Abstract
The damaged zebrafish retina replaces lost neurons through a regenerative response that initiates with the asymmetric cell division of Müller glia to produce neuronal progenitor cells that proliferate and migrate to the damaged retinal layer, where they differentiate into the lost neuronal cell types. Because Müller glia are known to phagocytose apoptotic retinal cells during development, we tested if Müller glia engulfed apoptotic rod cell bodies in light-damaged retinas. After 24 hours of constant intense light, damaged retinas revealed both a strong nuclear TUNEL signal in photoreceptors and a weak cytoplasmic TUNEL signal in Müller glia, although Müller glial apoptosis is not observed in the light-damaged retina. Light damage of a rod-specific transgenic reporter line, Tg(XlRho:EGFP)fl1, resulted in some Müller glia containing both TUNEL signal and EGFP, which indicated that this subset of Müller glia engulfed apoptotic photoreceptor cell bodies.
To determine if phagocytosis induced the Müller glial proliferative response in the light-damaged retina, we utilized O-phospho-L-serine (L-SOP), a molecule that mimics the phosphatidylserine head group and partially blocks microglial phagocytosis of apoptotic cells. Intravitreal injection of L-SOP immediately prior to beginning constant intense light treatment: i) did not significantly reduce light-induced photoreceptor cell death, ii) significantly reduced the number of PCNA-positive Müller glia, and iii) significantly reduced the number of cone photoreceptors in the regenerated retina relative to control retinas. Because L-SOP is also a specific group III metabotropic glutamate receptor (mGluR) agonist, we also tested if the more potent specific group III agonist, L-2-amino-4-phosphonobutyrate (L-AP4), the specific group III antagonist (RS)-α-Methylserine-O-phosphate (MSOP) or the specific group I antagonist, L-2-amino-3-phophonopropanoic acid (L-AP3) affected Müller glial proliferation. We found no changes with any of these factors compared to control retinas, revealing that metabotropic glutamate receptors were not necessary in the Müller glia proliferative response. Furthermore, ascl1a and stat3 expression were unaffected in either the L-SOP or MSOP-injected retinas relative to controls, suggesting L-SOP disrupts Müller glia proliferation subsequent to or in parallel with ascl1a and stat3 activation. This implies that at least one signaling mechanism, in addition to the process disrupted by L-SOP, is required to activate Müller glia proliferation in the light-damaged retina.
Keywords: regeneration, Müller glia, retina, phosphatidylserine, type III metabotropic glutamate receptor, phagocytosis
INTRODUCTION
A wide variety of vertebrates (fish, amphibians, reptiles, and birds) regenerate various tissues that mammals cannot. Adult zebrafish can rapidly and accurately regenerate its damaged fin, heart and retina (Poss et al., 2007; Iovine et al., 2007; Vihtelic and Hyde, 2000), but the molecular signals that initiate these responses is largely unknown. Determining the source and identity of these signals in the adult zebrafish may reveal mechanisms that will facilitate tissue regeneration in humans.
Various models of retinal damage have been described in zebrafish (Bernardos et al., 2007; Fimbel et al., 2007; Sherpa et al., 2007; Otteson and Hitchcock, 2003; Yurco and Cameron, 2005; Fausett and Goldman, 2006), including constant intense light (Vihtelic and Hyde, 2000; Kassen et al., 2007). These insults result in the loss of different classes of retinal neurons. For example, intravitreal injection of a low concentration of ouabain causes the loss of ganglion cells and inner nuclear layer (INL) neurons (Fimbel et al., 2007), while constant intense light causes apoptosis of the rod and cone photoreceptors in the dorsal and central retina (Vihtelic et al., 2006). In all the retinal damage models, new neurons regenerate from a subset of Müller glia, which are induced to divide asymmetrically and produce a population of transit-amplifying neuronal progenitor cells that migrate to the proper retinal layer and differentiate into the retinal neurons that were lost (Bernardos et al., 2007; Vihtelic and Hyde, 2000; Kassen et al., 2007; Fimbel et al., 2007; Sherpa et al., 2007; Otteson and Hitchcock, 2003; Yurco and Cameron, 2005; Fausett and Goldman, 2006). In the light-damage model, the number of proliferating Müller glia is proportional to the amount of cell death (Vihtelic et al., 2006). Thus, the extent and location of photoreceptor cell death appears to be communicated to the associated Müller glia.
A variety of different molecules and pathways influence Müller glia proliferation either in the damaged or undamaged retina. For example, CNTF induces Müller glial proliferation in the absence of damage in the zebrafish retina (Kassen et al., 2009), while insulin and Fgf2 stimulates a similar response in the undamaged chick retina (Fischer et al., 2004). In contrast, some molecules increase Müller glial cell proliferation in the damaged retina, such as Wnt and Shh in the rodent retina (Osakada et al., 2007; Das et al., 2006; Wan et al., 2007; Close et al., 2006). Inhibition of either Wnt (Das et al., 2006) or Shh (Wan et al., 2007) signaling pathways reduces Müller glial cell-based regeneration in the rat. Inhibition of Notch signaling in the NMDA-damaged chick retina, however, either inhibits neuronal regeneration if blocked early or increases the numbers of regenerated neurons if blocked after neuronal progenitors are produced (Hayes et al., 2007).
In addition to responding to many extracellular signaling molecules, the Müller glia also phagocytose a variety of different substances. Foreign molecules that are injected into the vitreous of the rodent eye are phagocytosed and distributed throughout the whole Müller cell body (Friedenwald and Chan, 1932; Rosenthal and Appleton, 1975). Additionally, Müller glia phagocytose dying cells, such as RPE cell bodies upon disruption of the outer limiting membrane (Francke et al., 2001), degenerated rod photoreceptors in a dominant transgenic zebrafish line (Morris et al., 2005), and during normal retinal development (Egensperger, et al, 1996). This suggests that Müller glia possess the necessary receptors and molecular pathways to phagocytose apoptotic cell bodies (Friedenwald and Chan, 1932; Rosenthal and Appleton, 1975; Wagner and Raymond, 1991).
We examined whether Müller glia engulf apoptotic photoreceptor bodies in the light-damaged zebrafish retina and if this process regulated Müller glial proliferation in regeneration. Specifically, we tested the role of the potential engulfment signal phosphatidylserine (PtdSer) (Fadok et al., 1992) by intravitreal injection of O-phospho-L-serine (L-SOP), which mimics the structure of the PtdSer headgroup. L-SOP was shown to significantly reduce the number of phagocytes that internalize apoptotic cell bodies (Witting et al., 2000). Because L-SOP also mediates group III metabotropic glutamate receptor (mGluR) function (Tizzano et al., 1995, Faden et al., 1997), we verified that the L-SOP effects were not mediated through mGluRs by injecting chemicals that stimulate or antagonize mGluRs. These studies demonstrated that L-SOP disrupts a process that is necessary for the maximal number of Müller glia to proliferate and regenerate cone photoreceptors in the light-damaged retina.
MATERIALS AND METHODS
Animal care and use
Adult albinob4 zebrafish (Danio rerio), which were 6–14 months of age (2.5–4 cm in length), were raised at 28.5°C in a 14 hours of light: 10 hours of dark regime under an average luminance of 200 lux using standard husbandry techniques (Westerfield, 1993). All experiments were conducted in accordance with the protocols approved by the animal use committee at the University of Notre Dame and the ARVO statement on the use of animals in vision research.
Transgenic Lines
The transgenic line, Tg(XlRho:EGFP)fl1, a gift from Dr. James Fadool (Florida State University), was outcrossed into the albb4 background (Fadool, 2003). The Müller glial-specific transgenic line, Tg(gfap:EGFP)nt11, was described previously (Kassen et al., 2007). The 3.2 kb guanine nucleotide binding protein (G protein), alpha transducing activity polypeptide 2 (gnat2) promoter was amplified from a plasmid template (Kennedy et al., 2007) using Platinum Taq DNA polymerase High Fidelity (Invitrogen; Carlsbad, CA) and primers (5'-GGATCCTTCCGGCAGCAACCTA-3' and 5'-CCATGGTCGTCTGACGCTTCC-3') that incorporated BamHI and NcoI restriction sites (underlined). The PCR product was cloned into the pCR8/GW/TOPO vector (Invitrogen) for DNA sequencing. A BamHI/NcoI digest removed the preproinsulin (insa) promoter from the T2KXIG-ins:nfsB-EGFP plasmid (Pisharath et al., 2007) and the gnat2 promoter was ligated upstream of nfsB-EGFP. The SP6 mMessage mMachine kit (Ambion; Foster City, CA) was used to in vitro transcribe Tol2 transposase mRNA from the pCSTZ2.8 plasmid (Kawakami et al., 1998). The gnat2:nfsB-EGFP plasmid and transposase mRNA were combined at a concentration of 25 ng/μl each and co-injected into 1–4 cell stage AB wild-type embryos. Injected F0 adults were out-crossed to the AB strain and F1 carriers were again out-crossed to establish independent transgenic lines. The Tg(gnat2:nfsB-EGFP)nt21 line was crossed into the albb4 background for constant light damage experiments. NTR-EGFP was expressed in the majority of offspring of Tg(gnat2:nfsB-EGFP)nt21 out-crosses, consistent with multiple insertions of the transgene.
Intravitreal injections and constant intense light treatment
Intravitreal injections were described previously (Fimbel et al., 2007; Thummel et al., 2008). Briefly, 14-day dark-adapted adult albino zebrafish were anesthetized in 2-phenoxyethanol and an incision was made in the cornea adjacent to the iris using a sapphire blade. A Hamilton syringe was used to intravitreally inject 0.5 μL of either control saline (0.65% NaCl) or 0.65% saline containing either 20 mM O-phospho-L-serine (L-SOP), 20 mM (RS)-α-Methylserine-O-phosphate (MSOP), 20 mM L-2-amino-4-phosphonobutyrate (L-AP4), or 20 mM L-2-amino-3-phophonopropanoic acid (L-AP3) into the left eye, while the right eye was not injected. Immediately following injection, the fish were revived in system water and subjected to constant intense light. All chemicals were obtained from Sigma (St. Louis, MO), excepting MSOP (Tocris Bioscience; Ellisville, MO). Light treatment was performed as previously described (Vihtelic and Hyde, 2000; Vihtelic et al., 2006; Kassen et al., 2007). Control and experimental groups were treated simultaneously in different tanks under the same lamps. Fish were subjected to either 12, 24 or 51 hours of constant light before they were anesthetized, enucleated and processed for further analysis. Some fish were injected with either 0.65% NaCl, 20 mM L-SOP, 20 mM MSOP, 20 mM L-AP4, or 20 mM L-AP4, but subjected to standard (14 hours light: 10 hours dark) light for either 24 or 51 hours of normal light as controls.
Cryosectioning
Eyes were fixed in either 3.7% formaldehyde in 1× phosphate-buffered saline (PBS) or a 9:1 mixture of ethanol:37% formaldehyde overnight at 4°C. The eyes were rinsed with 1× PBS, cryoprotected in 30% sucrose/PBS (pH 7.4) overnight at 4°C and frozen embedded in 100% Tissue Freezing Medium (Triangle Biomedical Sciences; Durham, NC). Serial sections (14–20μm) were cut tangentially through the central retina to obtain sections containing the optic stalk. Cryosections were mounted onto Superfrost/Plus glass slides, air dried for at least 20 minutes at 50°C and then stored at −80°C until use.
TUNEL
Frozen tissue sections were permeabilized with either Neuropore (R&D Systems, Inc.; Minneapolis, MN) for 75 minutes at room temperature or 0.1% sodium citrate buffer/0.1% Triton X-100 on ice for 2 minutes. The fragmented DNA was labeled enzymatically according to the manufacturer's protocol (TACS™ TdT Kit; R&D Systems, Inc.; Minneapolis, MN or ApoAlert DNA Fragmentation Assay Kit; Clontech; Mountain View, CA). Apoptotic cells were also labeled with biotinylated dUTP (NEB; Ipswich, MA) and detected by a 1:200 dilution of streptavidin-AlexaFluor conjugates (Invitrogen). Retinal TUNEL-positive nuclei were quantified at either 0 or 24 hours of constant, intense light treatment, when maximal retinal damage is observed (Vihtelic and Hyde, 2000). Positive and negative controls included adding TACS™-nuclease and omitting the TdT enzyme, respectively.
Immunohistochemistry
Retinal tissue sections were rehydrated in 1× PBS (pH 7.4), outlined with a Pap Pen (Ted Pella, Inc.; Redding, CA), and blocked with 1× PBS, 0.2% normal goat serum, 0.2% Triton X-100, and 1% DMSO for 60 minutes at room temperature. The sections were incubated overnight at 4 C with either a 1:1000 dilution of mouse anti-PCNA monoclonal antibody (clone PC10; Sigma Chemical), a 1:250 dilution of mouse monoclonal antibody 4C4 (kind gift of Dr. Jonathan Scholes, University College London), or a 1:500 dilution of rabbit anti-PCNA polyclonal antiserum (Abcam; Cambridge, MA) in blocking solution. Müller glia were immunodetected using a 1:500 dilution of mouse anti-glutamine synthetase monoclonal antibody (Chemicon International; Temecula, CA). Secondary antibodies, conjugated to either Alexa-Fluor 488 or 594 (Molecular Probes; Eugene, OR), were diluted 1:500 in PBS/2% normal goat serum/0.2% Triton X-100 and incubated for 1 hour at room temperature.
Images were collected with a Leica TCS SP5 Broadband Confocal Microscopy System (Leica Microsystems; Germany) using sequential laser scanning during colocalization analysis to eliminate spectral contamination.
5-ethynyl-2'-deoxyuridine (EdU) labeling of proliferating cells during light treatment
Light-damaged albino zebrafish were subjected to constant intense light treatment for 96 hours. The fish were intravitreally injected with 200 nmol EdU (Invitrogen) in 1× PBS after 48, 72 and 96 hours after starting the constant light treatment (Salic and Mitchison, 2008; Sugiyama et al., 2009). After the third EdU injection, the fish were transferred to normal lighting conditions until enucleation. Eyes were cryosectioned as described above and processed for EdU labeling by the Click-iT EdU Alexa Fluor 488 Imaging kit (Invitrogen) according to the manufacturers instructions and subjected to immunohistochemistry.
Statistical analysis
For both TUNEL and PCNA labeling, the central-most 700 μm of the dorsal retina, equidistant from the margin and the optic stalk, was quantified. Experiments were statistically compared by two-tailed Student's t-test assuming unequal variance. Due to variability in the light damage between groups of fish, experimental cohorts were compared only to saline-injected controls that were treated, with or without light, at the same time using the same light sources.
Quantitative real-time polymerase chain reaction analysis
Zebrafish retinas were collected after 12 hours of constant light treatment, dissected and RNA from a pool of three retinas for each test condition was isolated by TRIzol (Invitrogen). Single-stranded cDNA was generated with the SuperScript III reverse transcriptase (Invitrogen) using random hexamer primers. Transcript levels were analyzed using the SYBR Green PCR Master Mix on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems; Foster City, CA). Primers to detect ascl1a (previously called ash[a]) and stat3 were described previously (Kassen et al., 2007). The reaction mix contained 10 μl SYBR Green master mix (Applied Biosystems), various primer pairs (1.2 μl of each primer at 10 μM each), and several cDNA dilutions (7.6 μl) in a total volume of 20 μl. The reactions were incubated for 2 minutes at 50°C and 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C, and 1 minute at 60°C. The comparative CT method was used for data analysis (Johnson et al., 2000; Vong et al., 2003). Two serial dilutions of cDNA from each of the time points were run in triplicate and the median CT value was compared against the corresponding median 18S rRNA CT values. The normalized CT values for each gene from each treated retina were compared to untreated control retinas to obtain the log2-fold change in gene expression levels.
RESULTS
Healthy Müller glia cell bodies become TUNEL-positive upon light-induced photoreceptor cell death
TUNEL-positive, apoptotic cells are detected in the outer nuclear layer (ONL) after only 6 hours of constant intense light treatment and increase in number until 12 hours, when they reach a maximum number that is maintained through 24 hours of treatment (Vihtelic and Hyde, 2000). In addition, a weak and diffuse TUNEL label was also observed in the inner nuclear layer (INL) and extended across both plexiform layers (Fig. 1, panels B and C, arrows). Furthermore, this INL labeling was most often found basal to a cluster of TUNEL-positive photoreceptors (Fig. 1C, asterisks) and exhibited the morphology of Müller glial cells. No labeling was detected in the absence of TdT enzyme (not shown), which confirmed that the INL signal resulted from specific labeling of fragmented DNA. Because this TUNEL labeling appeared to fill the cytoplasm of the Müller glial cells, including the processes (Fig. 1C, arrows), it likely did not represent apoptotic Müller glia.
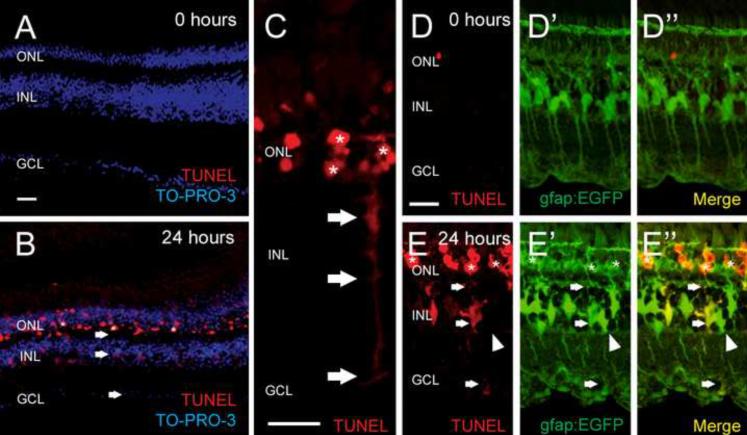
Figure 1. Light-induced TUNEL labeling of dying photoreceptors are detected in both the outer nuclear layer and Müller glia.
Both albino (A–C) and albino Tg(gfap:EGFP)nt11 transgenic (D–E) zebrafish were treated with constant intense light for either 0 (A and D) or 24 hours (B, C, and E). Retinal sections were stained for DNA fragmentation (TUNEL, red) and nuclei (TO-PRO-3, blue). At 0 hours, no significant TUNEL signal was detected (A, D). After 24 hours, the light-damaged albino retina exhibited TUNEL signal in both the ONL and INL (B, arrows). Higher magnification of a 24-hour light-damaged retina shows a TUNEL-positive INL cell with Müller glia morphology (C, arrows) directly adjacent apoptotic photoreceptors (asterisks). Sections of 24-hour light-treated albino Tg(gfap:EGFP)nt11 fish, which express EGFP in Müller glia (E'), revealed TUNEL signal (E) colabeled with a subset of Müller glia in only the light-damaged retina (E”, arrows). The remaining EGFP-positive Müller glia lacked the TUNEL signal (arrowheads). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bars represent 25 μm.
To confirm that the diffuse INL TUNEL signal was associated specifically with Müller glia cells, we repeated the TUNEL analysis with light-damaged Tg(gfap:egfp)nt11 transgenic zebrafish. The gfap:egfp transgene expresses EGFP under the transcriptional control of the Müller glial-specific glial fibrillary acidic protein promoter (Kassen et al., 2007). Non-light treated Tg(gfap:egfp)nt11 retinas displayed EGFP in Müller glia (Fig. 1D' and D”), but little TUNEL labeling (Fig. 1D and D”). After 24 hours of constant intense light, all TUNEL-positive INL cells colabeled with EGFP (Fig. 1E–E”, arrows), whereas not all EGFP-positive Müller cells colabeled with the TUNEL signal (Fig. 1E–E”, arrowhead). This confirmed that Müller glia are the predominant, if not the only, TUNEL-positive INL cell in the light-damaged retina. Furthermore, only a subset of the Müller glia were TUNEL-positive, which is similar to the previous observation that not all Müller glia proliferate during the regeneration response (Kassen et al., 2007; Thummel et al., 2008).
Müller glia engulf apoptotic, but not healthy, rod photoreceptor cell bodies
If the TUNEL-positive Müller glia were not dying, they could be phagocytosing apoptotic rod photoreceptor cell bodies. Therefore, we immunolabeled light-damaged retinas for various photoreceptor-specific antigens and analyzed their potential colocalization with TUNEL expression in the INL. The Tg(XlRho:EGFP)fl1 transgenic zebrafish line expresses EGFP throughout the rod photoreceptor cell body (Fadool et al., 2003). In undamaged Tg(XlRho:EGFP)fl1 controls, EGFP expression was restricted to the ONL and outer segments, even after signal amplification using an anti-GFP antibody (Fig. 2A). Further, there was no visible TUNEL labeling in either the INL or ONL in these undamaged retinas (Fig. 2B). In contrast, the light-damaged retinas exhibited the rod-derived EGFP in both the ONL and INL, where it displayed a characteristic Müller glial-like morphology (Fig. 2E). TUNEL labeling of the light-treated Tg(XlRho:EGFP)fl1 transgenic retinal sections revealed that EGFP colabeled with the TUNEL-positive cells (Fig. 2, panels F and H) and the Müller glial-specific glutamine synthetase immunolabeling (Fig 2, panels G and H). This confirmed the Müller glia in the light-damaged retina internalized remnants from the dying rod photoreceptors, which were likely apoptotic rod photoreceptor cell bodies that contained both rod-derived proteins and fragmented DNA.
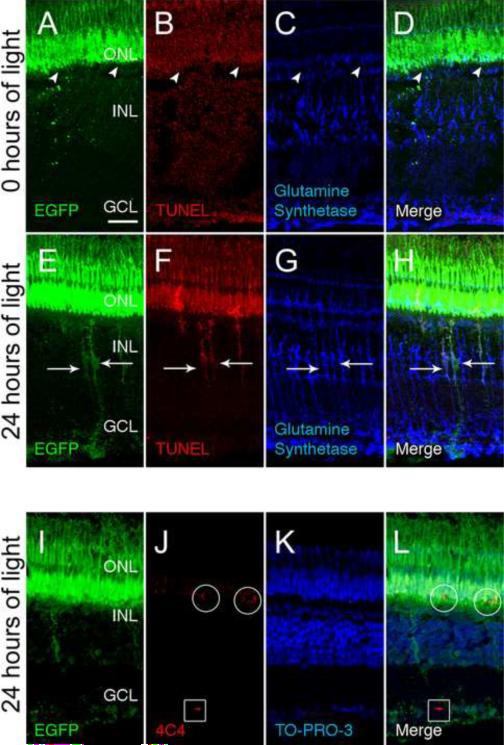
Figure 2. Müller glia engulf dying photoreceptor cell bodies in the ONL.
The Tg(XlRho:EGFP)fl1 transgenic fish express EGFP in rod photoreceptors from the Xenopus rhodopsin promoter. At 0 hours of light (A–D), control retinas exhibited EGFP expression (A and D, green) only in rods in the ONL (arrowheads) and the outer segments. There was no detectable TUNEL labeling (B) and low levels of Glutamine synthetase was restricted to the Müller glia (C and D). After 24 hours of light, EGFP was detected in both the ONL and INL (E and H, arrows), as was TUNEL labeling (F and H, arrows). Glutamine synthetase, which is expressed in Müller glia (G and H), colocalized with the rod-derived EGFP and TUNEL signal in the INL (H, arrows). An adjacent tissue section to that of panels E–H revealed that mAb 4C4-positive microglial/macrophage cells (J and L) were detected in both the ONL (circles) and GCL (boxes). These microglial/macrophage cells were EGFP-positive (I and L) in the ONL, but not in the GCL. The nuclear layers were identified by TO-PRO-3 staining (K and L). GCL ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar represents 25 μm.
We tested whether other photoreceptor proteins colocalized with the Müller glia in the light-damaged retina. Rhodopsin, normally restricted to the rod outer segments, but often mislocalized to the rod inner segments during photoreceptor damage was not detected in the INL of light-damaged retinas (Vihtelic, et al., 2000, Kassen, et al., 2007). Similarly, the zs-4 monoclonal antibody, which labels the rod inner segments (Trevarrow, et al., 1990, Vihtelic et al., 1999), did not label the INL of light-damaged retinas (Supplemental figure 1). We also tested if Müller glia engulfed apoptotic cone cells in the light-damaged retina by using the Tg(gnat2:nfsB-EGFP)nt21 transgenic zebrafish line that expresses EGFP specifically in cones under the control of the cone-specific transducin subunit promoter (Kennedy et al., 2007). We did not, however, detect cone cell-derived EGFP expression in the INL of light-damaged Tg(gnat2:nfsB-EGFP)nt21 transgenic fish (Supplemental figure 2).
Proliferating Müller glia colabel with TUNEL
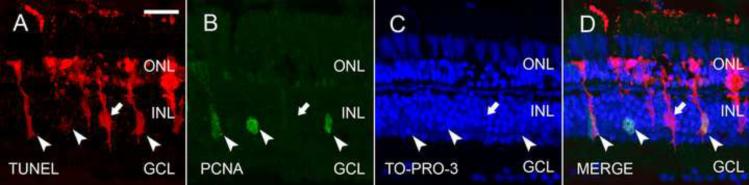
To test whether the proliferating Müller glia had engulfed the apoptotic photoreceptor cell bodies, we labeled light-damaged retinal sections with TUNEL and immunostained for proliferating cell nuclear antigen (PCNA), which is associated with replicating DNA. After 24 hours of constant intense light, a subset of the Müller glia had entered S phase of the cell cycle based on PCNA immunostaining (Fig. 3, panels B and D). Strikingly, the majority of the PCNA-positive Müller glia co-labeled with the TUNEL signal (Fig. 3, panels A, B, and D, arrowheads), while a small percentage of the TUNEL-positive Müller glia did not coexpress PCNA (Fig. 3, panels A, B, and D, arrow). Thus, the same Müller glia responded to cell death by phagocytosing the TUNEL-positive apoptotic rod photoreceptor cell bodies and proliferating. This suggests that the engulfment of apoptotic photoreceptor debris might stimulate the Müller glia to proliferate in the regenerative response.
Figure 3. Proliferating Müller glia engulf dying photoreceptor cell bodies.
Dark-adapted albino zebrafish were exposed to constant intense light for 24 hours and retinal sections were analyzed for cell death by TUNEL (A and D, red), proliferation by PCNA expression (B and D, green), and stained for cell nuclei by TO-PRO-3 (C and D, blue). D represents the merged image of panels A–C. All of the PCNA-positive Müller glia (B, arrowheads) were also TUNEL-positive (D), while some TUNEL-positive Müller glia (A, arrow) were not PCNA-positive (D). GCL ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar represents 25 μm.
Phosphatidylserine mimetic O-phospho-L-serine inhibits Müller glia proliferation in the light-damaged retina
To determine the role of engulfment of apoptotic debris on the proliferative response of Müller glia, we tested the ability of O-phospho-L-serine (L-SOP) to inhibit Müller glial cell proliferation. Apoptotic cells display phosphatidylserine (PtdSer) on the outer leaflet of the lipid bilayer as a signal to attract phagocytes and facilitate their engulfment and removal (Fadok et al., 1992). L-SOP mimics the structure of the PtdSer headgroup (Witting et al., 2000) and competes with PtdSer for binding to both PtdSer receptors and bridging proteins. Previous studies showed that L-SOP reduced microglial binding and phagocytosis of both apoptotic cells and lipid vesicles by 40% in cell culture (Witting et al., 2000).
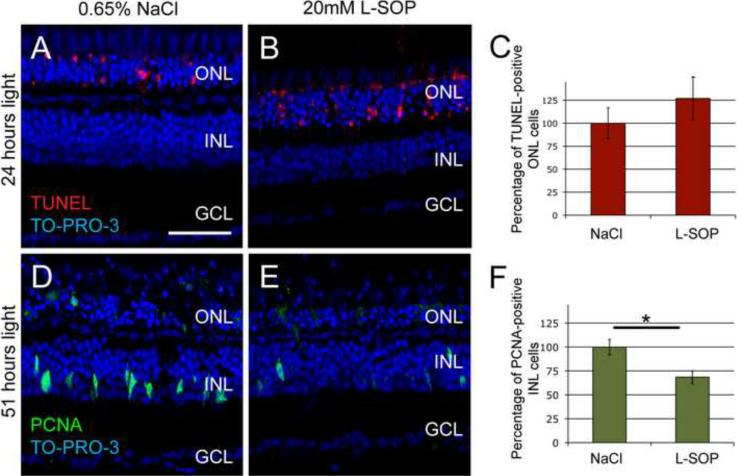
We intravitreally injected either 0.65% NaCl or 20 mM L-SOP immediately prior to initiating the constant intense light treatment. We selected 20 mM L-SOP because higher doses proved neurotoxic in the retina (data not shown). After 24 hours of light, the control retinas possessed a statistically equivalent number of TUNEL-positive ONL cells as the L-SOP-injected retinas (Fig. 4, panels A–C, p > 0.35, n=17). After 51 hours of light treatment, however, the L-SOP-injected retinas exhibited significantly fewer (69.5% +/− 6.8, p < 0.005, n=25) PCNA-positive INL cells relative to the saline-injected control retinas (Fig. 4, panels D–F). No change in the number of PCNA-positive ONL cells was observed between the control and the L-SOP-injected retinas after 51 hours of constant light (data not shown). These data suggest that L-SOP inhibited Müller glia proliferation, without affecting light-induced photoreceptor cell death.
Figure 4. O-phospho-L-serine (L-SOP) inhibits Müller glial cell proliferation in the light-damaged retina.
Dark-adapted albino zebrafish were injected intravitreally with either 0.65% NaCl (A and D) or 20 mM L-SOP (B and E) immediately prior to starting constant intense light treatment. After 24 hours of constant light (A and B), the retinas were assayed for cell death by TUNEL (red) and counterstained with TO-PRO-3 (blue). The number of TUNEL-positive ONL photoreceptors in the L-SOP-injected retina was normalized to the 0.65% NaCl control retinas (C). There was no statistically significant difference in TUNEL-positive photoreceptors between control and L-SOP-injected retinas (p>0.35, n=17). After 51 hours of constant light (D and E), the retinas were assayed for cell proliferation by PCNA immunostaining (green) and counterstained with TO-PRO-3 (blue). The L-SOP-injected retinas contained significantly less PCNA-expressing Müller glia (69.5% +/− 6.8) than the 0.65% saline-injected eyes (F, p < 0.005, n=25). GCL ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar represents 50 μm.
Photoreceptor apoptosis and Müller glial cell proliferation in light-damaged retinas are not regulated by the metabotropic glutamate receptors
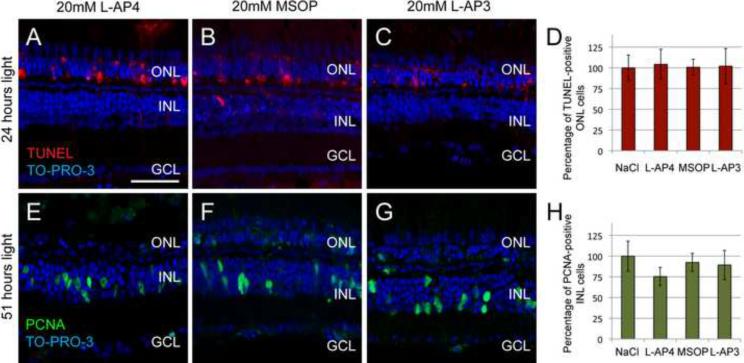
In addition to binding phosphatidylserine receptors, L-SOP is also an agonist of group III metabotropic glutamate receptors (group III mGluRs), although less potent than the structurally related L-2-amino-4-phosphonobutyrate (L-AP4) (Faden et al., 1997). Both compounds are neuroprotective during stress, whereas group I mGluR antagonists, such as L-2-amino-3-phosphonopropanoic acid (L-AP3), exacerbate neurotoxicity (Maiese et al., 2000). We tested whether the L-SOP-mediated suppression of Müller glial proliferation was due to potential interactions with mGluRs by comparing the effects of L-AP4 (group III agonist), (RS)-α-Methylserine-O-phosphate (MSOP, group III antagonist), and L-AP3 (group I antagonist) relative to 0.65% NaCl. We intravitreally injected L-AP4, MSOP, L-AP3, or saline into dark-adapted albino zebrafish and immediately introduced them to constant intense light for 24 hours. Retinal sections were then analyzed for neuroprotection of photoreceptor death by TUNEL (Figure 5, panels AC). L-AP4, MSOP, and L-AP3 did not induce photoreceptor cell death in non-light treated retinas (data not shown) and were not neuroprotective in light-treated retinas relative to the saline-injected control (Figure 5D, L-AP4, p>0.85, n = 13; MSOP, p>0.95, n=17; L-AP3, p>0.45, n = 20, respectively). Similar to L-SOP, these three compounds had neither a neurotoxic effect on the non-light-treated retina nor a neuroprotective effect in the light-treated retina.
Figure 5. Metabotropic glutamate receptor agonists and antagonists fail to affect either light-induced photoreceptor cell death or Müller glial cell proliferation.
Dark-adapted albino zebrafish were injected intravitreally with either 0.65% NaCl (Fig. 4, panels A and D), 20 mM L-2-amino-4-phosphonobutyrate (L-AP4, A and E), 20 mM (RS)-α-Methylserine-O-phosphate (MSOP, B and F) or 20 mM L-2-amino-3-phophonopropanoic acid (L-AP3, C and G) immediately prior to 24 hours of constant light treatment and then assayed for cell death by TUNEL (A–C, red) and counterstained with the nuclear dye TO-PRO-3 (blue). (D) The number of TUNEL-positive ONL bodies, which was normalized to 0.65% NaCl-injected controls, was not statistically different between either L-AP4, MSOP, or L-AP3 injected eyes and the control (D, L-AP4, p>0.85, n=13; MSOP, p>0.95, n=17; L-AP3, p>0.45, n=20). Zebrafish, similarly treated, but exposed to constant intense light for 51 hours (E–G) were assayed for Müller glia proliferation by immunohistochemistry using anti-PCNA antibodies (green) and counterstained with TO-PRO-3 (blue). The number of PCNA-expressing Müller glia, which was normalized to 0.65% NaCl-injected control eyes, was not statistically different between either L-AP4, MSOP, or L-AP3 retinas and the control (H, L-AP4, p>0.11, n=37; MSOP, p>0.63, n=27; L-AP3 p>0.64, n=33). GCL ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar represents 50 μm.
To determine whether Müller glia-mediated proliferation was dependent on mGluR signaling, L-AP4, MSOP, and L-AP3-treated fish were submitted to 51 hours of constant light treatment and analyzed for PCNA expression (Fig. 5, panels E–H). At this time, the majority of Müller glia that will respond to light damage are PCNA-positive. Light-treated L-AP4, MSOP, or L-AP3 retinas showed no significant differences in the number of PCNA-positive Müller glia relative to saline-injected light-damaged control retinas (Fig. 5H, L-AP4, p > 0.11, n = 37; MSOP, p>0.63, n=27; L-AP3, p > 0.64, n = 33, respectively). At this same time point, L-SOP significantly reduced the number of PCNA-positive Müller glia relative to the saline-injected control retinas. Thus, the L-SOP-mediated suppression of Müller glial proliferation in the light-damaged retina is not due to the action of L-SOP on the group III mGluRs.
Distribution of microglia in L-SOP-treated retinas
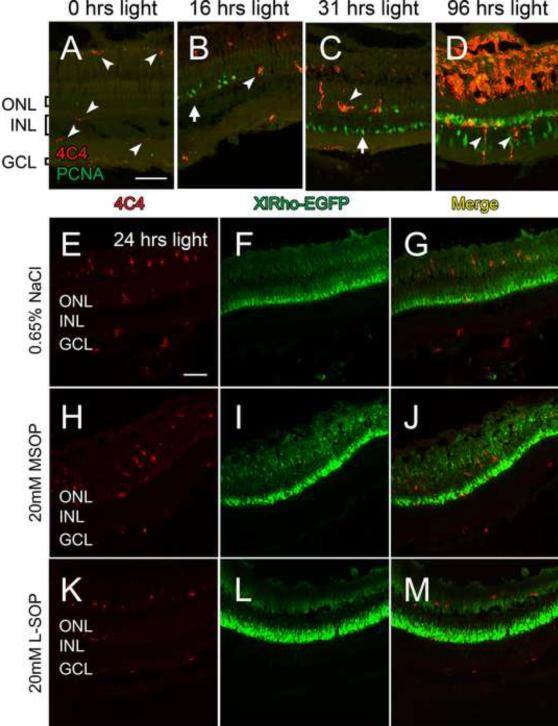
We also examined if the L-SOP-mediated suppression of Müller glia proliferation in the light-damaged retina was through the microglia, a known pleiomorphic phagocytic cell type in the retina (Hume, et al., 1983; Egensperger, et al, 1996). Microglia are detected in the retina by the 4C4 monoclonal antibody (Becker and Becker, 2001.). In the undamaged retina, microglia were present at relatively low numbers primarily in association with the rod outer segments, the INL, and the GCL (Fig. 6A, arrowheads). By 16 hours of constant light, the morphology of the 4C4-positive cells suggested that the microglia had become activated (Fig. 6B, arrowhead). However, large numbers of PCNA-positive rod precursor cells in the ONL were not associated with any microglia (Fig. 6B, arrow). By 31 hours of constant light, when the Müller glia began proliferating, the majority of the Müller glia were still not associated with the microglia (Fig. 6C, arrow). Only by 96 hours of constant light did we find increased numbers of activated microglia in the INL, as well as near the rod outer segment region (Fig. 6D, arrowheads).
Figure 6. Microglial activity during light damage.
Dark-adapted albino zebrafish were placed in constant intense light for various lengths of time, sacrificed, and retinal sections were colabeled with the 4C4 monoclonal antibody, which detects microglia (red), and anti-PCNA monoclonal antibody (green). Undamaged retinas possessed a limited number of microglia in the plexiform layers and associated with the rod outer segment region (A, arrowheads). After 16 ours of constant-intense light, larger, activated microglia (arrowhead) were found in the ONL (B). At 31 hours of constant light, activated microglia were restricted to the apical regions of the INL and the ONL (C, arrowhead), whereas PCNA-positive Müller glia were present in the basal INL (C, arrow). At 96 hours of constant light, activated microglia filled much of the ONL and the space formerly occupied by the rod outer segments, as well as some infiltration in the INL (D, arrowheads). (E–M) Immunohistochemistry of microglia in albino Tg(XlRho:EGFP)fl1 transgenic retinas. Dark-adapted albino Tg(XlRho:EGFP)fl1 zebrafish were treated with either 0.65% NaCl (E–G), 20 mM MSOP (H–J), or 20 mM L-SOP (K–M), subjected to 24 hours of constant intense light, sectioned, and labeled with 4C4 monoclonal antibody (E, G, H, J, K, and M, red). EGFP was detected throughout the rods (F, G, I, J, L, and M, green). Despite abundant numbers in the outer segments, an average of fewer than 5 4C4-positive microglia were observed in 400 μm of the ONL and INL of the L-SOP, MSOP, and saline-injected retinas. GCL ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar represents 50 μm and is the same for panels A–D and E–M.
To examine if L-SOP mediated its suppression of Müller glia proliferation through the microglia, we observed the microglia after 24 hours of constant light, when maximal rod photoreceptor cell death occurred and immediately before Müller glia proliferation began. We utilized dark-adapted albino Tg(XlRho:EGFP)fl1 transgenic line, which expresses EGFP from the Xenopus rod opsin promoter (Fadool, 2000). Light-treated saline-injected albino Tg(XlRho:EGFP)fl1 control retinas possessed activated microglia near the rod outer segments and in both the ONL and GCL (Fig. 6E). While many of these microglia were present in the region of the rod outer segments, they did not appear to possess any significant amounts of engulfed EGFP (Fig.6, panels F and G). Intravitreal injection of either 20 mM MSOP (Fig. 6, panels H–J) or 20 mM L-SOP (Fig. 6, panels K–M) did not affect either the relative number or distribution of the 4C4-positive microglia, as well as their potential engulfment of EGFP. Thus, the L-SOP-mediated suppression of Müller glia proliferation did not appear to be through the microglia, although the microglia could regulate Müller glia proliferation through a paracrine-mediated mechanism.
L-SOP disrupts Müller glia proliferation subsequent to or in parallel with the activation of ascl1a and stat3 expression in the light-damaged retina
Because Müller glia proliferation is dependent on increased ascl1a and stat3 expression in light-damaged retinas (Fausett et al., 2008; Kassen et al., 2007), we tested whether L-SOP affected transcription of these genes. Dark-adapted, albino zebrafish were injected with either 0.65% NaCl, 20 mM L-SOP, or 20 mM MSOP and subjected to 12 hours of constant intense light and compared to undamaged albino retinas. The retinas were isolated by dissection and mRNA levels were evaluated by quantitative real-time PCR (Figure 7). Control undamaged retinas had low levels of ascl1a and stat3 transcripts when compared with light-treated retinas. Light treatment of 0.65% NaCl-injected retinas caused a significant increase in the expression of ascl1a (p<0. 00005, n=4) and stat3 (p<0.0005, n=4) relative to untreated controls. Treatment with MSOP at the start of constant light treatment had no effect on the levels of either ascl1a (p>0.76, n=4) or stat3 (p>0.26, n=4) relative to saline-injected control retinas. Similarly, treating light-damaged retinas with L-SOP- failed to affect the increased expression of either ascl1a (p>0.12, n=4) or stat3 (p>0.84, n=4) compared to saline-injected control retinas. Thus, the L-SOP-mediated suppression of Müller glial proliferation in the light-damaged retina is likely acting downstream or in parallel of the activation of ascl1a and stat3 transcription and upstream of PCNA expression.
Figure 7. Expression levels of ascl1a and stat3 are unaltered in the L-SOP-treated retina.
Dark-adapted albino zebrafish were either untreated and housed at normal lighting conditions (no light) or injected intravitreally with either 0.65% NaCl, 20 mM L-SOP or 20 mM MSOP and subjected to 12 hours of constant intense light. RNA was isolated from the retinas and expression of ascl1a and stat3 were analyzed by quantitative real-time PCR. Constant intense light treatment caused a statistically significant increase in both ascl1a (p<0.05, n=4) and stat3 (p<0.005, n=4) expression, regardless of whether they were injected with saline, L-SOP, or MSOP, relative to undamaged controls (asterisks). There was no significant difference, however, between L-SOP and either saline or MSOP-treated control retinas in light-damaged eyes (p>0.10, n=4 for ascl1a, p>0.26, n=4 for stat3). Each n represents three pooled retinas harvested from different fish.
L-SOP inhibits cone cell regeneration in the light-damaged retina
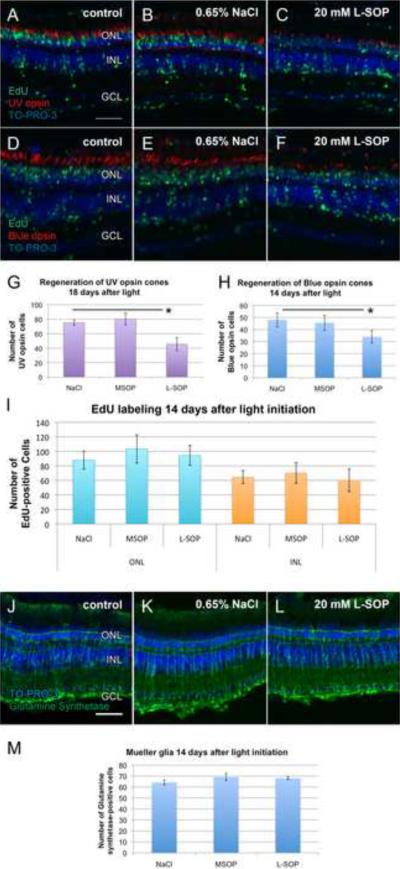
To assess the effects of L-SOP-mediated inhibition of Müller glial cell proliferation on photoreceptor cell regeneration, we injected dark-adapted albino zebrafish with 0.65% NaCl, 20mM MSOP, or 20mM L-SOP. The fish were intravitreally injected with EdU at 48, 72, and 96 hours of constant light to label dividing cells and their progeny. After the third EdU injection, the fish were transferred to normal light conditions for an additional 10 days. Eyes were enucleated and processed for cryosectioning to assess cone cell regeneration. Reduced numbers of both short single cones expressing UV opsin (Fig. 8, panels A–C) and long single cones expressing blue opsin (Fig. 8, panels D–F) were found in L-SOP-treated retinas relative to controls (Fig. 8, panels G and H, respectively).
Figure 8. L-SOP-mediated inhibition of UV and blue cone cell regeneration.
Dark-adapted albino zebrafish were intravitreally injected with EdU after 48, 72, and 96 hours of constant intense light. After the third injection, the fish were placed in standard light conditions for 10 days and then sacrificed. Retinal tissue sections were immunostained for EdU incorporation (A–F, green) and either the short single cone UV opsin (A–C, red) or the long single cone blue opsin (D–F, red). Eyes were either uninjected (A and D) or injected with either 0.65% NaCl (B and E) or 20 mM L-SOP (C and F) prior to initiating the constant light treatment. All sections were costained with TO-PRO-3 (A–F, blue). Statistically significant fewer short single cones (p<0.05, n=9) and long single cones (p<0.05, n=12) were present in the L-SOP-injected retinas relative to the control retinas (G and H, respectively). There was no significant difference in the number of EdU-labeled ONL and INL cells between the L-SOP-treated eyes relative to the controls (p>0.05, n=19). The same retinas described above were immunolabeled with anti-Glutamine synthetase monoclonal antibody (J–L, green) and counterstained with TO-PRO-3 (J–L, blue) to quantify the number of Müller glia present in the regenerated retinas (M). There was no statistically significant difference between the number of glutamine synthetase-positive Müller glia over a 400 μm linear retinal distance in the L-SOP-treated retinas relative to the controls (M, p>0.10, n=12) GCL ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar represents 50 μm and is the same for panels A–F and J–L.
This suppressed regeneration of cones could result from either reduced numbers of Müller glia, as observed by fewer PCNA-positive Müller glia (Fig. 4), or L-SOP acting directly on PCNA, which would result in the Müller glia undergoing apoptosis (Thummel et al., 2008). To examine the latter possibility, we immunostained the retinas described above for glutamine synthetase expression (Fig. 8, panels J–M). We found no statistical difference in the number of glutamine synthetase-positive Müller glia in the L-SOP-treated retinas relative to controls (p>0.1, n=10). Thus, L-SOP did not directly affect PCNA expression or activity, which would have resulted in the loss of Müller glia. Therefore, we examined EdU incorporation to confirm that L-SOP suppressed the number of proliferating Müller glia. Surprisingly, a statistically equivalent number of EdU-labeled cells was observed in the INL, as well as the ONL, of all three treatments (Fig. 8, panels A–F and I). We examined if these EdU-labeled cells were microglia that had invaded the INL upon light damage. However, the majority of the EdU-labeled cells in both the L-SOP-treated and the control retinas did not colabel with the anti-4C4 antibody (data not shown), suggesting that these EdU-labeled INL cells were not residual microglia. While the identity of the EdU-labeled INL cells in the L-SOP-treated retina remains unknown, it is clear that L-SOP suppressed the regeneration of cone photoreceptors in the light-damaged zebrafish retina.
DISCUSSION
In the retina, a rapid and robust regeneration response is detectable within a few hours after retinal damage, as Müller glial cells hypertrophy and begin to express ascl1a (Fausett et al., 2008) and stat3 (Kassen et al., 2007). A subset of these Müller glia proliferate to produce neuronal progenitor cells that migrate radially into the damaged nuclear layer and differentiate into the lost neurons. Little is known about the molecular mechanisms that initiate this regeneration response in the Müller glia. Here we show that some Müller glia either phagocytosed apoptotic photoreceptors or that they, in fact, were apoptotic. This latter hypothesis was rejected as the TUNEL labeling was cytoplasmic and no pyknotic nuclei were found in the INL, in striking contrast to the strongly TUNEL-labeled and pyknotic nuclei of the apoptotic photoreceptors. Furthermore, a loss of Müller glial cells during the normal regeneration response was not found (Fig. 8; Vihtelic et al., 2000; Kassen et al., 2007). Finally, we found that rod-specific EGFP in the transgenic Tg(XlRho:EGFP)fl1 zebrafish colocalized with the TUNEL signal in a subset of Glutamine synthetase-expressing Müller glial cells (Fig. 2A, panels E–H) only after light damage. These data demonstrate selective endocytosis of dying rod photoreceptors by Müller glia and support previous studies that Müller glia engulfed RPE debris and apoptotic neurons in both developing and damaged retinas (Egensperger et al., 1996; Francke et al., 2001; Morris et al., 2005; Friedenwald and Chan, 1932).
Surprisingly we did not detect either rhodopsin or the inner segment zs-4 antigen colabeling with the Müller glia (data not shown and Sup. Fig. 1, respectively). The lack of Müller glial engulfment of rod inner and outer segments likely results from these regions lying apical to the outer limiting membrane (OLM), which limits their contact with the Müller glia. It is more likely that they are phagocytosed by the retinal pigment epithelial cells and the highly-phagocytic and mobile microglia, which remove dying photoreceptor cell debris (Hume et al., 1983; Egensperger et al., 1996). While rhodopsin and other proteins become mislocalized in dying photoreceptors, engulfment of these proteins by the Müller glia may be at levels lower than those detectable by immunohistochemistry. The extremely high levels of rod-derived EGFP in the Tg(XlRho:EGFP)fl1 transgenic fish is at sufficiently large amounts in the regions basal to the OLM to be detected in the Müller glia if it was endocytosed. Although this EGFP expression in the Müller glia could be due to inadvertent expression of the XlRho:EGFP transgene in the Müller glia, the EGFP expression was often limited to the apical regions of the Müller glia, matching the subcellular distribution of TUNEL labeling rather than the expected expression throughout the cell body of autonomously expressed EGFP. Thus, we conclude it is more likely that the EGFP was engulfed by Müller glia.
We also failed to detect cone-derived EGFP in the Müller glia from the light-damaged Tg(gnat2:ntr-EGFP)nt21 transgenic line (Sup. Fig. 2). Indeed, no colabeling of TUNEL and cone-derived EGFP was found, despite obvious cone death and INL streaming of TUNEL label (likely from rod cells) at 51 hours of light damage (Sup. Fig. 2). This suggests that either these cone cell-specific proteins were below our level of detection or the Müller glia selectively engulfed dying rod cell bodies. If only dying rods were engulfed by the Müller glia, then a different signal must be produced by the dying cones to ensure their regeneration. The necessity of multiple signals is consistent with the inability of L-SOP to suppress the increased expression of the ascl1a and stat3 genes, which were shown to be required for both Müller glial cell proliferation and regeneration (Fausett et al., 2008; Kassen et al., 2007; Kassen et al., manuscript in preparation). Thus, another, yet to be identified, signal must be required to induce the expression of the ascl1a and stat3 genes and only more detailed molecular analysis will elucidate all of the signals required to regenerate neurons in the damaged zebrafish retina.
We found that the 4C4-labeled microglia infiltrated and hypertrophied in response to the light damage (Figure 6). However, they did not amplify in number and become the predominant phagocytic cell in the retina until later in the constant light treatment (Fig. 6D). Their presence in association with the rod outer segments is consistent with previous reports that the microglia engulfed dying photoreceptors in the light-damaged retina (Ng and Streilein, 2001). However, we failed to detect 4C4-positive microglia in close proximity to the Müller glia that were either engulfing dying rod cell debris or proliferating (Figs. 2 and 6, respectively). While this suggests that L-SOP was acting directly on Müller glial cell proliferation, it does not eliminate the possibility that L-SOP suppressed microglial phagocytosis of the dying rods, which in turn activated the proliferation of the Müller glia through a paracrine-mediated signaling mechanism.
If phagocytosis of dying rod cells signals the Müller glia to proliferate, then all PCNA-positive Müller glia should coexpress TUNEL signal. We found that all PCNA-positive Müller glia colabeled with TUNEL, however, not all Müller glia expressed PCNA at 24 hours of constant light (Fig. 3). This is consistent with our previous finding that approximately 50% of the Müller glia were PCNA-positive in the light-damaged retina (Thummel et al., 2008). This suggests that either only a subset of Müller glia are capable of proliferating or that a regenerative paracrine-like signal, which acts over a limited spatial range, induces a specific predetermined subset of Müller glia to proliferate. Because all of the PCNA-positive Müller glia had phagocytosed TUNEL-positive rod cell debris (Fig. 3), it is more likely that proximity to the cell death, not intrinsic qualities, determined which Müller glial cells responded to damage.
One important engulfment signal of apoptotic cell bodies is phosphatidylserine (PtdSer), which is normally maintained on the inner leaflet of healthy cells and exposed to the extracellular environment upon apoptosis (Fadok et al., 1992; Fadok et al., 2001). Blocking PtdSer-based cell-cell interaction with small molecules inhibits microglial phagocytosis of apoptotic cells in vitro (Witting et al., 2000). We found that intravitreal injection of the phosphatidylserine headgroup mimetic, L-SOP, significantly reduced the number of PCNA-positive Müller glia after 51 hours of constant intense light. In contrast, L-SOP failed to reduce the number of EdU-labeled INL cells 10 days after terminating the constant light treatment (Fig. 8I). EdU, a thymidine analog, was shown to label the same proliferating cells and their progeny as BrdU (Salic and Mitchison, 2008; Sugiyama et al., 2009). Immunolabeling EdU-treated retinas with the microglial-4C4 monoclonal antibody revealed that most of the EdU-labeled INL cells were not microglia. If the EdU-labeled INL cells represent Müller glia and their neuronal progenitor progeny, it is possible that the L-SOP-mediated suppression of Müller glial cell proliferation was only transient. Thus, the number of EdU-labeled cells in the L-SOP-treated retinas may have reached control levels by 10 days after terminating the light treatment (Fig. 8), but the L-SOP-treated retinas were still delayed in differentiating the regenerated short and long single cone cells relative to the control retinas (Fig. 8, panels A–H).
Because L-SOP binds both the phosphatidylserine receptor and the group III metabotropic glutamate receptor (mGluR), we examined if L-SOP exerted its effects through the group III mGluRs by comparing L-SOP to another agonist and two antagonists of mGluRs. While L-SOP is a group III mGluR agonist, it is only one-tenth as efficient as the related L-AP4 agonist (Tizzano et al., 1995, Faden et al., 1997). Group III mGluR agonists attenuate in vivo neurotoxicity of foreign substances introduced into the brain (Zhou et al., 2006) and in vitro neurotoxicity of physical trauma in mixed neuron and glia cultures (Faden et al., 1997). However, neither L-SOP nor L-AP4 were neuroprotective in the light-damaged retina. The Group I selective antagonist L-AP3, which protects against nitric oxide-induced apoptosis (Maiese et al., 2000) and hypoxia-induced apoptosis (Opitz et al., 1991), also was not neuroprotective in the light-damaged retina. In contrast, the Group III mGluR antagonist, MSOP, exacerbates neurotoxicity (Faden et al., 1997; Zhou et al, 2006), but was not neurotoxic in the zebrafish retina at the concentration we used, whereas a higher concentration (100 mM) was neurotoxic to all retinal cells (data not shown).
While L-SOP significantly reduced the number of PCNA-positive Müller glia in the light-damaged retina (Fig. 4F), L-AP4, L-AP3, or MSOP did not have any significant effect (Fig. 5H). This suggests that L-SOP was not acting through the mGluR to repress Müller glia proliferation. The inability to detect either engulfed rod cell debris in the microglia or increased numbers of activated microglia closely associated with the PCNA-positive Müller glia at 31 hours of constant light, further suggests that L-SOP is not acting on the microglia, which in turn, stimulated the Müller glia in a paracrine-like fashion.
Surprisingly, L-SOP suppressed Müller glial proliferation without decreasing the number of TUNEL–positive Müller glia in L-SOP-treated retinas relative to saline-injected retinas (data not shown). We also found no difference in the number of EGFP-positive Müller glia in the light-damaged Tg(XlRho:EGFP)fl1 transgenic retinas treated with saline, MSOP, or L-SOP (data not shown). Because L-SOP did not suppress the number of Müller glia engulfing dying rod cell debris, we tested if L-SOP decreased the relative amount of debris engulfed by the Müller glia. Due to the high variability in TUNEL labeling intensity in the tissue sections, however, we were unable to distinguish any difference in the amount of TUNEL signal in the L-SOP-treated Müller glia and the controls. Because we were unable to detect a difference in the number of Müller glia that engulfed dying rod cell debris or the amount that was engulfed, we tested if another compound that blocks the endocytic pathway had an analogous effect as L-SOP. We found that chlorpromazine, which inhibits clathrin-mediated endocytosis (Wang et al., 1993), also reduced the number of PCNA-positive Müller glia in the light-damaged retina (Bailey and Hyde, manuscript in preparation). This leaves open the mechanism of L-SOP-mediated suppression of Müller glia proliferation. First, L-SOP (and chlorpromazine) may block Müller glial cell proliferation by inhibiting the quantity of dying rod cell debris that is engulfed by Müller glia. Second, L-SOP (and chlorpromazine) may suppress microglial engulfment of dying rod cell debris, which activate Müller glial cell proliferation in a paracrine-like manner. Third, L-SOP may act through an unidentified process (possibly related to or different from chlorpromazine) in reducing Müller glial cell proliferation. We are currently performing experiments to try to differentiate between these three possibilities.
Increased expression of both ascl1a and stat3 are required for the proliferation of Müller glia in the regenerating retina (Fausett et al., 2008; Kassen et al., 2006). However, neither L-SOP nor MSOP altered the increased expression of ascl1a and stat3, which suggests that the L-SOP-mediated repression of Müller glia proliferation is either downstream or in a parallel pathway to ascl1a and stat3 transcription activation. Preliminary results in our lab suggest that apoptotic photoreceptors produce a diffusible molecule that induces this regeneration response (Nelson and Hyde, personal communication). This is consistent with an unknown signaling pathway activating the expression of ascl1a and stat3 either upstream of or in parallel with the Müller glia phagocytosis of apoptotic rod cell bodies. Further studies are needed to determine if either of these hypotheses correctly describes the underlying mechanism involved in activating the Müller glia proliferation response in the light-damaged retina.
Supplementary Material
Section from a retina of a dark-adapted albino Tg(gfap:EGFP)nt11 zebrafish that was subjected to 24 hours of constant intense light contained many TUNEL-positive Müller glia (See Fig. 1E). An adjacent retinal section was immunolabeled with monoclonal antibody zs-4 (B and C, red), which labels rod inner segments. EGFP-positive Müller glia (A and C) failed to colabel with the monoclonal antibody zs-4. Scale bar represents 50 μm.
Dark-adapted albino Tg(gnat2:nfsB-EGFP)nt21 zebrafish were subjected to 24 (A–C) hours of constant intense light and then analyzed for cell death by TUNEL (A and C, red). TUNEL labeled apoptotic rods (arrows) and Müller glial cell bodies (arrowheads). EGFP (B and C), which is expressed specifically in cone cells (green), was not detected in the Müller glia (C, Merge, arrowheads). Dark-adapted albino Tg(gnat2:nfsB-EGFP)nt21 zebrafish were subjected to a further 27 hours of constant light and analyzed by TUNEL (D and F, red). Cell death (TUNEL-postive cells) was marked in rod (EGFP-negative), as well as cone (EGFP-positive) cells (D and F, arrows). Although weaker than at 24 hours, TUNEL labeling was found in Müller glia (D and F, arrowheads), but no colabeling of EGFP was found in the INL, despite the obvious cone death. Scale bar represents 50 μm.
ACKNOWLEDGEMENTS
We thank D. Bang and the staff of the Freimann Life Science Center for providing zebrafish husbandry and care. We thank Jonathan Scholes for the 4C4 monoclonal antibody. This work was funded by National Institutes of Health grant R01EY018417 (D.R.H.) and the Center for Zebrafish Research at the University of Notre Dame.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Becker T, Becker CG. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J Comp Neurol. 2001;433:131–147. doi: 10.1002/cne.1131. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54:94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Egensperger R, Maslim J, Bisti S, Hollander H, Stone J. Fate of DNA from retinal cells dying during development: uptake by microglia and macroglia (Muller cells) Brain Res Dev Brain Res. 1996;97:1–8. doi: 10.1016/s0165-3806(96)00119-8. [DOI] [PubMed] [Google Scholar]
- Faden AI, Ivanova SA, Yakovlev AG, Mukhin AG. Neuroprotective effects of group III mGluR in traumatic neuronal injury. J Neurotrauma. 1997;14:885–895. doi: 10.1089/neu.1997.14.885. [DOI] [PubMed] [Google Scholar]
- Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258:277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Eubanks J, McGuire CR, Dierks BD, Reh TA. Different aspects of gliosis in retinal Muller glia can be induced by CNTF, insulin, and FGF2 in the absence of damage. Mol Vis. 2004;10:973–986. [PubMed] [Google Scholar]
- Francke M, Makarov F, Kacza J, Seeger J, Wendt S, Gartner U, Faude F, Wiedemann P, Reichenbach A. Retinal pigment epithelium melanin granules are phagocytozed by Muller glial cells in experimental retinal detachment. J Neurocytol. 2001;30:131–136. doi: 10.1023/a:1011987107034. [DOI] [PubMed] [Google Scholar]
- Friedenwald JS, Chan E. Pathogenesis of retinitis pigmentosa with a note on the phagocytic activity of Meller's fibers. Arch of Opthamol. 1932;8:173. [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312:300–311. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA, Perry VH, Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983;97:253–257. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovine MK. Conserved mechanisms regulate outgrowth in zebrafish fins. Nat Chem Biol. 2007;3:613–618. doi: 10.1038/nchembio.2007.36. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Wang K, Smith JB, Heslin MJ, Diasio RB. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem. 2000;278:175–184. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Ramanan V, Montgomery JE, C TB, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Thummel R, Campochiaro LA, Harding MJ, Bennett NA, Hyde DR. CNTF induces photoreceptor neuroprotection and Muller glial cell proliferation through two different signaling pathways in the adult zebrafish retina. Exp Eye Res. 2009;88:1051–1064. doi: 10.1016/j.exer.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Koga A, Hori H, Shima A. Excision of the tol2 transposable element of the medaka fish, Oryzias latipes, in zebrafish, Danio rerio. Gene. 1998;225:17–22. doi: 10.1016/s0378-1119(98)00537-x. [DOI] [PubMed] [Google Scholar]
- Kennedy BN, Alvarez Y, Brockerhoff SE, Stearns GW, Sapetto-Rebow B, Taylor MR, Hurley JB. Identification of a zebrafish cone photoreceptor-specific promoter and genetic rescue of achromatopsia in the nof mutant. Invest Ophthalmol Vis Sci. 2007;48:522–529. doi: 10.1167/iovs.06-0975. [DOI] [PubMed] [Google Scholar]
- Maiese K, Vincent A, Lin SH, Shaw T. Group I and group III metabotropic glutamate receptor subtypes provide enhanced neuroprotection. J Neurosci Res. 2000;62:257–272. doi: 10.1002/1097-4547(20001015)62:2<257::AID-JNR10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Morris AC, Schroeter EH, Bilotta J, Wong RO, Fadool JM. Cone survival despite rod degeneration in XOPS-mCFP transgenic zebrafish. Invest Ophthalmol Vis Sci. 2005;46:4762–4771. doi: 10.1167/iovs.05-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TF, Streilein JW. Light-induced migration of retinal microglia into the subretinal space. Invest Ophthalmol Vis Sci. 2001;42:3301–3310. [PubMed] [Google Scholar]
- Opitz T, Reymann KG. Blockade of metabotropic glutamate receptors protects rat CA1 neurons from hypoxic injury. Neuroreport. 1991;2:455–457. doi: 10.1097/00001756-199108000-00011. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–936. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD. Getting to the heart of regeneration in zebrafish. Semin Cell Dev Biol. 2007;18:36–45. doi: 10.1016/j.semcdb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Rosenthal AR, Appleton B. Histochemical localization of intraocular copper foreign bodies. Am J Ophthalmol. 1975;79:613–625. doi: 10.1016/0002-9394(75)90801-6. [DOI] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherpa T, Fimbel SM, Mallory DE, Maaswinkel H, Spritzer SD, Sand JA, Li L, Hyde DR, Stenkamp DL. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2007 doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M, Sakaue-Sawano A, Iimura T, Fukami K, Kitaguchi T, Kawakami K, Okamoto H, Higashijima SI, Miyawaki A. Illuminating cell-cycle progression in the developing zebrafish embryo. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano JP, Griffey KI, Schoepp DD. Induction or protection of limbic seizures in mice by mGluR subtype selective agonists. Neuropharmacology. 1995;34:1063–1067. doi: 10.1016/0028-3908(95)00083-i. [DOI] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci. 1999;16:571–585. doi: 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006;82:558–575. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Vong QP, Chan KM, Cheng CH. Quantification of common carp (Cyprinus carpio) IGF-I and IGF-II mRNA by real-time PCR: differential regulation of expression by GH. J Endocrinol. 2003;178:513–521. doi: 10.1677/joe.0.1780513. [DOI] [PubMed] [Google Scholar]
- Wagner EC, Raymond PA. Muller glial cells of the goldfish retina are phagocytic in vitro but not in vivo. Exp Eye Res. 1991;53:583–589. doi: 10.1016/0014-4835(91)90216-2. [DOI] [PubMed] [Google Scholar]
- Wan J, Zheng H, Chen ZL, Xiao HL, Shen ZJ, Zhou GM. Preferential regeneration of photoreceptor from Muller glia after retinal degeneration in adult rat. Vision Res. 2007 doi: 10.1016/j.visres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. 3 edn Univeristy of Oregon Press; Eugene, OR: 1993. [Google Scholar]
- Witting A, Muller P, Herrmann A, Kettenmann H, Nolte C. Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem. 2000;75:1060–1070. doi: 10.1046/j.1471-4159.2000.0751060.x. [DOI] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Muller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Zhou F, Yao HH, Wu JY, Yang YJ, Ding JH, Zhang J, Hu G. Activation of Group II/III metabotropic glutamate receptors attenuates LPS-induced astroglial neurotoxicity via promoting glutamate uptake. J Neurosci Res. 2006;84:268–277. doi: 10.1002/jnr.20897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Section from a retina of a dark-adapted albino Tg(gfap:EGFP)nt11 zebrafish that was subjected to 24 hours of constant intense light contained many TUNEL-positive Müller glia (See Fig. 1E). An adjacent retinal section was immunolabeled with monoclonal antibody zs-4 (B and C, red), which labels rod inner segments. EGFP-positive Müller glia (A and C) failed to colabel with the monoclonal antibody zs-4. Scale bar represents 50 μm.
Dark-adapted albino Tg(gnat2:nfsB-EGFP)nt21 zebrafish were subjected to 24 (A–C) hours of constant intense light and then analyzed for cell death by TUNEL (A and C, red). TUNEL labeled apoptotic rods (arrows) and Müller glial cell bodies (arrowheads). EGFP (B and C), which is expressed specifically in cone cells (green), was not detected in the Müller glia (C, Merge, arrowheads). Dark-adapted albino Tg(gnat2:nfsB-EGFP)nt21 zebrafish were subjected to a further 27 hours of constant light and analyzed by TUNEL (D and F, red). Cell death (TUNEL-postive cells) was marked in rod (EGFP-negative), as well as cone (EGFP-positive) cells (D and F, arrows). Although weaker than at 24 hours, TUNEL labeling was found in Müller glia (D and F, arrowheads), but no colabeling of EGFP was found in the INL, despite the obvious cone death. Scale bar represents 50 μm.