Abstract
Although variations of response time (RT) within a particular experimental condition are typically ignored, they may sometimes reflect meaningful changes in the efficiency of cognitive and neural processes. In the present study, we investigated whether trial-by-trial variations of response time (RT) in a cross-modal selective attention task were associated with variations of functional connectivity between brain regions that are thought to underlie attention. Sixteen healthy young adults performed an audiovisual selective attention task, which involved attending to a relevant visual letter while ignoring an irrelevant auditory letter, as we recorded their brain activity using functional magnetic resonance imaging (fMRI). In line with predictions, variations of RT were associated with variations of functional connectivity between the anterior cingulate cortex and various other brain regions that are posited to underlie attentional control, such as the right dorsolateral prefrontal cortex and bilateral regions of the posterior parietal cortex. They were also linked to variations of functional connectivity between anatomically early and anatomically late regions of the relevant-modality visual cortex whose communication is thought to be modulated by attentional control processes. By revealing that variations of RT in a selective attention task are linked to variations of functional connectivity in the attentional network, the present findings suggest that variations of attention may contribute to trial-by-trial fluctuations of behavioral performance.
Keywords: attention, visual, auditory, functional connectivity, fMRI, response time
Introduction
An axiom of human behavior is that it varies appreciably from one moment to the next even in the same behavioral context. Although typically chalked up to “noise”, such variance is increasingly thought to reflect meaningful fluctuations in the efficiency of cognitive and neural processes underlying task performance (Bellgrove et al., 2004; Castellanos et al., 2005; Gilbert et al., 2006; Gilden, 2001; Hahn et al., 2006; Weissman et al. 2006). For example, excessive response time (RT) variability in selective attention tasks is thought to reflect problems with attentional control in people who have frontal lobe damage (Stuss et al., 2003), attention-deficit and hyperactivity disorder (ADHD) (Castellanos et al., 2005), sleep-deprivation (Chee et al., 2008), or advanced age (West et al., 2002). Further developing our understanding of the psychological and neurological sources of RT variability is therefore important from both theoretical and clinical perspectives.
To this end, we have begun using functional magnetic resonance imaging (fMRI) to investigate whether variations of attention contribute to variations of RT across trials in tasks requiring selective attention (Chee et al., 2008; Weissman et al., 2006; Weissman et al., 2009). In our studies, participants were instructed to identify a relevant stimulus (e.g., a visual letter) as quickly as possible without making mistakes while ignoring an irrelevant distractor (e.g., an auditory letter). Thus, increases of RT across trials might, in part, have reflected reductions of attention to the relevant stimulus and/or failures to suppress the irrelevant distractor. Of importance, the nature of trial-by-trial relationships between brain activity and RT in these studies was consistent with this hypothesis. First, increases of RT were linked to reductions of pre-stimulus activity in brain regions that are thought to underlie the control of attention, such as the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate cortex (ACC) (Chee et al., 2008; Weissman et al., 2006). Second, increases of RT were associated with (a) reduced activity in sensory regions that processed relevant stimuli and (b) increased activity in sensory regions that processed irrelevant stimuli, suggesting that increases of RT reflected, to some extent, failures of attention to enhance the sensory processing of relevant stimuli and to limit the sensory processing of irrelevant stimuli (Weissman et al., 2009). Third, increases of RT were linked to greater stimulus-evoked activity in both the ACC and DLPFC, suggesting increased processing demands on these regions, possibly as a consequence of receiving low-quality perceptual representations of relevant stimuli from the sensory cortices (Chee et al., 2008; Chee & Tan, in press; Weissman et al., 2006). Together, these findings supported our hypothesis that variations of attention contributed to variations of RT in the selective attention tasks that we employed (Weissman et al. 2006; Weissman et al. 2009).
A major limitation of our prior studies, however, is that they did not investigate whether variations of RT in selective attention tasks were linked to variations of functional connectivity between brain regions that support attentional processing. Prior findings indicate that the ACC and the DLPFC are not only more activated (Milham et al., 2001; Pessoa et al., 2002; Weissman et al., 2004), but also more functionally connected (Fan et al., 2008; Wang et al., 2009) when attentional processes are recruited than when they are not, consistent with the view that interactions between brain regions make critical contributions to attention (Mesulam, 1981; Miller and Cohen, 2001; Posner and Petersen, 1990). Thus, if reductions of attention contribute to increases of RT, then increases of RT should be linked to reductions of functional connectivity between the ACC and the DLPFC. Previous work also indicates that anatomically early and anatomically late sensory regions that process relevant stimuli are more functionally connected when attentional control processes are recruited to enhance the sensory processing of relevant stimuli than when they are not recruited (Friston and Buchel, 2000). Therefore, if reductions of attention contribute to increases of RT, then increases of RT should be linked to reductions of functional connectivity between anatomically early and anatomically late sensory regions that process relevant stimuli.
To investigate these predictions, we asked participants to perform an audiovisual selective attention task (Fig. 1) while we recorded their brain activity using functional magnetic resonance imaging (fMRI). In each trial, participants identified a relevant letter in the visual modality while ignoring an irrelevant letter in the auditory modality. Since sensory information from the visual and the auditory modalities is processed in different brain regions (Kandel et al., 2000), this task allowed us to distinguish the sensory regions that processed the relevant visual letter from those that processed the irrelevant auditory letter. We tested our predictions about variations of RT by conducting a novel investigation of how functional connectivity varies with RT on a trial-by-trial basis. The present findings confirmed our predictions, showing for the first time that the moment-to-moment variations of RT that characterize our everyday experience are mirrored by corresponding variations of functional connectivity between brain regions that support attentional processing.
Figure 1.
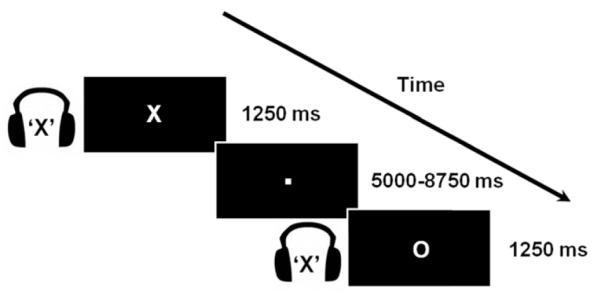
Illustration of the experimental task. In each trial (1250 ms), participants identified a centrally-presented visual letter (“X” or “O”; duration, 350 ms) while ignoring an auditory letter spoken in headphones (“X” or “O”; duration, 350 ms). The auditory letter was either congruent or incongruent with the visual letter. Variable periods of visual fixation were added between trials (ranging from 5000 ms to 8750 ms, in units of 1250 ms).
Materials and Methods
Participants
Eighteen healthy adults participated in the study. All were right-handed and had normal hearing, normal or corrected-to-normal vision, and no history of neurological or psychiatric disorders. Each participant gave informed written consent before the experiment and was paid $20 per hour. Two participants were excluded due to excessive head movement (i.e., greater than 3mm), such that 16 participants were included in our final analyses (6 men, 10 women; age range, 18–23 years; mean age, 21 years). All experimental procedures were approved by the University of Michigan Biomedical and Health Sciences Institutional Review Board.
Task and procedure
Participants performed an audiovisual selective attention task. In each trial, they identified a centrally-presented visual letter (“X” or “O”; 1.72° × 2.15° of visual angle; white against a black background; duration, 350 ms) while ignoring an auditory letter that was spoken in headphones (“X” or “O”; duration, 350 ms). The auditory letter was equally likely to be congruent or incongruent with the visual letter. Variable periods of fixation were added after each trial (in units of the 1250 ms TR, ranging from 5000 ms to 8750 ms). The duration of these fixation periods was varied using a pseudo-exponential distribution that favored short inter-trial intervals (Ollinger et al., 2001). The experiment was divided into seven runs of 48 trials each (24 congruent, 24 incongruent). The trials within each run were presented in a pseudo-random order such that each trial type was preceded equally often by every trial type in the design.
Half of the participants responded with their left index finger if the visual letter was an X and with their right index finger if the visual letter was an O; this mapping was reversed in the second half of participants. Behavioral responses were recorded using two MR-compatible keypads placed below each hand. Visual stimuli were generated using Presentation software (Neurobehavioral Systems, www.neurobs.com) and projected onto a translucent screen that was viewed by the participants through a mirror attached to the head-coil. Auditory stimuli were presented binaurally via GE MR-compatible, pneumatic sound transmission headphones. The same, clearly audible, volume level was used for all participants.
Imaging procedures
Images were collected using a 3-T GE Signa scanner (General Electric, Milwaukee, WI) equipped with a standard quadrature head coil. The fMRI blood oxygenation level dependent (BOLD) signal was measured with a reverse spiral imaging sequence (repetition time [TR] = 1250 ms, echo time [TE] = 30 ms). Twenty-seven contiguous axial slices (4.50-mm thick, field of view, 22 cm; in-plane resolution, 3.44×3.44 mm) were acquired per functional image. In each run, we collected 303 functional images. The first six images contained no trials and were discarded to allow for T1 equilibration effects.
Following functional image acquisition, a 3D spoiled gradient echo (SPGR), high-resolution, T1-weighted anatomical image was collected for each subject (TR = 10.5 ms, TE = 3.4 ms, FOV = 24 mm, flip angle = 25°, slice thickness = 1.5 mm).
FMRI data analysis
A number of preprocessing steps were performed on the fMRI data before trial-related activity was estimated. First, physiologic fluctuations were corrected using waveforms of respiration and cardiac cycles that were collected while participants performed the task in the scanner (Hu et al., 1995). Second, using SPM5 software (Wellcome Department of Cognitive Neurology, London, UK, www.fil.ion.ucl.ac.uk), the functional images were corrected for slice acquisition delays, spatially realigned to the first image of the first run to correct for head movements, normalized to the Montreal Neurological Institute (MNI) template (normalized voxel size, 3.75×3.75×4.5 mm), and spatially smoothed with an isotropic Gaussian filter (8-mm full width at half maximum).
Event-related regression analyses (conducted separately in each participant) were performed using a version of the general linear model in which the fMRI signal in each trial is modeled with a standard hemodynamic response function (Josephs et al., 1997). In each run, correct trials with RTs more than three standard deviations from the mean of their corresponding trial type (i.e., outliers) were excluded from behavioral and fMRI analyses (2% of all trials). Errors (1.25% of trials) were also excluded from the analysis. Correct trials were sorted by trial type (congruent, incongruent) and responding hand (left, right), yielding four regressors per run (congruent left hand, congruent right hand, incongruent left hand, and incongruent right hand).
The relationship between stimulus-evoked BOLD activity and RT is primarily linear when BOLD activity is modeled without assuming a standard hemodynamic response function (HRF) (Chee et al., 2008). However, it is unclear whether this linear relationship also holds when a standard HRF is assumed as in the present study. Therefore, we used a polynomial regression approach to determine the nature of the relationship (e.g., linear, quadratic, quartic) between variations of RT and stimulus-evoked BOLD activity. In particular, for each voxel the brain response (y) was modeled by a general linear model of the following form:
In this equation, the coefficient α0 models the average response to each trial type (independent of variation in RT) and the coefficients α1, α2, α3 and α4, respectively, model the linear (first-order), quadratic (second-order), cubic (third-order) and quartic (fourth-order) contribution of RT to the average hemodynamic response for each trial type. β0 is the y-intercept term (a column of ones), and ε represents the residual error term after each component has been fitted to the data. Following Weissman et al. (2006) and Chee et al. (2008), the RT for each trial was mean-centered by subtracting the mean RT (i.e., ) for all correct trials of the corresponding trial type (i.e., congruent left hand, congruent right hand, incongruent left hand, or incongruent right hand) within the same functional run. The parameter estimate for the linear polynomial regressor was therefore calculated in units of change in parameter estimate per second of increased RT.
Regressors of no interest reflecting head motion were also included in the model. Moreover, the time series data from each run was high-pass filtered (1/128 Hz), and serial correlations were corrected using an autoregressive AR(1) model. Finally, random effects analyses on the beta values from each participant were used to account for between-participants variance and to ensure that any findings concerning stimulus-evoked activity would generalize to the population.
Stepwise Regression Analysis
We performed a serial forward stepwise regression analysis to assess the fit of each of the polynomial regressors to the data. In particular, starting with the zero-order component, we progressively added higher-order components (i.e., linear, quadratic, cubic, and quartic) to the regression equation. F-tests were used to determine which, if any, of these higher-order relationships between RT and activity improved the overall fit of the model.
Functional connectivity analyses
We tested our hypotheses about functional connectivity using psychophysiological interaction (PPI) analyses (Friston et al., 1997; Gitelman et al., 2003). Such analyses assess whether interactions between brain regions vary with an experimental parameter. More specifically, they identify brain regions whose activity varies more or less strongly with activity in a seed region across the different levels of a psychological factor (Friston et al., 1997; Gitelman et al., 2003). Our PPI analyses determined whether functional connectivity changed as a function of RT, trial type (congruent, incongruent), or with an interaction between these factors. To make this possible, we extended the standard PPI analysis implemented in SPM5 to include both of these psychological factors and their interaction within the same multiple regression model.
PPI analyses in each participant were conducted in the following manner. To begin, we extracted the first eigenvariate time series from a sphere that was 6 mm in radius and centered around coordinates that were either previously published (ACC; Fan et al., 2008) or functionally defined in the present study (right MOG). Each regional time series served as the first regressor in a distinct PPI analysis (i.e., the “physiological” part of the PPI). Next, we entered the mean-centered RT and congruency value (1 or -1) in each trial, after they had each been convolved with a standard HRF, as the second and third regressors (the “psychological” parts of the PPI). Lastly, we entered regressors reflecting interactions between the physiological and psychological factors (i.e., the “interaction” parts of the PPI). To compute these interaction regressors, we first deconvolved the the BOLD signal in the seed region by using a Bayesian estimation algorithm (Gitelman et al., 2003). We then multiplied various combinations of the RT, congruency, and deconvolved seed activity regressors (Gitelman et al., 2003) to produce three interaction terms: Seed x RT, Seed x Congruency, and Seed x RT x Congruency. These interaction terms were then convolved with a standard HRF. Random effects analyses on the beta values from each participant were used to account for between-participants variance and to ensure that any findings concerning functional connectivity would generalize to the population.
Voxelwise analyses
Unless otherwise noted, regions identified in voxelwise analyses were considered to be significant if they survived a combined height (p < 0.005, uncorrected) and cluster extent (20 contiguous voxels) threshold. All coordinates are reported in MNI space.
Region of interest analyses
Region of interest (ROI) analyses were conducted using the SPM toolbox Marsbar (http://marsbar.sourceforge.net/). ROIs included all voxels within a 6 mm radius of each coordinate of interest. For each participant, we calculated the average activity for each trial type within an ROI by averaging the fMRI signal across all voxels within that ROI. Unless otherwise noted, one-tailed p values were reported because most of our hypotheses were directional. P values less than 0.05 were considered to be significant.
Results
Overall behavior
Consistent with prior findings (Weissman et al., 2004), mean RT was significantly longer in incongruent (M = 669 ms, SD = 137 ms) than in congruent (M = 655 ms, SD = 136 ms) trials, t(15) = 4.39, p = 0.0003. Analogously, mean error rate was significantly greater in incongruent (M = 1.86%, SD = 2.86%) than in congruent (M = 0.72%, SD = 1.44%) trials (t(15) = 1.99, p = 0.031). Neither of these effects was modulated by the congruency of the previous trial. Specifically, the Previous Trial Type (congruent, incongruent) x Current Trial Type (congruent, incongruent) interaction did not achieve significance for mean RT (F(1,15) = 1.15, p = 0.298) or for mean error rate (F(1,15) = 0.05, p = 0.818).
We hypothesize that, to some extent, increases of RT in our task reflect reductions of attention (Chee et al., 2008; Weissman et al. 2006; Weissman et al. 2009). Consistent with this hypothesis, in incongruent trials the slowest 20% of responses were associated with a higher error rate than the fastest 20% of responses (5.41% vs. 0.39%; t(15) = 1.84, p = 0.043). No corresponding difference in error rate was observed between the slowest 20% and the fastest 20% of responses in congruent trials (0.80% vs. 0.71%; t(15) = 0.14, p = 0.445), likely because error rates were extremely low in this trial type. Nonetheless, the findings from incongruent trials are consistent with our hypothesis that reductions of attention contributed to increases of RT in our task (Chee et al., 2008; Weissman et al., 2006; Weissman et al., 2009).
FMRI
Stimulus-evoked BOLD activity varies linearly with RT
An important methodological assumption underlying our work is that stimulus-evoked BOLD activity varies linearly with RT across trials. To test this assumption, we used a polynomial regression model to characterize the relationship between stimulus-evoked activity and trial-to-trial variability in RT (see Materials and Methods). Next, using a serial forward stepwise regression analysis, we sequentially tested whether various possible relationships (i.e., linear, quadratic, cubic, and quartic) between stimulus-evoked activity and RT uniquely accounted for significant proportions of variance in the BOLD signal.
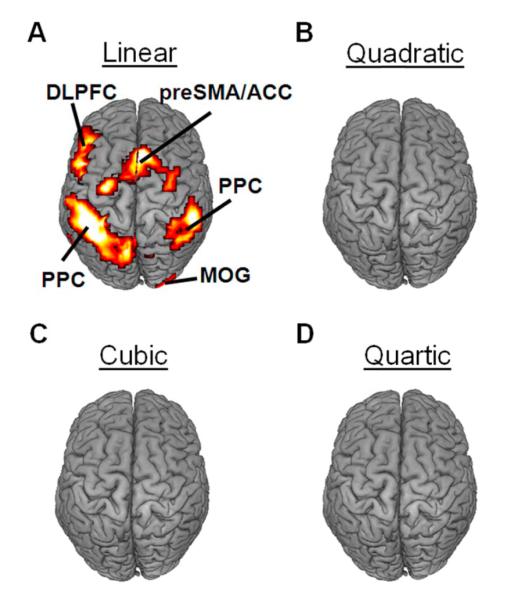
The first-order term modeled the linear relationship between activity and RT across trials after accounting for the zero-order term, which modeled the average response to each trial type. Adding a linear term to the regression significantly improved the model fit in several brain regions, including bilateral dorsolateral prefrontal cortex (DLPFC), bilateral posterior parietal cortex (PPC), the right ventrolateral prefrontal cortex (VLPFC), the pre-supplementary motor area (preSMA), the anterior cingulate cortex (ACC), and the right middle occipital gyrus (MOG) (Fig. 2a).
Figure 2.
Stimulus-evoked BOLD activity varied with RT in a predominantly linear fashion. (A) A linear relationship between stimulus-evoked activity and RT accounted for a significant proportion of signal variance in the bilateral posterior parietal cortex (PPC), the left dorsolateral prefrontal cortex (DLPFC), the pre-supplementary motor area / anterior cingulate cortex (preSMA/ACC), and the middle occipital gyrus (MOG). In contrast, quadratic (B), cubic (C), and quartic (D) relationships between stimulus-evoked activity and RT did not account for significant proportions of signal variance in any brain regions. All activations are overlaid on a 3D rendering of the MNI-normalized anatomical brain.
The second-order term modeled the quadratic relationship between stimulus-evoked activity and RT across trials after accounting for the zero- and first- order terms. Adding a quadratic term to the model did not significantly improve the statistical fit of the model in any brain regions (Fig. 2b). Similarly, no improvement was observed by adding third-order (cubic) (Fig. 2c) and fourth-order (quartic) (Fig. 2d) terms. Although we did not model terms higher than the fourth-order term, these findings would appear to support our assumption that the relationship between stimulus-evoked activity and RT is predominantly linear.
Increases of RT are linked to opposing variations of activity in sensory regions that process relevant and irrelevant stimuli
We hypothesize that variations of attention contribute to variations of RT in our task. In considering how to perform an initial test of this hypothesis, we noted that attention is thought to enhance the perceptual processing of relevant stimuli while limiting the perceptual processing of irrelevant stimuli (Desimone and Duncan, 1995). Thus, we reasoned that if reductions of attention contributed to increases of RT in our task, then increases of RT should be associated with reductions of activity in sensory regions that process relevant stimuli, but with increases of activity in sensory regions that process irrelevant stimuli. Moreover, increases of RT should be most strongly linked to increases of activity in sensory regions that process irrelevant stimuli in incongruent trials, in which the auditory distractor is mapped to a different response than the visual target.
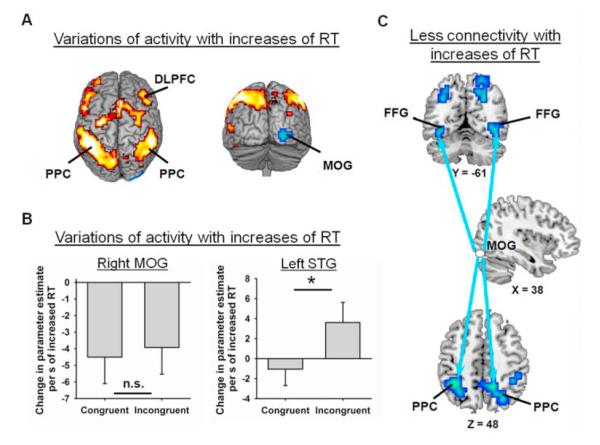
Replicating one of our prior studies (Weissman et al., 2009), whole-brain and ROI analyses confirmed both of our predictions above. First, voxelwise analyses revealed that increases of RT were associated with reduced activity in the right middle occipital gyrus (MOG; right: x = 28, y = -100, z = 0), which likely processed perceptual aspects of the relevant visual stimuli (Fig. 3a and Table 1). Follow-up ROI analyses revealed that the magnitude of this reduction did not vary with congruency (congruent vs. incongruent; t(15) = 0.25, p = 0.809, two-tailed t-test) (Fig. 3b, left). Thus, failing to enhance the perceptual processing of the relevant visual letter was uniformly detrimental to performance.
Figure 3.
Increases of RT were associated with both variations of activity and variations of functional connectivity in the sensory cortices. (A) Increases of RT were linked to reductions of activity in bilateral regions of the middle occipital gyrus (MOG; blue), but to increases of activity in a bilateral fronto-parietal network (red), which included the dorsolateral prefrontal cortex (DLPFC) and the posterior parietal cortex (PPC). (B) In the right MOG (MNI coordinates: x = 28, y = −100, z = 0), increases of RT were associated with statistically equivalent reductions of activity in incongruent and congruent trials (left). In the left superior temporal gyrus (STG, MNI coordinates: x = −59, y = 35, z = −1), increases of RT were associated with larger increases of activity in incongruent than in congruent trials (right). The extent to which activity varied with RT is plotted in units of change in parameter estimate per second of increased RT. (C) Increases of RT were associated with reductions of functional connectivity between the right MOG and bilateral regions of the (a) fusiform gyrus (FFG) and (b) PPC. The arrows indicate reductions of functional connectivity between the right MOG (white) and these FFG and PPC regions (blue). All activations are overlaid on a 3D rendering or slices of the MNI-normalized anatomical brain.
Table 1.
Brain regions for which reductions of attention (i.e., increases of RT) were associated with changes of activity.
| Anatomical location | ~ BA | MNI coordinates |
t-score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Increased activity | |||||
| L. Posterior Parietal Cortex | 40 | −48 | −41 | 54 | 8.71 |
| R. Supplementary Motor Area | 6 | 7 | 21 | 54 | 7.77 |
| L. Precentral Gyrus | 6 | −48 | 3 | 32 | 6.63 |
| R. Posterior Parietal Cortex | 40 | 52 | −38 | 45 | 6.61 |
| L. Middle Frontal Gyrus | 9 | −38 | 34 | 27 | 6.19 |
| L. Middle Temporal Gyrus | 22 | −48 | −52 | 9 | 5.01 |
| R. Inferior Frontal Gyrus | 45 | 31 | 28 | 0 | 4.65 |
| R. Middle Frontal Gyrus | 8 | 45 | 24 | 45 | 4.42 |
| L. Superior Frontal Gyrus | 9 | −34 | 55 | 22 | 3.76 |
| Reduced activity | |||||
| L. Caudate | - | 10 | 28 | 0 | 5.15 |
| R. Middle Occipital Gyrus | 18 | 28 | −100 | 0 | 3.92 |
Notes. L., left; R. right; ~ BA, approximate Brodmann Area; RT, reaction time; MNI, Montreal Neurological Institute
Second, ROI analyses in a region of the left auditory cortex (i.e., the superior temporal gyrus, STG; x = −59, y = −35, z = −1), which was identified in one of our prior studies of audiovisual selective attention (Weissman et al., 2004), indicated that increases of RT were associated with significantly larger increases of activity in incongruent than in congruent trials (t(15) = 2.24, p = 0.0203) (Fig. 3b, right). Further analyses revealed that increases of RT were linked to significant increases of activity in incongruent trials (t(15) = 1.80, p = 0.0459), but not in congruent trials (t(15) = 0.64, p = 0.265). This result is consistent with the view that increases of RT were associated with failing to suppress the perceptual processing of the auditory distracter, which was deleterious to performance when the distracter was mapped to a different response than the visual target. In sum, increases of RT were associated with failures to (a) enhance activity in the relevant-modality visual cortex and (b) suppress activity in the irrelevant-modality auditory cortex. Thus, the nature of trial-by-trial relationships between BOLD activity and RT in the visual and auditory cortices supported our hypothesis that variations of attention contributed to variations of RT in our selective attention task.
Increases of RT are linked to reductions of functional connectivity between the ACC and the right DLPFC
Our first prediction was that increases of RT would be associated with reductions of functional connectivity between the ACC and the DLPFC: two regions that are thought to make crucial contributions to attentional control (Desimone and Duncan, 1995; Posner and Petersen, 1990). To localize a region of the ACC that was involved in attentional control, we contrasted activity in incongruent and congruent trials in a voxelwise analysis. However, in line with the relatively small difference in RT between incongruent and congruent trials (14 ms), no regions of the ACC were significantly activated in this contrast. Thus, we could not functionally define an ACC seed region that was involved in attentional control using the present data set. We therefore defined a seed region in the ACC (x = 6, y = 36, z = 26) that Fan and colleagues (2005, 2008) have previously implicated in attentional control. Specifically, both the level of activity (Fan et al., 2005) and the level of functional connectivity with the DLPFC (Fan et al., 2008) were higher in incongruent than in congruent trials. For these reasons, this region of the ACC (termed RCZa by Fan et al., 2008) appeared to be a good choice for investigating whether increases of RT were linked to reductions of functional connectivity between brain regions that support attentional processing.
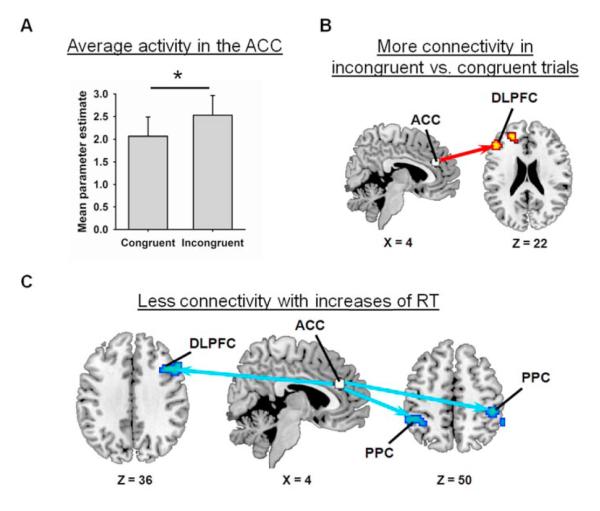
Before investigating our first prediction, however, we tested our assumption that this ACC region contributed to attentional control in the present study. Of importance, two findings verified this assumption. First, ROI analyses, which are often more sensitive than voxelwise analyses because less stringent statistical thresholds can be employed, revealed greater activity in the ACC seed in incongruent than in congruent trials (t(15) = 1.82, p = 0.0445) (Fig. 4a), consistent with various proposals that the ACC contributes to attentional control under conditions of distraction by detecting response conflict (Orr & Weissman, 2008), facilitating response selection (Rushworth et al., 2007), or increasing attention to relevant stimuli (Botvinick et al., 2004; Posner and Petersen, 1990). Second, in line with Fan et al. (2008), we observed greater functional connectivity between the ACC seed and the left DLPFC (x = −48, y = 31, z = 22; this effect did not vary with RT) in incongruent than in congruent trials (Fig. 4b; voxelwise significance threshold for search restricted to dorsolateral prefrontal regions: p < 0.005, 15 contiguous voxels), consistent with the view that the ACC works with the DLPFC to increase control when irrelevant stimuli threaten to undermine performance (Kerns et al., 2004). Together, these findings support our assumption that the ACC seed region participates in the control of attention.
Figure 4.
(ACC). (A) Average activity in the ACC was greater in incongruent than in congruent trials. (B) The ACC exhibited greater functional connectivity with the left dorsolateral prefrontal cortex (DLPFC) in incongruent than in congruent trials. (C) Increases of RT were associated with reductions of functional connectivity between the ACC and several regions of the attentional network including the right DLPFC and bilateral regions of the posterior parietal cortex (PPC). The arrows indicate reductions of functional connectivity between the ACC (white) and these DLPFC and PPC regions (blue). All activations are overlaid on slices of the MNI-normalized anatomical brain.
Next, we investigated our first prediction. As hypothesized, voxelwise analyses revealed that increases of RT were associated with reductions of functional connectivity between the ACC seed and (a) the right DLPFC and (b) bilateral regions of the posterior parietal cortex (PPC) (Fig. 4c and Table 2). The magnitude of these reductions did not differ significantly in congruent and incongruent trials possibly because, as we mentioned earlier, behavioral effects of response conflict were relatively small (14 ms) in the present study. Nonetheless, consistent with our first prediction, increases of RT were associated with reductions of functional connectivity between the ACC and the right DLPFC.
Table 2.
Brain regions for which reductions of attention (i.e., increases of RT) were associated with changes of functional connectivity involving either the ACC or the right MOG.
| Anatomical location | ~ BA | MNI coordinates |
t-score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| ACC seed: Increased functional connectivity | |||||
| No suprathreshold clusters | |||||
| ACC seed: Reduced functional connectivity | |||||
| R. Precentral Gyrus | 6 | 34 | 3 | 22 | 4.87 |
| R. Middle Frontal Gyrus | 9 | 55 | 21 | 40 | 4.30 |
| R. Middle Temporal Gyrus | 22 | 69 | −31 | 4 | 4.20 |
| R. Posterior Parietal Cortex | 40 | 48 | −31 | 50 | 3.89 |
| L. Posterior Parietal Cortex | 40 | −45 | −41 | 50 | 3.86 |
| Right MOG seed: Increased functional connectivity | |||||
| No suprathreshold clusters | |||||
| Right MOG seed: Reduced functional connectivity | |||||
| L. Fusiform Gyrus | 37 | −48 | −45 | −18 | 5.84 |
| R. Precuneus | 7 | 14 | −52 | 45 | 5.35 |
| L. Inferior Parietal Lobule | 40 | −38 | −48 | 45 | 5.44 |
| R. Superior Parietal Lobule | 7 | 28 | −69 | 45 | 5.11 |
| L. Anterior Cingulate Cortex | 25 | −3 | 7 | −9 | 5.05 |
| L. Inferior frontal Gyrus | 44 | −52 | 10 | 22 | 5.04 |
| R. Cerebellum | − | 17 | −38 | −45 | 4.77 |
| R. Superior Frontal Gyrus | 10 | 24 | 58 | 22 | 4.41 |
| R. Fusiform Gyrus | 37 | 41 | −62 | −9 | 4.32 |
| L. Superior Frontal Gyrus | 10 | −21 | 58 | 18 | 4.00 |
| R. Medial Frontal Gyrus | 10 | 3 | 58 | 0 | 3.55 |
| L. Lingual Gyrus | 17 | −17 | −100 | −14 | 3.34 |
Notes. L., left; R. right; ~ BA, approximate Brodmann Area; RT, reaction time; MNI, Montreal Neurological Institute
Finally, we investigated whether the effects above were specific to the ACC seed region or might occur even for a left primary motor cortex seed region that is not thought to contribute to attentional processing. Importantly, voxelwise analyses confirmed that increases of RT were not associated with variations of functional connectivity between the left primary motor cortex (defined in the contrast overall stimuli vs. baseline; x=−38 y=−24 z=68) and either the DLPFC or the PPC. Therefore, the functional connectivity results involving the DLPFC and the PPC were specific to the ACC seed region.
Increases of RT are linked to increases of activity in the ACC and the right DLPFC
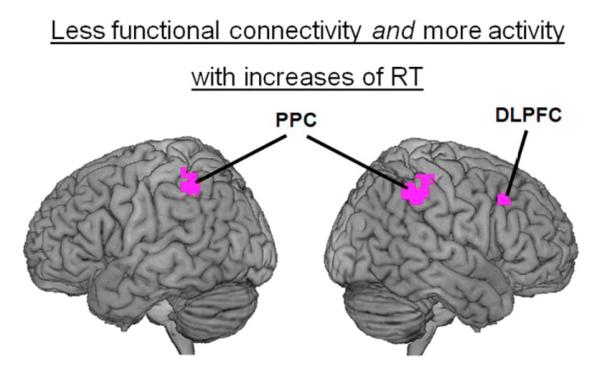
Reductions of functional connectivity between task-relevant frontal and temporal regions have been linked to increases of activity in these regions, suggesting greater processing demands on these regions when their interactions with each other are more sparse (Ghuman et al., 2008). Here, we investigated whether an analogous effect might be observed with the ACC and the right DLPFC. Consistent with prior studies (Chee et al., 2008; Weissman et al., 2006), whole-brain analyses revealed that increases of RT were associated with increased activity in several regions that are thought to underlie attentional control including bilateral DLPFC, bilateral PPC, the right ventrolateral prefrontal cortex (VLPFC), and the pre-supplementary motor area (preSMA) (Fig. 3a and Table 1). Critically, a conjunction analysis revealed that these regions included the same right DLPFC (x = 48, y = 22, z = 42) and bilateral PPC (left: x = −46, y = 44, z = 50; right: x = 51, y = −37, z = 48) regions for which increases of RT were linked to reductions of functional connectivity with the ACC (voxelwise significance threshold for both contrasts: p < 0.005, 20 contiguous voxels) (Fig 5). Thus, increases of RT were linked to increases of activity in the same right DLPFC and bilateral PPC regions that exhibited reduced functional connectivity with the ACC, consistent with increased processing demands on these regions when their interactions with the ACC were reduced.
Figure 5.
Overlapping regions of the attentional network were implicated in the activity and functional connectivity analyses. Increases of RT were associated not only with increases of activity in the right dorsolateral prefrontal cortex (DLPFC) and in bilateral regions of the posterior parietal cortex (PPC), but also with reductions of functional connectivity between each of these regions and the ACC. These regions are overlaid on a 3D rendering of the MNI-normalized anatomical brain.
Increases of RT are linked to reductions of functional connectivity between anatomically early and anatomically late sensory regions that process relevant stimuli
Attention is thought to enhance the flow of perceptual information from anatomically early to anatomically late sensory regions that process relevant stimuli (Friston and Buchel, 2000). Consequently, our second prediction was that if variations of attention contribute to variations of RT in our task, then increases of RT should be linked to reduced functional connectivity between these sensory regions. To test this prediction, we contrasted all stimuli to baseline in order to identify two seed regions located in the early visual cortex that were highly activated by our relevant visual stimuli. These regions were located in the left and the right middle occipital gyrus (MOG) (left: x = −28, y = −82, z = 18; right: x = 38, y = −83, z = −14).
As hypothesized, voxelwise analyses revealed that increases of RT were associated with reductions of functional connectivity between the right MOG and anatomically late sensory regions along both the ventral and the dorsal visual pathways (Fig. 3c and Table 2; no effects were observed for the left MOG). Along the ventral pathway, increases of RT were associated with reductions of functional connectivity between the right MOG and bilateral regions of the fusiform gyrus (FFG), which overlap with a previously described visual letter processing area (Polk et al., 2002). Along the dorsal pathway, increases of RT were associated with reductions of functional connectivity between the right MOG and bilateral regions of the inferior parietal cortex. These inferior parietal regions have been implicated in various aspects of letter recognition (Joseph et al., 2003) including spatial processing of letters and words (Kuriki et al., 1998). In sum, consistent with our second prediction, increases of RT were linked to reductions of functional connectivity between anatomically early and anatomically late sensory regions which, respectively, likely processed basic and higher-order perceptual aspects of the relevant visual stimuli.
Unmodeled BOLD activity in error trials does not appear to have driven our findings
Because error rates were extremely low in this experiment (1.29%), there were not enough trials to accurately estimate brain activity associated with errors. Therefore, error trials were not included in the regression model. This raises the possibility that some of our results may have been driven by unmodeled BOLD activity in error trials. To test this hypothesis, we investigated whether our findings varied with mean error rate across subjects.
Critically, a series of across-subjects voxelwise correlations provided no evidence to support this view. First, we correlated mean error rate with the voxelwise map relating RT to functional connectivity with the ACC seed. This analysis revealed no significant correlations in the right DLPFC or in bilateral PPC regions that were reported in the original analysis. Second, we correlated mean error rate with the voxelwise map relating RT to functional connectivity with the MOG seed. This analysis revealed no significant correlations anywhere in the brain. Third, we correlated mean error rate with the voxelwise map that resulted from the analysis relating RT to stimulus-evoked BOLD activity. This analysis revealed no significant correlations in any of the frontal, parietal, or sensory regions that were reported in the original analysis. In sum, our findings do not appear to have been driven by unmodeled BOLD activity in error trials.
Discussion
Variations of response time (RT) in selective attention tasks are often associated with changes of activity in the fronto-parietal attentional network and in sensory regions that process relevant stimuli (Chee et al., 2008; Hahn et al., 2007; Leber et al., 2008; Weissman et al., 2006). Such findings have led us to hypothesize that variations of RT reflect, to some extent, variations of attention. In the present study, we further investigated this hypothesis by determining whether variations of RT are associated with variations of functional connectivity between brain regions that are thought to underlie attentional processing. As predicted, variations of RT were linked to variations of functional connectivity between the ACC and several brain regions that are thought to underlie attentional control including the right DLPFC and bilateral regions of the PPC. Also as predicted, variations of RT were linked to variations of functional connectivity between anatomically early and anatomically late sensory regions of the visual cortex that likely processed perceptual aspects of the relevant visual stimuli. These findings show that the moment-to-moment variations of RT that ubiquitously characterize human performance are accompanied by corresponding variations of functional connectivity between brain regions that support attentional processing.
Increases of RT are linked to reduced functional connectivity in the attentional network
Our first main finding was that increases of RT were associated with reductions of functional connectivity between the ACC and (a) the right DLPFC and (b) bilateral regions of the PPC. Prior work indicates that these regions work together as part of an attentional network that enables goal-directed behavior. Indeed, the ACC is co-activated with the right DLPFC and/or the PPC when attentional control processes are recruited to voluntarily direct attention to an upcoming stimulus (Weissman et al., 2004; Woldorff et al., 2004), to overcome response conflict (Milham et al., 2001), or to maintain information in visual working memory (Pessoa et al., 2002). Moreover, functional connectivity between the ACC and (a) the right DLPFC (Fan et al., 2008) and (b) the PPC (Wang et al., 2009) increases with demands on attentional control processes. In line with these prior results, our findings suggest that when control processes are effectively recruited during trials with relatively fast responses, there is enhanced functional connectivity between the ACC and (a) the right DLPFC and (b) bilateral regions of the PPC. In contrast, when control processes are less effectively recruited during trials with relatively slow responses, there is reduced functional connectivity between the ACC and these other regions. Although these findings fit nicely with prior work, to our knowledge they are the first to reveal that across-trial variability in RT is linked to changes in functional connectivity between frontal and parietal regions within the attentional network.
How might communication between the ACC and (a) the right DLPFC and (b) bilateral PPC influence RT? One possibility is that such communication facilitates response selection, consistent with models in which the right DLPFC is involved in maintaining stimulus-response associations in working memory (Curtis and D’Esposito, 2003; Miller and Cohen, 2001), the PPC plays a role in planning responses (Andersen and Cui, 2009), and the ACC participates in selecting a relevant response (Rushworth et al., 2007). A second possibility is that such communication increases an attentional bias toward relevant stimuli, consistent with models in which the PPC and the ACC play roles in biasing attention toward relevant stimuli (Corbetta et al., 2008; Posner and Petersen, 1990) while the right DLPFC plays a role in sustaining attention (Rueckert and Grafman, 1996). Additional research will be needed to determine which of these, or other, interpretations best accounts for our data. Thus, at present, we simply note that our findings are consistent with multiple models of attentional control.
Interestingly, increases of RT were associated not only with reductions of functional connectivity between the ACC and (a) the right DLPFC and (b) bilateral regions of the PPC, but also with increases of activity in these same DLPFC and PPC regions. This result supports the view that communication between brain regions makes contributions to cognitive processes that are not merely the sum of processing that occurs within individual brain regions (Banich and Karol, 1992; Weissman and Banich, 1999). It also fits with findings from a recent magnetoencephalography (MEG) study (Ghuman et al., 2008). In this study, trials with longer (versus shorter) RT in an object classification task were linked to decreased functional connectivity between prefrontal and temporal regions, which was quickly followed by increased activity in these same regions. The authors suggested that reductions of communication between task-relevant frontal and temporal regions increased processing demands on those regions. The present findings would appear to indicate an analogous effect within the fronto-parietal attentional network.
Increases of RT are linked to reduced functional connectivity between sensory regions that underlie the perceptual processing of relevant stimuli
Our second main finding was that increases of RT were linked to reductions of functional connectivity between the right early visual cortex (i.e., the MOG) and multiple downstream regions that process sensory information from the visual modality including the FFG and the PPC. This finding complements prior work indicating that increases of RT in selective attention tasks are linked to reductions of activity in sensory regions that process perceptual aspects of relevant stimuli (Weissman et al., 2009). It also provides converging evidence for the view that attention normally enhances the flow of perceptual information between anatomically early and anatomically late sensory regions which, respectively, process basic and higher-order perceptual features of relevant stimuli (Friston and Buchel, 2000). Critically in this respect, the FFG and the PPC are both thought to process higher-order perceptual features of relevant visual stimuli (Joseph et al., 2003; Polk et al., 2002; Price et al., 1996). Specifically, the FFG exhibits domain-specific responses for words (McCandliss et al., 2003) and letters (Polk et al., 2002), while the PPC has been implicated in the spatial processing of letter strings (Kuriki et al., 1998). In line with prior findings (Haynes et al., 2005), we speculate that reductions of functional connectivity between task-relevant sensory regions may have impaired participants’ ability to quickly perceive the relevant visual stimuli.
Broader implications of our findings
Our findings both complement and extend prior work suggesting that reductions of functional connectivity are linked to reductions in the quality of behavioral performance. For instance, Heekeren and colleagues (Heekeren et al., 2004) demonstrated that reduced functional connectivity between the DLPFC and posterior sensory cortices that process perceptual aspects of relevant stimuli is linked to less accurate decisions about the nature of a stimulus (e.g., face or house). As another example, reduced synchronization of neuronal oscillations within a fronto-parietal-temporal attentional network, which likely reflects reduced functional connectivity within this network (Fries, 2005), is associated with less successful detection of the second of two rapidly-presented targets in the attentional blink paradigm (Gross et al., 2004). The present findings relating functional connectivity to reaction time on a trial-by-trial basis extend these previous results by showing that behavior varies with functional connectivity continuously rather than discretely. Put simply, the subtle fluctuations of behavioral performance that characterize human behavior are mirrored by equally subtle changes of functional connectivity in the human brain.
Limitations
In line with prior work (Ghuman et al., 2008), we have argued that reductions of functional connectivity between brain regions in the attentional network leads to increased processing demands on the associated regions. However, it is important to consider whether the increases of activity that we have observed in the right DLPFC and bilateral regions of the PPC simply reflect greater time on task. Although we cannot exclude this possibility, we have shown previously that a unit increase of RT (e.g., 100 ms) is associated with a larger increase of activity in both the DLPFC and the PPC when study participants are well-rested (and have greater spare attentional capacity with which to respond to increased processing demands) than when they are sleep-deprived (Chee et al., 2008; Weissman et al., 2006). Moreover, this effect was probably not due to a general suppression of overall activity in the sleep-deprived state because it was also observed in regions (e.g., the thalamus) that exhibited greater activity in the sleep-deprived state than in the well-rested state (Chee et al., 2008). Thus, in the present study, it is likely that the increases of activity in the right DLPFC and bilateral PPC that were linked to increases of RT reflected, at least partially, increased processing demands on those regions.
Our findings are generally consistent with current neurological models of attention. However, RT variability in our task likely stems from numerous sources. For example, variations in arousal, response strategies, decision-making processes (e.g., changes in speed-accuracy tradeoffs or stimulus expectations), and even voluntary switches of attention (i.e., switching between focusing on relevant vs. irrelevant stimuli) may all contribute to RT variability in selective attention tasks. Nevertheless, none of these factors provides a better explanation of our findings in the sensory cortices than variations of attention. In particular, we found that increases of RT in incongruent trials were associated with linear decreases of activity in the task-relevant visual cortex, but with linear increases of activity in the task-irrelevant auditory cortex. First, these effects are not consistent with variations in arousal, which should produce similar changes of activity in task-relevant and task-irrelevant sensory ****regions (Spitzer, Desimone, & Moran, 1998). Second, these effects are not consistent with variations in response strategies or decision-making processes (e.g., changes in speed-accuracy tradeoffs or stimulus expectancies), which are typically not associated with changes of activity in the sensory cortices (van Veen et al., 2008). Third, these effects are not consistent with repetition priming, a phenomenon in which faster RT is linked to reduced activity in task-relevant sensory cortices (Buckner et al., 1998), rather than with increased activity as we observed. Fourth, although these effects might be consistent with voluntary switches of attention from the relevant visual modality to the irrelevant auditory modality, such switches would nonetheless constitute reductions of attention to the primary task, which impair the processing of relevant stimuli and/or lead to difficulties with ignoring irrelevant stimuli. Thus, although variations of RT likely stem from numerous sources, the brain-behavior relationships that we have observed are most easily and most parsimoniously explained by variations of attention.
Conclusion
The present findings indicate that variations of RT in a cross-modal selective attention task are associated with variations of functional connectivity between brain regions that support attentional processing. Moreover, the specific nature of our findings suggests that, to some extent, increases of RT in our task reflect reductions of attention. Together, these findings suggest that variations of attention are an important contributor to variations of RT in selective attention tasks. They also provide novel support for the view that interactions between brain regions make important contributions to attentional processing (Corbetta et al., 2008; Desimone and Duncan, 1995; Fries, 2005; Friston and Buchel, 2000; Ghuman et al., 2008). Future studies relating functional connectivity to RT on a trial-by-trial basis may therefore continue to advance our understanding of how interactions between brain regions contribute to cognitive processing.
Acknowledgements
This research was supported by an NIH grant (1R03DA021345-01) and by startup funds from the University of Michigan awarded to Daniel Weissman. We thank Keith Newnham and Colleen Hammond for their assistance in collecting the fMRI data. We also thank Rebecca Compton, Scott Huettel, John Jonides, Kristina Visscher, Vince Wu and two anonymous reviewers for their helpful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Banich MT, Karol DL. The sum of the parts does not equal the whole: evidence from bihemispheric processing. J Exp Psychol Hum Percept Perform. 1992;18:763–784. doi: 10.1037//0096-1523.18.3.763. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Tan JC. Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. Neuroimage. doi: 10.1016/j.neuroimage.2010.02.031. (in press) doi:10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buchel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc Natl Acad Sci U S A. 2000;97:7591–7596. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Bueschel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. Proc Natl Acad Sci U S A. 2008;105:8405–8409. doi: 10.1073/pnas.0710674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low demand tasks. J Exp Psychol Hum Percept Perform. 2006;32:45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- Gilden DL. Cognitive emissions of 1/f noise. Psych Rev. 2001;108:33–56. doi: 10.1037/0033-295x.108.1.33. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci U S A. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Driver J, Rees G. Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron. 2005;46:811–821. doi: 10.1016/j.neuron.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Piper GA. Shared and dissociated cortical regions for object and letter processing. Brain Res Cogn Brain Res. 2003;17:56–67. doi: 10.1016/s0926-6410(03)00080-6. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston K. Event-related fMRI. Human Brain Mapping. 1997;5:1–7. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. Fourth ed McGraw-Hill; 2000. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kuriki S, Takeuchi F, Hirata Y. Neural processing of words in the human extrastriate visual cortex. Brain Res Cogn Brain Res. 1998;6:193–203. doi: 10.1016/s0926-6410(97)00030-x. [DOI] [PubMed] [Google Scholar]
- Leber AB, Turk-Browne NB, Chun MM. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci U S A. 2008;105:13592–13597. doi: 10.1073/pnas.0805423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Orr JM, Weissman DH. Anterior cingulate cortex makes 2 contributions to minimizing distraction. Cerebral Cortex. 2009;19:703–711. doi: 10.1093/cercor/bhn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D’Esposito M, Detre JA, Farah MJ. Neural specialization for letter recognition. J Cogn Neurosci. 2002;14:145–159. doi: 10.1162/089892902317236803. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Frackowiak RS. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Grafman J. Sustained attention deficits in patients with right frontal lesions. Neuropsychologia. 1996;34:953–963. doi: 10.1016/0028-3932(96)00016-4. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- van Veen V, Krug MK, Carter CS. The neural and computational basis of controlled speed-accuracy tradeoff during task performance. J Cogn Neurosci. 2008;20:1952–1965. doi: 10.1162/jocn.2008.20146. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J. Effective Connectivity of the Fronto-parietal Network during Attentional Control. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Banich MT. Global-local interference modulated by communication between the hemispheres. J Exp Psychol Gen. 1999;128:283–308. doi: 10.1037//0096-3445.128.3.283. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. J Neurosci. 2004;24:10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. Momentary reductions of attention permit greater processing of irrelevant stimuli. Neuroimage. 2009;48:609–615. doi: 10.1016/j.neuroimage.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armillo ML, Craik F, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cogn. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hazlett CJ, Fichtenholtz HM, Weissman DH, Dale AM, Song AW. Functional parcellation of attentional control regions of the brain. J Cogn Neurosci. 2004;16:149–165. doi: 10.1162/089892904322755638. [DOI] [PubMed] [Google Scholar]