Abstract
Evidence suggests that physical activity (PA) is associated with the maintenance of cognitive function across the lifespan. In contrast, the apolipoproteinE-ε4 (APOE-ε4) allele, a genetic risk factor for Alzheimer’s disease (AD), is associated with impaired cognitive function. The objective of this study was to examine the interactive effects of PA and APOE-ε4 on brain activation during memory processing in older (ages 65–85) cognitively intact adults. A cross-sectional design was used with four groups (n = 17 each): (1) Low Risk/Low PA; (2) Low Risk/High PA; (3) High Risk/Low PA; and (4) High Risk/High PA. PA level was based on self-reported frequency and intensity. AD risk was based on presence or absence of an APOE-ε4 allele. Brain activation was measured using event-related functional magnetic resonance imaging (fMRI) while participants performed a famous name discrimination task. Brain activation subserving semantic memory processing occurred in 15 functional regions of interest. High PA and High Risk were associated with significantly greater semantic memory activation (famous > unfamiliar) in 6 and 3 of the 15 regions, respectively. Significant interactions of PA and Risk were evident in 9 of 15 brain regions, with the High PA/High Risk group demonstrating greater semantic memory activation than the remaining three groups. These findings suggest that PA selectively increases memory-related brain activation in cognitively intact but genetically at-risk elders. Longitudinal studies are required to determine whether increased semantic memory processing in physically active at-risk individuals is protective against future cognitive decline.
Keywords: Leisure-time physical activity, exercise, Alzheimer’s disease, APOE-epsilon4 allele genetic risk, age-related cognitive decline, fMRI
1. Introduction1
Emerging evidence suggests that increased leisure-time physical activity (PA) is associated with enhanced cognitive function and preservation of brain tissue volume (Colcombe and Kramer, 2003; Kramer et al., 2006) in healthy older adults. In contrast, possession of one or more ApolipoproteinE-ε4 (APOE-ε4) alleles is a risk factor for Alzheimer’s disease (AD) and has been associated with accelerated cognitive decline, reduced neurite outgrowth, and amyloid β aggregation (Kim et al., 2009). Interestingly, the protective effects of PA on neurocognitive test performance appear to be greater in carriers than non-carriers of the APOE-ε4 allele. Specifically, high PA APOE-ε4 carriers experience less cognitive decline and a lower risk of being diagnosed with mild cognitive impairment (MCI) (Etgen et al., 2010; Geda et al., 2010) and AD (Laurin et al., 2001) than more sedentary carriers and non-carriers (Etnier et al., 2007; Schuit et al., 2001). Thus, PA may protect against cognitive decline, especially among healthy older adults at genetic risk for dementia (Kivipelto et al., 2008).
The precise mechanisms by which PA influences brain structure and function are unclear. One hypothesis, based on animal research, posits that PA produces neurogenic (van Praag et al., 1999) and angiogenic (Pereira et al., 2007) brain changes. In a functional magnetic resonance imaging (fMRI) study, greater activation in response to an executive control task was observed in the prefrontal and parietal cortices of high functioning older adults who were more physically active at study entry and in persons who had become more physically fit over the course of the study.(Colcombe et al., 2004) These limited findings contrast with a complete absence of data addressing the brain mechanisms influencing the possible interaction of PA and APOE status.
The purpose of this study, therefore, was to examine the interactive effect of PA and APOE status on brain activation patterns, as measured by task-activated fMRI. Four groups of cognitively intact older adults were studied based on self-reported frequency and intensity of PA (active versus inactive) and genetic risk for AD (presence versus absence of an APOE-ε4 allele). The fMRI task involved a low-effort, high accuracy semantic memory task that involved discrimination of famous from unfamiliar names. Previous work by our group (Seidenberg et al., 2009) has shown that healthy elders with the APOE-ε4 allele demonstrate greater semantic memory activation (famous > unfamiliar names) than non-carriers in the posterior cingulate/precuneus, lateral temporal-parietal and medial prefrontal regions. Furthermore, greater semantic memory activation at study entry was shown to be protective against future cognitive decline measured 18 months post-scanning (Woodard et al., in press). Based on our previous work, we hypothesized that brain activation patterns would show an interaction between PA and APOE status, with the greatest amount of semantic memory brain activation occurring in physically active, genetically at-risk older participants.
2. Methods
This study was approved by the institutional review board at the Medical College of Wisconsin and conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all participants.
2.1 Participants
Healthy adults between the ages of 65 and 85 were recruited from newspaper advertisements. A telephone screen was administered initially to 459 individuals to determine eligibility based on inclusion/exclusion criteria (see section 2.4). Of those who met criteria, 109 agreed to undergo APOE genotyping from blood samples, a physical activity questionnaire, neuropsychological testing, and an fMRI scanning session. From this initial pool, four subgroups of equal sample size (n = 17), carefully matched on demographic variables (age, sex, education), were formed based on the presence/absence of at least one APOE-ε4 allele and self-reported amounts of leisure-time physical activity: (1) Low Risk/Low PA, (2) Low Risk/High PA, (3) High Risk/Low PA, and (4) High Risk/High PA. Potential Low Risk participants were excluded if they reported a family history of AD. The sample sizes were equated to avoid potential biases in the group image analyses.
2.2 Physical Activity Status
Frequency and intensity of leisure time PA was measured using the Stanford Brief Activity Survey (SBAS) (Taylor-Piliae et al., 2006). The SBAS has demonstrated validity for assessing habitual PA (Taylor-Piliae et al., 2007; Taylor-Piliae et al., 2006). Those individuals endorsing items indicating two or fewer days of low intensity PA (ranging from no PA to slow walking or light chores) were classified as physically inactive (Low PA). Participants who endorsed items indicating moderate to vigorous intensity PA three or more days per week (ranging from brisk walking, jogging or swimming for 15 minutes or more, or moderately difficult chores for 45 minutes, to regular jogging, running, bicycling or swimming for 30 minutes or more, or playing sports such as handball or tennis for an hour or more) were classified as physically active (High PA).
2.3 Genetic Risk
APOE genotype was determined using a PCR method described by Saunders et al. (Mayeux et al., 1998; Saunders et al., 1996). DNA was isolated with Gentra Systems Autopure LS for Large Sample Nucleic Acid Purification. Participants with one or both APOE-ε4 alleles were classified as at-risk for developing AD (High Risk); the remaining participants were classified as not at-risk (Low Risk). APOE genotype results for the four groups were as follows: Low Risk/Low PA (1 ε2/ε3; 16 ε3/ε3); Low Risk/High PA (1 ε2/ε3; 16 ε3/ε3); High Risk/Low PA (2 ε2/ε4; 15 ε3/ε4); and High Risk/High PA (15 ε3/ε4; 2 ε4/ε4).
2.4 Inclusion and Exclusion Criteria
Potential participants were excluded if they reported a history of cognitive deterioration and/or dementia, neurological disease, medical illnesses, major psychiatric disturbance meeting DSM-IV Axis I criteria, a Geriatric Depression Scale score greater than 15 and substance abuse meeting DSM-IV Axis I criteria. Participants were allowed to take cardiovascular drugs. No between-group differences were observed in the percent of participants taking blood pressure medications. A blood chemistry screen (TSH, homocysteine, vitamin B12, folate, and creatinine) indicated all participants were within normal limits, and there were no significant differences between the groups on these measures. Only right-handed participants, based on the Edinburgh Handedness Inventory (Oldfield, 1971), were included.
2.5 Procedures
Neuropsychological testing and the fMRI scanning were conducted on the same day. Participants were asked to refrain from alcohol use 24 hours and caffeine use 12 hours prior to testing. All participants received financial compensation.
2.6 Neuropsychological Testing
The neuropsychological test battery consisted of the Mini-Mental State Examination (Folstein et al., 1975), Mattis Dementia Rating Scale 2 (DRS-2) (Jurica et al., 2001); Rey Auditory Verbal Learning Test (RAVLT) (Rey, 1964), Geriatric Depression Scale (GDS) (Yesavage, 1988), and Lawton Activities of Daily Living (ADLs) (Lawton and Brody, 1969).
2.7 Functional MRI
2.7.1 Famous Name Recognition Task
The task stimuli consisted of 30 names of easily recognized famous persons (e.g., Frank Sinatra) and 30 names of unfamiliar individuals chosen from a local phone book. Only names with a high rate of identification (> 90% correct for targets and foils) were selected from an original pool of 784 names (Douville et al., 2005). A trial consisted of the visual presentation of a single name for 4 s. Participants were instructed to make a right index finger key press if the name was famous and a right middle finger key press if the name was unfamiliar. Both accuracy (% correct) and reaction time (in ms) were recorded. The 60 name trials were randomly interspersed with 20 4-s trials in which the participant was instructed to fixate on a single centrally placed crosshair in order to introduce “jitter” into the fMRI time course. The imaging run began and ended with 12 s of fixation. Total time for the single imaging run was 5 min and 24 s.
2.7.2 fMRI Acquisition
Whole-brain, event-related fMRI was conducted on a General Electric (Waukesha, WI) Signa Excite 3.0 Tesla short bore scanner equipped with a quad split quadrature transmit/receive head coil. Images were collected using an echoplanar pulse sequence (TE = 25 ms; flip angle = 77 degrees; field of view (FOV) = 240 mm; matrix size = 64 × 64). Thirty-six contiguous axial 4-mm-thick slices were selected to provide coverage of the entire brain (voxel size = 3.75 × 3.75 × 4 mm). The interscan interval (TR) was 2 s. High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired (TE = 3.9 ms; TR = 9.5 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12 degrees; number of excitations (NEX) = 2; slice thickness = 1.0 mm; FOV = 240 mm; resolution = 256 × 224). Foam padding was used to reduce head movement within the coil.
2.7.3 Image Analysis
Functional images were generated with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Each image time series was time shifted to the beginning of the TR and then spatially registered to reduce the effects of head motion using a rigid body iterative linear least squares method. A deconvolution analysis was used to extract separate hemodynamic response functions (HRFs) for famous and unfamiliar names from the time-series. HRFs were modeled for the 0–16 s period post-stimulus onset. Motion parameters were incorporated into the model as nuisance regressors. The HRFs were also transposed so that the value of the HRF at trial onset was zero. Despite the high task accuracy rate (see Table 1), estimation of the HRFs for identification of famous names and rejection of unfamiliar names was restricted to correct trials. Area under the curve (AUC) was calculated by summing the hemodynamic responses at time points 4, 6, and 8 s post trial onset. Individual anatomical and functional scans were transformed into standard stereotaxic space (Talairach and Tournoux, 1988). To compensate for normal variation in anatomy across subjects, functional images were blurred using a 6 mm Gaussian full-width half-maximum filter.
Table 1.
Participant Characteristics and Task Performance. Mean (SD) demographic characteristics, neuropsychological test results, and fMRI task performances for the four participant groups. Results of one-way ANOVAs are summarized by p-values and associated effect sizes (η2). No significant group differences emerged on any of the variables.
| Variables | Low Risk | High Risk | p | η2 | ||
|---|---|---|---|---|---|---|
| Low PA (n = 17) | High PA (n = 17) | Low PA (n = 17) | High PA (n = 17) | |||
| Demographics | ||||||
| Age (yrs) | 73.3 (5.2) | 74.3 (4.6) | 72.9 (5.2) | 70.7 (4.2) | .18 | .07 |
| Education (yrs) | 14.1 (2.5) | 14.7 (2.6) | 15.8 (2.6) | 15.7 (3.2) | .22 | .07 |
| Gender | 3M, 14F | 4M, 13F | 6M, 11F | 5M, 12F | .69 | .02 |
| Neuropsychological Testing | ||||||
| MMSE | 29.1 (1.0) | 29.3 (0.8) | 29.1 (1.0) | 29.2 (1.5) | .94 | <.01 |
| DRS Attention | 36.5 (0.7) | 36.5 (0.7) | 36.2 (1.0) | 36.5 (0.8) | .57 | .03 |
| DRS I/P | 36.5 (1.0) | 36.8 (0.4) | 36.6 (0.8) | 36.2 (2.5) | .69 | .02 |
| DRS Memory | 23.8 (1.3) | 24.3 (0.9) | 24.3 (1.6) | 24.0 (1.3) | .56 | .03 |
| DRS Total | 140.1 (1.9) | 141.1 (2.3) | 140.4 (3.7) | 139.5 (3.6) | .52 | .04 |
| AVLT Trials 1–5 | 47.3 (7.0) | 48.9 (8.1) | 47.6 (8.1) | 46.4 (9.0) | .84 | .01 |
| AVLT DR | 9.8 (1.6) | 9.7 (2.3) | 9.5 (2.9) | 9.4 (2.7) | .96 | <.01 |
| AVLT LTPR | 84.9 (13.1) | 84.4 (15.0) | 82.6 (19.8) | 80.1 (17.3) | .83 | .01 |
| GDS | 3.8 (4.3) | 2.3 (3.4) | 1.8 (2.2) | 2.4 (3.1) | .34 | .05 |
| fMRI Task Performance | ||||||
| % Correct Famous | 91.6 (9.7) | 91.8 (6.1) | 95.9 (3.8) | 92.2 (7.1) | .24 | .06 |
| % Correct Unfamiliar | 97.8 (3.5) | 96.1 (6.2) | 96.5 (5.1) | 94.7 (10.9) | .63 | .03 |
| RT Famous | 1291 (207) | 1289 (182) | 1263 (150) | 1337 (215) | .72 | .02 |
| RT Unfamiliar | 1614 (317) | 1570 (279) | 1595 (326) | 1602 (242) | .98 | <.01 |
Notes: All indices represent raw scores. High Risk = APOE-ε4 allele carrier; PA = Physical Activity; M = male; F = female; MMSE = Mini-Mental State Exam; DRS = Mattis Dementia Rating Scale; I/P = Initiation/Perseveration; AVLT = Rey Auditory Verbal Learning Test; DR = Delayed Recall; LTPR = Long-term percent retention; GDS = Geriatric Depression Scale; RT = reaction time.
2.7.4 Spatial Extent Analysis
This analysis was performed to examine between group differences in the spatial extent of activation comparing the Famous and Unfamiliar name conditions. For each group, statistical parametric maps were generated to identify voxels where the AUC for famous names differed significantly from the AUC for unfamiliar names. An individual voxel probability threshold (t (22) = 3.12, p = .005) was coupled with a minimum cluster volume threshold of 0.731 ml. This combination of individual voxel probability and minimum cluster size thresholds is equivalent to a whole brain family-wise error threshold of p < .05 based on 3,000 Monte Carlo simulations (Ward, 2000).
2.7.5 Functional Region of Interest (fROI) Analysis
A fROI analysis was conducted to evaluate potential group differences in the magnitude of the BOLD response in functionally active regions. A fROI map was generated by conjoining activated regions identified in the spatial extent analysis (see section 2.4.4) across the four groups. Any voxel deemed “activated” by the Famous-Unfamiliar name subtraction in at least one of the four groups contributed to the final fROI map. For each participant, an “averaged HRF” was calculated for all voxels within a fROI. AUC (4, 6, and 8 s post stimulus onset) served as the dependent variable in a two-way analysis of variance (ANOVA) in each fROI to examine main effects of physical activity (High vs. Low PA) and risk (High vs. Low Risk), and the interaction between PA and Risk. For significant interaction effects, follow-up contrasts were performed to identify group differences within each fROI, with the level of significance set at p < .01.
2.8 Voxel-Based Morphometry (VBM)
VBM was conducted using SPM8(Ashburner and Friston, 2005) to determine if significant group differences were observed in cortical grey matter density, which is influenced by regional brain atrophy (Good et al., 2001). Modulated grey matter density maps for each subject were blurred using a 12 mm FWHM spatial filter. Multiple regression with regressors for risk, PA, and the risk by PA interaction was conducted with total intracranial volume as a covariate. A family-wise probability threshold of p < .05 was established using an individual voxel probability threshold of p < .005 in combination with a cluster volume threshold of 2.76 ml.
3. Results
No statistically significant group differences were observed on demographic variables (age, education, or gender), neuropsychological testing, or fMRI task performance (Table 1). All groups correctly identified greater than 91% of the names as either famous or unfamiliar. A family history of AD was present in 12 of 17 participants in the High Risk/Low PA group and in 11 of 17 participants in the High Risk/High PA group; as noted above, none of the Low Risk participants had a family history of AD.
3.1 Spatial extent analysis
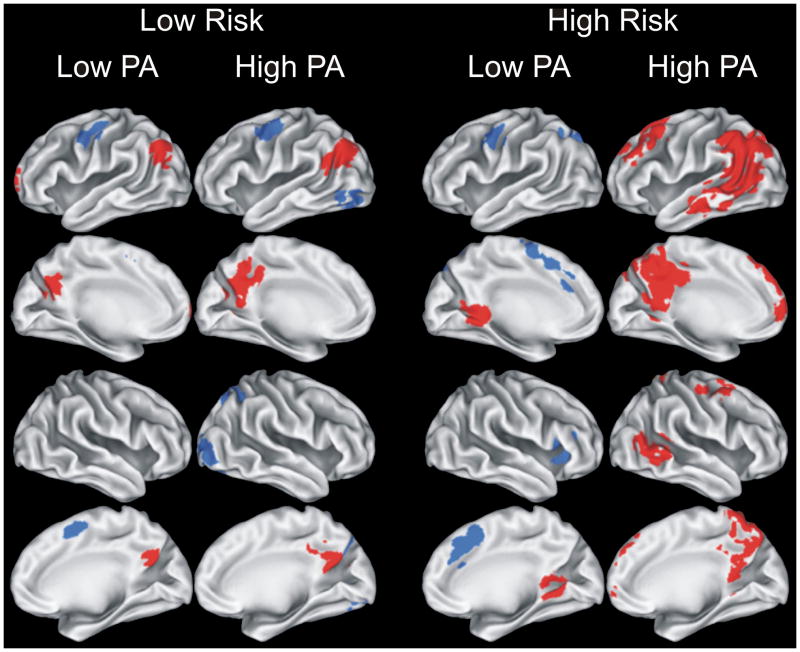
Results of the voxelwise analysis comparing the Famous and Unfamiliar name conditions are presented in Figure 1 and Table 2. Of note, the Famous > Unfamiliar subtraction (shown in red on Figure 1) resulted in a greater volume of semantic processing-related activation in the High Risk/High PA group (39.7 ml) compared to the other three groups: Low Risk/Low PA (4.0 ml), Low Risk/High PA (7.8 ml), and High Risk/Low PA (2.6 ml). As expected, greater semantic memory processing (Famous > Unfamiliar) was associated with a greater spatial extent of activation in the left than right hemisphere. Additionally, all groups, except the High Risk/High PA group, showed areas of activation where the contrast was greater for Unfamiliar compared to Famous names (shown in blue on Figure 1).
Figure 1.
Results of voxelwise analysis showing brain regions demonstrating significant differences between Famous and Unfamiliar name conditions for each of the four groups (Low Risk/Low PA; Low Risk/High PA; High Risk/Low PA; and High Risk/High PA). Areas in red indicate Famous > Unfamiliar; blue areas indicate Unfamiliar > Famous. Location and volume of activation foci delineated in Table 2. PA = physical activity.
Table 2.
Activation Foci and Volumes. Location and spatial extent of activated regions based on a voxelwise analysis comparing the Famous and Unfamiliar name conditions (see Methods for details). Activation foci are shown separately for each of the four groups, with Famous > Unfamiliar regions shown in the upper table half and Unfamiliar > Famous regions shown in lower half. Activated regions are depicted in Figure 1.
| Low Risk | High Risk | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low PA | High PA | Low PA | High PA | |||||||||||||||
| Side | Region | BA | x | y | z | vol | x | y | z | vol | x | y | z | vol | x | y | z | vol |
| Famous > Unfamiliar | ||||||||||||||||||
| Frontal Lobes | ||||||||||||||||||
| L | SFG/MFG | 6,8 | −28 | 17 | 49 | 4.3 | ||||||||||||
| L | SFG/MFG | 8,9 | −33 | 38 | 38 | 2.2 | ||||||||||||
| R | SFG/MFG | 4,6,32 | 30 | 4 | 50 | 1.3 | ||||||||||||
| B | SFG/MdFG | 8,9 | −2 | 49 | 44 | 1.3 | ||||||||||||
| L | SFG/MdFG | 9,10 | −24 | 63 | 13 | 1.1 | −6 | 61 | 12 | 0.9 | ||||||||
| Parietal/Temporal Lobes | ||||||||||||||||||
| B | PC/Precuneus | 7,23,31 | −2 | −58 | 25 | 1.3 | −3 | −52 | 28 | 3.6 | ||||||||
| R | PC/Retrosplenial | 23,29,30 | 10 | −48 | 5 | 1.3 | ||||||||||||
| L | PC/Retrosplenial | 23,29,30 | −11 | −46 | 3 | 1.3 | ||||||||||||
| L | STG/MTG/AG/SMG | 7,22,39,40 | −44 | −65 | 29 | 1.6 | −42 | −67 | 30 | 4.2 | −28 | −54 | 28 | 26.3 | ||||
| R | STG/MTG | 22,37,39 | 52 | −53 | 6 | 2.1 | ||||||||||||
| L | MTG/ITG | 21 | −54 | −22 | −11 | 1.5 | ||||||||||||
| Total Volume of Activated Tissue (ml) | 4.0 | 7.8 | 2.6 | 39.7 | ||||||||||||||
| Unfamiliar > Famous | ||||||||||||||||||
| Frontal Lobes | ||||||||||||||||||
| L | PreG/MFG | 4,6 | −45 | −8 | 44 | 0.8 | −40 | −6 | 47 | 1.1 | −47 | −2 | 40 | 0.8 | ||||
| B | SMA/MdFG/AC | 6,8,24,32 | 6 | 4 | 53 | 0.8 | 1 | 17 | 44 | 3.5 | ||||||||
| R | Insula/IFG | 44,45 | 38 | 20 | 10 | 1.7 | ||||||||||||
| Parietal/Temporal Lobes | ||||||||||||||||||
| L | Precuneus/SPL | 7 | −22 | −65 | 42 | 1.4 | ||||||||||||
| R | Precuneus/SPL | 7 | 24 | −58 | 42 | 2.1 | ||||||||||||
| Occipital Lobes | ||||||||||||||||||
| R | MOG/IOG/LG/FG | 18,19 | 32 | −80 | 1 | 1.8 | ||||||||||||
| L | MOG/IOG/LG/FG | 18,19 | −37 | −71 | −9 | 0.8 | ||||||||||||
| Total Volume of Activated Tissue (ml) | 1.6 | 5.8 | 7.4 | 0.0 | ||||||||||||||
Notes: BA = Brodmann areas; Risk = +/− APOE-ε4; PA = Physical Activity; L = left; R = right, B = bilateral; positive = right (x), anterior (y), and superior (z), representing center of mass in Talairach coordinates; vol = volume in ml; PreG = precentral g.; MFG = middle frontal g.; SFG = superior frontal g.; MdFG = Medial Frontal g.; SMA = supplementary motor area; AC = anterior cingulate; IFG = inferior frontal g.; SPL = superior parietal lobule; PC = posterior cingulate; STG = superior temporal g.; MTG = middle temporal g.; AG = angular g.; SMG = supramarginal g.; MOG = middle occipital g.; IOG = inferior occipital g.; LG = lingual g.; FG = fusiform g.
3.2 fROI Analysis
The conjunction analysis produced 15 fROIs (Table 3 and Figures 2 and 3). A 2 (PA) × 2 (Risk) ANOVA was performed on the average AUC of all voxels within each fROI.
Table 3.
Functional Regions of Interest (fROIs) Analysis. fROIs derived by conjoining the voxelwise maps of the four groups (Table 2, Figure 1). For each fROI, the three rightmost columns represent results of a 2 × 2 ANOVA for the main effects of Physical Activity (PA) and Risk, and their interaction (Int) effect. Results of the ANOVA are represented in Figures 2 and 3.
| # | Side | Region | BA | x | y | z | vol | PA: p (ηp2) | Risk: p (ηp2) | Int: p (ηp2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Lobes | ||||||||||
| 1 | L | SFG/MFG | 6, 8 | −28 | 17 | 49 | 4.3 | .145 (.033) | .178 (.028) | .022 (.079) |
| 2 | B | MdFG/SMA | 6,32 | 2 | 14 | 46 | 4.3 | .028 (.073) | .779 (.001) | .067 (.051) |
| 3 | L | PreG/MFG | 6 | −43 | −6 | 44 | 2.3 | .045 (.062) | .096 (.043) | .286 (.018) |
| 4 | L | SFG/MFG | 8,9 | −33 | 38 | 38 | 2.2 | .044 (.062) | .059 (.054) | .004 (.120) |
| 5 | L | SFG/MFG | 10,32 | −16 | 62 | 13 | 2.0 | .339 (.014) | .331 (.015) | < .001 (.175) |
| 6 | R | Insula/IFG | 44,45 | 38 | 20 | 10 | 1.7 | .136 (.034) | .327 (.036) | .007 (.109) |
| 7 | L | SFG/MdFG | 8,9 | −2 | 49 | 44 | 1.3 | .948 (.000) | .023 (.078) | .008 (.105) |
| 8 | R | SFG/MFG | 4,6,32 | 30 | 4 | 50 | 1.3 | .039 (.065) | .016 (.087) | .180 (.028) |
| Parietal/Temporal Lobes | ||||||||||
| 9 | L | MTG/AG/SMG, Precuneus | 7,22,39,40 | −44 | −57 | 24 | 19.2 | .052 (.058) | .703 (.002) | .013 (.092) |
| 10 | B | PC, Retrosplenial, Precuneus | 7,23,29,30 | −2 | −54 | 30 | 16.6 | .332 (.015) | .104 (.041) | .248 (.021) |
| 11 | R | Precuneus/SPL | 7 | 24 | −58 | 42 | 2.1 | .845 (.001) | .075 (.054) | .035 (.068) |
| 12 | R | STG/AG | 22,37,39 | 52 | −53 | 6 | 2.1 | .311 (.016) | .127 (.036) | .052 (.058) |
| 13 | L | MTG/ITG | 21 | −54 | −21 | −11 | 1.5 | .001 (.152) | .005 (.112) | .675 (.003) |
| Occipital Lobes | ||||||||||
| 14 | R | MOG/IOG/LG/FG | 18,19 | 32 | −80 | 1 | 1.8 | .719 (.002) | .063 (.053) | .112 (.039) |
| 15 | L | MOG/IOG/LG/FG | 18,19 | −37 | −71 | −9 | 0.8 | .232 (.022) | .373 (.012) | .013 (.092) |
Notes: ηp2 = partial eta2 (effect size); # corresponds with regions shown in the left panel and panels A, B & C of Fig. 3; BA = Brodmann areas; Risk = +/− APOE-ε4; positive = right (x), anterior (y), and superior (z), representing center of mass in Talairach coordinates; vol = volume in ml; PreG = precentral g.; MFG = middle frontal g.; SFG = superior frontal g.; MdFG = Medial Frontal g.; SMA = supplementary motor area; IFG = inferior frontal g.; SPL = superior parietal lobule; PC = posterior cingulate; STG = superior temporal g.; MTG = middle temporal g.; AG = angular g.; SMG = supramarginal g.; ITG = inferior temporal g.; MOG = middle occipital g.; IOG = inferior occipital g.; LG = lingual g.; FG = fusiform g.
Figure 2.
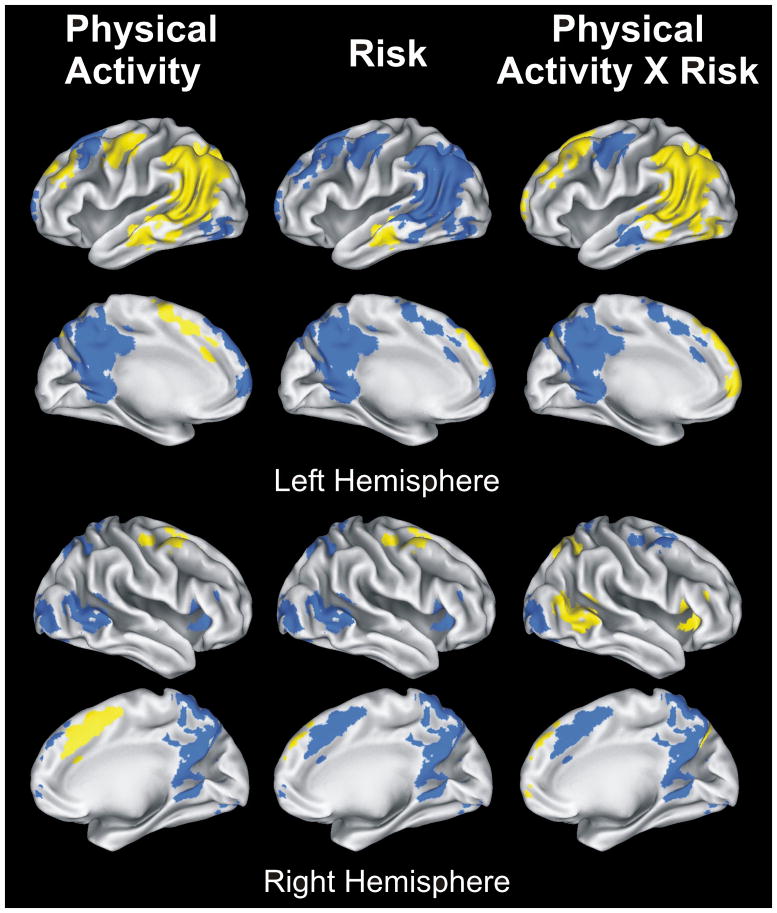
Results of 2 (Physical Activity) × 2 (Risk) ANOVA conducted on 15 fROIs (p-values shown in 3 rightmost columns of Table 3). Yellow regions indicate significant effects; blue areas indicate non-significant effects. Main effects of Physical Activity and Risk are shown in left and middle panels; interaction effect (Physical Activity × Risk) in right panel. See Figure 3 for bar graphs illustrating significant main and interaction effects.
Figure 3.
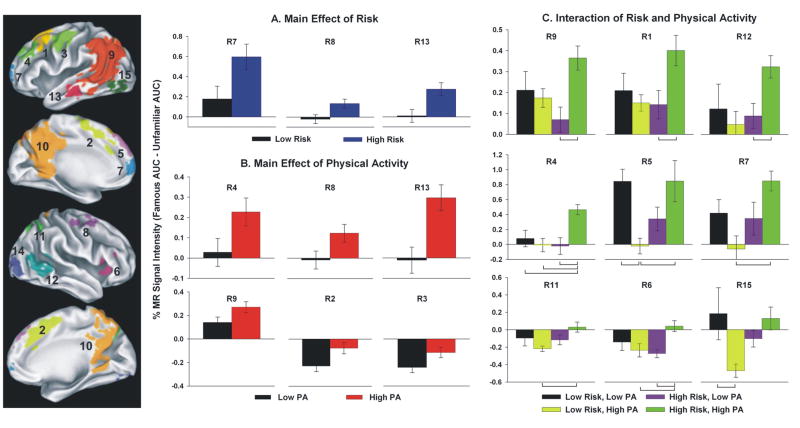
The 15 functional regions of interest (fROIs) are identified in the left panel with region numbers (R#) corresponding with activation foci delineated in Table 3. Bar graphs represent mean percent MR signal intensity change for main effects of Physical Activity and Risk (panels A and B, respectively) and interaction effect of Physical Activity × Risk (panel C). Post-hoc group differences are indicated by brackets in panel C (p < .01). Error bars = S.E.M.
The main effect of Risk produced significant differences in 3 of 15 fROIs: left superior frontal gyrus (SFG)/medial frontal gyrus (MdFG), right middle frontal gyrus (MFG)/SFG, and left middle temporal gyrus (MTG)/inferior temporal gyrus (ITG). In all cases, High Risk was associated with significantly greater semantic memory activation (Famous > Unfamiliar) than the Low Risk group (Figure 3, panel A), consistent with our previously reported findings (Seidenberg et al., 2009).
The main effect of PA resulted in significant differences in 6 of 15 fROIs. In all cases, greater positive activity (Famous > Unfamiliar) or less negative activity (Unfamiliar > Famous) was observed in the physically active group as opposed to the physically inactive group (Figure 3, panel B). fROIs showing this main effect included: bilateral MdFG/supplementary motor area (SMA); left precentral gyrus (PreG)/MFG; left MFG/SFG; right MFG/SFG; left MTG/angular gyrus (AG)/supramarginal gyrus (SMG)/precuneus; and left MTG/inferior temporal gyrus (ITG).
Nine of 15 fROIs produced significant PA × Risk interaction effects. Confirming the results of the spatial extent analysis, the High Risk/High PA group typically exhibited greater semantic processing (Famous > Unfamiliar) or less ‘negative’ activity (Unfamiliar > Famous) than one or more of the remaining three groups (Figure 3, panel C). These regions included: three regions of the left SFG/MFG; right insula/inferior frontal gyrus (IFG); left SFG/MdFG; left MTG/angular gyrus (AG)/supramarginal gyrus (SMG)/precuneus; right precuneus/superior parietal lobule (SPL); right STG/AG; and left middle occipital gyrus (MOG)/inferior occipital gyrus (IOG)/lingual gyrus (LG)/fusiform gyrus (FG). Post-hoc group differences are indicated by brackets in Figure 3 (panel C).
There are two important features of the interaction between PA and Risk. First, the High Risk/High PA group had the greatest spatial extent of activation. Additionally, differences in the spatial extent of activation based on PA status were more apparent in the high than low risk groups (Figure 1, Table 2). Second, the greatest degree of separation between activation for famous and unfamiliar names was consistently found in the High Risk/High PA group. Moreover, the effects of PA on task-related activation were reliably more pronounced in APOE-ε4 carriers (Figure 3, panel C). Only two out of 15 task-activated regions were different between High and Low PA groups among non-carriers of the APOE-ε4 allele (Low Risk).
3.3 Voxel-Based Morphometry (VBM)
No significant regions were identified based on the main effects of risk and PA and the interaction of risk and PA.
4. Discussion
Our results provide the first evidence that leisure-time PA can influence brain activation patterns within memory-related brain circuits in cognitively intact elders at risk for developing AD. Specifically, we have shown that enhanced semantic memory processing is observed in more physically active elders and this activation difference in memory circuits is most pronounced in those individuals who possess one or both APOE-ε4 alleles. A fundamental interpretive question remains: is enhanced semantic memory processing, characterized by greater fMRI response to famous than unfamiliar names, a positive or negative indicator of future cognitive decline? In a longitudinal study of cognitively intact individuals at varying risk for developing AD, we (Woodard et al., in press) have shown that baseline fMRI brain activation to the famous name recognition task was protective against a decline in neuropsychological test performance after a follow-up period of 18 months. Taken together, results from these two studies suggest that PA enhances memory circuit performance in individuals at-risk for AD in a manner that is protective against future cognitive decline.
Our results concur with two recent population-based studies of the effects of PA on the diagnosis of cognitive impairment in elders. In a two year prospective study, Etgen and colleagues (Etgen et al., 2010) reported significant reductions in incident cognitive impairment for cognitively intact elders who engaged in either moderate or high intensity physical activity at baseline. Geda and colleagues (Geda et al., 2010) conducted a case-control study in 198 patients diagnosed with mild cognitive impairment (MCI), a prodromal condition with a high rate of conversion to AD, and 1126 healthy controls. After controlling for age, sex, education, comorbid medical conditions, and depression, the odd ratios for being diagnosed with MCI were significantly reduced for those who engaged in moderate intensity PA during both mid-life (50–65 years; OR = 0.61) and late-life (>65 years; OR = 0.68).
Previous reports have shown that performance on standardized neuropsychological tests can be influenced by both APOE-ε4 status and PA. In one study, a nearly four-fold risk of cognitive decline was reported among APOE-ε4 allele carriers who engaged in less than one hour of PA compared to carriers who were more physically active (Schuit et al., 2001). In another study, the protective effects of PA on future cognitive decline was specific to APOE-ε4 carriers (Rovio et al., 2005). Significant positive relationships specific to homozygous ε4 carriers have been reported between greater cardiorespiratory fitness and performance on neuropsychological tests (Etnier et al., 2007). In contrast, one study found that the beneficial effects of PA on cognition were found to be independent of APOE-ε4 status (Lindsay et al., 2002). Another group reported that PA reduced the risk for dementia, but only in APOE-ε4 non-carriers (Podewils et al., 2005). We did not observe between group differences on either neuropsychological test scores or fMRI task performance. This finding is not surprising because we purposely excluded potential participants with evidence of cognitive impairment in order to focus on possible intergroup differences in brain activation patterns. Our fMRI results nonetheless complement the previously published neuropsychological studies by showing that moderately physically active APOE-ε4 carriers are able to engage semantic processing networks to a greater extent than those individuals engaging in little or no leisure-time physical activity.
Previous fMRI studies conducted in older individuals at-risk for AD have typically used episodic memory activation tasks (Johnson et al., 2006; Pihlajamaki et al., 2009) because episodic memory is the most pronounced cognitive deficit observed in MCI and the early stages of AD (Dubois et al., 2007). Episodic memory tasks, however, are effortful and result in numerous errors even in cognitively intact elders. These errors can have a detrimental impact on the interpretation of brain activation patterns, since extraneous non-memory brain regions associated with error detection and arousal will become activated (Carter et al., 2000). In contrast, our group has employed a low effort, high accuracy semantic memory task involving the discrimination between famous and unfamiliar names (Douville et al., 2005; Nielson et al., 2006; Seidenberg et al., 2009; Woodard et al., 2009; Woodard et al., 2007). This task activates key memory regions, including the lateral temporal-parietal, posterior cingulate/precuneus, and medial prefrontal regions. These regions overlap extensively with a predominantly left lateralized semantic processing system described in a recent meta-analysis (Binder et al., 2009) as well as with the so-called default mode network (DMN) observed in individuals scanned during rest (Buckner et al., 2008). It has been suggested that this overlap occurs because the resting state invokes random semantic processes (Binder et al., 2009). Previous research on the DMN has identified compromised neuronal efficiency in asymptomatic individuals at-risk for AD (Buckner, 2004). Furthermore, cognitively intact APOE-ε4 carriers demonstrate increased amyloid accumulation in brain areas that overlap with the semantic processing system and the DMN (Reiman et al., 2009). Our study identified an interaction of PA with APOE-ε4 status involving nine of 15 fROIs comprising the semantic memory system. The current findings, therefore, suggest that PA promotes engagement of the extended cortical networks related to semantic memory system and the DMN in individuals at genetic risk for developing AD.
Several fMRI studies have suggested that brain activation patterns in otherwise healthy older adults may be predictive of future cognitive decline, although the results have been contradictory. Some have shown that increased episodic memory activation may be predictive of future cognitive decline (Bookheimer et al., 2000; O’Brien et al., in press). Other studies have shown the opposite pattern: increased activation predicts cognitive stability (Lind et al., 2006a; Lind et al., 2006b). Our recent longitudinal fMRI study (Woodard et al., in press), which used the same activation task as in the current study, showed that increased semantic memory activation is protective against future cognitive decline after an 18 month test-retest interval. Two factors may explain these apparent contradictory findings. One is the choice of activation task: previous studies have used effortful episodic memory tasks that are prone to large numbers of errors. Errors can influence the pattern of activation especially in blocked design administration formats that can not easily remove error trials from the resulting brain maps. In contrast, our event-related task, reported here and in Woodard et al. (in press), used a low effort, high accuracy (>90% correct) semantic memory task in which the few error trials that do occur were excluded from the final image analyses. A second factor is the brain activation regions selected to predict future cognitive decline. Sperling and colleagues (Miller et al., 2008; O’Brien et al., in press) have demonstrated that increased hippocampal activation during the encoding of episodic information predicts cognitive decline over the course of two to six years. In the current study, we observed increased activation within cortical, as opposed to hippocampal memory regions. We speculate that exercise promotes greater neural efficiency in cortical memory areas that compensate for inefficiencies associated with medial temporal lobe dysfunction (Cabeza et al., 2002). Our results go one step further and suggest that the PA-mediated cortical compensatory response is observed predominantly in individuals at-risk for AD.
Our research begs the question as to why semantic memory activation might be differentially greater in high PA individuals at risk for AD. Previous research has demonstrated that PA increases high-density lipoprotein cholesterol and reduces triglyceride levels to a greater extent among ε4 than non-ε4 carriers (Bernstein et al., 2002). Exercise is also known to produce strong anti-inflammatory effects through the release of IL-6 from skeletal muscle (Petersen and Pedersen, 2005). Markers of oxidative stress and pro-inflammation (Glodzik-Sobanska et al., 2009; Licastro et al., 2007), along with lipid dysregulation (Jarvik et al., 1995), are more apparent in ε4 carriers and may contribute to their increased risk for AD. Although it has been suggested that the anti-inflammatory effects of exercise extend to brain (Ang and Gomez-Pinilla, 2007), it is unclear whether these effects interact with the presence of the ε4 allele. We speculate that PA may preserve compensatory neural responses by reducing inflammation and enhancing oxidation of fatty acids in ε4 positive elders. The neural effects of PA may be attenuated in individuals at lower genetic risk for AD.
Regardless of AD risk, physically active, cognitively asymptomatic older adults showed greater semantic memory processing in six of 15 fROIs. These results are consistent with previous brain imaging studies of the effects of PA. Colcombe and colleagues (Colcombe et al., 2004), using a flanker task, showed a differential activation pattern among older adults depending on their physical fitness level. Specifically, more physically fit adults showed greater functional activation in the right middle frontal gyrus and superior frontal gyrus and less activation in the anterior cingulate cortex than less physically fit participants (Colcombe et al., 2004). Reduced activation in the anterior cingulate cortex is frequently associated with better levels of task performance (Carter et al., 2000). A magnetoencephalography study conducted during the performance of the Sternberg working memory task found that the M170 component in the right temporal lobe was larger in amplitude among physically active APOE-ε4 carriers compared to their less physically active counterparts (Deeny et al., 2008). Based on these brain imaging studies and our current study, we speculate that PA delays age-related cognitive decline by promoting greater cognitive/neural reserve (Stern et al., 2008).
It is important to consider possible neurophysiological evidence that may underlie the effects of PA on cognition and brain function. Exercise has been shown to stimulate transcription and expression of neural growth factors in rodents and exercise leads to neurogenesis in the dentate gyrus (van Praag et al., 2005). In addition, the cholinergic effects of exercise may increase perfusion, enhance neural recruitment, and possibly attenuate the accumulation of beta-amyloid. In a transgenic mouse model of AD, voluntary exercise decreased amyloid burden in the frontal and temporal cortices and in the hippocampus (Adlard et al., 2005). Exercise training also reversed the activation of AChE in the hippocampus and cerebral cortex of ovariectomized rats (Ben et al., 2009), similar to the mechanism of action for prescribed AchE-inhibitors in AD patients. In healthy adults, exercise training increased cerebral blood volume in the dentate gyrus (Pereira et al., 2007). Whether these potential mechanisms are more potent among APOE-ε4 carriers is unknown.
PA and enhanced cardiorespiratory fitness in healthy older adults are associated with attenuation of age-related brain tissue atrophy, especially within the hippocampus (Colcombe et al., 2006; Erickson et al., 2009; Gordon et al., 2008). Attenuation of whole brain atrophy is also associated with greater cardiorespiratory fitness in early AD patients (Burns et al., 2008). In the current cross-sectional study, the VBM analysis revealed no differences in gray matter density between groups. Our inability to replicate the previous findings may result from the observation that our high PA participants were less physically fit than in previous studies. Only a small minority of our participants reported strenuous exercise (e.g., running more than three days per week). In addition, precise measurements of the rate of atrophy require longitudinal data.
The brain maps generated from our semantic memory task involve a subtraction of the blood oxygen level dependent (BOLD) contrast between two active states: famous versus unfamiliar name discrimination. An alternative design would be to compare each task to the resting state baseline. This latter method would create maps that not only incorporate brain regions specific to semantic memory processing, but also regions subserving primary sensory and motor regions that are less relevant for examining the cognitive effects of aging. In addition, the subtraction of two active cognitive states can minimize the potential influence of increased cerebral perfusion and cerebral blood volume associated with greater PA (Pereira et al., 2007). Future studies may wish to employ perfusion MRI techniques to examine its impact on BOLD activation as a function of PA.
In the present study, risk was defined as possession of at least one APOE-ε4 allele. All participants were cognitively intact despite a fairly wide age range. While it is expected that some of the High Risk participants will go on to show the symptoms of AD, it is clear that the possession of one or both APOE-ε4 alleles is an imperfect predictor of AD (Devanand et al., 2005; McConnell et al., 1999). Other genetic factors (Roses et al., 2009) and lifestyle characteristics (Wu et al., 2008) may confer protection from AD such that some or even all of our High Risk individuals may never develop AD. Fortunately, all the participants are part of a longitudinal study that will allow us to determine which of them eventually develop AD and which of several factors are most associated with decline or protection from AD.
The High PA/Low Risk group included two participants with the ε2ε4 genotype. Although the APOE ε2 allele has been suggested to confer a neuroprotective effect, case-control data from the Rotterdam Study (Slooter et al., 1998) revealed that the this effect is clearest for the ε2ε3 genotype. The adjusted odds ratios for AD and for all dementia for the ε2ε3 genotype was 0.4 and 0.5, respectively. In contrast, the same odds ratios for the ε2ε4 genotype were 1.3 and 3.6 for AD and for all dementia, respectively. Thus, there remains an increased risk for both AD and all dementia in persons with the ε2ε4 genotype. Exclusion of these two participants did not substantially alter the observed effect sizes in the main and interaction fROI analyses.
Additional limitations of this study include the cross-sectional design, lack of control for other health-related behaviors (e.g., diet), and the use of a self-report measure of leisure-time PA rather than an assessment of cardiorespiratory fitness. It is important to note, however, that responses on the SBAS were related to cardiovascular risk biomarkers and estimated energy expenditure in a dose-dependent fashion in a large sample (Taylor-Piliae et al., 2006). In addition, self-reported PA, in comparison to estimates of cardiorespiratory fitness, has been shown to be an equally important predictor of all-cause mortality (Myers et al., 2004) and cardiovascular events (Talbot et al., 2002) in older adults. The positive effects of PA on the cognitive function of older adults are more consistently observed using self-report than objective fitness outcome measures (Paterson and Warburton, 2010). In a clinical setting, self-report assessments are more easily translated into specific recommendations for patients to increase PA (Nelson et al., 2007). Nevertheless, it is conceivable that an objective measure of aerobic capacity, such as a graded exercise test to determine maximal rate of oxygen consumption (VO2max), may provide greater sensitivity to the interactions between APOE status and PA than our subjective measure.
In summary, our fMRI results provide preliminary evidence that leisure-time PA may serve as an effective lifestyle intervention for maintaining memory function among older APOE-ε4 carriers. These results may provide the impetus for future large-scale follow-up studies. A controlled clinical trial, using fMRI as a possible outcome measure, may determine if exercise intervention is effective in improving brain activation patterns in sedentary at risk individuals. Long-term longitudinal studies may also determine if PA synergizes with other environmental or behavioral factors to reduce the likelihood of conversion to AD (Hertzog et al., 2009).
Acknowledgments
The authors thank Qi Zhang and Amelia Gander for their help with subject recruitment and data collection. This project was supported by the National Institute on Aging (R01 AG022304), Medical College of Wisconsin General Clinical Research Center (M01 RR00058), and the Medical College of Wisconsin Advancing a Healthier Wisconsin Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Abbreviations: PA = physical activity; APOE-ε4 = ApolipoproteinE-epilson4; AD = Alzheimer’s disease; MCI = mild cognitive impairment; SBAS = Stanford Brief Activity Survey
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J. Carson Smith, Email: jcarson@uwm.edu.
Kristy A. Nielson, Email: kristy.nielson@marquette.edu.
John L. Woodard, Email: john.woodard@wayne.edu.
Michael Seidenberg, Email: michael.seidenberg@rosalindfranklin.edu.
Sally Durgerian, Email: sallyd@mcw.edu.
Piero Antuono, Email: pantuono@mcw.edu.
Alissa M. Butts, Email: alissa.butts@marquette.edu.
Nathan C. Hantke, Email: nathan.hantke@marquette.edu.
Melissa A. Lancaster, Email: melissa.lancaster@my.rfums.org.
Stephen M. Rao, Email: raos2@ccf.org.
References
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14:2564–2571. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ben J, Soares FM, Cechetti F, Vuaden FC, Bonan CD, Netto CA, Wyse AT. Exercise effects on activities of Na(+),K(+)-ATPase, acetylcholinesterase and adenine nucleotides hydrolysis in ovariectomized rats. Brain Res. 2009;1302:248–255. doi: 10.1016/j.brainres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bernstein MS, Costanza MC, James RW, Morris MA, Cambien F, Raoux S, Morabia A. Physical activity may modulate effects of ApoE genotype on lipid profile. Arterioscler Thromb Vasc Biol. 2002;22:133–140. doi: 10.1161/hq0102.101819. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cereb Cortex. 2009;12:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burns JM, Mayo MS, Anderson HS, Smith HJ, Donnelly JE. Cardiorespiratory fitness in early-stage Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:39–46. doi: 10.1097/WAD.0b013e31815a9ddc. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Deeny SP, Poeppel D, Zimmerman JB, Roth SM, Brandauer J, Witkowski S, Hearn JW, Ludlow AT, Contreras-Vidal JL, Brandt J, Hatfield BD. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78:179–187. doi: 10.1016/j.biopsycho.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Pelton GH, Zamora D, Liu X, Tabert MH, Goodkind M, Scarmeas N, Braun I, Stern Y, Mayeux R. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62:975–980. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Franczak M, Antuono P, Rao SM. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170:186–193. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, McLemore EC. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39:199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Pirraglia E, Brys M, de Santi S, Mosconi L, Rich KE, Switalski R, Saint Louis L, Sadowski MJ, Martiniuk F, Mehta P, Pratico D, Zinkowski RP, Blennow K, de Leon MJ. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer’s disease. Neurobiol Aging. 2009;30:672–681. doi: 10.1016/j.neurobiolaging.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Sci Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Jarvik GP, Wijsman EM, Kukull WA, Schellenberg GD, Yu C, Larson EB. Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer’s disease: a case-control study. Neurology. 1995;45:1092–1096. doi: 10.1212/wnl.45.6.1092. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML, Asthana S, Chen K, Reiman EM, Alexander GE. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006;27:1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2 professional manual. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Rovio S, Ngandu T, Kareholt I, Eskelinen M, Winblad B, Hachinski V, Cedazo-Minguez A, Soininen H, Tuomilehto J, Nissinen A. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12:2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Licastro F, Porcellini E, Caruso C, Lio D, Corder EH. Genetic risk profiles for Alzheimer’s disease: integration of APOE genotype and variants that up-regulate inflammation. Neurobiol Aging. 2007;28:1637–1643. doi: 10.1016/j.neurobiolaging.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lind J, Ingvar M, Persson J, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Parietal cortex activation predicts memory decline in apolipoprotein E-epsilon4 carriers. Neuroreport. 2006a;17:1683–1686. doi: 10.1097/01.wnr.0000239954.60695.c6. [DOI] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Backman L, Nilsson LG, Petersson KM, Nyberg L. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain. 2006b;129:1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, Hyman BT, Crain B, Tang MX, Phelps CH. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med. 1998;338:506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- McConnell LM, Sanders GD, Owens DK. Evaluation of genetic tests: APOE genotyping for the diagnosis of Alzheimer disease. Genet Test. 1999;3:47–53. doi: 10.1089/gte.1999.3.47. [DOI] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, Yamazaki T, Froelicher V. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;117:912–918. doi: 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, Antuono P, Rao SM. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol Aging. 2006;27:1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JL, O’Keefe KM, Laviolette PS, Deluca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. doi: 10.1212/WNL.0b013e3181e3966e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s Physical Activity Guidelines. Int J Behav Nutr Phys Act. 2010;7:38. doi: 10.1186/1479-5868-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, O’Keefe K, Bertram L, Tanzi RE, Dickerson BC, Blacker D, Albert MS, Sperling RA. Evidence of Altered Posteromedial Cortical fMRI Activity in Subjects at Risk for Alzheimer Disease. Alzheimer Dis Assoc Disord. 2009;24:28–36. doi: 10.1097/WAD.0b013e3181a785c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen clinique en psychologie. Presses Universitaires; de France Paris: 1964. [Google Scholar]
- Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, Huentelman MJ, Welsh-Bohmer KA, Reiman EM. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2009 doi: 10.1038/tpj.2009.69. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Hulette O, Welsh-Bohmer KA, Schmechel DE, Crain B, Burke JR, Alberts MJ, Strittmatter WJ, Breitner JC, Rosenberg C. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer’s disease. Lancet. 1996;348:90–93. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–777. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, Zhang Q, Rao SM. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73:612–620. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooter AJ, van Duijn CM, Bots ML, Ott A, Breteler MB, De Voecht J, Wehnert A, de Knijff P, Havekes LM, Grobbee DE, Van Broeckhoven C, Hofman A. Apolipoprotein E genotype, atherosclerosis, and cognitive decline: the Rotterdam Study. J Neural Transm Suppl. 1998;53:17–29. doi: 10.1007/978-3-7091-6467-9_3. [DOI] [PubMed] [Google Scholar]
- Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, Flynn J, Steffener J, Brown T. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18:959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart: 1988. [Google Scholar]
- Talbot LA, Morrell CH, Metter EJ, Fleg JL. Comparison of cardiorespiratory fitness versus leisure time physical activity as predictors of coronary events in men aged < or = 65 years and > 65 years. Am J Cardiol. 2002;89:1187–1192. doi: 10.1016/s0002-9149(02)02302-0. [DOI] [PubMed] [Google Scholar]
- Taylor-Piliae RE, Haskell WL, Iribarren C, Norton LC, Mahbouba MH, Fair JM, Hlatky MA, Go AS, Fortmann SP. Clinical utility of the Stanford brief activity survey in men and women with early-onset coronary artery disease. J Cardiopulm Rehabil Prev. 2007;27:227–232. doi: 10.1097/01.HCR.0000281768.97899.bb. [DOI] [PubMed] [Google Scholar]
- Taylor-Piliae RE, Norton LC, Haskell WL, Mahbouda MH, Fair JM, Iribarren C, Hlatky MA, Go AS, Fortmann SP. Validation of a new brief physical activity survey among men and women aged 60–69 years. Am J Epidemiol. 2006;164:598–606. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. AFNI User’s Manual. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Woodard J, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, Guidotti L, Zhang Q, Butts A, Hantke N, Lancaster M, Rao SM. Prediction of cognitive decline in healthy older adults using fMRI. J Alzheimer’s Dis. doi: 10.3233/JAD-2010-091693. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Zhang Q, Lancaster M, Hantke N, Butts A, Rao SM. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Miller SK, Franczak M, Antuono P, Douville KL, Rao SM. Temporally graded activation of neocortical regions in response to memories of diffferent ages. J Cog Neurosci. 2007;19:1–12. doi: 10.1162/jocn.2007.19.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]