Abstract
Aims
Previous neuropathological studies documented that small vascular and microvascular pathology is associated with cognitive decline. More recently, we showed that thalamic and basal ganglia lacunes are associated with post-stroke depression and may affect emotional regulation. The present study examines whether this is also the case for late-onset depression.
Methods
We performed a detailed analysis of small macrovascular and microvascular pathology in the postmortem brains of 38 patients with late-onset major depression (LOD) and 29 healthy elderly controls. A clinical diagnosis of LOD was established while the subjects were alive using the DSM-IV criteria. Additionally, we retrospectively reviewed all charts for the presence of clinical criteria of vascular depression. Neuropathological evaluation included bilateral semiquantitative assessment of lacunes, deep white matter and periventricular demyelination, cortical microinfarcts and both focal and diffuse gliosis. The association between vascular burden and LOD was investigated using Fisher’s exact test and univariate and multivariate logistic regression models.
Results
Neither the existence of lacunes nor the presence of microvascular ischaemic lesions was related to occurrence of LOD. Similarly, there was no relationship between vascular lesion scores and LOD. This was also the case within the subgroup of LOD patients fulfilling the clinical criteria for vascular depression.
Conclusions
Our results challenge the vascular depression hypothesis by showing that neither deep white matter nor periventricular demyelination is associated with LOD. In conjunction with our previous observations in stroke patients, they also imply that the impact of lacunes on mood may be significant solely in the presence of acute brain compromise.
Keywords: brain ischaemia, elderly, mood, neuropathology, vascular depression
Introduction
Clinically significant depressive syndromes can be found in 8% to 16% of community-dwelling, 25 % of primary care, and 23 % of hospitalized older adults [1, 2], and are associated not only with functional disability and decline in global health but also with an increase in the use of medical services and in mortality rate [3-6].
Late-onset depression (LOD) is a heterogeneous and broad concept that includes both individuals with early-onset recurrent depression and those who developed a first depressive episode after the age of 60. In contrast with major depression at younger ages, which is thought to have a strong genetic background, subcortical vascular pathology has consistently been pointed as a possible key substrate of LOD [7-12].
The concept of “vascular depression” postulates that the disruption of frontostriatal circuits by vascular lesions predisposes, perpetuates, or exacerbates depressive symptoms in some elderly individuals. These patients display a significant increase in the frequency of cardiovascular risk factors such as hypertension, dyslipidaemia or a history of cerebrovascular disease and predominant dysexecutive syndrome [13]. Krishnan and colleagues reported the substantial development of white matter hyperintensities (WMH) in the deep white matter and subcortical grey matter [14] of patients older than 60 presenting with a first depressive episode after the age of forty and in the absence of psychotic symptoms. Early neuroimaging findings revealed that WMH tend to be more frequent and more severe in LOD patients compared both to elders with early-onset depression [15-18] and age-matched controls [18, 19]. However, more recent contributions including the community-based Longitudinal Aging Study Amsterdam [20] challenged the validity of “vascular depression” as a clinically recognized subtype of depression phenotype [21]. In particular, the implication of cardiovascular risk factors in the pathogenesis of LOD remains controversial [22-27], and two recent studies failed to identify WMH burden differences between LOD and elderly controls [28, 29]. One main methodological limitation of the concept of “vascular depression” resides in the heterogeneous character of WMH that correspond to several distinct neuropathological changes including arteriosclerosis, perivascular demyelination, dilated perivascular spaces, vascular ectasia, ischaemia, incomplete infarction, partial loss of myelin and axons, gliosis, and infarction with necrosis [30-35].
As recently stated by Smith and Alexopoulos [36], a rigorous neuropathological investigation in clinically well-documented autopsy cases is crucial to shed some light on the role of cerebrovascular lesions in late-life affective disorders. The only currently available clinicopathological analyses were performed by a single research group and led to conflicting conclusions [35, 37-39]. Given the marked difficulty to obtain autopsy material from LOD cases, these observations have not been subsequently tested in an independent sample. To address this issue, we had the opportunity to examine in detail the small macrovascular and microvascular pathology in a postmortem series of 38 subjects having suffered from LOD and 29 age- and gender-matched controls.
Methods
Selection of cases
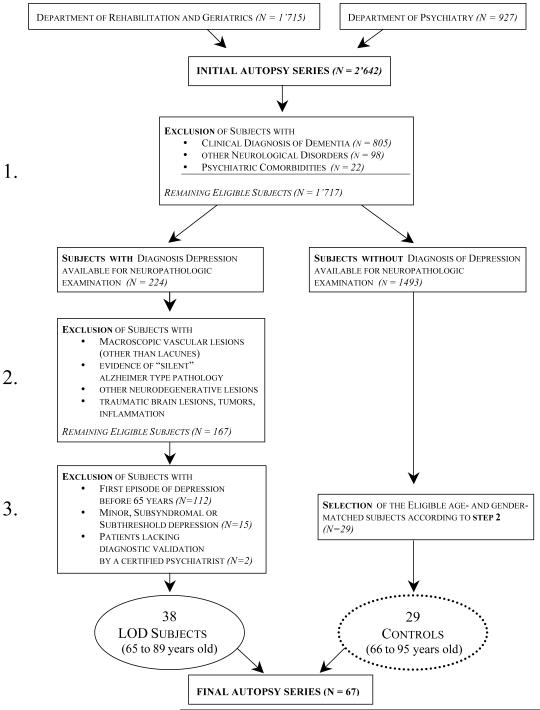
The initial autopsy series included 2,642 patients who were autopsied at the Department of Rehabilitation and Geriatrics and at the Department of Psychiatry of Geneva University Hospitals between 1998 and 2007 (see figure 1).
Figure 1.
Study profile and patient selection
The final sample was defined in a three-step process. First, exclusion criteria were applied by reviewing the clinical files of autopsied cases. All patients with a clinical diagnosis of dementia were excluded. We also excluded all cases with neurological disorders, as well as those presenting with psychiatric co-morbidities (19 cases with schizophrenia and 3 cases with chronic alcoholism). From the remaining 1,717 cases, 224 had a prospectively documented diagnosis of major depression [40].
Second, on the basis of the routine neuropathological assessment of these 224 cases, we excluded those presenting with macroscopic vascular pathology other than lacunes (brain infarcts, haemorrhage, venous sinus thrombosis), clinically silent but substantial Alzheimer type pathology (Braak neurofibrillary tangle staging >3), other neurodegenerative lesions (Pick bodies, ubiquitin-positive inclusions of frontotemporal dementia, argyrophilic grains and Lewy bodies), as well as traumatic brain lesions, tumours and inflammation. An intermediate series of 167 cases was considered for the last step of the inclusion process.
Third, subjects with a first depressive episode before age 65, minor, subsyndromal or subthreshold [40] depression or without DSM-IV diagnosis established by a certified psychiatrist were also excluded. The final sample of LOD cases included 38 patients aged 65 to 89 (16 men, mean age: 79 years; and 22 women, mean age: 80 years).
Twenty-nine age- and gender-matched control subjects (without history of depression) were selected from the initial autopsy series of the Department of Rehabilitation and Geriatrics (1,715 subjects). They also satisfied the exclusion criteria applied in the two first steps of the inclusion process for depressed cases (15 men aged 68 to 95, mean age 80 years old; and 14 women aged 66 to 95, mean age: 79 years old).
Clinical assessment
A fully trained senior resident established the clinical diagnosis while the subjects were alive, according to DSM-IV criteria for major depression. His assessment was confirmed by the independent evaluation of the senior attending. This diagnosis was confirmed in all of the cases by a Geriatric Depression Scale score higher than 5 [41]. Similarly, the clinical diagnosis of dementia was assessed according to the DSM-IV criteria and confirmed by a Clinical Dementia Rating score > 0.5 [42]. Cardiovascular risk factors (hypertension, diabetes and smoking) in LOD patients and controls were identified by retrospective chart review in all cases. In order to identify the presence of vascular depression we applied retrospectively the set of criteria proposed by Alexopoulos et al. [13]. This clinical definition requires an onset of depression after 65 years of age and clinical and/or laboratory evidence of vascular disease or vascular risk factors.
Our final sample included 38 cases with LOD autopsied cases and 29 age- and gender-matched controls. All procedures involving the use of postmortem human brain were conducted with the written consent of the next of kin and were approved by the relevant ethics committee of the University Hospitals of Geneva.
Neuropathological assessment
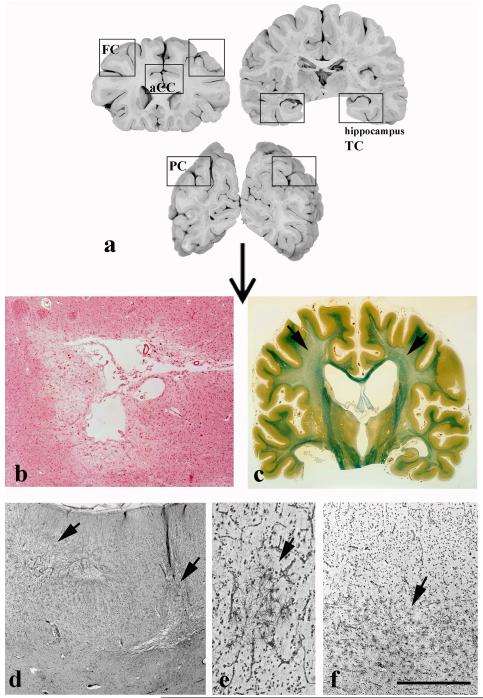
Brains obtained at autopsy were fixed in 15 % formaldehyde for at least 4 weeks, cut into 1-cm-thick coronal slices and examined for macroscopic vascular lesions including brain infarcts, cerebral haemorrhage and lacunes (table 1). The latter were defined as small definitive areas of ischaemic necrosis, ranging from 1 to 15 mm, located in the white matter, basal ganglia or thalamus (Fig. 2a). They were identified on macroscopic examination and confirmed on Luxol-van Gieson (LVG) stained coronal sections. Histologically, lacunes (Fig. 2b) were recognized as irregular cavities containing parenchymal fragments and lipid- or haemosiderin-laden macrophages [43]. To assess deep white matter and periventricular demyelination, whole coronal sections at the level of the anterior commissure were embedded in paraffin, cut into 20-μm-thick sections and stained with LVG (Fig. 2c). Cortical microinfarcts were defined as small areas of ischaemic necrosis in the cerebral cortex seen only on histological examination (Fig. 2d). In order to visualize cortical microinfarcts, focal cortical and diffuse white matter gliosis (Figs 2e, f), 1cm-thick tissue blocks from the anterior hippocampus, inferior temporal cortex (area 20), prefrontal cortex (area 9), parietal cortex (area 40) and anterior cingulate cortex (area 24) bilaterally were cut into 20-μm-thick serial sections of approximately 3×2cm (Fig. 2a). Every 50 sections, one section was stained with Globus silver impregnation for a total of ten sections per area which were subsequently considered for semiquantitative analysis [44].
Table 1. Neuropathologic assessment of vascular lesions.
| Area | Lesions | Methods |
|---|---|---|
| Thalamus | Lacunes | Macroscopy Luxol-van Gieson (LVG) |
| Basal ganglia | Lacunes | Macroscopy LVG |
| White matter | Lacunes Demyelination Diffuse gliosis |
Macroscopy LVG LVG Globus silver impregnation |
| Gray matter (Hippocampus, temporal, frontal, parietal and anterior cingulate cortex) |
Cortical microinfarcts Focal cortical gliosis |
Globus silver impregnation |
Figure 2.
Schematic representation of the assessed cortical areas (a) and representative examples of basal ganglia lacunes (b), deep white matter demyelination (c, arrow), multiple cortical microinfarcts in the frontal cortex (d, arrows), focal cortical gliosis (e, arrow), and diffuse white matter gliosis (f, arrow). Sections were stained with hematoxylin-eosin (b), Luxol-van Gieson staining (c), and Globus silver impregnation (d-f). Scale bar (on f): 1 mm (b and d), 250 μm (e), and 500 μm (f).
To assess the severity of Alzheimer type pathology and exclude other degenerative lesions such as Pick bodies, ubiquitin-positive inclusions of frontotemporal dementia, argyrophilic grains and Lewy bodies, additional blocks from hippocampus, temporal, frontal, parietal, and occipital cortex were embedded in paraffin, and 12-μm-thick sections were processed with highly specific and fully characterized antibodies to the phosphorylation-dependent tau AT8 (1:1,000, Innogenetics) [45], to the amino acid residues 17-24 of the human amyloid ß protein (Clone 4G8, 1:1000, Signet Laboratories) [46], alpha-synuclein (1:20,000 courtesy of Dr Y. Charnay) and ubiquitin (1:100, Sigma) as previously described [44]. The assessment of NFT and Aß pathology was made according to the Braak and Braak [47] and Thal Aß phase [48] staging systems. In routine neuropathological evaluation, the exclusion of argyrophilic grains and alpha-synuclein pathology was based on the procedure described by the DLB Consortium [49] and that of Pick bodies and ubiquitin pathology on the criteria of the Consortium for FLD [50].
The mean lacune severity in thalamus, basal ganglia and white matter was assessed semiquantitatively using the following score: 0 (absence of such lesions), 1 (< 3 lesions per slide), 2 (3-5 lesions per slide), 3 (> 5 lesions per slide). The same score was applied in ten sections per area studied for cortical microinfarcts and focal cortical gliosis. Semiquantitative assessment of diffuse white matter gliosis was made in the same number of sections using the following rating scale: 0 = absent, 1 = mild, 2 = moderate, 3 = severe. For these three microvascular lesions, an individual total score was obtained by adding the scores of each area. Subsequently, the scores of cortical microinfarcts, focal cortical and diffuse white matter gliosis for each hemisphere were added to obtain a total microvascular score for each case. The severity of deep white matter and periventricular demyelination in each hemisphere was estimated in Luxol-van Gieson stained sections using the following rating scale: 0 = absent, 1 = mild, 2 = moderate, 3 = severe. Scores for each hemisphere were added to obtain a total score for each type of demyelination. This semi-quantitative assessment of lacunes and microvascular pathology has already been used in our previous studies with a high inter-rater reliability [51, 52].
Statistics
Univariate analysis was performed to identify independent risk factors associated with the outcome (LOD), using Student’s t tests or Fisher exact test as appropriate. Demographic variables assessed as possible predictors of LOD were age, gender and main CVRF (hypertension, diabetes, and smoking). The relationship between survival time after the first episode of depression, number of depressive recurrences and vascular scores were assessed by Spearman’s rho correlation coefficient. All variables were coded as dichotomous except survival time after the first episode of depression, number of depressive episodes and age. For univariate regression analyses, Odds Ratios (OR) and 95% confidence intervals (CI) were calculated to evaluate the relationship between vascular scores and the clinical outcome. Multiple logistic regression models were built to explore the association between possible predictors and the presence of LOD and vascular depression. Only the predictors closer to significance in univariate analysis were used in this part of the statistical analysis. In order to limit statistical errors due to multiple comparisons, only P values < 0.01 were considered as statistically significant. Statistical analyses were performed with Stata software version 11.
Results
The demographic and clinical characteristics in our series are summarized in table 2. Among the 38 patients with LOD, 71 % survived at least one year after the first depressive episode and the average survival time was 3.3 years (Standard deviation, SD = 3.9). The average age at onset of depression was 75.8 years (SD = 7.6) and the average duration of the first depressive episode was one year (SD = 1.7). One third of the LOD patients had at least one recurrence of major depression and the average number of recurrences was 1 (SD = 0.8). Among the LOD patients, 14 were untreated (refusal of pharmacological treatment in the context of severe episodes of major depression but no suicidal thoughts) and 24 were treated with serotonin reuptake inhibitors or tricyclic antidepressants. No control cases received psychotropic medication. Within the 38 LOD cases, 25 (66%) fulfilled Alexopoulos et al. criteria for vascular depression [13]. There were no statistically significant differences in the frequency of hypertension (p=0.07), diabetes (p = 0.34) and smoking (p = 0.69) between the two diagnostic groups.
Table 2. Characteristics of LOD patients and controls.
| LOD N (%) |
Controls N (%) |
|
|---|---|---|
| N | 38 | 29 |
| Age, years (SD) | 79 (6.1) | 80 (8.6) |
| Male gender (%) | 16 (42) | 15 (52) |
| Braak stage I for NFT [47] | 25 (65.8) | 13 (44.8) |
| Braak stage II for NFT [47] | 12 (31.6) | 15 (51.7) |
| Braak stage III for NFT [47] | 1 (2.6) | 1 (3.5) |
| Phase 1 for SP [48] | 17 (44.7) | 19 (65.5) |
| Phase 2 for SP [48] | 12 (31.6) | 7 (24.1) |
| Phase 3 for SP [48] | 3 (7.9) | 3 (10.4) |
| Phase 4 for SP [48] | 6 (15.8) | 0 (0) |
| Hypertension (%) | 12 (32) | 14 (48) |
| Diabetes (%) | 6 (16) | 7 (24) |
| Smoking (%) | 5 (13) | 2 (7) |
NFT = neurofibrillary tangles; SP = senile plaques.
The frequency of all of the lesions studied did not differ between LOD and control groups (table 3). In LOD patients, the average length of survival after the first LOD episode and the number of subsequent depressive recurrences were not associated with the vascular scores (Spearman’s rho coefficients ranging from 0.15 to 0.02, p ranging from 0.11 to 0.74).
Table 3. Regional distribution of frequencies of small macrovascular and microvascular lesions in the present series.
|
Univariate Analysis |
|||
|---|---|---|---|
| Late-onset depression |
No Depression | Fisher exact p1 |
|
| N = 38 (%) |
N = 29 (%) |
||
| Lacunes | 53 | 45 | |
| Thalamus | 3 | 8 | 0.557 |
| Basal ganglia | 32 | 24 | 0.579 |
| Deep white matter | 18 | 20 | 1.000 |
| Demyelination | 55 | 86 | |
| Deep white matter demyelination | 50 | 76 | 0.065 |
| Periventricular demyelination | 5 | 24 | 0.050 |
| Cortical microinfarcts | 24 | 45 | |
| Hippocampus | 12 | 21 | 0.503 |
| Temporal cortex | 0 | 0 | -- |
| Frontal cortex | 4 | 5 | 1.000 |
| Parietal cortex | 13 | 12 | 1 |
| Anterior cingulate cortex | 0 | 0 | -- |
| Focal Gliosis | 37 | 21 | |
| Hippocampus | 3 | 0 | 1.000 |
| Temporal cortex | 4 | 3 | 1.000 |
| Frontal cortex | 18 | 16 | 1.000 |
| Parietal cortex | 5 | 0 | 0.514 |
| Anterior cingulate cortex | 8 | 4 | 1.000 |
| Diffuse white matter gliosis | 74 | 79 | |
| Hippocampus | 13 | 12 | 1.000 |
| Temporal cortex | 11 | 20 | 0.463 |
| Frontal cortex | 34 | 36 | 1.000 |
| Parietal cortex | 5 | 16 | 0.204 |
| Anterior cingulate cortex | 11 | 8 | 1.000 |
Data from both hemispheres were pooled; see text for details.
In the present study, two independent investigators (EK and MS), blind to the clinical findings, assessed the severity of vascular pathology with a high inter-rater reliability (kappa values ranging from 0.88 to 0.95 for the severity score of the different neuropathological variables). In case of disagreement, the final determination was defined in a consensus meeting between the two raters.
The lacune scores (thalamic, basal ganglia, and white matter lesions) were not associated with the LOD diagnosis. Although controls displayed higher frequencies of deep white matter and periventricular demyelination compared to LOD cases, this difference did not reach the statistical significance threshold. No significant differences in focal gliosis, diffuse white matter gliosis or cortical microinfarcts’ scores were found between LOD patients and controls (table 3). This was also the case within the LOD subgroups fulfilling or not previously described clinical criteria for vascular depression (OR ranging from 0.23 to 6.67, p values between 0.092 and 0.899). In multivariate models including all variables close to statistical significance in univariate models (hypertension, deep white matter and periventricular demyelination, table 4), no association was found between vascular lesion burden and LOD (OR/CI: 0.36/0.11-1.12, 0.27/0.08-0.92 and 0.15/0.02-0.98, respectively). This was also the case for the diagnosis of vascular depression (OR/CI: 0.53/0.14-1.75 for hypertension, 0.24/0.06-0.86 for deep white matter demyelination and 0.22/0.03-1.44 for periventricular demyelination).
Table 4. Relationship between late-onset depression and vascular scores.
| Univariate Logistic Regression | |||
|---|---|---|---|
| Crude Odds Ratio |
|||
| OR | 95% CI | P1 | |
| Lacunes | |||
| Thalamus | 0.31 | 0.27-3.62 | 0.351 |
| Basal ganglia | 1.46 | 0.47-4.59 | 0.516 |
| Deep white mattera | 0.9 | 0.25-3.24 | 0.876 |
| Demyelination | |||
| Deep white matter demyelination | 0.32 | 0.10-0.96 | 0.043 |
| Periventricular demyelination | 0.17 | 0.32-0.96 | 0.044 |
| Cortical microinfarcts | |||
| Hippocampus | 1.96 | 0.46-8.22 | 0.360 |
| Temporal cortex | -- | -- | -- |
| Frontal cortex | 1.33 | 0.11-15.5 | 0.818 |
| Parietal cortex | 1.11 | 0.24-5.13 | 0.893 |
| Anterior cingulate cortex | -- | -- | -- |
| Focal Gliosis | |||
| Hippocampus | -- | -- | -- |
| Temporal cortex | 0.65 | 0.39-10.87 | 0.763 |
| Frontal cortex | 1.19 | 0.31-4.56 | 0.805 |
| Parietal cortex | -- | -- | -- |
| Anterior cingulate cortex | 2.06 | 0.20-10.98 | 0.543 |
| Diffuse white matter gliosis | |||
| Hippocampus | 1.11 | 0.24-5.13 | 0.893 |
| Temporal cortex | 0.47 | 0.11-1.96 | 0.300 |
| Frontal cortex | 0.92 | 0.32-2.66 | 0.884 |
| Parietal cortex | 0.29 | 0.05-1.73 | 0.175 |
| Anterior cingulate cortex | 1.35 | 0.23-8.01 | 0.739 |
Data from both hemispheres were pooled; see text for details.
Discussion
After the publications of O’Brien’s group [35, 37-39, 53], this is the first neuropathological report attempting to validate the concept of vascular depression in the elderly in an independent autopsy series. The present autopsy study is based on a relatively large number of prospectively documented LOD cases, with careful exclusion of both clinical and neuropathological conditions that could affect the specificity of the LOD sample, a systematic bilateral assessment of lacunes and each type of microvascular lesions, and controls for possible confounders such as survival time after the first episode of depression, number of depressive recurrences, and main CVRFs. Several limitations should, however, be considered. First, autopsy-related biases such as selection of younger, atypical or highly puzzling cases not representative of the whole spectrum of LOD cannot be formally ruled out. However, even the inclusion of such cases that usually display severe brain pathology [54] did not lead to positive clinicopathological correlations in the present series. Second, the temporal relationship between the occurrence of LOD and vascular pathology cannot be established on the basis of post-mortem observations. We thus cannot exclude the possibility that lacune and microvascular lesion formation may have taken place in part during the survival period after the first depressive episode biasing the relationship between vascular lesion scores and LOD onset. The fact that the survival time after first depressive episode and the number of depressive recurrences were not related to neuropathological parameters in LOD cases is, however, reassuring in this regard. Third, even though our previous work on Alzheimer’s disease neuropathology demonstrated that the use of stereological principles in lesion quantification might lead to a substantial improvement of clinicopathological correlations [55], in the present study lacunes and microvascular lesions were assessed with a semi-quantitative severity scale. Fourth, the number of cases was not sufficient to determine whether the location of microvascular lesions modulated their effect on mood. In addition, even though dysfunction in several prefrontal areas has consistently been related to depression in both neuroimaging [56-62] and neuropathologic [63-67] studies, we did not assess separately the vascular burden in this frontal lobe subdivision. Finally, the present investigation focuses on small macrovascular and microvascular lesions and did not address possible changes in vascular structure (i.e., capillary integrity or changes in vascular endothelial cells) that may participate in the pathogenesis of LOD and deserve further quantitative analysis.
At a first glance, our results appear to contradict the observations of the O’Brien’s group in 20 hospital-based LOD cases [35, 37-39, 53]. In these studies, radiologically confirmed deep WMHs were more frequent in LOD compared to control cases. However, the neuropathological assessment per se led to different conclusions. Amongst deep WMH, only those located in the dorsolateral prefrontal cortex were mainly of ischaemic origin and were associated with increased expression of intercellular cell adhesion molecule and glial fibrillary acidic protein in depressed patients compared to controls [39]. However, LOD was associated neither with small vascular or microvascular lesions [35, 37] nor with an increase of vascular cell adhesion molecule [53]. There are two main methodological differences between the present study and these contributions. First, our hospital-based autopsy series included only cases with major depression from two primary care county hospitals whereas the cases analyzed by Thomas and coworkers were from referrals to a secondary care unit. Unlike these studies, we have examined separately each type of microvascular lesions and subcortical lacunes. Moreover, the bilateral assessment of microvascular pathology throughout the brain makes it possible to define semi-quantitative scores that reflect at least partly the global microvascular burden of each case. Our results complete the observations of Thomas et al. [35, 37, 39] in that they indicate that small macrovascular and microvascular burden is not a major determinant of LOD. One could argue that the paucity of the observed relationships may be due to the heterogeneity of the LOD group in respect of the presence of vascular co-morbidities. However, this is highly unlikely given that similar results were found in the subgroup of LOD patients that satisfied the clinical criteria for vascular depression [13].
The present observations should be interpreted in the light of the current debate regarding the deleterious role of lacunes and cortical microinfacrts in brain aging (for review see [68]). Our earlier neuropathological work documented that cortical microinfarcts and, to a lesser degree, thalamic and basal ganglia lacunes are independent predictors of cognitive decline [51, 52, 69]. More recently, we found that the chronic accumulation of lacunar infarcts within the thalamus, basal ganglia and deep white matter was strongly related to the occurrence of post-stroke depression and explained 25% of the variability of this occurrence [70]. Based on these data we proposed that the development of subcortical lacunes may be a common denominator of cognitive and mood disorders in old age. In contrast to our initial expectations, the present data did not confirm this hypothesis in LOD cases, supporting the idea that the chronic accumulation of lacunes may exert a negative impact on mood exclusively in the presence of acute brain compromise. Furthermore, they indicate that both deep white matter and periventricular demyelination are not associated with LOD. Recent prospective studies have proposed a shift of focus towards clinical and psychological determinants of LOD such as global medical burden [22, 23, 71-73], intensity of treatment for depression [74], social support [75-78], functional disability [79, 80], and personal characteristics such as resilience [81] and locus of control [76], comorbid anxiety [82], and psychiatric functional status [22, 75]. Future neuropathological studies in large community-based autopsy series including rigorous assessment of vascular lesion volumes in mood regulation-related cortical areas, as well as morphological analyses of microvascular structure are warranted to explore the complex relationships between vascular burden and LOD.
Acknowledgements
This work was supported by an unrestricted grant of the Vachoux Foundation (MS) and NIH grant AG02219 (PRH).
References
- 1.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 2.Schwenk TL. Diagnosis of late life depression: the view from primary care. Biol Psychiatry. 2002;52:157–163. doi: 10.1016/s0006-3223(02)01389-6. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulos GS, Vrontou C, Kakuma T, Meyers BS, Young RC, Klausner E, Clarkin J. Disability in geriatric depression. Am J Psychiatry. 1996;153:877–885. doi: 10.1176/ajp.153.7.877. [DOI] [PubMed] [Google Scholar]
- 4.Beekman AT, Deeg DJ, Braam AW, Smit JH, Van Tilburg W. Consequences of major and minor depression in later life: a study of disability, well-being and service utilization. Psychol Med. 1997;27:1397–1409. doi: 10.1017/s0033291797005734. [DOI] [PubMed] [Google Scholar]
- 5.Jacoby RJ, Levy R, Bird JM. Computed tomography and the outcome of affective disorder: a follow-up study of elderly patients. Br J Psychiatry. 1981;139:288–292. doi: 10.1192/bjp.139.4.288. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ, Lin E, Russo J, Unutzer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RE, Alexopoulos GS. The Vascular Depression Concepts and Its Implications. In: Ellison JM, Kyomen HH, Verma S, editors. Mood Disorders in Later Life. 2nd edn. Informa Healthcare; New York: 2009. pp. 161–178. [Google Scholar]
- 8.Alexopoulos GS, Kelly RE., Jr. Research advances in geriatric depression. World Psychiatry. 2009;8:140–149. doi: 10.1002/j.2051-5545.2009.tb00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodaty H, Luscombe G, Parker G, Wilhelm K, Hickie I, Austin MP, Mitchell P. Early and late onset depression in old age: different aetiologies, same phenomenology. J Affect Disord. 2001;66:225–236. doi: 10.1016/s0165-0327(00)00317-7. [DOI] [PubMed] [Google Scholar]
- 10.Camus V, Kraehenbuhl H, Preisig M, Bula CJ, Waeber G. Geriatric depression and vascular diseases: what are the links? J Affect Disord. 2004;81:1–16. doi: 10.1016/j.jad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Janssen J, Beekman AT, Comijs HC, Deeg DJ, Heeren TJ. Late-life depression: the differences between early- and late-onset illness in a community-based sample. Int J Geriatr Psychiatry. 2006;21:86–93. doi: 10.1002/gps.1428. [DOI] [PubMed] [Google Scholar]
- 12.Kendler KS, Fiske A, Gardner CO, Gatz M. Delineation of two genetic pathways to major depression. Biol Psychiatry. 2009;65:808–811. doi: 10.1016/j.biopsych.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 15.Fujikawa T, Yamawaki S, Touhouda Y. Incidence of silent cerebral infarction in patients with major depression. Stroke. 1993;24:1631–1634. doi: 10.1161/01.str.24.11.1631. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan KR, Goli V, Ellinwood EH, France RD, Blazer DG, Nemeroff CB. Leukoencephalopathy in patients diagnosed as major depressive. Biol Psychiatry. 1988;23:519–522. doi: 10.1016/0006-3223(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 17.Tupler LA, Krishnan KR, McDonald WM, Dombeck CB, D’Souza S, Steffens DC. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53:665–676. doi: 10.1016/s0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- 18.Lesser IM, Boone KB, Mehringer CM, Wohl MA, Miller BL, Berman NG. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 19.Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- 20.Naarding P, Veereschild M, Bremmer M, Deeg D, Beekman AT. The symptom profile of vascular depression. Int J Geriatr Psychiatry. 2009;24:965–969. doi: 10.1002/gps.2203. [DOI] [PubMed] [Google Scholar]
- 21.McDougall F, Brayne C. Systematic review of the depressive symptoms associated with vascular conditions. J Affect Disord. 2007;104:25–35. doi: 10.1016/j.jad.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Cui X, Lyness JM, Tang W, Tu X, Conwell Y. Outcomes and predictors of late-life depression trajectories in older primary care patients. Am J Geriatr Psychiatry. 2008;16:406–415. doi: 10.1097/JGP.0b013e3181693264. [DOI] [PubMed] [Google Scholar]
- 23.Cui X, Lyness JM, Tu X, King DA, Caine ED. Does depression precede or follow executive dysfunction? Outcomes in older primary care patients. Am J Psychiatry. 2007;164:1221–1228. doi: 10.1176/appi.ajp.2007.06040690. [DOI] [PubMed] [Google Scholar]
- 24.Firbank MJ, Lloyd AJ, Ferrier N, O’Brien JT. A volumetric study of MRI signal hyperintensities in late-life depression. Am J Geriatr Psychiatry. 2004;12:606–612. doi: 10.1176/appi.ajgp.12.6.606. [DOI] [PubMed] [Google Scholar]
- 25.Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI. White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am J Psychiatry. 1999;156:1602–1607. doi: 10.1176/ajp.156.10.1602. [DOI] [PubMed] [Google Scholar]
- 26.Lyness JM, Caine ED, Cox C, King DA, Conwell Y, Olivares T. Cerebrovascular risk factors and later-life major depression. Testing a small-vessel brain disease model. Am J Geriatr Psychiatry. 1998;6:5–13. [PubMed] [Google Scholar]
- 27.Lyness JM, King DA, Conwell Y, Cox C, Caine ED. Cerebrovascular risk factors and 1-year depression outcome in older primary care patients. Am J Psychiatry. 2000;157:1499–501. doi: 10.1176/appi.ajp.157.9.1499. [DOI] [PubMed] [Google Scholar]
- 28.Dalby RB, Chakravarty MM, Ahdidan J, Sorensen L, Frandsen J, Jonsdottir KY, Tehrani E, Rosenberg R, Ostergaard L, Videbech P. Localization of white-matter lesions and effect of vascular risk factors in late-onset major depression. Psychol Med. 2009:1–11. doi: 10.1017/S0033291709991656. [DOI] [PubMed] [Google Scholar]
- 29.Videbech P, Ravnkilde B, Gammelgaard L, Egander A, Clemmensen K, Rasmussen NA, Gjedde A, Rosenberg R. The Danish PET/depression project: performance on Stroop’s test linked to white matter lesions in the brain. Psychiatry Res. 2004;130:117–130. doi: 10.1016/j.pscychresns.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. Am J Roentgenol. 1988;151:559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- 31.Chimowitz MI, Awad IA, Furlan AJ. Periventricular lesions on MRI. Facts and theories. Stroke. 1989;20:963–967. doi: 10.1161/01.str.20.7.963. [DOI] [PubMed] [Google Scholar]
- 32.Chimowitz MI, Estes ML, Furlan AJ, Awad IA. Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging. Arch Neurol. 1992;49:747–752. doi: 10.1001/archneur.1992.00530310095018. [DOI] [PubMed] [Google Scholar]
- 33.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 34.Marshall VG, Bradley WG, Jr., Marshall CE, Bhoopat T, Rhodes RH. Deep white matter infarction: correlation of MR imaging and histopathologic findings. Radiology. 1988;167:517–522. doi: 10.1148/radiology.167.2.3357964. [DOI] [PubMed] [Google Scholar]
- 35.Thomas AJ, Perry R, Barber R, Kalaria RN, O’Brien JT. Pathologies and pathological mechanisms for white matter hyperintensities in depression. Ann N Y Acad Sci. 2002;977:333–339. doi: 10.1111/j.1749-6632.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith GS, Alexopoulos GS. Neuroimaging in geriatric psychiatry. Int J Geriatr Psychiatry. 2009;24:783–787. doi: 10.1002/gps.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas AJ, Ferrier IN, Kalaria RN, Perry RH, Brown A, O’Brien JT. A neuropathological study of vascular factors in late-life depression. J Neurol Neurosurg Psychiatry. 2001;70:83–87. doi: 10.1136/jnnp.70.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas AJ, O’Brien JT, Barber R, McMeekin W, Perry R. A neuropathological study of periventricular white matter hyperintensities in major depression. J Affect Disord. 2003;76:49–54. doi: 10.1016/s0165-0327(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 39.Thomas AJ, O’Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 40.APA . In: Diagnostic and statistical Manual of Mental Disorders. 4th edn. American Psychiatric Association, editor. American Psychiatric Association; Washington: 2000. text revision. [Google Scholar]
- 41.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 42.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A New Clinical Scale for the Staging of Dementia. Brit J Psychiat. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 43.Lammie GA, Brannan F, Wardlaw JM. Incomplete lacunar infarction (Type Ib lacunes) Acta Neuropathol. 1998;96:163–171. doi: 10.1007/s004010050877. [DOI] [PubMed] [Google Scholar]
- 44.Vallet PG, Guntern R, Hof PR, Golaz J, Delacourte A, Robakis NK, Bouras C. A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer’s disease. Acta Neuropathol. 1992;83:170–178. doi: 10.1007/BF00308476. [DOI] [PubMed] [Google Scholar]
- 45.Goedert M, Jakes R, Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 46.Bussière T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, Giannakopoulos P, Robakis NK, Morrison JH, Perl DP, Hof PR. Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer’s disease. Neuroscience. 2002;112:75–91. doi: 10.1016/s0306-4522(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 48.Thal DR, Rub U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H. Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. 2000;59:733–748. doi: 10.1093/jnen/59.8.733. [DOI] [PubMed] [Google Scholar]
- 49.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 50.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold G, Kovari E, Hof PR, Bouras C, Giannakopoulos P. Sorting out the clinical consequences of ischemic lesions in brain aging: a clinicopathological approach. J Neurol Sci. 2007;257:17–22. doi: 10.1016/j.jns.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- 53.Thomas AJ, Ferrier IN, Kalaria RN, Davis S, O’Brien JT. Cell adhesion molecule expression in the dorsolateral prefrontal cortex and anterior cingulate cortex in major depression in the elderly. Br J Psychiatry. 2002;181:129–134. doi: 10.1017/s0007125000161847. [DOI] [PubMed] [Google Scholar]
- 54.Lloyd AJ, Ferrier IN, Barber R, Gholkar A, Young AH, O’Brien JT. Hippocampal volume change in depression: late- and early-onset illness compared. Br J Psychiatry. 2004;184:488–495. doi: 10.1192/bjp.184.6.488. [DOI] [PubMed] [Google Scholar]
- 55.Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 56.Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia--focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 57.Dolan RJ, Bench CJ, Brown RG, Scott LC, Friston KJ, Frackowiak RS. Regional cerebral blood flow abnormalities in depressed patients with cognitive impairment. J Neurol Neurosurg Psychiatry. 1992;55:768–773. doi: 10.1136/jnnp.55.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 59.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 60.Mountz JM, Liu HG, Deutsch G. Neuroimaging in cerebrovascular disorders: measurement of cerebral physiology after stroke and assessment of stroke recovery. Semin Nucl Med. 2003;33:56–76. doi: 10.1053/snuc.2003.127293. [DOI] [PubMed] [Google Scholar]
- 61.Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 62.Ishizaki J, Yamamoto H, Takahashi T, Takeda M, Yano M, Mimura M. Changes in regional cerebral blood flow following antidepressant treatment in late-life depression. Int J Geriatr Psychiatry. 2008;23:805–811. doi: 10.1002/gps.1980. [DOI] [PubMed] [Google Scholar]
- 63.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 64.Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- 65.Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O’Brien JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry. 2003;18:7–13. doi: 10.1002/gps.720. [DOI] [PubMed] [Google Scholar]
- 66.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 67.Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santos M, Kovari E, Hof PR, Gold G, Bouras C, Giannakopoulos P. The impact of vascular burden on late-life depression. Brain Res Rev. 2009;62:19–32. doi: 10.1016/j.brainresrev.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gold G, Kövari E, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 70.Santos M, Gold G, Kövari E, Herrmann FR, Bozikas VP, Bouras C, Giannakopoulos P. Differential impact of lacunes and microvascular lesions on Poststroke depression. Stroke. 2009;40:3557–3562. doi: 10.1161/STROKEAHA.109.548545. [DOI] [PubMed] [Google Scholar]
- 71.Lyness JM, Heo M, Datto CJ, Ten Have TR, Katz IR, Drayer R, Reynolds CF, 3rd, Alexopoulos GS, Bruce ML. Outcomes of minor and subsyndromal depression among elderly patients in primary care settings. Ann Intern Med. 2006;144:496–504. doi: 10.7326/0003-4819-144-7-200604040-00008. [DOI] [PubMed] [Google Scholar]
- 72.Lyness JM, Niculescu A, Tu X, Reynolds CF, 3rd, Caine ED. The relationship of medical comorbidity and depression in older, primary care patients. Psychosomatics. 2006;47:435–439. doi: 10.1176/appi.psy.47.5.435. [DOI] [PubMed] [Google Scholar]
- 73.Sanders ML, Lyness JM, Eberly S, King DA, Caine ED. Cerebrovascular risk factors, executive dysfunction, and depression in older primary care patients. Am J Geriatr Psychiatry. 2006;14:145–152. doi: 10.1097/01.JGP.0000192482.27931.1e. [DOI] [PubMed] [Google Scholar]
- 74.Bogner HR, Cary MS, Bruce ML, Reynolds CF, 3rd, Mulsant B, Ten Have T, Alexopoulos GS. The role of medical comorbidity in outcome of major depression in primary care: the PROSPECT study. Am J Geriatr Psychiatry. 2005;13:861–868. doi: 10.1176/appi.ajgp.13.10.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyness JM, Chapman BP, McGriff J, Drayer R, Duberstein PR. One-year outcomes of minor and subsyndromal depression in older primary care patients. Int Psychogeriatr. 2009;21:60–68. doi: 10.1017/S1041610208007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harris T, Cook DG, Victor C, DeWilde S, Beighton C. Onset and persistence of depression in older people - results from a 2-year community follow-up study. Age Ageing. 2006;35:25–32. doi: 10.1093/ageing/afi216. [DOI] [PubMed] [Google Scholar]
- 77.Smit F, Ederveen A, Cuijpers P, Deeg D, Beekman A. Opportunities for cost-effective prevention of late-life depression: an epidemiological approach. Arch Gen Psychiatry. 2006;63:290–296. doi: 10.1001/archpsyc.63.3.290. [DOI] [PubMed] [Google Scholar]
- 78.Travis LA, Lyness JM, Shields CG, King DA, Cox C. Social support, depression, and functional disability in older adult primary-care patients. Am J Geriatr Psychiatry. 2004;12:265–271. [PubMed] [Google Scholar]
- 79.Licht-Strunk E, Bremmer MA, van Marwijk HW, Deeg DJ, Hoogendijk WJ, de Haan M, van Tilburg W, Beekman AT. Depression in older persons with versus without vascular disease in the open population: similar depressive symptom patterns, more disability. J Affect Disord. 2004;83:155–160. doi: 10.1016/j.jad.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Friedman B, Lyness JM, Delavan RL, Chunyu L, Barker WH. Major depression and disability in older primary care patients with heart failure. J Geriatr Psychiatry Neurol. 2008;21:111–122. doi: 10.1177/0891988707311563. [DOI] [PubMed] [Google Scholar]
- 81.Mehta M, Whyte E, Lenze E, Hardy S, Roumani Y, Subashan P, Huang W, Studenski S. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. Int J Geriatr Psychiatry. 2008;23:238–243. doi: 10.1002/gps.1868. [DOI] [PubMed] [Google Scholar]
- 82.Andreescu C, Butters M, Lenze EJ, Venkatraman VK, Nable M, Reynolds CF, 3rd, Aizenstein HJ. fMRI activation in late-life anxious depression: a potential biomarker. Int J Geriatr Psychiatry. 2009;24:820–828. doi: 10.1002/gps.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]