Abstract
Objectives
To determine patient compliance and effectiveness of antiarrhythmic treatment by the wearable cardioverter-defibrillator (WCD).
Background
Effectiveness of the WCD for prevention of sudden death is dependent on event type, patient compliance and appropriate management of ventricular tachycardia/fibrillation (VT/VF).
Methods
Compliance and events were recorded in a nation-wide registry of post-market release WCDs. Survival, using the Social Security Death Index, was compared with survival in implantable cardioverter-defibrillator (ICD) patients.
Results
Of 3569 patients wearing the WCD (age 59.3±14.7, duration 52.6±69.9 days), daily use was 19.9±4.7 hours (>90% of the day) in 52% of patients. More days of use correlated with higher daily use (p<0.001). Eighty sustained VT/VF events occurred in 59 patients (1.7%). First shock success was 76/76 (100%) for unconscious VT/VF and 79/80 (99%) for all VT/VF. Eight patients died after successful conversion of unconscious VT/VF (survival 89.5% of VT/VF events). Asystole occurred in 23 (17 died), pulseless electrical activity in 2 and respiratory arrest in 1 (3 died), representing 24.5% of sudden cardiac arrests. During WCD use, 3541/3569 patients (99.2%) survived overall. Survival occurred in 72/80 (90%) VT/VF events and 78/106 (73.6%) for all events. Long-term mortality was not significantly different from first ICD implant patients but highest among patients with traditional ICD indications.
Conclusions
Compliance was satisfactory with 90% wear time in >50% of patients and low sudden death mortality during usage. Survival was comparable to that of implantable ICD patients. However, asystole was an important cause of mortality in sudden cardiac arrest events.
Keywords: Wearable cardioverter defibrillator, Implantable cardioverter defibrillator, Outcomes, Compliance
Several studies have demonstrated implantable cardioverter defibrillator (ICD) devices to be effective in primary and secondary SCD prevention in high risk populations(1-5). These studies led to expanding indications for ICD implantation.
However, despite identification of risk factors indicating a high risk for sudden cardiac death (SCD), ICD implantation is commonly deferred in a variety of clinical situations. Several primary prevention studies of SCD excluded patients early after acute myocardial infarction (MI) or coronary revascularization(4,5), leading to exclusion of such patients from early prophylactic ICD implantation. Moreover, early ICD implantation 6-40 days after acute MI with left ventricular ejection (LVEF) ≤35% has not been shown to improve early overall survival, despite a reduction in arrhythmic deaths, as patients receiving ICD shocks are at high risk for nonarrhythmic deaths during this early period(6). In Medicare patients with LVEF≤35% and New York Heart Association class II or III heart failure, ICD implantation is not indicated for nonischemic dilated cardiomyopathy within 3 months of diagnosis and for ischemic cardiomyopathy within 40 days of acute MI or 3 months of revascularization. Nevertheless, SCD risk may be highest early after diagnosis or MI, as VALIANT demonstrated by reporting SCD risk appears highest in the first 30 days after MI in patients with left ventricular dysfunction or heart failure(7). ICD implantation may also need to be deferred in patients with surgical contraindications, ventricular thrombi, treatable comorbidities, vascular obstructions, infections, or awaiting clearance of infection for re-implantation after extraction.
Once discharged from the hospital, these patients generally rely on emergency medical services for resuscitation should they have a sudden cardiac arrest (SCA). Additional protection has been proposed through means including bridging with an automatic external defibrillator, antiarrhythmic drugs, or a wearable cardioverter-defibrillator (WCD). However, the automatic external defibrillator is limited by requiring a bystander to apply the defibrillator, and antiarrhythmic drugs, including amiodarone, may not be better than placebo in the prevention of SCD(1,2).
The WCD is an external device capable of automatic ventricular tachycardia (VT)/fibrillation (VF) detection and defibrillation. It has been shown to improve SCA survival over reliance on emergency medical services(8), but the efficacy of the WCD in the prevention of arrhythmic SCD would seem to be highly dependent on patient compliance as well as appropriate detection and treatment of VT/VF. We sought to determine the efficacy of the WCD in the detection and treatment of VT/VF, to assess compliance of patients in wearing of the device, and to compare the long term survival of patients who wore the WCD compared to a population of patients who underwent ICD implants.
METHODS
All patients issued a WCD after market release in the United States are entered into a database maintained by the manufacturer (ZOLL Cardiac Management Solutions, Pittsburgh, PA) for regulatory, reimbursement, and tracking purposes. The database contains indications, baseline demographics (age and sex), compliance, and events. All patients signed consent to use their data for quality monitoring, healthcare operation activities, and/or research. Patients in the United States who wore the WCD from August 2002 through December 2006 were included in this study. Survival of these patients was compared to a population of patients who underwent first ICD implantation at the Cleveland Clinic. Mortality outcomes of WCD patients with Social Security numbers were determined from the Social Security Death Index (SSDI) and de-identified survival data compared to SSDI-determined survival data in an ICD population studied under a retrospective medical records review protocol approved by the Cleveland Clinic Institutional Review Board.
WCD Description
The WCD is composed of four dry, non-adhesive capacitive electrodes for monitoring two surface electrocardiogram leads, three non-adhesive defibrillation electrodes incorporated into a chest strap assembly, and a 1.7 pound defibrillator unit carried on a waist belt. The monitoring electrodes are positioned circumferentially around the chest and held in place by about 1.5 lb of tension from an elastic belt. The defibrillation electrodes are positioned for apex-posterior defibrillation. If an arrhythmia is detected, an escalating alarm sequence starts, including a vibration against the skin, audible tones, and a voice cautioning bystanders of an impending shock. Patients are trained to hold a pair of response buttons during these alarms. Responding acts as a test of consciousness; if no response occurs the device charges, extrudes gel from the defibrillation electrodes, and delivers up to five biphasic shocks of pre-programmable energy levels with maximum output of 150 joules. The WCD records time/date stamps for device on/off switching, monitor connection to the electrodes, and electrode-to-skin contact.
Definitions
For this study compliance was defined as the time during a day that a WCD user had the device on, the belt connected, and at least one electrocardiogram lead contacting the skin. Days were determined as any day with at least some WCD use. During the compliance calculation one day was subtracted from the total number of days to compensate for the expected partial use on the first and last days.
Electrocardiogram and defibrillation electrode contact was determined by μampere AC signals similar to conventional monitoring systems. The device also recorded electrocardiogram data for rhythms greater than a pre-programmed rate threshold that did not match a baseline template and defined such arrhythmias as ventricular arrhythmias. A buffer in the monitoring software captured 30 seconds of electrocardiogram signal prior to the determination of VT/VF. Treatment event outcomes were determined through phone contact with patients or medical personnel. In addition to recording potential VT/VF, asystole recordings occurred when the ventricular rate dropped below 20 BPM (minimum detected QRS height: 100 μvolts). The buffer incorporated into the monitoring software stored five minutes of electrocardiogram prior to the asystole determination.
For the purposes of this study, all potentially lethal arrhythmias (sustained VT/VF or asystole) occurring within 24 hours were considered a single SCA event. In addition to reviewing electrocardiogram records stored by the WCD, patient call reports were reviewed for reports of deaths while wearing a WCD. All such reports were explored to further isolate the cause of death if possible. A ZOLL physician determined WCD shocks to be appropriate if occurring on sustained VT/VF and inappropriate if not. Inappropriate shocks were further analyzed for inappropriate detection cause from electrocardiogram recordings and lack of response button use from patient call reports. Two-lead electrocardiograms from all shocks and baseline tracings were reviewed by two authors (SJS, EZ) and differences adjudicated by consensus with the first author (MKC).
Although the WCD did not have pacing capability in this version, it recorded asystole events and broadcast “device disabled, call ambulance” to enlist bystander help once asystole was detected.
Indications for WCD use
Indications for WCD were based on Medicare Durable Medical Equipment Regional Carrier local coverage policies for use. Because no coverage policy was in effect prior to January 2005, additional categories were added for patients not fitting into the coverage policies. Indications included:
ICD explantation with delayed re-implantation (e.g., extended antibiotic therapy for infections).
Delays in ICD implantation (e.g., co-morbidities) following a VF or sustained VT event.
Delays in ICD implantation for genetic arrhythmogenic syndromes or congenital heart disease.
Prior to ICD evaluation following MI with LVEF≤35%.
Prior to ICD evaluation following revascularization with LVEF≤35% and past MI.
Prior to ICD evaluation after diagnosis with non-ischemic cardiomyopathy and LVEF≤35%.
Unspecified cardiomyopathy with LVEF≤35%.
MI with LVEF>35% or unspecified
Other temporary or fluctuating SCD risk conditions (e.g., waiting for cardiac transplant).
If more than one category was specified, patients were grouped according to the following heirarchy: ICD explant > VF or sustained VT event > genetic or congenital SCA risk > nonischemic cardiomyopathy with low LVEF > CABG with low LVEF and prior MI > MI with low LVEF > MI with high or unknown LVEF > unknown low LVEF > other.
Survival outcomes were also analyzed by WCD indications, grouped by whether patients met traditional vs. non-traditional ICD indications. Traditional ICD indications included patients who underwent ICD explants, had VT/VF while awaiting ICD implant, genetic predisposition to SCD, or LVEF ≤35% with unspecified cardiomyopathy. Non-traditional ICD indications included recent MI, post-CABG, recent nonischemic cardiomyopathy, miscellaneous or unknown indications.
ICD population
Patients undergoing first ICD implants at the Cleveland Clinic between August 1996 and May 2004 were identified from a prospectively collected database in the Cleveland Clinic Electrophysiology Laboratory. Mortality was determined by the SSDI.
Statistical Analysis
Data are reported as mean±standard deviation unless otherwise stated. Data were compared using Student's t tests for continuous variables and Chi square tests for discrete variables. Kaplan-Meier survival analysis was performed for the WCD and ICD groups. Cox proportional hazards modeling was performed for adjusted survival analyses. Data were considered statistically significant at two-sided p-value <0.05. All analyses were conducted using SAS 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Patient Population
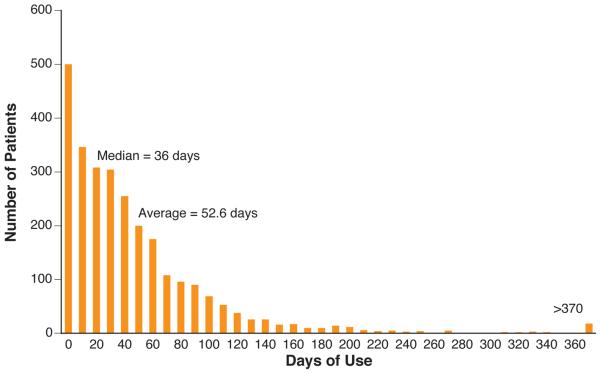
A total of 3569 patients wore the WCD for at least one day. Baseline demographics were available in 2731 patients (Table 1). Mean age was 59.3±14.7 (range 12-93 years, N=2723). Indications were unknown in 838 (23%) due to missing links between clinical and reimbursement datasets, primarily during early commercial use. Use totaled 143,643 patient-days. Mean duration of use was 52.6±69.9 days, range 1-1590 days (Figure 1A).
TABLE 1.
Patient Characteristics
| Sex, male | 2000/2720 (74%) |
|
| |
| Age, years | 59.3 ± 14.6 |
|
| |
| Indications | N=2731 |
| ICD explants | 638 (23.4%) |
| VT/VF prior to ICD implant | 439 (16.1%) |
| Genetic predisposition to SCD | 12 (0.4%) |
| LVEF ≤ 35% | |
| Recent MI | 341 (12.5%) |
| Post-CABG | 243 (8.9%) |
| Nonischemic cardiomyopathy | 546 (20.0%) |
| Unspecified cardiomyopathy | 222 (8.1%) |
| LVEF >35%, Recent MI | 104 (3.8%) |
| Miscellaneous or unknown | 186 (6.8%) |
|
| |
| Duration of Monitoring, days | 52.6 ± 69.9 (range 1-1590) |
CABG = coronary artery bypass graft surgery, ICD = implantable cardioverter defibrillator, LVEF = left ventricular ejection fraction, MI = myocardial infarction, SCD = sudden cardiac death, VT/VF = ventricular tachycardia/ventricular fibrillation.
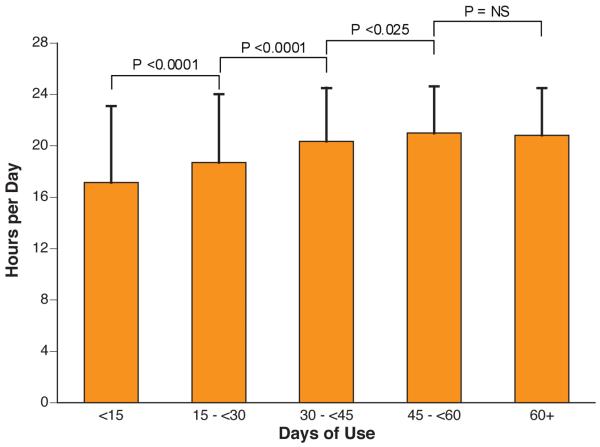
Figure 1. Actual WCD Use.
A. Duration of Monitoring. Distribution of patients by duration of WCD use. B. Daily hours of use by overall duration of WCD use.
Compliance
Daily use for each patient (available in 2208 patients) was calculated by dividing the total hours worn by the number of days worn minus one, to adjust for partial use on the first and last days worn. Median daily use was 21.7 hours (91% of time available). Mean daily use was 19.9 ± 4.7 hours, range .4 to 25.9 hours (>24 hours possible due to the methodology used to adjust for partial days at the beginning and end of use, although internal clocking errors cannot be completely excluded). Daily use was >90% in 52% of patients and >80% in 71% of patients. Of 2169 patients with recorded data, 307 (14.2%) stopped wearing the WCD prematurely because of comfort issues or adverse reactions, primarily complaining of size and weight of the monitor.
Longer duration of monitoring correlated with higher compliance rates (Figure 1B). Patients using the device over 60 days (n=599) averaged 20.8±3.7 hours per day, significantly more than all other groups except those using it between 45 and 60 days (p<0.05). Patients using it < 15 days (n=160) averaged 17.2±5.9 hours, significantly less than all other groups (p<0.001).
Arrhythmia Events, Treatment Efficacy, and Mortality During WCD Use
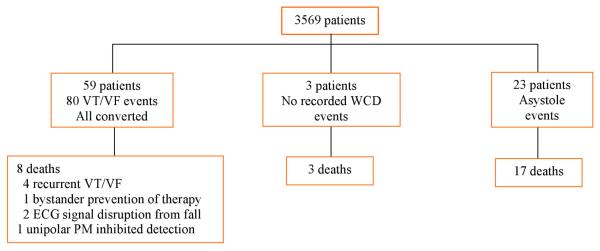
The WCD was designed to treat sustained VT/VF and to record sustained VT/VF and asystole. Thus, most SCA events were expected to have an associated electrocardiogram record. Events are shown in Figure 2 and Table 2. There were three reports of deaths while a WCD was worn that did not have an associated WCD electrocardiogram recording. Two were from pulseless electrical activity confirmed by hospital telemetry recordings, and one was due to a respiratory arrest, documented by a physician present at the event (a prison medical ward), although telemetry recordings were not documented to determine presence or absence of an arrhythmia. The number of patients who died not wearing the WCD was not recorded in the database but determined using the SSDI. During the time the WCD was worn, 80 sustained VT/VF events occurred in 59 patients (1.7% of the total number of patients). Four patients reported being conscious during the shock. First shock success was 76/76 (100%) among unconscious patients and 79/80 (99%) for all patients. The single failure to halt VT/VF on the first attempt occurred when a conscious patient with sustained VT allowed himself to be shocked after ten minutes of using the WCD response buttons to delay the shock. He was later shocked while conscious during his ambulance ride to the hospital (two shocks, 100 and 200 joules) and in the emergency room (two shocks, 50 and 200 joules). All shocks were ineffective although pharmacologic therapy restored a normal rhythm within hours. This patient was using a WCD after ICD explantation due to device infection; the original indication for ICD implantation is unknown. Eight patients died after successful conversion of unconscious VT/VF; survival occurred in 89.5% of events. Four patients died due to recurrent arrhythmias after initially recovering consciousness and the arrival of professional medical care (one ambulance death, one emergency department death, two telemetry deaths). One patient's spouse prevented a second WCD shock for a recurrent ventricular tachyarrhythmia minutes after a first successful shock. Two patients with recurrent arrhythmias were prevented or delayed from getting a second shock due to electrocardiogram signal disruption believed secondary to falling and wedging their bodies in an unfavorable position for proper electrode contact. The last patient had a unipolar pacemaker that paced during an episode of ventricular fibrillation, and the large pacing artifact prevented WCD arrhythmia detection from proceeding to shock(14).
Figure2. Events while wearing the WCD.
ECG=electrocardiogram, PM=pacemaker. VT/VF = ventricular tachycardia/ventricular fibrillation, WCD = wearable cardioverter defibrillator,
Table 2.
Events by Indication
| Indications for WCD Use | n (%) | Duration of Use (Days) |
Patients With Shocked VT/VF Events While Wearing the WCD* |
VT/VF Deaths While Wearing the WCD |
Non-VT/VF SCA Events While Wearing the WCD |
Non-VT/VF SCA Deaths While Wearing the WCD |
|---|---|---|---|---|---|---|
| Traditional ICD Indications | ||||||
| ICD explants | 638 (23.4%) | 56.1 ± 77.7 | 33 (49 events) | 2 | 9 | 7 |
| VT/VF before to ICD implant | 439 (16.1%) | 49.6 ± 71.1 | 6 (9 events) | 1 | 0 | 0 |
| Genetic predisposition to SCD | 12 (0.4%) | 56.2 ± 75.6 | 0 (0 events) | 0 | 0 | 0 |
| LVEF ≤ 35%, cardiomyopathy | 222 (8.1%) | 56.6 ± 117.4 | 0 (0 events) | 0 | 2 | 2 |
| Nontraditional ICD Indications | ||||||
| LVEF ≤ 35% | ||||||
| Recent MI | 341 (12.5%) | 7.8 ± 38.9 | 10 (12 events) | 2 | 7 | 7 |
| Post-CABG | 243 (8.9%) | 46.5 ± 44.0 | 2 (2 events) | 1 | 3 | 1 |
| Recent NICM | 546 (20.0%) | 56.5 ± 59.1 | 4 (4 events) | 1 | 2 | 1 |
| LVEF >35%, Recent MI | 104 (3.8%) | 50.0 ± 48.8 | 0 (0 events) | 0 | 0 | 0 |
| Miscellaneous or unknown | 186 (6.8%) | 49.3 ± 73.1 | 4 (4 events) | 1 | 3 | 2 |
To be counted as a separate event, the VT/VF shock had to occur at least 24 h from the last shock. Other SCA events included asystole and pulseless electrical activity.
NICM = nonischemic cardiomyopathy: SCA = sudden cardiac arrest: WCD = wearable cardioverter-defibrillator: other abbreviations as in Table 1.
Non tachy-arrhythmia events
There were 23 asystole events recorded (17 deaths) and 3 pulseless electrical activity or respiratory arrests (3 deaths) while wearing the WCD, all confirmed by medical personnel present at the time. These non-VT/VF events accounted for 26/106 (24.5%) of SCAs. One patient who was treated for VT/VF several times died four days after the last treatment with asystole as the presenting arrhythmia. Two treatments for VT/VF shocked the rhythm into asystole (no cardiac signal for at least 15 seconds), but both survived with return of rhythm. Inappropriate shocks (not occurring on sustained VT or VF) occurred in 67/3569 (1.9%) of patients during 4788 months of use (1.4%/month). Inappropriate shocks are a combination of sustained inappropriate detections by the WCD and failure to use the response buttons by the patient. Reasons for inappropriate detection were (multiple reasons possible during a detection): ECG signal loss 4.4%, multicounting on normal cardiac signal 4.4%, signal artifact 67.6%, supraventricular tachycardia 26.5%, and nonsustained VT 5.9%. Patient-reported reasons for failure to use the response buttons were (one reason recorded per episode): inconsistent or unconcerned 32.4%, physically or mentally unable to respond 11.8%, sleeping 26.5%, didn't remember why 10.3%, didn't remember training 10.3%, mental or physical obstacle at the time 4.4%, and didn't hear alarms 4.4%.
Overall acute survival
Overall acute survival during the time of WCD use was 99.2% (3541/3569 patients) with 0.78% sudden death mortality over a mean usage time of 53 days. Survival was 72/80 (90%) for ventricular tachyarrhythmia events and 78/106 (73.6%) for all events, including non-VT/VF events.
Survival and events in WCD patients by device indication
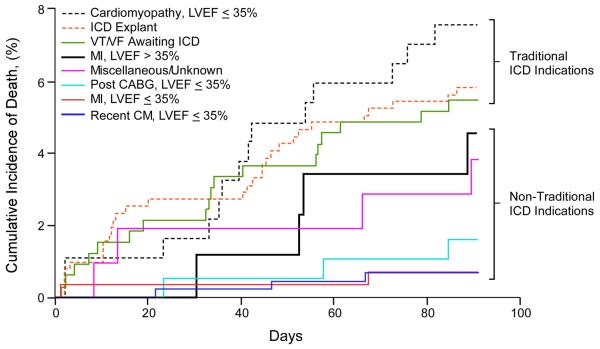
Events in individual traditional vs. non-traditional ICD indications are shown in Table 2. Among patients with traditional ICD indications, the WCD recorded events in the groups with ICD explant and history of VT/VF while awaiting ICD implant. No shocked events occurred in the genetic predisposition to SCD and LVEF ≤35% cardiomyopathy groups, although two SCA events and deaths occurred in the LVEF≤35% cardiomyopathy group. Shocked events occurred in all non-traditional ICD indications groups, except the recent MI with LVEF>35% group.
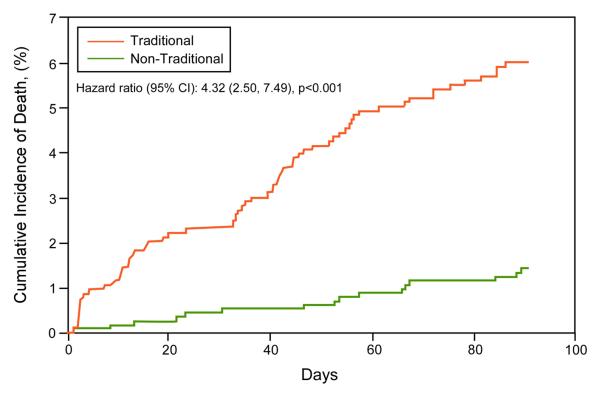
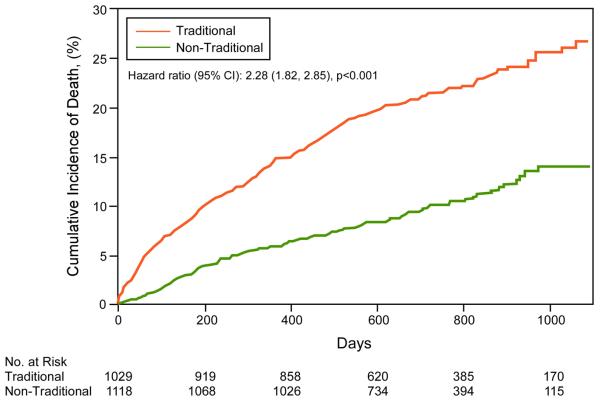
Survival by traditional vs. non-traditional ICD indications was analyzed based on SSDI mortality data (N=2147). Long-term (3-year) and short-term (3-month) mortality was significantly worse in the group with traditional ICD indications (Figure 3). Short-term Kaplan-Meier curves for individual indications are shown in Figure 4, and long-term mortality estimates for the composite traditional ICD indications group and individual non-traditional indications groups in Table 3. The highest mortality was observed in three of the four traditional ICD indications groups: cardiomyopathy with LVEF≤35%, ICD explanted awaiting re-implantation, and VT/VF awaiting ICD implantation. Similar long-term mortality was observed in patients surviving a recent MI with LVEF>35%, a non-traditional ICD indication. Other non-traditional ICD indication groups demonstrated lower mortality compared to traditional ICD indication groups. These non-traditional groups included LVEF≤35% with recent MI, post-CABG, and recent non-ischemic cardiomyopathy.
Figure 3. Short- and Long-term Kaplan-Meier Survival Analyses of WCD patients by Traditional vs. Non-Traditional ICD Indications.
A. 3-month cumulative mortality. B. Long-term cumulative mortality.
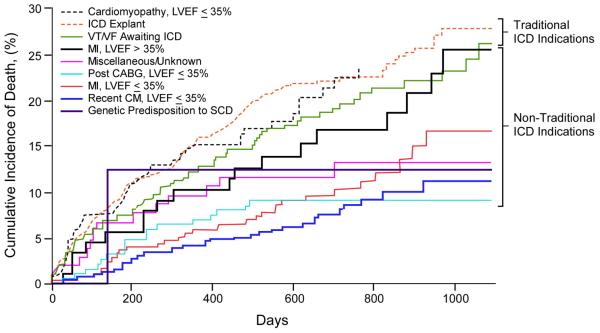
Figure 4. Kaplan-Meier Survival Analyses of WCD patients by Specific ICD Indications.
A. Short term (3-month). B. Long term (3-year). Traditional ICD indications include cardiomyopathy with LVEF ≤35%, ICD explant awaiting reimplantation, and ventricular tachycardia/ventricular fibrillation (VT/VF) awaiting ICD implantation, and genetic predisposition to sudden cardiac death (SCD). Non-traditional indications include recent MI with LVEF >35%, post-CABG with LVEF ≤35%, recent MI with ≤35, recently diagnosed cardiomyopathy (CM) with LVEF ≤35%.
Table 3.
Estimated 3-year mortality rates in the composite traditional ICD indications group compared to individual non-traditional ICD indication groups. Adjusted hazard ratios are adjusted for age and gender.
| N | Estimated mortality rate, % (95% CI) |
Hazard Ratio (95% CI) |
p value |
Adjusted* Hazard Ratio (95% CI) |
p value |
|
|---|---|---|---|---|---|---|
| Traditional ICD indications |
1029 | 26.8 (23.3, 30.4) | -- | -- | -- | -- |
| LVEF ≤ 35%, Recent MI |
273 | 16.7 (10.1, 23.3) | 0.49 (0.33, 0.71) |
<.001 | 0.69 (0.57, 0.83) | <.001 |
| LVEF ≤ 35%, Post- CABG |
184 | 9.3 (5.1, 13.5) | 0.40 (0.25, 0.66) |
<.001 | 0.72 (0.61, 0.85) | <.001 |
| LVEF ≤ 35%, Recent NICM |
428 | 11.3 (7.0, 15.5) | 0.34 (0.24, 0.49) |
<.001 | 0.42 (0.29, 0.61) | <.001 |
| LVEF >35%, Recent MI |
87 | 25.6 (14.7, 36.5) | 0.84 (0.52, 1.36) |
0.48 | 0.94 (0.84, 1.06) | 0.34 |
| Miscellaneous or unknown |
104 | 13.2 (6.4, 20.0) | 0.54 (0.31, 0.95) |
0.03 | 0.91 (0.82, 1.02) | 0.11 |
CABG = coronary artery bypass graft surgery, ICD = implantable cardioverter defibrillator, LVEF = left ventricular ejection fraction, MI = myocardial infarction, NICM = nonischemic cardiomyopathy, 95% CI = 95% confidence interval.
Adjusted for age and gender.
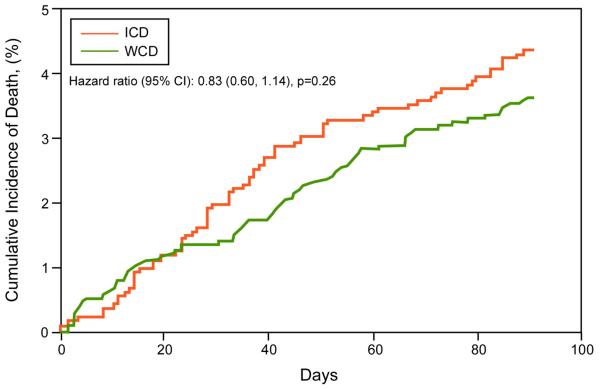
Survival in WCD vs. ICD groups
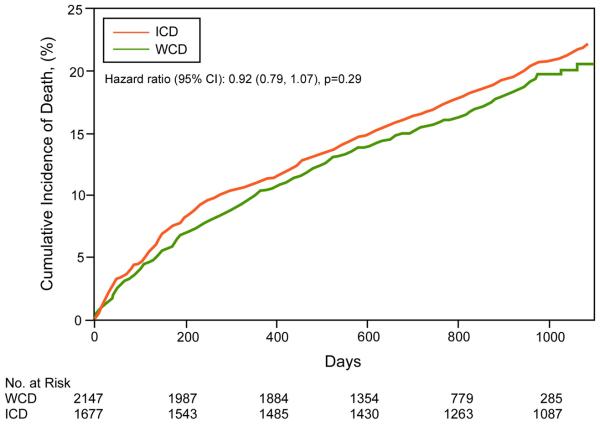
Patient characteristics of WCD and ICD groups are shown in Table 4. Mean age was higher in patients with ICDs compared to WCDs. Kaplan-Meier survival curves for the WCD and ICD groups (Figure 5), including 3 year and 3 month survival curves, show no significant survival differences between the groups, including upon Cox proportional hazards analyses adjusting for age and gender (p=0.707). Estimated mortality rates in WCD and ICD groups (Table 3) were not significantly different.
Table 4.
Patient characteristics and estimated mortality rates of WCD and ICD patients
| WCD N=2207 |
ICD N=1677 |
p value | |
|---|---|---|---|
| Age, years | 59.3±14.7 | 62.9±12.5 | <0.001 |
|
| |||
| Sex, male | 74.6% | 77.6% | 0.028 |
|
| |||
| Estimated mortality rates | |||
| 3 months | 3.6% | 4.4% | 0.256 |
| 6 months | 6.3% | 7.6% | 0.134 |
| 1 year | 10.1% | 11.0% | 0.379 |
| 2 years | 15.6% | 16.7% | 0.341 |
| 3 years | 20.5% | 22.1% | 0.288 |
| 4 years | 22.2% | 27.9% | 0.279 |
ICD = implantable cardioverter defibrillator, WCD = wearable cardioverter defibrillator.
Figure 5. Short- and long-term Kaplan-Meier survival analysis of WCD and ICD patients.
A. 3-month cumulative mortality. B. 3-year cumulative mortality. ICD=implantable cardioverter defibrillator. WCD = wearable cardioverter defibrillator.
DISCUSSION
This study reports the United States outcomes with the WCD. Indicated for temporary or changing SCA risk, the WCD may be useful in situations in which ICD implantation is warranted but deferred. Use has been limited by perceptions that patient compliance is low. The data reported here shows compliance of at least 90% for the majority of users. Survival was comparable to that of a population of implanted ICD patients.
In this study, 14.2% stopped wearing the WCD because of comfort issues or adverse reactions. In a prior published investigational study of the WCD, 68 of 289 patients (24.5%) stopped wearing an earlier version of the WCD. Although patients who stopped using the WCD prematurely complained mostly of size and weight of the monitor/defibrillator unit, improved compliance may have been seen because the WCD used in this study was 40% smaller in size and weight. In a recent paper of aspirin, ACEI, and beta-blocker use following MI, 15% of patients stopped using all three medications within 30 days of hospital discharge, despite the known survival benefits of these medications(9). The rate of WCD discontinuation appears similar.
The shock efficacy results reported here are comparable to those reported in investigational studies of the WCD(8,10). In electrophysiology laboratory testing of the WCD, 100% of 20 shocks given for VF were terminated by a single shock of either 70 or 100 joules(10). In 289 patients who wore the WCD, 6/8 (75%) defibrillation attempts were successful(8). The two unsuccessful defibrillations occurred in patients who had incorrectly reversed the defibrillating electrodes, such that shocks were not directed to the skin. A high first-shock conversion rate was observed in this study by the WCD, indicating efficacy in conversion of VT/VF that appears comparable to that of ICDs. First shock efficacy has been reported to be 80-90% with ICDs,(11), which delivers therapies rapidly but which can also deliver shocks to conscious and/or potentially hemodynamically stable or nonsustained VT/VF episodes. Only 10% of appropriately treated patients reported syncope during AVID. In fact, up to 40% of VF detected arrhythmias terminate during charging but before shock delivery(12). In contrast WCD shocks are delivered to unresponsive patients who have had at least 30 seconds of VT/VF and who have failed to respond to the alarms by pressing the response buttons. Despite the programmed longer time to shock, shock efficacy appears to be similar to shocks reported with ICDs.
During pre-market release studies, 12 deaths occurred, including six sudden deaths. Of these, five occurred in patients not wearing the device and one in a patient who reversed a defibrillation electrode (the current version alarms when any of the defibrillation electrodes are not touching the skin properly)(8,10). Thus, despite use of the WCD, SCD can occur. However, as reported here, some SCD may have occurred due to bystander interference, electrocardiogram signal disruption, or unipolar permanent pacing inhibiting arrhythmia detection by the WCD. The latter instance emphasizes the relative contraindication to use of the WCD with unipolar pacing, as is the case with ICDs.(13) Future device improvements, such as the already implemented detection of electrode contact and improved warnings to bystanders, may help to reduce but is unlikely to completely eliminate these issues. These results highlight the importance of patient education in use of the WCD, as well as in the critical need to promote compliance in using the WCD for the prevention of SCD.
Proper instruction on use of the WCD is also important to the avoidance of inappropriate shocks. The incidence of inappropriate shocks was relatively low and comparable to ICDs. Inappropriate or unnecessary shocks occurred in 6/289 (2.1%) of patients during 901 months of use (0.7% per month) in a prior reported study(8). In the current study, inappropriate shocks occurred 1.9% of patients at a rate of 1.4% per month. In contrast, studies in ICDs report that 0.6 to 1.5% inappropriate shocks occur per month over the first six months of use (14). The ability to prevent shocks by holding response buttons in WCD patients may have contributed to keeping a lower inappropriate shock incidence as the sustained inappropriate detection rate was five times the inappropriate shock rate. It is interesting to speculate from these results that this type of shock-prevention feature might be useful for selected low-risk arrhythmia treatment strategies in patients with ICDs.
There are few reported data on actual arrhythmic causes of SCD. This study documented that asystole or pulseless electrical activity accounted for 24.5% of SCA events with high associated mortality rates. These data indicate the need to incorporate pacing therapies into future WCD systems, although it is unclear whether pacing would prevent deaths in these patients.
In the analysis of events and mortality by traditional vs. non-traditional ICD indications, patients with traditional ICD indications had the highest long-term mortality, reinforcing these groups as higher risk populations. Among non-traditional indications groups, however, the groups with LVEF≤35% and recent cardiovascular events, including recent MI, CABG, and new cardiomyopathy diagnosis, had lower long-term mortality, perhaps reinforcing the appropriate deferral of ICD implantation after acute cardiac events and use of the WCD while awaiting potential improvement in cardiac function. Paradoxically, the recent MI group with LVEF >35% demonstrated similar mortality to groups with traditional ICD indications. It is possible that there were other high risk indicators that may have prompted WCD prescription, although shocked events did not occur in this group. Accordingly, these individuals may have died from nonarrhythmic causes and may not have benefited from the WCD.
The long-term survival data comparing WCD and ICD survival are reassuring that WCD therapy may be comparable to ICD therapy, rationalizing use of the WCD as an acceptable temporary alternative or bridge to long-term ICD implantation. Short term, 3-month survival estimates were also performed, as the mean WCD use duration was 53 days, and were not significantly different from that of ICD patients.
With limitations inherent to a device that is external and interactive, it is evident that should an ICD be indicated, implantation of an ICD would be clearly preferable and perhaps significantly more efficacious over the WCD, as patient compliance is irrelevant to ICD therapy and asystolic events could be treated. Should ICD implantation need to be deferred, however, a WCD may be elected, and this study demonstrated comparable survival between WCD and ICD patients. However, intensive patient and family education may be required to reinforce the importance of compliance in achieving effective prevention of SCD with this therapy.
LIMITATIONS
Because of the nature of the registry, availability of demographic and daily use information was not complete and thus could lead to potential bias in these data. Similarly, the comparability of WCD to ICD patients in terms of demographics and disease comorbidities cannot be assessed.
CONCLUSIONS
The WCD was worn with >90% compliance by most patients. Sudden death mortality was 0.78% over a mean usage time of 53 days. Survival occurred in 73.6% of events and in 90% of VT/VF events. Survival was comparable to that of ICD patients and highest in patients with traditional ICD indications, rationalizing use of the WCD as an acceptable temporary alternative or bridge to long-term ICD implantation. However, as sudden death mortality occurred in patients who were not wearing the WCD or who had bystander interference, electrocardiogram signal disruption, or unipolar pacing artifacts, patient instruction regarding proper use and compliance of the WCD is vital to insuring efficacy of the WCD in preventing SCD. Also, asystole or pulseless electrical activity accounted for 24.5% of SCA events with high associated mortality rates, rationalizing the need to incorporate pacing therapies into future WCD systems.
Abbreviations
- WCD
Wearable cardioverter defibrillator
- VT
Ventricular tachycardia
- VF
Ventricular fibrillation
- ICD
Implantable cardioverter defibrillator
- LVEF
Left ventricular ejection fraction
- SCA
Sudden cardiac arrest
- SSDI
Social Security Death Index
- SCD
Sudden cardiac death
- MI
Myocardial infarction
- CABG
Coronary artery bypass graft surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Bokhari F, Newman D, Greene M, Korley V, Mangat I, Dorian P. Long-term comparison of the implantable cardioverter defibrillator versus amiodarone: eleven-year follow-up of a subset of patients in the Canadian Implantable Defibrillator Study (CIDS) Circulation. 2004;110:112–6. doi: 10.1161/01.CIR.0000134957.51747.6E. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–8. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 8.Feldman AM, Klein H, Tchou P, et al. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol. 2004;27:4–9. doi: 10.1111/j.1540-8159.2004.00378.x. [DOI] [PubMed] [Google Scholar]
- 9.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–7. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 10.Reek S, Geller JC, Meltendorf U, Wollbrueck A, Szymkiewicz SJ, Klein HU. Clinical efficacy of a wearable defibrillator in acutely terminating episodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol. 2003;26:2016–22. doi: 10.1046/j.1460-9592.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 11.Klein RC, Raitt MH, Wilkoff BL, et al. Analysis of implantable cardioverter defibrillator therapy in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Trial. J Cardiovasc Electrophysiol. 2003;14:940–8. doi: 10.1046/j.1540-8167.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- 12.Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97:1255–61. doi: 10.1016/j.amjcard.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 13.LaPage MJ, Canter CE, Rhee EK. A fatal device-device interaction between a wearable automated defibrillator and a unipolar ventricular pacemaker. Pacing Clin Electrophysiol. 2008;31:912–5. doi: 10.1111/j.1540-8159.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- 14.Friedman PA, McClelland RL, Bamlet WR, et al. Dual-chamber versus single-chamber detection enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation. 2006;113:2871–9. doi: 10.1161/CIRCULATIONAHA.105.594531. [DOI] [PubMed] [Google Scholar]