bladders were studied in each group. Numbers in parentheses represent urine samples studied.
Percentage of positive cultures. The lowest number of organisms detectable by the method was 50 CFU per g (kidney or bladder) or per ml (urine).
Values represent the mean log10 CFU per gram or per milliliter ± standard deviation of the positive cultures. All the assays were done at least in triplicate.
Virulence was tested in two different models: (i) a septicemia model in mice by intraperitoneal injection and recording the mortality (LD50) and (ii) a murine model of pneumonia by intratracheal injection. When we measured the virulence of the strains in the septicemia model, 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwabG mutants showed a strong increase (approximately 5 log) in their LD50s in comparison with that of the wild-type strain (Table 3). The 52145ΔwaaL mutant showed only a 3-log increase in its LD50 compared to that of the wild-type strain. When the plasmid vectors harboring the corresponding wild-type gene were introduced in the mutant strains, all of them recovered LD50s similar to that of the wild-type strain in this virulence model, while no changes were observed in the LD50s of the mutant strains transformed with the plasmid vector alone (Table 3).
TABLE 3.
LD50s of mice inoculated intraperitoneally with different K. pneumoniae strains
| Strain | LD50a |
|---|---|
| 51245 (O1:K2, wild type) | 102.1 |
| 52145ΔwaaC | 107.8 |
| 52145ΔwaaC + pGEMT | 107.6 |
| 52145ΔwaaC + pGEMT-WaaC | 102.5 |
| 52145ΔwaaF | 107.9 |
| 52145ΔwaaF + pGEMT | 107.8 |
| 52145ΔwaaF + pGEMT-WaaF | 102.6 |
| 52145ΔwabG | 107.5 |
| 52145ΔwabG + pGEMT | 107.7 |
| 52145ΔwabG + pGEMT-WabG | 102.4 |
| 52145ΔwaaL | 105.1 |
| 52145ΔwaaL + pGEMT | 105.7 |
| 52145ΔwaaL + pGEMT-WaaL | 102.3 |
LD50s were calculated as previously described (22).
When the virulence of the strains was assayed in the murine pneumonia model, we obtained the results showed in Table 4. The 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwabG mutants were completely avirulent in this model, while the 52145ΔwaaL mutant and the wild-type strain showed similar values. Introduction of the corresponding gene(s) in the mutants rendered them as virulent as the wild-type strain or the 52145ΔwaaL mutant. Mutants 52145ΔwaaC, 52145ΔwaaF, and 52145ΔwabG, which were transformed with the plasmid vector (pGEMT) alone, remained avirulent in this animal model.
TABLE 4.
Experimental pneumonia induced by different K. pneumoniae strainsa
| Strain | Mean inoculum (range) | Mean lung wt (g) | Mean log CFU/g | No. dead/total no. (%) | No. positive culture/total no. (%) |
|---|---|---|---|---|---|
| 52145 | 1.0 × 107 (±1.4) | 0.29 ± 0.11 | 6.11 | 21/36 (58) | 19/36 (53) |
| 52145ΔwaaC | 1.8 × 107 | 0.16 ± 0.07 | <1.0 | 0/9 (0) | 0/9 (0) |
| 52145ΔwaaC + pGEMT | 1.5 × 107 | 0.17 ± 0.08 | <1.0 | 0/9 (0) | 0/9 (0) |
| 52145ΔwaaC + pGEMT-WaaC | 2.0 × 107 (±0.3) | 0.27 ± 0.12 | 6.02 | 10/18 (56) | 9/18 (50) |
| 52145ΔwaaF | 1.6 × 107 | 0.15 ± 0.05 | <1.0 | 0/9 (0) | 0/9 (0) |
| 52145ΔwaaF + pGEMT | 1.3 × 107 | 0.16 ± 0.04 | <1.0 | 0/9 (0) | 0/9 (0) |
| 52145ΔwaaF + pGEMT-WaaF | 1.8 × 107 (±0.9) | 0.28 ± 0.09 | 6.05 | 10/18 (56) | 10/18 (56) |
| 52145ΔwabG | 1.9 × 107 | 0.18 ± 0.05 | <1.0 | 0/9 (0) | 0/9 (0) |
| 52145ΔwabG + pGEMT | 1.8 × 107 | 0.16 ± 0.07 | <1.0 | 0/9 (0) | 0/9 (0) |
| 52145ΔwabG + pGEMT-WabG | 2.2 × 107 (±0.5) | 0.27 ± 0.12 | 5.98 | 10/18 (56) | 9/18 (50) |
| 52145ΔwaaL | 1.0 × 107 (±2.1) | 0.35 ± 0.09 | 6.31 | 10/18 (56) | 10/18 (56) |
| 52145ΔwaaL + pGEMT | 1.7 × 107 (±1.6) | 0.37 ± 0.05 | 6.42 | 10/18 (56) | 10/18 (56) |
| 52145ΔwaaL + pGEMT-WaaL | 1.3 × 107 (±1.1) | 0.37 ± 0.11 | 6.09 | 10/18 (56) | 9/18 (50) |
P was <0.01 for all comparisons (lung weight, log CFU per gram of lung, mortality, and blood or spleen positive cultures) between the strains, two-tailed t test.
DISCUSSION
Structural studies of the core region of LPS from K. pneumoniae have revealed that all of them showed very similar core structures with minor changes among different serogroups (29). In all the studied K. pneumoniae strains, the LPS core structure is characterized by the substitution of l,d-HeppI at the O-4 position by a Glcp [β-d-Glcp-(1→4)-α-l,d-HeppI] and by the substitution of the l,d-HeppII at the O-3 position by an α-Kdo-(2→6)-α-d-GlcN-(1→4)-α-d-GalA trisaccharide (29) (Fig. 1). Since the six genes involved in the biosynthesis of the K. pneumoniae inner core LPS (gmhD, waaC, -F, -Q, -A, and -E) have been previously identified by us (14, 24), we set up experiments to begin the identification and characterization of the genes involved in outer core LPS biosynthesis. Among the four unassigned genes in the K. pneumoniae waa gene cluster, the orf4- and orf6-encoded products showed similarity to the E. coli K-12 WaaZ and enterobacterial ADP-heptose-LPS heptosyltransferases, respectively. In addition, results of a previous analysis of a K. pneumoniae orf10 (yibD) mutant suggested that it could be involved in capsule attachment (24). Thus, only orf8 and orf9 remained as candidates to be involved in the transfer of the first residue of the outer core LPS. In the five E. coli core types and S. enterica serovar Typhimurium, the l,d-HeppII residue is substituted at the O-3 position by a d-Glc residue (Glc I), while a d-GalA residue is found in this position in K. pneumoniae. In addition, the orf8-encoded protein (WabG) showed low albeit significant similarity to the E. coli and S. enterica WaaG protein, and no cross-complementation between wabG and waaG was detected.
To study the function of the wabG, two nonpolar mutants were constructed and characterized by using K. pneumoniae wild-type strains 889 (O:8 K:69) and 52145 (O:1 K:2). SDS-tricine-PAGE analysis of LPS samples from both 889ΔwabG and 52145ΔwabG mutants suggested that these LPS are devoid of O antigen and contain a truncated-core LPS (Fig. 2). The comparative chemical and structural analyses (Fig. 3 and 4) of the LPS from wild-type and mutant strains allow us to conclude that WabG is involved in the linkage of the first outer core residue (d-GalA) to the O-3 position of the l,d-HeppII residue. The complementation achieved when the mutant 52145ΔwabG was transformed with plasmid pGEMT-Orf9Sm strongly suggests that the first residue in the S. marcescens N28b core LPS would be an α-d-GalpA residue linked to the l,d-HeppII by an α1,3 linkage.
In Enterobacteriaceae organisms containing phosphoryl modifications in their inner core LPS, such as E. coli and S. enterica serovar Typhimurium, truncation of the outer core results in alterations in cell permeability to hydrophobic compounds (21). Mutation in the waaG gene of E. coli strain F470 results in loss of the outer core, absence of l,d-HeppII phosphorylation, a 60% reduction in l,d-HeppI phosphorylation, and decrease in MICs of SDS (34). An E. coli waaP mutant produces core LPS totally devoid of phosphoryl modifications; this mutant was found to be even more sensitive to SDS and other hydrophobic compounds than the corresponding waaG mutant (34). The two K. pneumoniae wabG mutants totally devoid of the outer core LPS obtained in this study were more sensitive to SDS and polymyxin B than the wild-type strains. In addition, for K. pneumoniae waaC and waaF mutants, MICs of these hydrophobic compounds were essentially similar to those for the wabG mutants; however, K. pneumoniae waaC and waaF mutants were more sensitive to deoxycholate, nalidixic acid, erythromycin, novobiocin, and rifampin. In K. pneumoniae there are no phosphoryl modifications of the inner core l,d-HeppI and -II residues, the negative charges being contributed by the GalA residue(s) (29). Thus, the absence of the outer core GalA residue in the wabG mutation results in the loss of the stoichiometric core LPS negative charge and might explain why the levels of sensitivity to SDS and polymyxin B were essentially the same as those of the waaC and -F mutants but different from those of E. coli and S. enterica serovar Typhimurium (32, 33).
The fact that the wabG as well as waaC and -F mutants are unencapsulated but able to biosynthesize specific antiserum cross-reacting polysaccharide (K−) may be explained in two different ways. Since deep LPS core mutants (like waaC and -F) in Enterobacteriaceae are altered in different outer membrane components, one of them could be the attachment site for capsule linkage. A second possibility is a direct linkage of the capsule to LPS core. If the capsular polysaccharide is linked either to the LPS core directly or to some other outer membrane molecules, the waaC, -F, and wabG mutants may be sufficiently altered in these outer membrane components to preclude the capsular polysaccharide attachment. Furthermore, the lack of cell-bound capsule was found in all the Klebsiella waaC, -F, and wabG mutants isolated belonging to different K serotypes (unpublished data). Nonpolar K. pneumoniae 52145 mutants have been constructed for all the nonessential genes of the waa cluster (our unpublished results). Only the waaC, -F, and wabG mutants lacked K2 capsule, and the NC18 (yibD) mutant showed a drastic reduction of K2 capsule, as previously described (24). All the other K. pneumoniae 52145 mutants showed the presence of K2 capsule.
The effects of the wabG mutation on colonization and virulence experiments were studied in the K. pneumoniae 52145 background because this strain is highly virulent and is able to colonize different surfaces. The wabG mutation drastically reduces the colonization ability of K. pneumoniae in experimental UTIs (Table 2). In addition, this mutation also results in a 5-log-fold increase in LD50 in mice inoculated intraperitoneally (Table 3) and is completely avirulent in an experimental model of pneumonia (Table 4). Similar levels of reduction in colonization and virulence were observed in the corresponding waaC and -F mutants. On the other hand, a K. pneumoniae waaL mutant, with a full inner and outer core but devoid of O antigen, showed a smaller reduction in colonization and virulence when tested in mice inoculated intraperitoneally and showed no reduction in the pneumonia model when compared to the wild-type strain. The effect of the waaL mutant could be fully attributed to the O-antigen deficiency, since this mutant still contains capsule, as judged by EM, sensitivity to phage Φ2, and reaction with anti-K2-specific polyclonal serum. From these results we can conclude that the capsule is essential in the K. pneumoniae experimental model of pneumonia, while the colonization of the UT by K. pneumoniae requires a complete LPS with O antigen. The K. pneumoniae virulence tested as LD50 in mice inoculated intraperitoneally seems to be dependent on the capsule and the complete LPS (probably full-core LPS and O-antigen molecules). Finally, all the changes observed in the K. pneumoniae waaC, -F, -L, and wabG mutants are ameliorated by introduction of the corresponding single wild-type gene, while the introduction of the plasmid vector alone is unable to accomplish this.
Acknowledgments
This work was supported by Plan Nacional de I + D grants (Ministerio de Ciencia y Tecnología, Spain) and from Generalitat de Catalunya. L.I., N.C., B.H., and S.F. are supported by predoctoral fellowships from Ministerio de Ciencia y Tecnología (Spain), Generalitat de Catalunya, and Universitad de Barcelona.
We also thank Maite Polo for her technical assistance.
REFERENCES
- 1.Camprubí, S., S. Merino, and J. M. Tomás. 1993. The role of the O-antigen lipopolysaccharide and capsule on an experimental Klebsiella pneumoniae infection of the rat urinary tract. FEMS Microbiol. Lett. 111:9-14. [DOI] [PubMed] [Google Scholar]
- 2.Ciucanu, I., and F. Kerek. 1984. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 131:209-217. [Google Scholar]
- 3.Corsaro, M. M., R. Lanzetta, E. Parrilli, M. Parrilli, and M. L. Tutino. 2001. Structural investigation on the lipooligosaccharide fraction of psychrophilic Pseudoalteromonas haloplanktis TAC 125 bacterium. Eur. J. Biochem. 268:5092-5097. [DOI] [PubMed] [Google Scholar]
- 4.Cortés, G., N. Borrell, B. Astorza, C. Gómez, J. Sauleda, and S. Albertí. 2002. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect. Immun. 70:2583-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domon, B., and C. E. Costello. 1988. A systematic nomenclature for carbohydrate fragmentations in FABMS/MS of glycoconjugates. Glycoconj. J. 5:397-409. [Google Scholar]
- 6.Forsberg, L. S., U. Ramadas Bhat, and R. W. Carlson. 2000. Structural characterization of the O-antigenic polysaccharide of the lipopolysaccharide from Rhizobium etli strain CE3. J. Biol. Chem. 275:18851-18863. [DOI] [PubMed] [Google Scholar]
- 7.Galanos, C., O. Lüderitz, and O. Westphal. 1969. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 9:245-249. [DOI] [PubMed] [Google Scholar]
- 8.Garozzo, D., V. Nasello, E. Spina, and L. Sturiale. 1997. Discrimination of isomeric oligosaccharides and sequencing of unknowns by post source decay matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 11:1561-1566. [DOI] [PubMed] [Google Scholar]
- 9.Gibson, B. W., J. J. Engstrom, C. M. John, W. Hines, and A. M. Falick. 1997. Characterization of bacterial lipooligosaccharides by delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Am. Soc. Mass Spectrom. 8:645-658. [Google Scholar]
- 10.Guasch, J. F., N. Piqué, N. Climent, S. Ferrer, S. Merino, X. Rubirés, A. Aguilar, J. M. Tomás, and M. Regué. 1996. Cloning and characterization of two Serratia marcescens genes involved in core lipopolysaccharide biosynthesis. J. Bacteriol. 178:5741-5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen, D. S., F. Mestre, S. Albertí, S. Hernández-Allés, D. Alvarez, A. Domenech Sanchez, J. Gil, S. Merino, J. M. Tomás, and V. J. Benedí. 1999. Klebsiella pneumoniae lipopolysaccharide O typing: revision of prototype strains and O-group distribution among clinical isolates of different sources and countries. J. Clin. Microbiol. 37:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrichs, D. E., J. A. Yethon, and C. Whitfield. 1998. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 30:221-232. [DOI] [PubMed] [Google Scholar]
- 13.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo, L., N. Abitiu, N. Coderch, B. Hita, S. Merino, R. Gavin, J. M. Tomás, and M. Regué. 2002. The inner-core lipopolysaccharide biosynthetic waaE gene: function and genetic distribution among some Enterobacteriaceae. Microbiology 148:3485-3496. [DOI] [PubMed] [Google Scholar]
- 15.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Nassif, X., J. M. Fournier, J. Arondel, and P. J. Sansonetti. 1989. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect. Immun. 57:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsthoorn, M. M. A., J. Haverkamp, and J. E. Thomas-Oates. 1999. Mass spectrometric analysis of Klebsiella pneumoniae ssp. pneumoniae rough strain R20 (O1−: K20−) lipopolysaccharide preparations: identifications of novel core oligosaccharide components and three 3-deoxy-d-manno-oct-2-ulopyranosonic artifacts. J. Mass Spectrom. 34:622-636. [DOI] [PubMed] [Google Scholar]
- 19.Pradel, E., and C. A. Schnaitman. 1991. Effect of the rfaH (sfrB) and temperature on the expression of the rfa genes of Escherichia coli K-12. J. Bacteriol. 173:6428-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radziejewska-Lebrecht, J., A. S. Shashkov, V. Stroobant, K. Wartenberg, C. Wart, and H. Mayer. 1994. The inner core region of Yersinia enterocolitica. Eur. J. Biochem. 221:343-351. [DOI] [PubMed] [Google Scholar]
- 21.Raetz, C. R. H., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed, L. J., and C. H. Muench. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 23.Reeves, P. R., M. Hobbs, M. A. Valvano, M. Skurnik, C. Whitfield, D. Coplin, N. Kido, J. Klena, D. Maskell, C. R. H. Raetz, and P. D. Rick. 1996. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 4:495-503. [DOI] [PubMed] [Google Scholar]
- 24.Regué, M., N. Climent, N. Abitiu, N. Coderch, S. Merino, L. Izquierdo, M. Altarriba, and J. M. Tomás. 2001. Genetic characterization of the Klebsiella pneumoniae waa gene cluster, involved in core lipopolysaccharide biosynthesis. J. Bacteriol. 183:3564-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roantree, R. J., T. T. Kuo, and D. G. MacPhee. 1977. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J. Gen. Microbiol. 103:223-234. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Spina, E., R. Cozzolino, E. Ryan, and D. Garozzo. 2000. Sequencing of oligosaccharides by collision-induced dissociation matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 35:1042-1048. [DOI] [PubMed] [Google Scholar]
- 28.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharide in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 29.Vinogradov, E., and M. B. Perry. 2001. Structural analysis of the core region of the lipopolysaccharides from eight serotypes of Klebsiella pneumoniae. Carbohydr. Res. 335:291-296. [DOI] [PubMed] [Google Scholar]
- 30.Vinogradov, E., J. Radziejewska-Lebrecht, and W. Kaca. 2000. The structure of the carbohydrate backbone of core-lipid A region of the lipopolysaccharides from Proteus mirabilis wild-type strain S1959 (serotype O3) and its Ra mutant R110/1959. Eur. J. Biochem. 267:262-268. [DOI] [PubMed] [Google Scholar]
- 31.Williams, P., and J. M. Tomás. 1990. The pathogenicity of Klebsiella pneumoniae. Rev. Med. Microbiol. 1:196-204. [Google Scholar]
- 32.Yethon, J. A., J. S. Gunn, R. K. Ernst, S. I. Miller, L. Laroche, D. Malo, and C. Whitfield. 2000. Salmonella enterica serovar Typhimurium waaP mutants show increased susceptibility to polymyxin and loss of virulence in vivo. Infect. Immun. 68:4485-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yethon, J. A., D. E. Heinrichs, M. A. Monteiro, M. B. Perry, and C. Whitfield. 1998. Involvement of waaY, waaQ, and waaP in the modification of Escherichia coli lipopolysaccharide and their role in the formation of a stable outer membrane. J. Biol. Chem. 273:26310-26316. [DOI] [PubMed] [Google Scholar]
- 34.Yethon, J. A., E. Vinogradov, M. B. Perry, and C. Whitfield. 2000. Mutation of the lipopolysaccharide core glycosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J. Bacteriol. 182:5620-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]