Abstract
We examined normal emmetropization and the refractive responses to binocular plus or minus lenses in young (late infantile) and juvenile tree shrews. In addition, recovery from lens-induced myopia was compared with the response to a similar amount of myopia produced with plus lenses in age-matched juvenile animals. Normal emmetropization was examined with daily noncycloplegic autorefractor measures from 11 days after natural eye-opening (days of visual experience [VE]) when the eyes were in the infantile, rapid growth phase and their refractions were substantially hyperopic, to 35 days of VE when the eyes had entered the juvenile, slower growth phase and the refractions were near emmetropia. Starting at 11 days of VE, two groups of young tree shrews wore binocular +4 D lenses (n = 6) or −5 D lenses (n = 5). Starting at 24 days of VE, four groups of juvenile tree shrews (n = 5 each) wore binocular +3 D, +5 D, −3 D, or −5 D lenses. Non-cycloplegic measures of refractive state were made frequently while the animals wore the assigned lenses. The refractive response of the juvenile plus-lens wearing animals was compared with the refractive recovery of an age-matched group of animals (n=5) that were myopic after wearing a −5 D lens from 11 to 24 days of VE. In normal tree shrews, refractions (corrected for the small eye artifact) declined rapidly from (mean ± SEM) 6.6 ± 0.6 D of hyperopia at 11 VE to 1.4 ± 0.2 D at 24 VE and 0.8 ± 0.4 D at 35 VE. Plus 4 D lens treatment applied at 11 days of VE initially corrected or over-corrected the young animals’ hyperopia and produced a compensatory response in most animals; the eyes became nearly emmetropic while wearing the +4 D lenses. In contrast, plus-lens treatment starting at 24 days of VE initially made the juvenile eyes myopic (over-correction) and, on average, was less effective. The response ranged from no change in refractive state (eye continued to experience myopia) to full compensation (emmetropic with the lens in place). Minus-lens wear in both the young and juvenile groups, which initially made eyes more hyperopic, consistently produced compensation to the minus lens so that eyes reached age-appropriate refractions while wearing the lenses. When the minus lenses were removed, the eyes recovered quickly to age-matched normal values. The consistent recovery response from myopia in juvenile eyes after minus-lens compensation, compared with the highly variable response to plus lens wear in age-matched juvenile animals suggests that eyes retain the ability to detect the myopic refractive state, but there is an age-related decrease in the ability of normal eyes to use myopia to slow their elongation rate below normal. If juvenile human eyes, compared with infants, have a similar difficulty in using myopia to slow axial elongation, this may contribute to myopia development, especially in eyes with a genetic pre-disposition to elongate.
Keywords: myopia development, animal models, refractive error, emmetropization, plus lenses, axial elongation, age, recovery
1. INTRODUCTION
At birth (or hatching) the eyes of most young animals and human infants have substantial spherical refractive error. Typically, but not universally, they are hyperopic (Cook and Glasscock, 1951; Pickett-Seltner et al., 1988; Andison et al., 1992; Norton and McBrien, 1992; Troilo and Judge, 1993; Bradley et al., 1999). Within a few weeks or months, the refractive error distribution narrows such that most eyes become nearly emmetropic, usually retaining a slight hyperopia that can be readily compensated for with low levels of accommodation (Pickett-Seltner et al., 1988; Smith, III and Hung, 1999; Mayer et al., 2001; Norton et al., 2010). Evidence has accumulated that the narrowing of the refractive distribution during this “infantile” growth stage (Sorsby et al., 1961) involves visually-guided modulation of the axial elongation rate of the eye (Schaeffel et al., 1988; Wildsoet, 1997; Smith, III and Hung, 1999; Wallman and Winawer, 2004; Mutti et al., 2005; Norton et al., 2008; Howlett and McFadden, 2009; Norton et al., 2010). For example, between three and nine months of age when the human refractive distribution narrows, infants that are more hyperopic at three months increase their vitreous chamber depth more than do less hyperopic infants (Mutti et al., 2005).
Evidence for visually-guided emmetropization in animals has come primarily from studies in which plus or minus-power lenses have been used to affect refractive development (Schaeffel et al., 1988; Irving et al., 1992; Hung et al., 1995; Wildsoet, 1997; Smith, III and Hung, 1999; Wallman and Winawer, 2004; Shen and Sivak, 2007; Metlapally and McBrien, 2008; Troilo et al., 2009; Howlett and McFadden, 2009; Norton et al., 2010). Although generally consistent responses to plus lenses have been found in chick, the response to plus lenses in mammals has been more variable. An early report from this lab using lenses that covered just the temporal visual field in juvenile tree shrews found a robust response to minus lenses, but little effect with plus lenses (Siegwart and Norton, 1993). One purpose of this study was to re-examine the response of plus lenses in juvenile tree shrews using lenses that covered the entire visual field.
Additional evidence for visually guided emmetropization comes from animal eyes that have elongated in response to a minus lens or to form deprivation and are myopic when the treatment is removed. These eyes generally respond to the myopic refractive state by reducing their growth rate while the eye’s optics continue to mature, so that they recover from the induced myopia (Wallman and Adams, 1987; Qiao-Grider et al., 2004; Troilo and Nickla, 2005; Howlett and McFadden, 2006; Norton et al., 2010). In chicks, monkeys, and tree shrews, recovery is more rapid and consistent in young animals compared to older animals (Wallman and Adams, 1987; Siegwart, Jr. and Norton, 1998; Qiao-Grider et al., 2004; Norton et al., 2010) suggesting that the emmetropization mechanism may become less effective at using myopia to guide recovery in older animals. It is not known if this reduced effectiveness is due to sensory (retinal) changes or to changes in the ability to use myopic refractive error information to slow axial elongation.
After early emmetropization in infancy reduces initial refractive error, visually-guided adjustment of axial growth continues throughout the juvenile, slower-growth period (Sorsby et al., 1961) in order to maintain near-emmetropia as the eyes continue to grow and the optical power of the eye decreases. Visual control of axial elongation during the juvenile growth period is demonstrated by two observations: 1) chicks and mammals (tree shrew, monkey) can respond to minus lens wear or form deprivation throughout the juvenile period and into adulthood (Papastergiou et al., 1998; Smith, III et al., 1999; Troilo et al., 2000; Norton et al., 2010); 2) if visual guidance is removed by placing tree shrews or chicks in continuous darkness, refractive errors reappear (Gottlieb et al., 1987; Nickla et al., 2001; Norton et al., 2006a).
The response of the eye to myopic refractive error throughout the juvenile phase may be particularly relevant given that human juvenile-onset myopia typically appears as a failure to maintain emmetropia during the slower juvenile eye growth period, rather than a failure to initially emmetropize during rapid infantile growth (Gwiazda et al., 1993; Howland et al., 1993; Mutti et al., 2005). When a child first begins to develop myopia, before any refractive correction is prescribed, the eyes experience a myopic refractive state. In many such eyes the myopia continues to increase to the point where optical correction is needed for clear distance vision. Why do such eyes not respond to the emergence of myopic refractive error by slowing their growth rate and restoring emmetropia, in a manner similar to the “recovering” animal eyes? One possibility that has not been examined is that age is a factor and that, as juveniles, children’s eyes and animal eyes may still detect the myopia but be less able to utilize it to slow axial elongation than are eyes at a younger age.
In this study we examined the response in tree shrew eyes to binocular plus or minus lenses at an early age, when the eye is still growing rapidly and initial emmetropization is not yet complete, analogous to early infantile emmetropization in humans. We also examined the responses of the eyes of older animals to plus and minus lenses during the slower juvenile stage of growth more analogous to the stage when myopia emerges in children. Binocular treatment was used because it is closer to the condition typically experienced by children, and also to examine whether tree shrews might increase their accommodation in response to minus lenses and reduce the response to the lenses. We also compared the recovery of juvenile eyes that were myopic after compensating to a minus lens with the response of age-matched normal eyes made similarly myopic with plus lenses to learn if recovering eyes respond differently to myopia than do normal age-matched eyes treated with plus lenses.
MATERIALS AND METHODS
2.1. Subjects and Experimental Groups
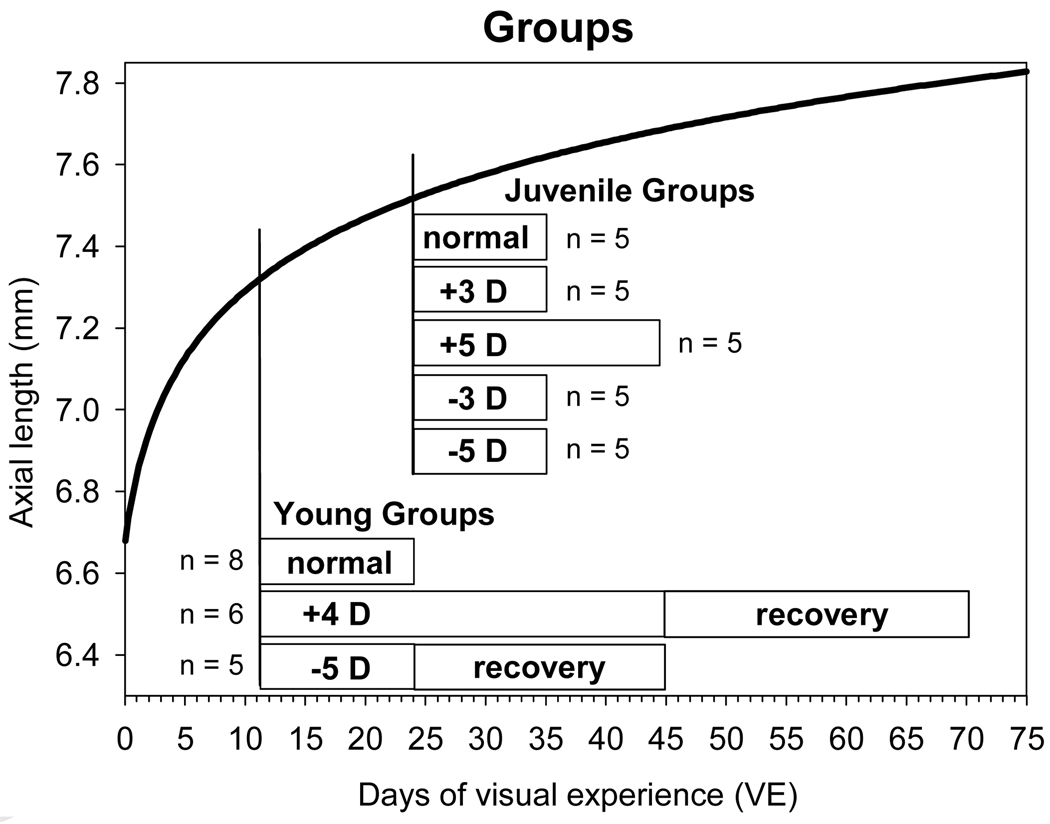
Maternally reared tree shrews (Tupaia glis belangeri), housed in our breeding colony on a 14 h/10 h light/dark cycle (Norton and McBrien, 1992) were the subjects in this study. All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. There were two normal groups and six experimental groups (Fig. 1). The primary measurement was frequent (often daily) non-cycloplegic measurement of refractive state with an autorefractor (Norton et al., 2003).
Figure 1.
Experimental groups as a function of days of VE. The dark curve shows the normal axial growth of tree shrew eyes from 1 to 75 days of VE (curve fitted to the data of Norton and McBrien, 1992).
Tree shrews are an altricial species; newborn pups have no hair and their eyes remain closed until approximately 21 postnatal days. We define the first day that both eyes are open as day 1 of visual experience (VE). The axial eye growth in normal animals (Norton and McBrien, 1992; Siegwart, Jr. and Norton, 1998) has an infantile rapid-growth phase (much of which occurs before the eyes open) and a slower, juvenile phase as shown in Figure 1. Susceptibility to form deprivation and minus lens wear begins late in the infantile phase (around 14 days of VE) and continues through the slow-growth phase (Siegwart, Jr. and Norton, 1998; Norton et al., 2010). Normal weaning age is at approximately 21 days of VE (6 weeks of age), but with supplemental feeding, pups have been weaned as early as 10 days after eye opening, which is near the end of the infantile growth period and is the youngest age at which lens experiments can be started. We compared the effects of plus and minus lenses in young (late infantile) animals starting at 11 days of VE with the effects of lenses in older, juvenile animals starting at 24 days of VE. Although separated by only 13 days, the eye growth rate and completeness of the emmetropization process are quite distinct at these ages (see Results).
Young Animals
A young normal group (n=8) had normal visual experience in their home cages and their refractions were measured daily between 11 and 24 days of VE, during the infantile period when the eyes are rapidly emmetropizing from the hyperopia that is present at eye opening (Norton and McBrien, 1992). A young plus-lens group (n=6) wore binocular +4 D lenses from 11 to 45 days of VE. The lenses were then removed and periodic refractive measurements continued until 65 – 70 days of VE. A young minus-lens group (n=5) wore binocular −5 D lenses from 11 to 24 days of VE. The lenses were then removed to initiate recovery and measurements continued until 45 days of VE.
Juvenile Animals
All juvenile groups contained 5 animals and had nearly reached emmetropia at the start of measurements. A juvenile normal group had normal visual experience in their home cages and their refractions were measured daily between 24 and 35 days of VE. Four juvenile groups wore binocular lenses of either +5 D, +3 D, −3 D or −5 D starting at 24 days of VE. Lens wear was continued until 35 days of VE (45 days of VE for the +5 D lens-wear group).
2.2. Goggle Procedures
A lightweight aluminum goggle that clipped onto a dental acrylic pedestal attached to the animal’s head was used to hold clear PMMA contact lenses (12 mm diameter, X-Cel Contact lenses) in front of the eyes at a vertex distance of approximately 3 mm. The dental acrylic pedestal was installed at 10 ± 1 days of VE (young groups) or 21 ± 1 days of VE (juvenile groups) as previously described (Siegwart and Norton, 1994). As in a previous study (Norton et al., 2010), the animals were allowed one (young groups) or three days (juvenile groups) to recover from the minor surgical procedure required to install the goggle pedestal before visual treatment and measurements were begun. The differing delay before the onset of lens treatment occurred because all animals were weaned and housed separately after pedestal installation and it was desired to let the young animals remain with their mothers as long as possible before the pedestal was installed. The animals in the normal groups received the pedestal to control for any effects from the pedestal installation but did not wear a goggle.
2.3. Refractive and Ocular Measurements
A-scan ultrasonography (Norton and McBrien, 1992) was performed under anesthesia (17.5 mg ketamine, 1.2 mg xylazine) immediately before the pedestal was installed. During treatment, non-cycloplegic refractive measures were taken at approximately 9:30 AM with an autorefractor (Nidek, Gamagori, Japan) in fully awake animals with the lenses removed (Norton et al., 2003; Norton et al., 2010). Measurements were made daily for the first 11 days of lens treatment, the first 10 days of recovery, and less frequently thereafter. Refractive measures were also made with the lenses in place to provide information on the refractive state experienced by the eyes. In the young groups, with-the-lens measures were made every time without-the-lens measures were made. In the juvenile groups, with-the-lens measures were made on all animals on the first day of treatment, midway, and on the last day of lens wear. For days on which only without-the-lens measurements were made, a calculated with-the-lens value was obtained by adding (or subtracting) the power of the lens that was worn. As shown in the Results, comparison of refractive measures with, and without, the lenses showed that this provided a good estimate (generally within 0.5 D) of the with-the-lens refraction. During lens treatment the lenses were cleaned twice each day at approximately 9:00 AM and 4:30 PM.
At the end of treatment (end of recovery in the 2 recovery groups), cycloplegic refractive measures were made in all groups (>1 hr after 2 drops of 1% ophthalmic atropine sulfate) both with, and without, the lenses in place and axial component dimensions were measured with A-scan ultrasonography under anesthesia. In the juvenile +5 D group, cycloplegic refractive measures were taken and A-scan ultrasonography was performed at 35 VE and 45 VE.
2.4. Statistics
All averaged values are reported as mean ± SEM. Paired t-tests were used to test for differences between the right and left eyes within each group. No statistically significant interocular differences were found in any group. Therefore, the average of the right and left eye for each animal was used for further data analysis and in most of the figures. ANOVAs and HSD post-hoc tests or unpaired t-tests were used to test for differences across groups.
3. RESULTS
To better reflect the refractive state experienced by the eyes during lens wear, the refractive values in the figures are the refractions measured or calculated with the lenses in place. In all figures, the 4 D small eye artifact of retinoscopy (Glickstein and Millodot, 1970; Norton et al., 2003) has been subtracted and results plotted relative to estimated emmetropia.
3.1. Normal Animals
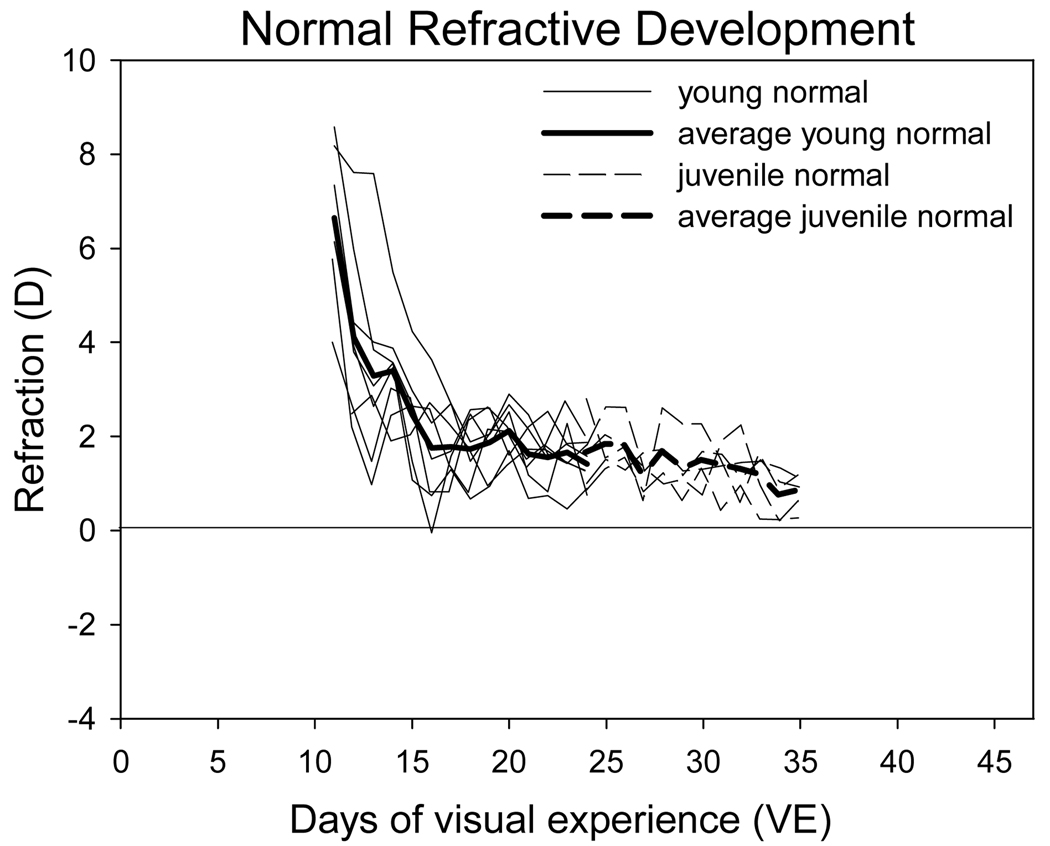
The refractive measures of the two normal groups are shown in Figure 2. As suggested by the data of Norton and McBrien (1992) who reported that tree shrew eyes at the time of eye opening were ~25 D hyperopic, the young animals at 11 days of VE were still substantially hyperopic (6.6 ± 0.6 D after subtracting the 4 D artifact of retinoscopy for small eyes) (Glickstein and Millodot, 1970; Norton et al., 2003). Over the next 5 days, the hyperopia decreased substantially to 1.8 ± 0.4 D at 16 days of VE and by 24 days of VE was 1.4 ± 0.2 D. The juvenile normal animals, first measured at 24 days of VE, had nearly completed emmetropization when first measured (1.6 ± 0.3 D) and showed a small (−0.8 +/− 0.4 D) reduction in hyperopia to 0.8 ± 0.2 D by 35 days of VE, as seen in other studies (Norton et al., 2006b; Norton et al., 2010). Together these normal groups show that rapid emmetropization occurs from eye opening to approximately 16 days of VE followed by continued but much slower reduction in the residual hyperopia. Thus the young animals began lens wear during a period of rapid emmetropization, while the juvenile animals began treatment when emmetropization was close to completion.
Figure 2.
Refractive development of the eight animals in the young normal group (solid lines) and the five animals in the juvenile normal group (dashed lines). Right and left eyes of individual animals were averaged and are shown with the thin lines. The dark lines are the group averages. In tree shrews, an autorefractor value of +4D is estimated to be emmetropia based on a prior study that used visual evoked potentials to measure the refractive state (Norton et al., 2003). Therefore, in this and other figures, 4 diopters have been subtracted from the autorefractor value.
3.2. Young Plus-lens Animals
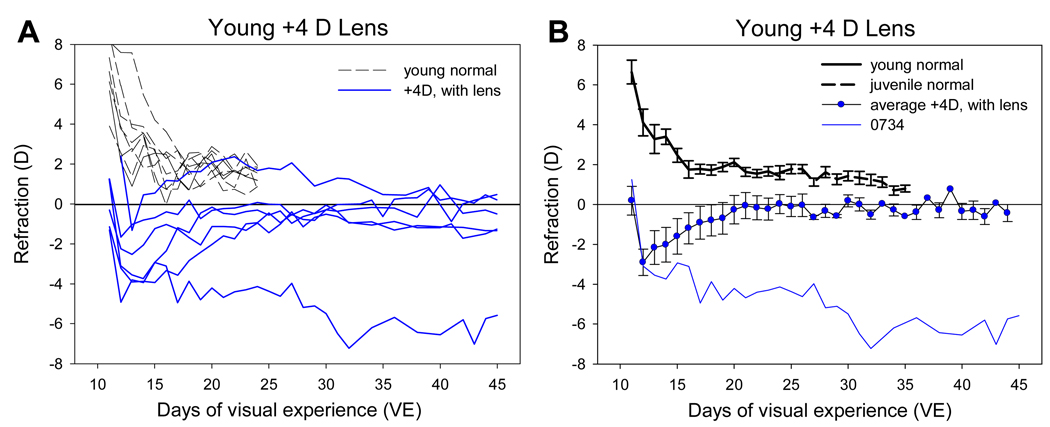
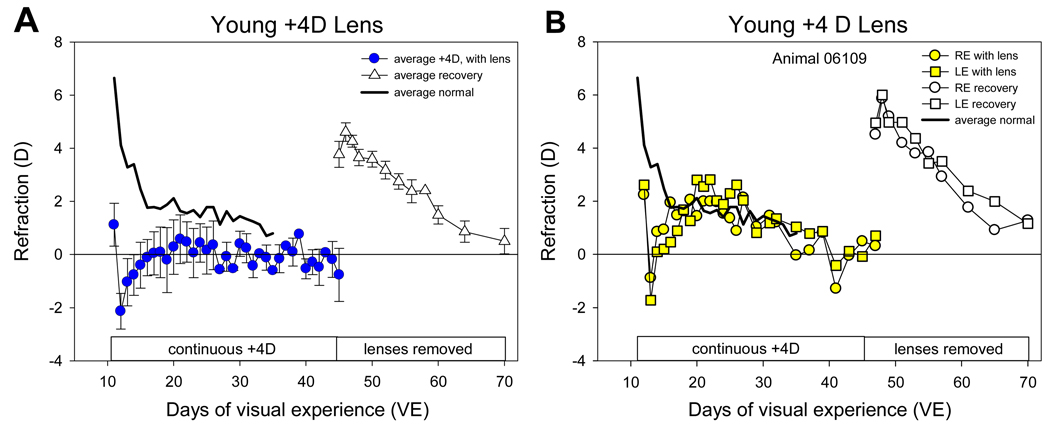
The average refractive state at the beginning of treatment (11 days of VE) in the +4 D lens treatment group was 4.2 ± 1.0 D relative to estimated emmetropia. The +4D lenses thus reduced, and in some cases over-corrected the hyperopia (Fig. 3). For the first 1 – 2 days after the onset of treatment, the eyes continued the rapid decrease in refractive state that occurs in young normal animals (Fig. 2). As a result, refractions in all eyes became myopic with the lenses in place. Continued plus-lens wear then produced a compensatory response in five of the six animals. The myopia in the eyes decreased so that they became emmetropic (0.0 ± 0.5 D) with the lenses in place by 24 days of VE. With the lenses removed, the eyes of the five animals (nonresponder excluded) were 3.8 ± 0.5 D hyperopic relative to estimated emmetropia (P < 0.05 vs normal), indicating that they had almost fully compensated for the plus lenses. The eyes remained near emmetropia with continued lens wear out to 45 days of VE. A-scan ultrasound measures were not made at the end of the plus lens treatment in these animals (to allow assessment of recovery unaffected by the anesthesia needed for axial measurements). However, based on the axial measures of similar binocular +4 D lens treated tree shrews studied by Metlapally and McBrien (2008), it is reasonable to assume that these young plus lens animals achieved compensation by slowing their axial elongation rate. When the +4D lenses were removed at 45 days of VE the eyes were 3.8 ± 0.8 D hyperopic (P < 0.05 vs normal, unpaired t-test). The eyes then reestablished emmetropia (Fig 4), presumably by increasing their axial elongation rate. The rapid “recovery” from plus lens wear shows that the emmetropization mechanism was actively restraining the refractions to maintain emmetropia with the lenses in place.
Figure 3.
Refractive development of the six animals in the young plus-lens group compared with normal refractive development. A. Individual animals. B. Group average responses excluding the non-responding animal (0734).
Figure 4.
Recovery from early plus-lens treatment. A. Group data for the 5 animals that compensated for the plus lens. B. Plots for animal 06109 showing the similar response in both eyes. After emmetropizing to the plus lenses, eyes were nearly emmetropic (filled symbols). When the lenses were removed at day 45 of VE, the eyes initially experienced hyperopia which rapidly decreased toward emmetropia (open symbols).
3.3. Juvenile Plus-lens Animals
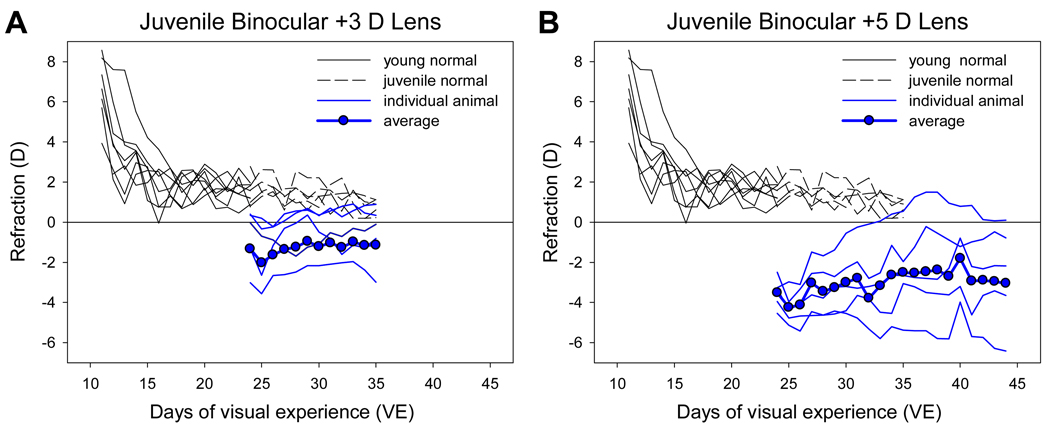
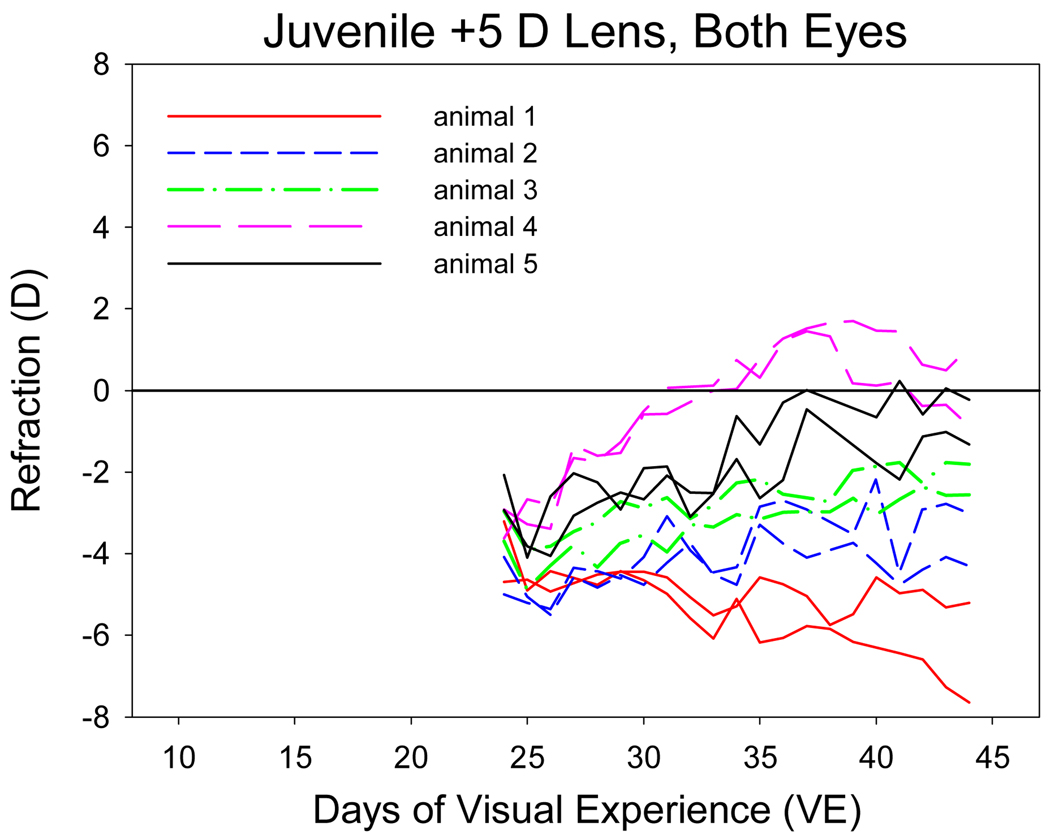
The daily non-cycloplegic autorefractor measures of the juvenile +3 D and +5 D lens groups are shown in Figure 5. Because these animals had nearly completed emmetropization at 24 VE, the +3 D lenses made two of the five animals myopic and the others nearly emmetropic at the start of treatment. In contrast to the young plus-lens animals, most of the juvenile eyes remained myopic while wearing the lenses. Two of the three animals in which the +3 D lenses corrected the eyes to emmetropia remained emmetropic with the lenses in place. The third animal at first became more myopic and then returned to nearly its starting refraction. The two animals made myopic by the +3 D lenses also showed variations in their daily refractive state, but were nearly as myopic at the end of treatment as at the start.
Figure 5.
Refractive development of the animals in the juvenile plus-lens treatment groups compared with normal refractive development. A. plus 3 D lenses. B. plus 5 D lenses. Values are calculated with the lens in place.
The +5 D lenses made all the eyes myopic at the start of treatment. One animal compensated for the +5 D lenses and became emmetropic with the lenses in place. Three others showed considerable daily variation and were slightly less myopic at the end of treatment. The fifth animal, which also showed variation in its daily refractions, was more myopic at the end of treatment than at the beginning.
After 12 days of treatment, measured with the lenses removed, the +3 D group was 1.3 D more hyperopic (cycloplegic measure) (p = 0.39) and the +5D group was 1.8 D more hyperopic (p = 0.69) than the age-matched normal animals (ANOVA, HSD). Thus, the average response to the +3D and +5D lenses was a small hyperopic shift that was not statistically significant due to high variability.
3.4. Young Minus-lens Animals
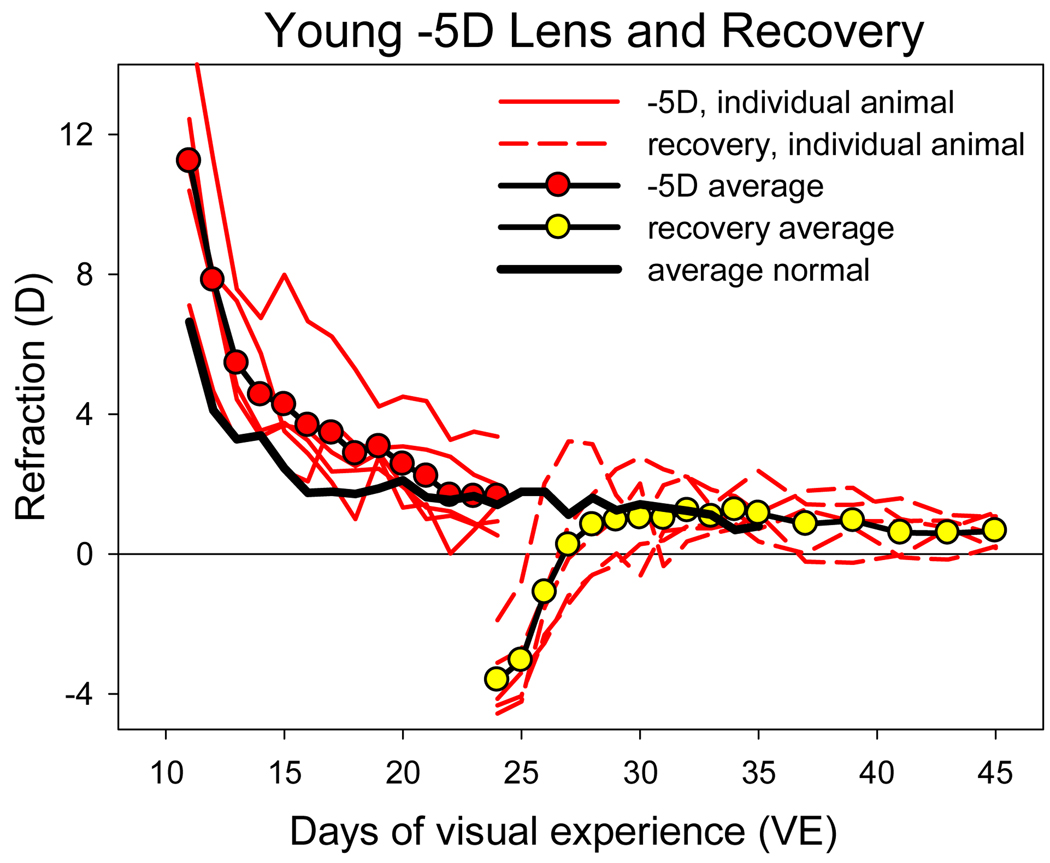
The animals in the young −5 D lens group were on average 5.6 ± 2.2 D hyperopic at the start of treatment (11 VE). The lenses increased this hyperopia by approximately five diopters (Fig. 6). During the 13-day treatment period, the eyes of all animals emmetropized from the higher starting point. The average refraction at 24 VE with the lens in place was 1.6 ± 0.5 D, compared with a refraction of 1.4 ± 0.4 D in age-matched normal animals. Thus, the minus lenses produced a rapid and consistent compensatory response.
Figure 6.
Refractive development and recovery in the young binocular −5 D group. The young minus lens-treated animals began recovery at 24 days of VE. The −5 D values are with the lens in place.
When recovery was begun at 24 VE by unclipping the goggle frame, the eyes were approximately 4 to 6 diopters myopic relative to age-matched normal eyes (Fig. 6). Over the next several days there was a rapid refractive recovery such that the refractions of all animals were within the range of age-matched normal refractions after 3 to 8 days of recovery.
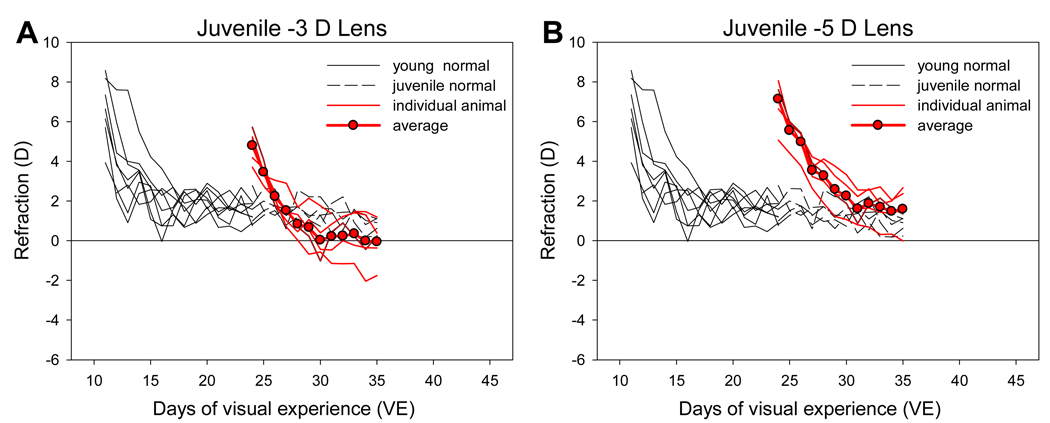
3.5. Juvenile Minus-lens Animals
Minus 3 D and −5 D lens treatments in juvenile animals starting at 24 days of VE also produced a consistent compensatory response (Fig. 7). Most eyes were similar to age-matched normal eyes at the end of treatment measured with the lenses in place. The eyes appeared to overcompensate for the −3D lenses relative to age-matched normal values, but the ending non-cycloplegic refractive difference was not statistically significant (unpaired t test) and the lower average values were largely due to overcompensation of approximately 2 D in one animal. The final cycloplegic refractive state measured with the lenses in place was 0.9 ± 0.6 D for the −3 D lens group, and 1.6 ± 0.4 D for the −5 D lens group. These values were not significantly different from the cycloplegic refraction of the juvenile normal group at 35 days of VE (1.4 ± 0.3 D).
Figure 7.
Refractive development of the animals in the juvenile minus lens groups, compared with the normal groups. A. −3 D lenses. B. −5 D lenses. Values are with the lens in place.
3.6. Juvenile Plus-lens Wear Compared to Age-matched Recovery from Minus-lens Wear
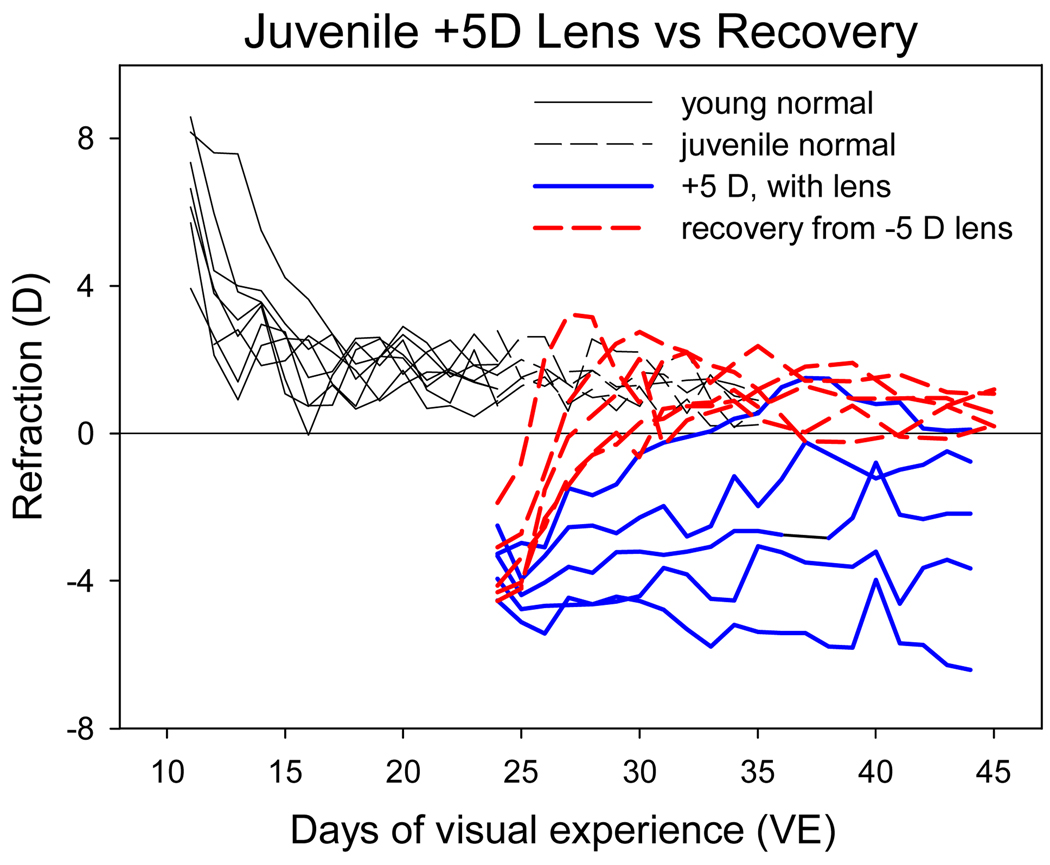
The juvenile animals that began to wear a +5 D lens starting at 24 days of VE experienced approximately the same amount of myopia (−3.5 ± 0.3 D) relative to estimated emmetropia as did the animals that began recovery from −5 D lens compensation at the same age (−3.6 ± 0.5 D). As may be seen in Figure 8, the similar refractive condition in these two age-matched groups produced, in general, distinctly different responses: a rapid consistent return to age-matched refractive values in recovering animals vs. a highly variable, persistent myopia in the plus lens-wearing animals. There was, however, a range of responses in both groups, with one of the +5D animals responding as well as the recovering animals.
Figure 8.
Comparison of recovery from myopia induced by −5 D lens wear (data from Fig. 6) with the response to +5 D lens-wear in age-matched normal animals (Fig. 5B). Both the recovery and the plus lens wear began at 24 days of VE.
3.7. Individual Variability
The high variability in the juvenile +5 D lens group raised the question of whether this variability was similar, or different, in both eyes of individual animals. The daily autorefractor values for both eyes of the animals in the +5 D group are shown in Figure 9. Both eyes in each animal followed a similar course, suggesting that the variability of the response to juvenile plus-lens wear resided in the animal, not in the individual eyes.
Figure 9.
Refractive development of both eyes in the juvenile +5 D plus-lens treatment group showing the similar response of the two eyes in each animal.
3.8. Cycloplegic vs. non-cycloplegic responses
Cycloplegic refractive measures were made at 35 days of VE in all animals in the juvenile groups both with and without the treatment lenses. Similar to previous studies (Norton et al., 2003) the cycloplegic refractions were shifted toward hyperopia (Table 1) with an average value of 0.7 ± 0.3 D. The shift was similar across groups. In addition, the with-the-lens vs. lens-removed measures were, on average, similar to the power of the lenses used. These measures suggest that, at least while held in position for autorefractor measures, the animals did not change their accommodation significantly while wearing either the plus or the minus lenses.
Table 1.
Refractive measures after 12 days of lens treatment in the juvenile groups
| Lens | Non Cycloplegic | Cycloplegic | Shift | |
|---|---|---|---|---|
| −5D | without lens | −3.4 | −2.9 | 0.5 |
| with lens | 1.4 | 1.6 | 0.2 | |
| difference | 4.8 | 4.5 | ||
| −3D | without lens | −3.1 | −2.3 | 0.8 |
| with lens | 0.2 | 0.9 | 0.7 | |
| difference | 3.3 | 3.2 | ||
| normal | no lens | 0.8 | 1. 3 | 0.5 |
| +3D | without lens | 1.9 | 2.7 | 0.8 |
| with lens | −1.9 | −0.9 | 1.0 | |
| difference | −3.8 | −3.6 | ||
| +5D | without lens | 2.5 | 3.2 | 0.7 |
| with lens | −3.6 | −2.4 | 1.2 | |
| difference | −6.1 | −5.6 |
3.9. Axial Elongation Rate
Axial component dimensions in the juvenile lens-wear groups were measured after 11 days of treatment (35 days of VE) (Table 2). There were no significant differences between the right and left eyes in any group. Therefore, the average of the right and left eye was used for statistical analysis and is shown in Table 2. Although corneal power was not measured in this study, previous studies examining cornea after minus lens or plus-lens wear have not detected significant changes in corneal curvature during lens compensation in tree shrews (McBrien and Norton, 1992; Norton and Rada, 1995; Metlapally and McBrien, 2008). Relative to the juvenile normal values, the average axial length (and vitreous chamber depth) was longer after minus-lens treatment and shorter after plus lens treatment although the differences were not statistically significant due to variation in starting axial length. Based on measures of many tree shrews in this laboratory, a change in vitreous chamber depth of 100 µm produces a 4 to 5 D refractive change. Thus, compared to the normal values, the difference in vitreous chamber depth with each lens power approximately matched the shift in refractive state (Table 1). Although the −3D group on average overcompensated for the power of the lens, the difference was primarily due to overcompensation of approximately 2 D by one animal in the group (Figure 7A).
Table 2.
Axial dimensions of the juvenile groups
| End of Treatment Values (mm) | Treated - Normal Difference (mm) | |||
|---|---|---|---|---|
| Group | Vitreous Chamber | Axial Length | Vitreous Chamber | Axial Length |
| −5D | 2.936 ± 0.021 | 7.358 ± 0.026 | 0.090 | 0.109 |
| −3D | 2.932 ± 0.027 | 7.347 ± 0.037 | 0.086 | 0.098 |
| Normal | 2.846 ± 0.013 | 7.249 ± 0.035 | ||
| +3D | 2.815 ± 0.032 | 7.217 ± 0.056 | −0.032 | −0.031 |
| +5D | 2.791 ± 0.027 | 7.207 ± 0.044 | −0.055 | −0.041 |
To examine the change in axial length during treatment and minimize the effect of variation in starting axial length across groups, axial elongation was normalized as percent change from the starting axial length at 21 days of VE when the pedestal was installed until 35 days of VE when measures were again made (Figure 10). Because A-scan was not performed at 24 days of VE when lens wear began, the change from 21 days of VE to 24 days of VE was estimated from the normal elongation rate in a previous study where tree shrews were measured both at 21 and 24 days of VE (Siegwart, Jr. and Norton, 2005). As shown in Figure 10, the eyes of the normal animals elongated 2.5 ± 0.2 %. The eyes of the animals treated with minus lenses elongated more and the eyes treated with plus lenses elongated less than the normal eyes. The percent elongation in all four lens treatment groups was significantly different than the elongation that occurred in the normal group (p < 0.05, ANOVA, HSD post hoc test).
Figure 10.
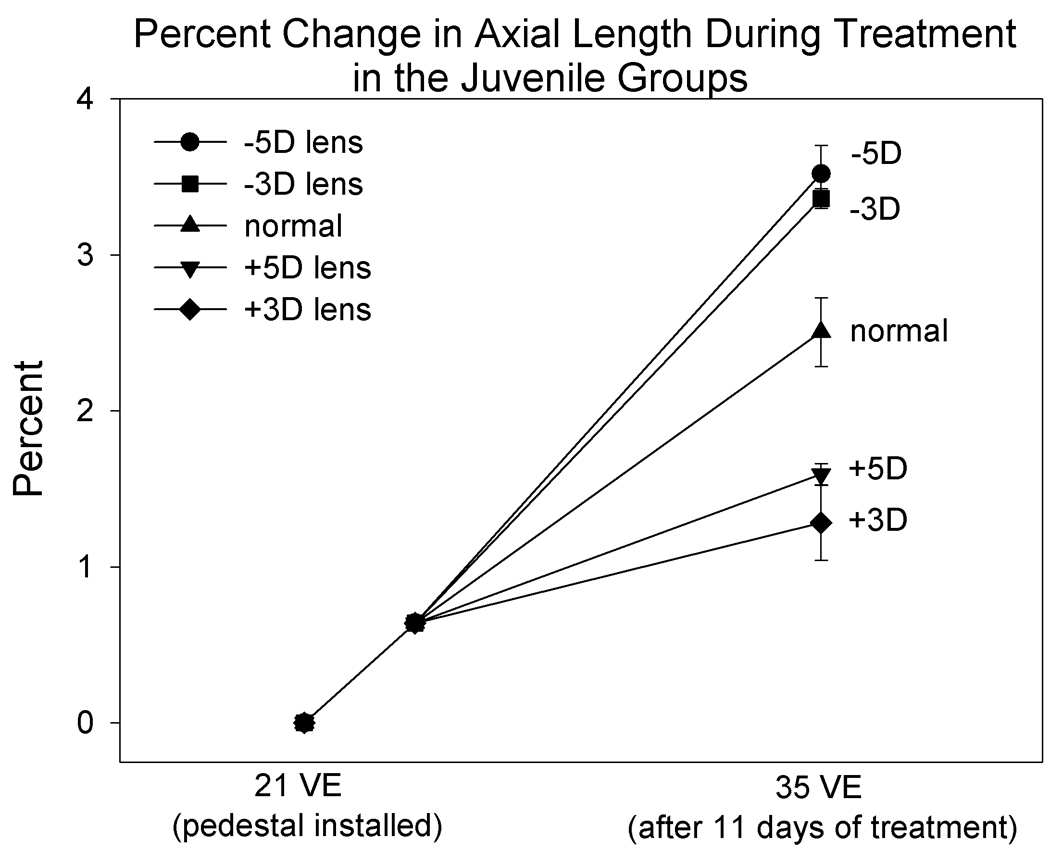
Normalized change in axial length in the juvenile groups based on measures made at pedestal installation (21 days of VE) and at 35 days of VE. The change from 21 to 24 VE is based on data from previous experiments in which animals were measured at 21 and 24 VE (Siegwart, Jr. and Norton, 2005).
It is interesting to note that, although axial elongation rate was slowed by plus lens wear compared to normal, the axial length of all juvenile plus-lens treated eyes in both the +3 D and +5 D groups was longer at the end of lens-wear than it was at the start. It thus appears that when juvenile normal eyes are subjected to myopia, they cannot completely stop their axial elongation which may contribute to the lack of full compensation for the plus lenses. However, note also that, in the +5D group, the individual percent elongation values were similar but were poorly correlated with the highly variable amount of refractive compensation (Fig. 8). This suggests that slowing growth to a minimum rate may produce different amounts of refractive compensation depending on other factors such as the rate of optical power change due to normal cornea and lens flattening.
4. DISCUSSION
This study using binocular lenses found four main results: 1) young tree shrews (11 days after natural eye opening, approximately 31 days old) generally responded to low-power plus lenses (+4 D) and most achieved a near-emmetropic refractive state while wearing the lenses; 2) juvenile tree shrews, just two weeks older, generally did not respond fully to plus-power lenses (+3 D, +5 D) and most remained myopic while wearing the lenses; 3) the response to minus lenses (−3 D, −5 D) was consistent both in young and juvenile tree shrews such that most animals achieved with-the-lens emmetropia during the lens-wear period; 4) juvenile tree shrews that had compensated for negative lenses showed a much stronger response to myopic refractive error than did age-matched tree shrews made similarly myopic with plus lenses.
4.1. Young vs. juvenile response to plus lenses
The ability of normal tree shrews to respond to plus lens-induced myopic refractive error decreased as a function of age. The decrease occurred over a relatively short period of time that corresponds to the transition from rapid emmetropization that reduces initial hyperopic refractive error to much slower emmetropization that further reduces low residual hyperopic refractive error. Young normal tree shrews, whose eyes were emmetropizing rapidly, were better able to emmetropize from myopia than were older juvenile tree shrews whose eyes had entered the slower juvenile growth phase. In both the young and juvenile groups there were exceptions. One young tree shrew did not emmetropize to the +4 D lenses and remained myopic while wearing the lenses. One juvenile tree shrew wearing +5 D lenses achieved emmetropia while wearing the lenses. Typically, however, the older animals did not respond as effectively to myopic refractive error as did the younger animals. Even the young animals did not precisely come to match normal eye refractions, remaining approximately 1 D lower compared to age matched normal eyes. This was, however, very close to estimated emmetropia given that the normal refractive state at that age is approximately 1 diopter hyperopic.
The response of the young tree shrews to plus lenses in this study is similar to that found by Metlapally and McBrien (2008), who began lens treatment at 14 days of VE. Their binocular +4 D lens group consistently became hyperopic when compared to a plano lens-wearing group of animals. Higher powered plus lenses (+6D and +9D) produced an increasingly variable response suggesting that the upper limit for a consistent response at this age is 4 to 5 diopters. It is interesting that their +9D lens treatment, which may have been outside the response range, caused unlinking of the response in the right and left eyes, while in this study, +5D lenses in the juvenile animals produced a variable, but linked, response. This suggests that the variable response to the +5 D lenses was not simply due to the myopic refractive error being out of sensory range. A linked response to binocular plus lenses has also been observed in monkeys (Smith, III and Hung, 1999).
Binocular plus lens treatment in young tree shrews was generally similar to binocular plus lens treatment in infant rhesus monkeys (Smith, III and Hung, 1999), which also typically are hyperopic at the start of lens wear and generally respond to plus lens treatment by slowing axial growth. In infant monkeys treated with low power binocular plus lenses that essentially corrected the existing hyperopia, no further emmetropization occurred so the eyes remained emmetropic with the lenses and were hyperopic when they were removed. Higher power plus lenses (+6D, +9D) which exceeded the initial hyperopic refractive error making the eyes myopic also typically eliminated further emmetropization but did not produce a hyperopic shift large enough to compensate for the effective myopic errors. There are currently no data on plus lens treatment in normal juvenile rhesus monkeys in which emmetropization is nearly complete so it is not known if they show a similar decrease in the ability to respond to plus lens induced myopic refractive error.
In a study using marmoset monkeys, treatment with plus power soft contact lenses starting at an average age of 76 days typically slowed eye growth (Troilo et al., 2009). Based on published eye growth curves (Troilo and Nickla, 2005), the marmosets, while not infants, were still in the late infantile growth phase of eye growth comparable to our young animals and had not entered the slower juvenile phase of eye growth. Infant guinea pigs several days old also show reduced eye growth to plus lens treatment relative to plano lens treatment (Howlett and McFadden, 2009). Like rhesus monkey and marmoset, there are currently no data on plus lens treatment at an older age after initial rapid eye growth has slowed and emmetropization is nearly complete. It would be informative to examine, in these other mammalian species and chicks, the characteristics of the response to plus lens-induced myopic refractive error in normal animals after the eyes have entered the slower juvenile growth phase analogous to the period during which myopia develops in humans.
Because viewing distance was not controlled, this study cannot speak to the issue of whether tree shrew eyes can use myopic defocus, per se, to control refractive development. While the plus lenses were in place, the animals were free to move about their home cages. As they did so, the myopic eyes presumably experienced myopic defocus for more distant objects, clear images for nearer objects and, perhaps even occasional hyperopic defocus for very nearby objects. All that can be known is that the eyes were myopic to some degree and that, in many of the young animals, the myopia appeared to contribute to the response of the eyes toward with-the-lens emmetropia. In the older animals, similar amounts of myopia were much less effective.
4.2. Response to minus lenses
Both young and juvenile tree shrews wearing minus lenses consistently moved toward re-establishing with-the-lens emmetropia during treatment. This is a very similar result to that found with monocular minus lens treatment in previous studies from this laboratory (Siegwart, Jr. and Norton, 1999; Siegwart, Jr. and Norton, 2005; Moring et al., 2007; Norton et al., 2010). At both ages, the response to binocular hyperopic refractive error, produced by the minus lenses, was more consistent and resulted in more complete compensation for the altered refractive state than was the response to myopic refractive error produced by the plus lenses in the juvenile animals. Although the response to hyperopic refractive error and form deprivation diminish with age in tree shrews (Siegwart, Jr. and Norton, 1998; Norton et al., 2010), normal tree shrew eyes clearly retain the ability to increase elongation in response to hyperopic refractive error longer than they retain the ability to restrain growth in response to myopic refractive error.
4.3. Possible Accommodation
With chemical stimulation, tree shrew eyes have been shown to be able to accommodate by as much as 8D (Cottriall and McBrien, 1996). One motivation for using binocular treatment in this study was to test the possibility that a mammal with linked accommodation might increase its accommodation during binocular minus lens wear and reduce the amount of minus-lens compensation. This did not appear to occur to a significant extent in this study. Although the final refractive difference between the −5 D group and the normal group was −4.2 diopters, less than the expected −5 diopters, it is not clear if it was due to accommodation that reduced the visual stimulus or simply due to chance. The final refraction in the binocular −5D group was not significantly different from the final refraction using monocular −5 D treatment in previous studies in this lab, suggesting that the effect on the eye was similar (Siegwart, Jr. and Norton, 2002). The lack of an accommodation effect is consistent with observations made during autorefractor measurements. As shown in Table 1, the difference between the no-lens and with-the-lens measure was close to the power of the lens both for plus and minus lenses, suggesting that the animals did not alter their accommodation, at least under those measurement conditions.
4.4. Recovery vs. Juvenile Plus Lens Wear
The eyes of the animals that began recovery from lens-induced myopia at 24 days of VE and the normal eyes that began to wear +5 D lenses at the same age experienced similar amounts of myopia, but generally responded quite differently. The consistent recovery response showed that the retina, at this age, is able to detect the myopic refractive error and guide refractive recovery. Thus, the highly variable response to the +5 D lenses is likely not due to retinal insensitivity to myopia. It is possible that the retina was in some way altered by minus lens treatment, but it is difficult to imagine how it would enhance the retina’s ability to respond to myopic refractive error compared to age-matched normal eyes. The primary difference between these two groups is the sclera: the animals that had emmetropized for the −5D lenses presumably had elongated eyes relative to normal (Siegwart, Jr. and Norton, 1999; Moring et al., 2007) whereas the plus lens group did not. The finding that an elongated eye responds more readily to myopia is consistent with results from previous studies (Nickla et al., 2005) lending support to previous suggestions (Troilo and Wallman, 1991; Schaeffel and Howland, 1991; Nickla et al., 2005) that an “eye-size” or “eye-shape” factor may be a variable in emmetropization. Together these data suggest that an eye that has increased its elongation to emmetropize retains the ability to respond to myopic refractive error to an older age.
4.5. Factors underlying the age-related reduction in the plus-lens response
There are several possible reasons, associated with either the retina or the sclera, that plus-lens wear might have been less effective in older animals than in young animals: 1) the retina in the juvenile animals might have become less sensitive to myopic refractive error; 2) the amount of myopia produced by the plus lenses might have been outside the operating range of the retina in the juvenile animals; 3) the sclera may be less able to respond to retinal signals to slow axial elongation in juvenile animals than in younger animals. The first two scenarios are unlikely for the same reason: all of the animals that, at the same age, began recovery from minus lens-induced myopia returned to emmetropia. That the eyes experienced myopia very similar in amount to that produced by the +3 and +5 D lenses and recovered suggests that the myopia was detected by the retina and was within the operating range at that age. Further, some of the juvenile plus-lens animals moved toward emmetropia, including a +5 D lens-wearing animal that became emmetropic while wearing the lens. This suggests that the retinas of the normal juvenile eyes could detect the myopic refractive error and send an appropriate signal, but that the scleras were less able to respond by slowing elongation.
This interpretation assumes that recovery requires the detection of a “STOP” visual stimulus rather than the removal of a “GO” visual stimulus produced by the minus lens. We have found previously that tree shrews do not recover from minus lens-induced myopia in the dark (Norton et al., 2006a) which suggests that removal of hyperopic refractive error produced by the minus lens is not sufficient to allow recovery and that a “STOP” visual stimulus is needed. However, we have also found that darkness is myopiagenic in visually experienced tree shrews making it uncertain whether darkness can simply be considered removal of the “GO” visual stimulus. Regardless of whether myopic refractive error produces a “STOP” visual stimulus or removes a “GO” visual stimulus the recovery in juvenile animals suggest that tree shrew eyes of that age can detect the change in visual stimulus.
To the extent that the retinal mechanism remains intact, it then appears that the young vs. juvenile difference may be due to changes in the scleral effector portion of the emmetropization mechanism. One possible underlying reason is that the sclera in the young animals was still undergoing rapid growth (Norton and Miller, 1995). In contrast, by about two weeks after eye opening (14 days of VE), the amount of type I collagen in the sclera reaches a peak (Norton and Miller, 1995) suggesting that the rate of growth slows in juvenile tree shrews. It is possible that retinal signals related to myopia may readily modulate the growth of the sclera during this young period. In chicks, the size of the eye is largely controlled by growth of the cartilaginous portion of the sclera (Rada et al., 1992; Marzani and Wallman, 1997), which may account for the strong response of chick eyes to myopia produced with plus lenses. Thus, chickens might continue to show a consistent response to plus lens out to a greater age.
In older, juvenile tree shrews, it appears that controlling growth may be less of a factor and controlling the biomechanical properties of the sclera may become more important. Evidence has accumulated that the scleral response to hyperopia involves biochemical changes that allow the scleral layers to slide across one another more easily, producing an increase in scleral viscoelasticity, measured by the creep rate (Phillips and McBrien, 1995; Siegwart, Jr. and Norton, 1999). During recovery, the elevated creep rate decreases rapidly, but only back to or slightly below normal levels. In age-matched normal animals treated with plus lenses, it may be difficult for myopia to decrease the creep rate enough below already low normal levels to significantly slow axial elongation. As noted in the Results (Fig. 10), the plus lens-treated juvenile eyes, though shorter than normal at the end of treatment, continued to elongate during the treatment period, suggesting that there may be a genetically determined minimum elongation rate in eyes with a fibrous sclera that cannot be further slowed by reducing growth or controlling scleral viscoelasticity.
4.6. Implications for human myopia development
While the bulk of human emmetropization typically occurs in infancy, the emmetropization mechanism must continue to operate throughout the juvenile period in order to maintain emmetropia. Juvenile-onset myopia is a failure to maintain emmetropia during the juvenile stage of eye growth. While myopic progression may be triggered and driven by an increase in myopiagenic visual stimuli such as hyperopic defocus, such stimuli are not continuously present. Studies have found that as little as 2 hours “relief” from hyperopia (or form deprivation) can completely block the effect of hyperopia present the remainder of the time (Schmid and Wildsoet, 1996; Shaikh et al., 1999; Smith, III et al., 2002; Norton et al., 2006b), and that 2 minutes of plus lens-induced myopia, 4 episodes per day, can cancel out the effects of minus lens-induced hyperopia the rest of the day (Zhu et al., 2003). Thus, failure to respond to the emerging myopic refractive error by slowing axial elongation also appears to be a factor. Although higher myopia susceptibility in some individuals might be due to increased sensitivity to myopiagenic visual stimuli, it might also be due to decreased ability to use visual stimuli that typically slow elongation. Data from this study suggests that the ability to slow elongation in response to myopic refractive error from infancy through the juvenile growth phase is strongly affected by age and previous elongation history while the ability to respond to hyperopic refractive error remains much more stable.
How might these factors observed in animal models, if present in humans, interact with genetics to cause some human eyes to become myopic in a visual environment in which other eyes do not? One scenario involves an interaction between genetically-programmed eye growth and an otherwise normal emmetropization mechanism. If, through inheritance, a child’s eye would gradually grow throughout the juvenile period to a length that exceeds the point where the cornea and lens focus images, then the emmetropization mechanism must use myopia to restrain that growth to maintain emmetropia. To the extent that eyes in older children may become less able to do this, the eyes might fail to use initial low levels of myopia to slow growth and would become increasingly myopic. The myopiagenic effect of an age-related reduction in the ability to restrain genetically programmed growth would be compounded if there is an increase in myopiagenic visual stimuli, such as hyperopic defocus, to which the eye still readily responds. This also might help to explain why refractive undercorrection, that leaves the eyes with low levels of myopia, did not slow myopia progression in children (Chung et al., 2002).
Conversely, if a child’s eye, without emmetropization, would become hyperopic (eye genetically short), the continued ability of the emmetropization mechanism to respond to hyperopia, even into adulthood, could produce elongation above the genetically-programmed level to maintain emmetropia. In such an eye, an increase in the prevalence of myopiagenic visual stimuli such as hyperopic defocus might actually help maintain emmetropia.
Acknowledgements
Supported by NIH grants RO1 EY-005922, P30 EY-003909 (CORE). We thank Joel Robertson for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andison ME, Sivak JG, Bird DM. The refractive development of the eye of the American kestrel (Falco sparverius): a new avian model. J Comp Physiol. 1992;170:565–574. doi: 10.1007/BF00199333. [DOI] [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol. Vis. Sci. 1999;40:214–229. [PubMed] [Google Scholar]
- Chung K, Mohidin N, O'Leary DJ. Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res. 2002;42:2555–2559. doi: 10.1016/s0042-6989(02)00258-4. [DOI] [PubMed] [Google Scholar]
- Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–1413. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- Cottriall CL, McBrien NA. The M1 muscarinic antagonist pirenzepine reduces myopia and eye enlargement in the tree shrew. Invest. Ophthalmol. Vis. Sci. 1996;37:1368–1379. [PubMed] [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest. Ophthalmol. Vis. Sci. 1987;28:1225–1235. [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vision Sci. 1993;8:337–344. [Google Scholar]
- Howland HC, Waite S, Peck L. Early focusing history predicts later refractive state: a longitudinal photorefractive study. Optical Society of America. 1993;3:210–213. [Google Scholar]
- Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Res. 2006;46:267–283. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat. Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–456. [PubMed] [Google Scholar]
- Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest. Ophthalmol. Vis. Sci. 1997;38:1726–1739. [PubMed] [Google Scholar]
- Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch. Ophthalmol. 2001;119:1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J. Vis. 2008;8:1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- Moring AG, Baker JR, Norton TT. Modulation of Glycosaminoglycan Levels in Tree Shrew Sclera during Lens-Induced Myopia Development and Recovery. Invest Ophthalmol. Vis. Sci. 2007;48:2947–2956. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial Growth and Changes in Lenticular and Corneal Power during Emmetropization in Infants. Invest Ophthalmol. Vis. Sci. 2005;46:3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Sharda V, Troilo D. Temporal integration characteristics of the axial and choroidal responses to myopic defocus induced by prior form deprivation versus positive spectacle lens wear in chickens. Optom. Vis. Sci. 2005;82:318–327. doi: 10.1097/01.opx.0000159368.31481.de. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet CF, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: implications for ocular growth regulation. Invest Ophthalmol. Vis. Sci. 2001;42:584–588. [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006a;47:4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Metlapally R, Young TL Myopia, Garner A, Klintworth GK. [Pt A, 27] Pt A. New York: Taylor & Francis; 2008. The Pathobiology of Ocular Disease; pp. 537–556. [Google Scholar]
- Norton TT, Miller EJ. Collagen and protein levels in sclera during normal development, induced myopia, and recovery in tree shrews. Invest. Ophthalmol. Vis. Sci. 1995;36(4):S760. [ARVO Abstract] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–1281. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006b;47:4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom. Vis. Sci. 2003;80:623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Laties AM, Pendrak K, Lin T, Stone RA. Induction of axial eye elongation and myopic refractive shift in one-year-old chickens. Vision Res. 1998;38:1883–1888. doi: 10.1016/s0042-6989(97)00347-7. [DOI] [PubMed] [Google Scholar]
- Phillips JR, McBrien NA. Form deprivation myopia: elastic properties of sclera. Ophthalmic Physiol Opt. 1995;15:357–362. [PubMed] [Google Scholar]
- Pickett-Seltner RL, Sivak JG, Paternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28:323–328. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., III Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3361–3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr. Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Res. 1991;31:717–734. doi: 10.1016/0042-6989(91)90011-s. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom. Vis. Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Shen W, Sivak JG. Eyes of a lower vertebrate are susceptible to the visual environment. Invest Ophthalmol. Vis. Sci. 2007;48:4829–4837. doi: 10.1167/iovs.06-1273. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Refractive and ocular changes in tree shrews raised with plus or minus lenses. Invest. Ophthalmol. Vis. Sci. 1993;34:S1208. [ARVO Abstract] [Google Scholar]
- Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Lab. Animal Sci. 1994;44:292–294. [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–3515. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol. Vis. Sci. 2002;43:2067–2075. [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol. Vis. Sci. 2005;46:3484–3492. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Bradley DV, Fernandes A, Boothe RG. Form deprivation myopia in adolescent monkeys. Optom. Vis. Sci. 1999;76:428–432. doi: 10.1097/00006324-199906000-00023. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis. Sci. 2002;43:291–299. [PubMed] [Google Scholar]
- Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA. Refraction and its components during the growth of the eye from the age of three. Med Res Counc Spec Rep Ser. 1961;301:1–67. [PubMed] [Google Scholar]
- Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol. Vis. Sci. 2005;46:1873–1881. doi: 10.1167/iovs.04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in mature common marmosets (Callithrix jacchus) Invest Ophthalmol. Vis. Sci. 2000;41:2043–2049. [PubMed] [Google Scholar]
- Troilo D, Totonelly K, Harb E. Imposed anisometropia, accommodation, and regulation of refractive state. Optom. Vis Sci. 2009;86:E31–E39. doi: 10.1097/OPX.0b013e318194072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthal Physiol Opt. 1997;17:279–290. [PubMed] [Google Scholar]
- Zhu X, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol. Vis. Sci. 2003;44:2818–2827. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]