Abstract
The role of IL-17 and the IL-17 producing Th17 cells in cancer has recently become the focus of extensive investigation. An expanding body of literature implicate Th17 cells and their hallmark cytokine in both pro and anti-tumourigenic processes. In this review we describe their biological activities and outline the reciprocal interactions between Th17 cells and other cells of the immune system. We also discuss the evidence regarding their dual role in the tumour microenvironment. An understanding of the processes that regulate the pro or anti-tumour activities of Th17 cell and IL-17 will allow the development of more effective means for cancer immunotherapy.
Keywords: IL-17, Th17, cancer, inflammation, tumour microenvironment
Introduction
Cancer cells are characterized by a great potential to proliferate and evade apoptosis as well as a capacity to develop blood vessels, invade tissues and metastasize (Hanahan and Weinberg, 2000). These complex processes reflect intrinsic properties of the malignant cells but are also markedly regulated by non-neoplastic components in the tumour microenvironment (Colotta et al., 2009). Innate and adaptive immune cells including macrophages, neutrophils, mast cells and lymphocytes are present in most solid tumours. These cells mediate inflammatory responses that are necessary for both tumour progression and the elimination of malignant cells (Coussens and Werb, 2002; Dunn et al., 2004). For instance CD8+ cytotoxic T lymphocytes, interferon-γ (IFN-γ)-producing T helper 1 (Th1) cells, dendritic cells (DCs) and ‘M1’ macrophages secreting interleukin-12 (IL-12) mediate anti-tumour immunity (Dunn et al., 2004; Boon et al., 2006). In contrast, M2 macrophages, type 2 helper T cells and regulatory T cells (Tregs) have been found to enhance tumour development (Beyer and Schultze, 2006; Roberts et al., 2007; Sica and Bronte, 2007; DeNardo et al., 2009). Moreover, it has become evident that tumour cells are able to breach the host’s immune defences and divert the immune response in order to achieve progressive growth (Dunn et al., 2004). The driving initiator is still an oncogenic mutation leading to autonomous cell growth; however several genetic mutations have been demonstrated leading to the expression of chemokines and cytokines by the malignant cells (Mantovani et al., 2008). The result is a picture similar to inflammation with contribution of this inflammatory milieu to cancer progression.
IL-17, a widely recognised inflammatory cytokine, and the IL-17 producing Th17 cells have recently gained prominence in cancer. Despite the rapid advances in understanding their role in inflammation and autoimmunity, their activity in cancer yields conflicting data. In this review we will focus on the biological functions of IL-17 and discuss the settings in which IL-17 and Th17 cells have been found to exert a pro- or anti-tumourigenic effect.
IL-17 expression, receptor signalling and biological activities
IL-17 (also called IL-17A) is the prototypic member of the IL-17 family composed of six cytokines, IL-17A-F (Aggarwal and Gurney, 2002; Gaffen, 2004; Huang et al., 2004; Kolls and Linden, 2004). IL-17 is the hallmark cytokine of Th17 cells and along with IL-17F, with which shares the greatest homology, is also produced by γδT cells, natural killer (NK) T cells, neutrophils and eosinophils (Molet et al., 2001; Starnes et al., 2001; Ferretti et al., 2003; Zhou et al., 2005; Lockhart et al., 2006; Liu et al., 2007). IL-17A/F signals through IL-17RA, a type I transmembrane protein ubiquitously expressed (Moseley et al., 2003; Yang et al., 2008a). IL-17RA activates mitogen activated protein kinases (MAPK) and nuclear factor-kB (NF-κB) via TNF receptor associated factor-6 (TRAF6) and has also been found to physically associate with the NF-κB activatory protein (Act1) (Shalom-Barak et al., 1998; Schwandner et al., 2000). Knock down of Act1 was subsequently shown to abrogate IL-17 induced inflammatory gene expression as well as NF-κB activation (Chang et al., 2006).
In vivo
A large body of evidence suggests that IL-17A and IL-17F mediate local tissue inflammation by inducing the release of pro-inflammatory and neutrophil mobilising cytokines and chemokines. In vivo administration of recombinant IL-17A causes significant accumulation of neutrophils in the bronchioalveolar and joint areas (Hoshino et al., 1999; Laan et al., 1999; Hoshino et al., 2000; Miyamoto et al., 2003) and leads to disease progression in a syngeneic model of ovarian cancer (Charles et al., 2009). CXC-chemokine ligand-1 (CXCL1), CXCL2, IL-6 and granulocyte monocyte-colony stimulating factor (GM-CSF) have been found to mediate the neutrophil recruitment caused by IL-17A in similar in vivo experimental systems (Hoshino et al., 1999; Laan et al., 1999; Ferretti et al., 2003; Kolls et al., 2003; Laan et al., 2003). IL-17A has also been found to increase neutrophil elastase and myeloperoxidase (MPO) activity in vivo (Hoshino et al., 2000). Interestingly, it has been observed that upon local administration, IL-1β and IL-17A can synergistically increase neutrophil activity but not neutrophil accumulation suggesting a negative regulatory role for IL-17A in the presence of an inflammatory stimulus promoting the late phase and resolution of inflammation (Hoshino et al., 2000). By regulating neutrophil response IL-17A is also a crucial element in host responses to infections. For instance, in response to infections of gram-negative bacteria such as Klebsiella pneumoniae IL-17A is induced in a dose dependent manner and is critical for neutrophil recruitment (Ye et al., 2001a; Ye et al., 2001b).
In vitro
In vitro experiments have shown that IL-17A and F stimulate the production of several CXC chemokines, CXCL1, CXCL2 and CXCL5 in mouse fibroblasts and epithelial cells (Fossiez et al., 1996; Laan et al., 1999; Kawaguchi et al., 2001; Laan et al., 2001; Jones and Chan, 2002; Prause et al., 2004). They also induce CXCL1, CXCL2, CXCL5 and IL-8 (also known as CXCL8) in human epithelial cells. IL-17A induced release of CXC chemokines has been shown to involve MAPK and the extracellular signal-regulated kinase (ERK) (Kawaguchi et al., 2001; Laan et al., 2001; Prause et al., 2004). In addition to CXC chemokines IL-17A can induce the release of granulocyte-colony stimulating factor (G-CSF) and GM-CSF (Jones and Chan, 2002; Starnes et al., 2002) as well as the monocyte chemotactic protein (MCP)-1 and IL-6 in epithelial cells and fibroblasts (Yao et al., 1995; Kawaguchi et al., 2001; Molet et al., 2001). More interestingly, in response to IL-17A monocytes isolated from human peripheral blood release tumour necrosis factor-α (TNF-α) and IL-1β (Jovanovic et al., 1998). In contrast, alveolar and peritoneal macrophages fail to produce TNF-α, CXCL2 or IL-6 in response to IL-17A (Kolls et al., 2003).
Responses to IL-17A can functionally cooperate with responses to other inflammatory cytokines and maximise their biological effects or, as indicated above, in case of e.g. IL-1β modulate inflammatory responses. For example, IL-17A markedly synergises with TNF-α in inducing G-CSF, CXCL1 and IL-8 production by epithelium (Jones and Chan, 2002; McAllister et al., 2005). Costimulation with IL-17A and IFN-γ enhances the IL-8 response in human bronchiolar epithelial cells and increases intercellular adhesion molecule-1 (ICAM-1) (Kawaguchi et al., 2001). In contrast, the Th2 cytokines IL-4 and IL-13 have no additional effect on ICAM expression (Kawaguchi et al., 2001). Overall, the response to IL-17 seems to be context dependent. The majority of research work currently focuses on the biological role of IL-17A and distinctions between the six IL-17 family members in their biological activity are missing. Further studies on the regulation and biological function of these cytokines will benefit our understanding of immune responses.
IL-17 in malignancy
IL-17 has been well studied over recent years in inflammatory diseases, but what about its role in tumour development and malignant progression? IL-17A expression has been detected in several human tumours including prostate, breast and gastric cancer (Haudenschild et al., 2002; Steiner et al., 2003; Sfanos et al., 2008; Zhang et al., 2008; Derhovanessian et al., 2009; Horlock et al., 2009). However, the specific role of IL-17 in cancer is still elusive. IL-17A ectopically overexpressed in murine fibrosarcoma or colon adenocarcinoma cell lines can significantly enhance in vivo tumour growth and increase tumour vascularity (Numasaki et al., 2003), (Table 1). It can also stimulate endothelial cell cord formation and up-regulate the production of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), prostaglandin E1 (PGE1) and PGE2 (Numasaki et al., 2003). Notably, both cell lines used in those studies are weakly immunogenic. In line with these findings, human cervical cancer cell lines stimulated with recombinant IL-17 up-regulate IL-6 and IL-8 and when transfected with IL-17 cDNA they show significantly higher tumour growth in athymic nude mice (Tartour et al., 1999). However, when immunogenic hematopoietic tumour cells overexpressing IL-17 are implanted into syngeneic immunocompetent mice tumour growth is significantly inhibited (Benchetrit et al., 2002). Although the basis of these differences is not well understood, it appears that IL-17 enhances anti-tumour immunity in immunocompetent mice but can increase tumour growth in the absence of an adaptive immune response (Martin-Orozco and Dong, 2009).
Table 1.
Immunopathological implications of IL-17 and Th17 cells in malignancies.
| Pro-tumourigenic effects of IL-17 | References | |
|---|---|---|
| Immunocompromised mouse models |
IL-17 overexpression in tumours leads to increased angiogenesis and tumour growth | (Numasaki et al., 2003) |
| MB49 bladder adenocarcinoma and B16 melanoma model |
Decreased tumour growth in IL-17−/− and IL-17R−/− mice | (Wang et al., 2009; He et al., 2010) |
| ID8 ovarian cancer model | TNF-dependent IL-17 release leads to increased myeloid cell recruitment and increased tumour burden | (Charles et al., 2009) |
| Anti-tumourigenic effects of IL-17 | ||
| Immunocompetent mouse models | IL-17 overexpression in tumours leads to decreased tumour growth associated with increased CTL influx | (Benchetrit et al., 2002) |
| MC38 colon cancer and B16-F10 melanoma model |
Accelerated tumour growth in IL-17−/− mice | (Kryczek et al., 2009b; Martin-Orozco et al., 2009b) |
| Human ovarian cancer | IL-17 positively predicts survival | (Kryczek et al., 2009a) |
| Th17 cells with potential pro-tumourigenic effects | ||
| Gastric cancer | Blood Th17 cells increased in advanced cancer | (Zhang et al., 2008) |
| Hepatocellular carcinoma | Increased Th17 cells in the tumour correlating with angiogenesis | (Zhang et al., 2009) |
| Melanoma | Increased Th17 cells in the tumour | (Kryczek et al., 2007) |
| Th17 cells with potential anti-tumourigenic effects | ||
| Non-Hodgkin B-cell lymphoma | Tumours inhibit Th17 formation and promote differentiation towards Treg | (Yang et al., 2009) |
| Prostate cancer | Responders to immunotherapy have higher levels of Th17 cells, inverse correlation between numbers of Th17 cells and tumour stage | (Sfanos et al., 2008; Derhovanessian et al., 2009) |
| Ovarian cancer | Reverse relationship between Th17 and Tregs | (Kryczek et al., 2009a) |
| Breast cancer | Inverse correlation between Th17 and Treg cells following immunotherapy | (Horlock et al., 2009) |
More recent studies evaluated the role of endogenous IL-17 in tumour immunopathogenesis. Wang et al. showed that growth of the B16 melanoma and the MB49 bladder carcinoma cells is reduced in IL-17−/− mice but accelerated in IFN-γ−/− mice due to elevated intratumoural IL-17 (Wang et al., 2009), (Table 1). Interestingly, IL-17/IFN-γ double knockout mice are resistant to tumour cell growth similarly to the IL-17−/− suggesting a minor role of IFN-γ in IL-17 mediated tumour promotion. IL-17 stimulated IL-6 production via signal transducer and activator of transcription 3 (Stat3) activation in B16 and MB49 tumour cells, as well as in tumour associated stromal cells such as fibroblasts, endothelial cells and dendritic cells. However, it only modestly increased tumour cell proliferation in vitro. IL-17 also increased the secretion of angiogenic factors by endothelial cells and induced endothelial cell migration in a Stat3-dependent manner. Tumours grown in IFN-γ−/− mice were characterised by markedly elevated levels of IL-17 and IL-6 produced mainly by tumour infiltrating immune cells but also by tumour cells. In vivo IL-6 blockade partially reversed tumour progression in this setting indicating that the tumourigenic effects of IL-17 are mediated in part via IL-6, in a Stat3-dependent pathway (Wang et al., 2009).
Similarly, tumour growth is inhibited in IL-17R−/− as well as IL-17R/IFNγR−/− mice (He et al., 2010). The IL-17R deficiency increased tumour-specific CD8+ T cell infiltration while it reduced recruitment of immature myeloid cells. Further analysis of the tumour infiltrating CD8+ T cells showed that IL17R deficiency did not impair their cytotoxic acivity or the expression of cytotoxic T lymphocyte (CTL)-related molecules such as perforin, granzyme B and FasL.
Kryczek and colleagues, however, demonstrated a protective role of IL-17 in tumour immunity (Kryczek et al., 2009b). They showed, accelerated growth and enhanced lung metastasis of the murine colon cancer cell line MC38 when inoculated in IL-17−/− mice (Table 1). The underlying mechanism for the increased tumour growth in IL-17 deficient mice was considered to be a reduction in IFN-γ producing NK cells and CD8+ T cells. Notably, Ngiow et al. failed to reproduce the MC38 enhanced growth in IL-17−/− mice and indicated that although T and NK cells were exerting some host control on tumour development, this was not dependent on IL-17 production {Ngiow, 2010 #128}. Nevertheless, accelerated tumour growth was also observed in IL-17−/− mice challenged with the B16-F10 melanoma cell line that colonizes the lung (Martin-Orozco et al., 2009b). The discrepancy between these studies and the report by Wang et al. is possibly due to distinct roles of IL-17 in different tumour models mirroring the tissue and context dependency described in inflammatory models.
The majority of the studies investigating the role of IL-17 in tumour development have been conducted on implanted tumour models. The limitation of such models is that they may only show the effects of IL-17 on established tumours that do not grow at their original anatomical sites. The study of IL-17 in genetic, chemical or microbial induced tumour models is limited and mainly studied in relation to Th17 immune responses as will be discussed below. Certainly, further investigations are required to determine the basis of these differences and to clarify the role of IL-17 in the tumour microenvironment.
Tumour infiltrating Th17 cells
IL-17 is the signature cytokine of Th17 cells. An expanding body of studies indicates that Th17 cells are present at tumour sites (Table 1). These cells appear to constitute a minor population in human peripheral blood and lymph nodes with no major frequency changes in cancer patients compared to healthy donors (Kryczek et al., 2009a). There is however a strikingly high frequency of tumour infiltrating IL-17+ T cells in patients with diverse cancer types, including ovarian and pancreatic cancer (Kryczek et al., 2007; Kryczek et al., 2009a; Su et al., 2010). Similar to human, Th17 cells are found to infiltrate tumours in murine models of cancer while being minimal in peripheral tissues of normal mice (Kryczek et al., 2007). Moreover, the levels of intratumoural Th17 cells are increased in advanced tumour stages but remain minimal in the draining lymph nodes (Kryczek et al., 2007). The presence of Th17 cells in the tumour microenvironment raises the question about their recruitment, in situ differentiation and, most importantly, their role in tumour immunity.
Phenotypic and tissue-homing features
Tumour-infiltrating Th17 cells exhibit a memory phenotype (CD45RA−CD45RO+) and a range of receptors that allow them to traffic in the periphery. They have been found to express high levels of CXCR4 and CCR6 as well as the tissue homing C type lectin CD161, a molecule found on NK cells and CD8+ T cells (Kryczek et al., 2008; Muranski et al., 2008; Kryczek et al., 2009a; Martin-Orozco and Dong, 2009). They also express the gut homing CD49 integrins but not CCR2, CCR5 and CCR7 and therefore have a limited capacity of lymph node trafficking (Kryczek et al., 2009a). This pattern of receptors may be associated with a selective Th17 cell migration and retention within tumour sites as high levels of CCL20 and CXCL12 are present in the tumour microenvironment (Zou et al., 2001; Kryczek et al., 2005; Rubie et al., 2006; Aspord et al., 2007; Ghadjar et al., 2009). Nevertheless Th17 cells have also been found to produce CCL20 and can therefore promote their own frequency at the tumour site (Muranski et al., 2008).
Kryczek et al. further analysed the phenotype of tumour infiltrating Th17 and reported low levels of the activation markers HLA-DR and CD25 as well as low levels of granzyme B suggesting that they may not be conventional effector T cells and may not mediate cytotoxic killing via the granzyme B pathway (Kryczek et al., 2009a). On the other hand reduced levels of forkhead box P3 (Fox3) and the B7-H1 receptor, programmed cell death-1 (PD-1), both of which contribute to immunosuppression in the tumour microenvironment (Kryczek et al., 2009a).
Th17 regulation in the tumour microenvironment
Cytokine networks
Malignant cells and associated stromal cells, such as fibroblasts and antigen presenting cells (APCs), secrete large amounts of IL-1β, IL-6, IL-23, TNF-α and transforming growth factor-β (TGF-β); key cytokines in Th17 differentiation and expansion (Hodge et al., 2005; Balkwill, 2006; Langowski et al., 2006; Miyahara et al., 2008; Su et al., 2010). Upon exposure to TGF-β in combination with IL-6 or IL-21 naïve T cells initiate the Th17 differentiation programme characterized by expression of IL-17 and IL-21 and the transcription factor retinoic acid-related orphan receptor-γt (RORγt) (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006), (Figure 1). IL-23 subsequently stabilises the Th17 phenotype. As far as human Th17 are concerned, the combination of TGF-β, IL-1β plus IL-6, IL-21 or IL-23 is essential for their polarisation from naïve cells whilst TGF-β plus IL-6 is sufficient to drive IL-17 production in murine T cells (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006; Manel et al., 2008; Volpe et al., 2008). A number of studies originally claimed that TGF-β may be dispensable in humans (Acosta-Rodriguez et al., 2007; Wilson et al., 2007). The experimental protocols used to isolate naïve T cells and a TGF-β contamination in the culture system are believed to account for this discrepancy (Manel et al., 2008; Volpe et al., 2008; Yang et al., 2008b).
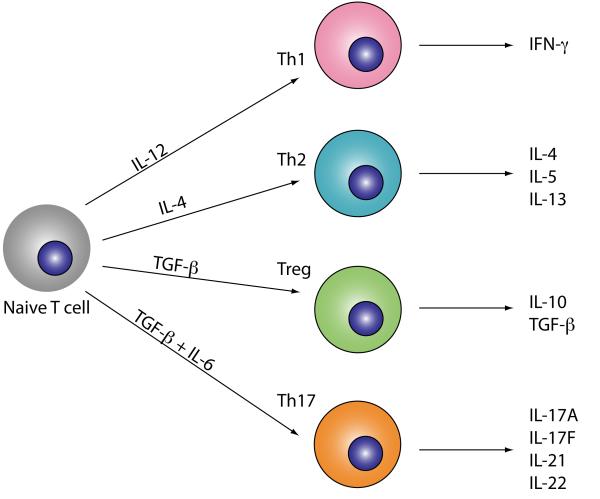
Figure 1. Differentiation of T cell subsets.
Upon activation naïve CD4+ T cells can differentiate into Th1, Th2, Th17 or Treg cells directed by the local inflammatory milieu. TGF-β and IL-6 induce Th17 cell polarisation characterised by the production of IL-17, IL-21 and IL-22 and the transcriptional factor RORγt.
TGF-β has a dual function in T cell polarisation by directing the differentiation of both Th17 cells and Tregs pending the polarising cytokines. Stimulation of naïve T cell with TGF-β alone induces expression of both Foxp3 – the transcription factor that guides Treg differentiation– and RORγt (Ivanov et al., 2006; Zhou et al., 2007). In TGF-β-induced naïve T cells, despite the presence of RORγt, the Foxp3-directed programme of Treg cell differentiation prevails. This is, at least in part, due to Foxp3 counteracting RORγt function (Zhou et al., 2008). However, in the presence of IL-6, IL-21 or IL-23, Foxp3 activity is inhibited while RORγt is upregulated and stabilised allowing the progression towards the IL-17 lineage (Zhou et al., 2008). The signalling pathways initiated after binding of both IL-6 and IL-21 to their receptors in naive CD4+ T cells are dependent on Stat3 (Zhou et al., 2007). Stat3 cooperates with RORγt to induce maximum IL-17 expression and optimal Th17 polarization. In addition to IL-17, it induces the transcription of IL-23 and IL-23R on naive T cells and stabilises the Th17 phenotype (Zhou et al., 2007).
TNF-α, present within the tumour microenvironment, has also been implicated in Th17 polarization. Although TNF-α is not essential for Th17 differentiation from naïve T cells, it synergises with IL-6 and IL-1β to amplify Th17 responses (Veldhoen et al., 2006). Furthermore, recent studies by our group indicate that TNF-α enhances Th17 differentiation by increasing IL-1R and IL-23R expression (Charles et al., 2009). More interestingly, microarray data generated from human ovarian cancer revealed significant association between high TNF-α signalling pathway gene expression and expression of genes of the Th17 pathways.
Interaction with the tumour microenvironment
Coculture of naïve and memory CD4+ T cells with tumour cells plus APCs can generate high percentages of Th17 cells (Miyahara et al., 2008). IL-1β appears to be critical in this setting while IL-6 and IL-23 is important only for the expansion of memory Th17 cells (Miyahara et al., 2008). Further studies dissect the contribution of tumour associated macrophages (TAMs) and DCs in Th17 polarization (Kryczek et al., 2009a). TAMs and myeloid DCs isolated from human ovarian cancers can polarize memory but not naïve T cells towards a Th17 phenotype. TAMs express higher levels of IL-1β and IL23p19 and are more efficient in inducing Th17 cells compared to normal macrophages (Kryczek et al., 2009a). Blocking IL-1β, but not IL-6 or TGF-β, reduced ΤAΜ mediated Th17 induction. Plasmacytoid DCs have minimal effect on Th17 polarization and as TAMs outnumber DCs in cancers they may be the predominant Th17 inducers in the tumour microenvironment (Kryczek et al., 2009a).
Tumour cells, fibroblasts and endothelial cells release several cytokines and C-C ckemokines such as MCP-1, MIP-1α and CSF-1 which contribute as major chemoattractants involved in monocyte/macrophage recruitment into the tumour (Coussens and Werb, 2002; Murdoch et al., 2004; Siveen and Kuttan, 2009). Th17 cells can in turn favour the recruitment of APCs by releasing CCL20 or inducing the release of this chemokine by resident cells (Martin-Orozco et al., 2009b). CCL20 leads to chemotactic recruitment of DCs via CCR6 which might in turn create a positive feedback loop between recruited DCs and Th17 cells promoting their frequency at the tumour site.
Tregs are found at high frequencies in the tumour microenvironment and have been shown to have a critical role in hampering anti-tumour immunity (Zou, 2006). More interestingly, the Th17 and Treg differentiation programmes are reciprocally related. This suggests a dynamic interaction between Treg and Th17 cells in the tumour microenvironment. Indeed, several studies have shown that naïve (CD45RO−) and memory (CD45RO+) Tregs can be induced to secrete IL-17 in the presence of IL-2, IL-1β, IL-6, IL-21, or IL-23. (maha ayyoub 26 106), (valmori 131 298) (Xu et al., 2007; Yang et al., 2008b; Voo et al., 2009). IL-2 alone has been shown to mediate the coversion of ovarian cancer-associed Tregs into IL-17 producers. (Leveque 32 101) . Notably, systemic administration of IL-2 in tumour bearing mice results in increased numbers of Foxp3+ T regs in the tumour and draining lymph nodes whilst tumour-associated IL-17+ cells are significantly reduced. Furthermore IL-2 appears to inhibit the differentiation of Th17 cells from mouse splenocytes in a Stat5-dependent manner but enhance development of Tregs (Laurence 26 371) (Kryczek et al., 2007). It is, therefore, possible that IL-2 has an opposite effect on differentiation of Th17 cells from conventional CD4+ T cells and Tregs (Leveque 32 101).
In line with the notion of a Treg-Th17 transition at tumour sites, a subpopulation of CD4+Foxp3+IL-17+ cells can be detected in humans (Leveque 32 101) (Voo et al., 2009). These cells co-express CD25, Foxp3, IL-17 and RORγt and maintain a suppressive function via a cell-cell contact mechanism (Voo et al., 2009). Whether these coexpressors derive from Treg or Th17 cells has not been demonstrated but they appear to represent a transition stage between Treg and Th17 cells. The plasticity of Tregs and the cytokine milieu at tumour sites may therefore allow an initial shift towards the IL-17 producing subpopulation and subsequently the development of Th17 cells.
Despite the high levels of IL-6, IL-1β and TGF-β in the tumour microenvironment, Th17 cells are present at lower frequencies compared to Tregs. High amounts of TGF-β can inhibit Th-17 differentiation even in the presence of pro-inflammatory cytokines (Manel et al., 2008). The relative amounts of these cytokines may therefore determine the Th17 or Treg lineage choice. Alternatively, other factors present at the tumour site may counteract the positive effect of pro-inflammatory cytokines on Th17 development. For instance, IL-2 inhibits Th17 differentiation while enhancing the Treg subset both in vitro and in vivo (Kryczek et al., 2007). Furthermore, retinoic acid, a vitamin A metabolite, inhibits the IL-6 mediated induction of IL-17 from Foxp3+ cells (Yang et al., 2008b). Whether retinoic acid plays a role in the Treg-Th17 balance in the tumour microenvironment is currently not known. Murine Tregs also directly repress Th17 responses in vivo in a Stat3 dependent manner. Ablation of Stat3 in Tregs leads to loss of their suppressive functions and their ability to control Th17 cells (Chaudhry et al., 2009).
The pro and anti-tumour activity of Th17 cells
Pro-tumour
Inflammatory can promote malignant cell transformation, tumour growth and metastasis (Mantovani et al., 2008). Th17 cells are characterised by potent pro-inflammatory activities mediated predominantly by their effector cytokines IL-17A and F, IL-21, IL-22 and IL-23. The major pro-tumour roles of Th17 cells rely on their capacity to induce angiogenesis, recruit inflammatory cells and activate tumour-promoting transcription factors (Figure 2). As already mentioned, by acting on stromal cells and fibroblasts IL-17 induces a wide range of angiogenic factors such as VEGF. Not surprisingly, the levels of IL-17 producing CD4+ T cells have been positively correlated with microvessel density in tumours (Numasaki et al., 2003). Interestingly, VEGF induces TGF-β while TGF-β can in turn upregulate expression of VEGFR receptor on endothelial cells thereby enhancing their responsiveness to VEGF (Huang and Lee, 2003).
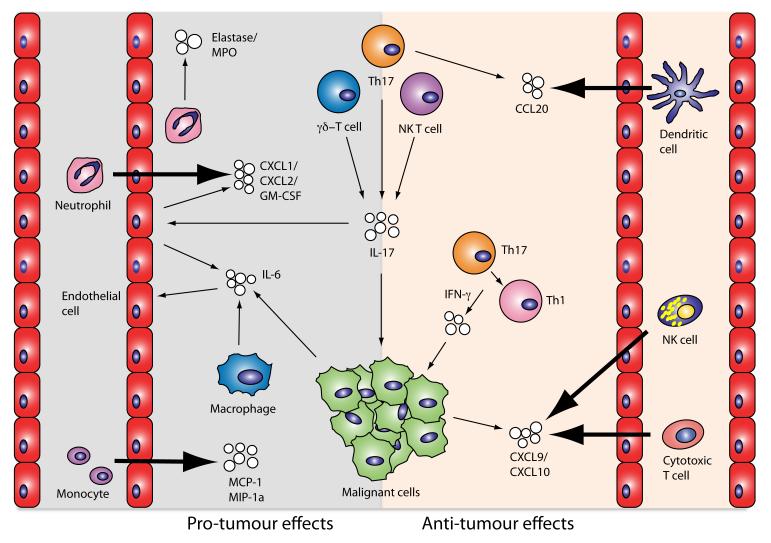
Figure 2. The pro and anti-tumour effects of Th17 cells and IL-17.
Interleukin-17 derived from Th17 cells, γδ-T cells and NK T cells exerts pro-tumourigenic activites by a range of actions. It has direct effects on angiogenesis mediated by endothelial cells, it also has indirect angiogenic effects by stimulating them to release of IL-6. Furthermore, it induces endothelial cells to secrete CXC chemokines and growth factors resulting in the recruitment of neutrophils. Infiltrating neutrophils respond to IL-17 within the tumour microenvironment by releasing inflammatory proteinases. Other inflammatory cells, such as monocytes, are also attracted to the tumour site by IL-17 induced MCP-1 release by epithelial cells and fibroblasts, and subsequently mature to tumour associated macrophages. The anti-tumour effects of IL-17 primarily come through its ability, in conjunction with IFN-γ, to stimulate tumour cells to release the chemokines CXCL9 and CXCL10, which recruit NK cells and cytotoxic CD8 cells to the tumour. Th17 cells have also been demonstrated to release CCL20, a chemoattractant for dendritic cells, potentially leading to an enhanced immune response.
Human Th17 cells can directly attract neutrophils through the production and release of IL-8 (Pelletier et al.). This may represent a more efficient mechanism for Th17 cells to rapidly recruit and interact with neutrophils as opposed to the indirect actions of IL-17A/F which mediate their effect via induction of chemokine secretion by epithelial and endothelial cells (Jones and Chan, 2002). The same study also shows that Th17 cells are able to modulate neutrophil responses via the release of GM-CSF, TNF-α and IFN-γ in an IL-17 independent manner. This Th17-neutrophil interaction appears to be bidirectional as activated neutrophils can also recruit Th17 in a CCL20/CCR6 dependent manner (Pelletier et al., 2010). Interestingly, tumour infiltrating neutrophils are associated with poor prognosis and their capacity to modify tumour growth and invasiveness led to suggestion of a N1/N2 phenotype differentiation similar to the M1/M2 polarisation (Haqqani et al., 2000; Sparmann and Bar-Sagi, 2004; Nozawa et al., 2006; Fridlender et al., 2009). A recent study demonstrates that neutrophil elastase is secreted upon neutrophil activation and can directly induce tumour cell proliferation both in human and mice. Moreover, in a murine model of lung adenocarcinoma, mice lacking neutrophil elastase have markedly decreased tumour burden (Houghton et al., 2010).
Stat3 has a central role in tumour immunity by promoting pro-oncogenic inflammatory pathways such as NF-κB and Jak pathways and by counteracting Stat1 and NF-κB mediated anti-tumour Th1 responses (Yu et al., 2009). Th17 derived IL-17, induces IL-6 production by malignant as well as tumour stromal cells which in turn activates Stat3 in both tumour and stromal cells in the tumour microenvironment (Wang et al., 2009). Beyond its role on malignant cells and the tumour stroma Stat3 is a critical transcription factor for Th17 differentiation. Its activation in the tumour microenvironment inhibits IL-12p35 while enhancing IL-23p19 transcription thereby shifting the balance from IL-12 to IL-23 (Langowski et al., 2006; Kortylewski et al., 2009). Given the close relation of IL-23 with Th17 cell development and along with the finding that chemical-induced skin carcinogenesis is diminished in IL-23p19−/− mice and enhanced in IL-12p35−/− mice it is suggested that Stat3 promotes a pro-carcinogenic Th17 response (Kortylewski et al., 2009). More recently, the notion of a crucial pro-tumorigenic effect of Th17 cells driven by Stat3 activation is further confirmed in infection induced colon carcinogenesis (Wu et al., 2009).
Anti-tumour
Although tumour infiltrating Th17 cells appear to have pro-tumour activity another line of evidence suggests that they may mediate protective anti-tumour immunity (Figure 2). Muranski and colleagues used T cell receptor (TCR) transgenic mice specific for a melanoma epitope and polarised CD4+ T cells towards Th17 in the presence of IL-6 and TGF-β. Upon adoptive transfer into mice with established cutaneous melanoma, these cells mediated effective tumour rejection, better than Th1 polarised cells (Muranski et al., 2008). Interestingly, their effect was critically dependent on IFN-γ whereas depletion of IL-17A and IL-23 had little impact (Muranski et al., 2008). In a similar study, CD8+ T cells were skewed to secrete IL-17 in Th17 polarising conditions and mediated efficient tumour destruction when adoptively transferred into tumour bearing mice. Again, the response was highly dependent on IFN-γ as these cells were found to convert into IFN-γ producers (Hinrichs et al., 2009). IL-17 derived from Th17 cells and IFN-γ can synergistically induce the secretion of the Th1 type CXCL9 and CXCL10 by tumour cells which in turn potentially attract effector T cells at the tumour site (Kryczek et al., 2009a). IFN-γ+IL17+ T cells have been reported in human tumours and in patients with autoimmune diseases (Kryczek et al., 2008; Kryczek et al., 2009a). Additionally Th17 cells have been reported to divert to Th1 under lymphopenic conditions in mice (Bending et al., 2009; Martin-Orozco et al., 2009a; Nurieva et al., 2009). It is therefore speculated that in the tumour microenvironment Th17 cells may be gradually converted into Th1 mediating tumour rejection (Zou and Restifo, 2010).
A recent study by Martin-Orozco indicates that tumour specific Th17 cells play a protective role against tumours by triggering strong CD8 immune responses (Martin-Orozco et al., 2009b). Th17 cells lack the ability to traffic to local regional lymph nodes (see above). However, Th17 cell therapy mediated DC recruitment in the tumour tissue in a CCL20/CCR6 dependent manner and further on the presentation of tumour antigens in tumour draining lymph nodes. The anti-tumour effects of Th17 were abrogated by CCR6 deficiency indicating the importance of the innate mediator. Notably, the Th17 cells retained their cytokine signature upon transfer in tumour bearing mice and exhibited stronger efficacy than Th1 cells. This finding suggests that tumour infiltrating Th17 cells may mediate protective immunity indirectly through DC recruitment and cytotoxic T cell activation.
Beside a pro- or anti-tumour function of Th17 cells clinical data from anti-CTLA4 treated melanoma patients has indicated that a post-dosing increase in Th17 cells within the peripheral blood mononuclear cell pool, associated with treatment-induced toxicity but not with an anti-tumour response (ribas 7 35) . Since Th17 cells have been so closely associated with inflammation and autoimmunity it is particularly important to investigate whether toxicities and responses could be differentially modulated in the context of cancer immunotherapy. The propagated plasticity of T cell populations is intriguing and the shift from Treg to Th17 cells might be beneficial in the right context.
Conclusions
The role of IL-17 and Th17 cells in the tumour microenvironment is not a clearcut case of pro- or anti-tumourigenic. The overall impact of IL-17 in cancer may also depend on other cellular sources of the cytokine, such as neutrophils, γδ T cells and NKT cells. Whether conventional anti-cancer therapies such as chemotherapy and radiotherapy modulate IL-17 secretion and/or Th17 polarisation and function is yet unexplored. Understanding the factors that regulate their pro- or anti-tumour activity will allow to use the functional properties of this exciting subpopulation for the development of more effective immune therapies in cancer.
Acknowledgements
This work was supported by the Medical Research Council (to T. Hagemann and R. Soper) and Cancer Research UK (to T. Hagemann and E.Maniati).
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhovanessian E, Adams V, Hahnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Biology of recently discovered cytokines: interleukin-17--a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res Ther. 2004;6:240–247. doi: 10.1186/ar1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadjar P, Rubie C, Aebersold DM, Keilholz U. The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. Int J Cancer. 2009;125:741–745. doi: 10.1002/ijc.24468. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Haqqani AS, Sandhu JK, Birnboim HC. Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia. 2000;2:561–568. doi: 10.1038/sj.neo.7900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild D, Moseley T, Rose L, Reddi AH. Soluble and transmembrane isoforms of novel interleukin-17 receptor-like protein by RNA splicing and expression in prostate cancer. J Biol Chem. 2002;277:4309–4316. doi: 10.1074/jbc.M109372200. [DOI] [PubMed] [Google Scholar]
- He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184:2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, Stebbing J. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br J Cancer. 2009;100:1061–1067. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H, Laan M, Sjostrand M, Lotvall J, Skoogh BE, Linden A. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol. 2000;105:143–149. doi: 10.1016/s0091-6749(00)90189-1. [DOI] [PubMed] [Google Scholar]
- Hoshino H, Lotvall J, Skoogh BE, Linden A. Neutrophil recruitment by interleukin-17 into rat airways in vivo. Role of tachykinins. Am J Respir Crit Care Med. 1999;159:1423–1428. doi: 10.1164/ajrccm.159.5.9806008. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Frydas S, Kempuraj D, Barbacane RC, Grilli A, Boucher W, et al. Interleukin-17 and the interleukin-17 family member network. Allergy Asthma Proc. 2004;25:17–21. [PubMed] [Google Scholar]
- Huang X, Lee C. Regulation of stromal proliferation, growth arrest, differentiation and apoptosis in benign prostatic hyperplasia by TGF-beta. Front Biosci. 2003;8:s740–749. doi: 10.2741/1093. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Kawaguchi M, Kokubu F, Kuga H, Matsukura S, Hoshino H, Ieki K, Imai T, Adachi M, Huang SK. Modulation of bronchial epithelial cells by IL-17. J Allergy Clin Immunol. 2001;108:804–809. doi: 10.1067/mai.2001.119027. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. Am J Respir Cell Mol Biol. 2003;28:9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009a;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009b;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- Laan M, Lotvall J, Chung KF, Linden A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133:200–206. doi: 10.1038/sj.bjp.0704063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M, Prause O, Miyamoto M, Sjostrand M, Hytonen AM, Kaneko T, Lotvall J, Linden A. A role of GM-CSF in the accumulation of neutrophils in the airways caused by IL-17 and TNF-alpha. Eur Respir J. 2003;21:387–393. doi: 10.1183/09031936.03.00303503. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol. 2007;82:354–360. doi: 10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009a;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs. 2009;10:543–549. [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009b;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol. 2009;182:2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- Prause O, Bozinovski S, Anderson GP, Linden A. Increased matrix metalloproteinase-9 concentration and activity after stimulation with interleukin-17 in mouse airways. Thorax. 2004;59:313–317. doi: 10.1136/thx.2003.008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, Ng BY, Filler RB, Lewis J, Glusac EJ, Hayday AC, Tigelaar RE, Girardi M. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:6770–6775. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubie C, Frick VO, Wagner M, Rau B, Weber C, Kruse B, Kempf K, Tilton B, Konig J, Schilling M. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand J Immunol. 2006;63:468–477. doi: 10.1111/j.1365-3083.2006.001766.x. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273:27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169:642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- Steiner GE, Newman ME, Paikl D, Stix U, Memaran-Dagda N, Lee C, Marberger MJ. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171–182. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008a;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008b;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2009;69:5522–5530. doi: 10.1158/0008-5472.CAN-09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001a;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001b;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Desta T, Fenton M, Graves DT, Amar S. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect Immun. 2005;73:935–943. doi: 10.1128/IAI.73.2.935-943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]