Abstract
Current theories of cognitive aging argue that neural representations become less distinctive in old age, a phenomenon known as dedifferentiation. The present study used multi-voxel pattern analysis (MVPA) to measure age differences in the distinctiveness of distributed patterns of neural activation evoked by different categories of visual images. We found that neural activation patterns within the ventral visual cortex were less distinctive among older adults. Further, we report that age differences in neural distinctiveness extend beyond the ventral visual cortex: older adults also showed decreased distinctiveness in early visual cortex, inferior parietal cortex, and medial and lateral prefrontal cortex. Neural distinctiveness scores in early and late visual areas were highly correlated, suggesting shared mechanisms of age-related decline. Finally, we investigated whether older adults can compensate for altered processing in visual cortex by encoding stimulus information across larger numbers of voxels within the visual cortex or in regions outside visual cortex. We found no evidence that older adults can increase the distinctiveness of distributed activation patterns, either within or beyond the visual cortex. Our results have important implications for theories of cognitive aging and highlight the value of MVPA to the study of neural coding in the aging brain.
Keywords: aging, fMRI, MVPA, dedifferentiation, compensation, ventral visual cortex
Introduction
Current models of aging argue that different cognitive and neural processes become more similar in old age, a phenomenon referred to as dedifferentiation. This term has been applied to patterns of cognitive aging observed across a range of research methods, including behavioral, neuroimaging, and computational modeling approaches. Behavioral studies have documented increased intercorrelations among perceptual and cognitive abilities in older adults (Baltes and Lindenberger, 1997; Li et al., 2004; Lindenberger and Baltes, 1994). Such results have been hypothesized to reflect a global decline in the integrity of the aging brain. The term dedifferentiation has also been applied to a ubiquitous finding in the cognitive aging literature: bilateral activation in older adults during tasks that evoke unilateral activation in younger adults (Cabeza, 2002; Dolcos et al., 2002; Reuter-Lorenz and Lustig, 2005). This additional recruitment has been hypothesized by different groups to reflect (1) compensation for age-related declines in neural resources (e.g., Cabeza et al., 2002) or (2) impaired neural processing (e.g., Duverne et al., 2009). Finally, dedifferentiation also refers to computational modeling work that links age-related performance declines to reduced distinctiveness of neural representations (Li et al., 2001; Li and Sikström, 2002). Consistent with this view, work from our lab and others shows that regional specialization within the ventral visual cortex (VVC) for different visual objects declines in old age (Chee et al., 2006; Park et al., 2004; Payer et al., 2006; Voss et al., 2008). The present study focuses on this variety of age-related dedifferentiation.
In this study, we reanalyzed the data from a previous report (Park et al., 2004) to address three novel questions about age-related dedifferentiation. First, we used multi-voxel pattern analysis (MVPA) to measure age differences in the distinctiveness of neural representations in the VVC. Following Li and Sikström (2002), we define a neural representation of a stimulus as the pattern of neural activity evoked by that stimulus; two neural representations are said to be distinctive if one can be distinguished from the other. Previous studies of neural dedifferentiation in visual cortex have focused on age differences in average regional activation (Grady et al., 1994; Park et al., 2004). However, these measures may not capture information encoded across multiple voxels within a region: patterns of activation that cannot be discriminated by univariate analysis may be discriminable by multivariate techniques (Haynes and Rees, 2006; Norman et al., 2006). Thus, we reasoned that MVPA would provide a more sensitive index of age differences in neural distinctiveness than measures used in previous work.
Second, we predicted that age-related dedifferentiation would extend beyond the visual cortex. Recent methodological advances have extended MVPA to map local changes in the distinctiveness of neural activation patterns throughout the brain using a multivariate searchlight procedure (Kriegeskorte et al., 2006). This method yields a voxel-by-voxel map of neural distinctiveness. Previous studies using univariate statistics have focused on brain regions in which the average response exceeded an arbitrary statistical criterion. In practice, these criteria have restricted analysis to the visual cortex (Grady et al., 1994; Park et al., 2004; Payer et al., 2006; Voss et al., 2008). However, multi-voxel activation patterns in subthreshold regions can also provide information about visual stimulus categories (Harrison and Tong, 2009; Serences et al., 2009). Thus, we used a multivariate searchlight analysis to measure age differences in neural distinctiveness throughout the brain.
Finally, we used MVPA to investigate the possibility that older adults compensate for altered processing in sensory cortex (Park et al., 2004). We asked whether older adults were able to increase the distinctiveness of multi-voxel activation patterns by (1) distributing stimulus codes across larger numbers of voxels in the visual cortex or (2) engaging brain regions outside the visual cortex. Previous studies of compensation that rely on univariate analysis often yield ambiguous results: age differences in overall activation in frontal areas have been hypothesized to reflect both compensation and impairment and are difficult to interpret (Reuter-Lorenz and Lustig, 2005). In contrast, MVPA measures the information present in patterns of neural activation (Kriegeskorte et al., 2006), simplifying the interpretation of age differences. If neural distinctiveness is reduced in older adults, we can conclude that activation patterns in these subjects convey relatively little information; if older adults exhibit enhanced distinctiveness, we can conclude that their activation patterns convey more information than those of younger subjects. We used both region-of-interest and whole-brain comparisons to assess compensation among older adults.
Materials and methods
Participants
Thirteen younger adults (age range 18 to 28 years; mean age 20.8 years; seven female) and 12 older adults (age range 64 to 79; mean age 69.9 years; seven female) were tested. All participants were right-handed and had 20/40 vision or better; participants who required vision correction wore corrective lenses in the fMRI scanner. Participants were also screened for disease, major depression, and artificial lens implants. Further details of the sample can be found in the original report of these data (Park et al., 2004).
Experimental design
Participants viewed static images while fMRI data were acquired. Images were drawn from four categories: faces, houses, pseudo-words, and chairs. Participants also viewed control images generated by phase-scrambling images from each category.
Stimuli were presented in three runs. Each run contained three 20-s blocks of each stimulus category, presented in pseudorandom order. Each block included 10 images from the same category presented for 1500 ms each, followed by a 500-ms inter-trial interval. Participants were instructed to view and try to remember each image. No additional tasks were given during scanning.
MRI acquisition
All participants were tested in a GE Signa 3T scanner. Neural activity was estimated based on the blood oxygenation level-dependent (BOLD) signal using a spiral acquisition sequence (2000 ms repetition time, 30 5-mm axial slices, 24-cm field of view, 30-ms echo time, 90° flip angle). These acquisition parameters yielded an in-plane resolution of 3.75 by 3.75 mm. High-resolution T1-weighted images were collected in 30 5-mm-thick axial slices parallel to the anterior commissure-posterior commissure line.
Data analysis
Data were preprocessed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK, www.fil.ion.ucl.ac.uk). All subsequent analysis was carried out using custom software implemented in MATLAB (MathWorks, Inc., Natick, MA) and the R statistical computing language.
Preprocessing
Functional data were corrected for differences in slice time acquisition and realigned to the first volume using SPM5. No normalization, spatial smoothing, or other transformation was applied before multivariate analysis (Haxby et al., 2001).
Multi-voxel pattern analysis
We used multi-voxel pattern analysis (MVPA) to test the hypothesis that patterns of neural activation evoked by different visual stimuli become less distinctive in old age. Following Haxby and colleagues (2001), we applied MVPA to individual subject data; results were subsequently averaged within age groups. First, we estimated the neural response to each category relative to phase-scrambled control images using the General Linear Model (Friston et al., 1995). Category-evoked activation was estimated separately for each of the three experimental runs. Within each run, the mean activation across all categories was subtracted from each category-evoked activation map (Haxby et al., 2001). Next, we compared within- and between-category correlations across activation maps for all pairs of categories and all pairs of runs. Neural distinctiveness was defined as the difference between the mean within- and between-category correlations, averaged over all such pairwise comparisons (Williams et al., 2007). As a difference between two correlation coefficients, this measure has a theoretical range of 2 to -2. In contrast to the univariate analysis methods used by previous studies of age-related dedifferentiation (Grady et al., 1994; Park et al., 2004), which focus on changes in average regional activation, MVPA reveals fine-grained differences in the distinctiveness of activation patterns (Norman et al., 2006).
Region-of-interest analysis
The ventral visual cortex (VVC) is specialized for the processing of object form and identity. Patterns of fMRI activation within the VVC reliably discriminate between different visual categories (Haxby et al., 2001). Therefore, we measured age differences in the distinctiveness of distributed category representations within this region. Regions of interest were constructed in two steps (described in further detail below). First, we defined an anatomical mask of the VVC for each subject based on his or her high-resolution structural scan. Second, we selected voxels within each subject’s anatomical mask that showed peak responses to visual object categories. Thus, ROIs were constructed using both anatomical and functional criteria, independently for each subject.
We first constructed single-subject anatomical masks of the VVC that included the parahippocampal gyrus, the inferior temporal gyrus, and the portion of the fusiform gyrus anterior to the anterior occipital sulcus (Park et al., 2004). Next, we identified the voxels within these masks that showed the most robust responses to objects relative to scrambled images. For each subject, we ranked VVC voxels according to their absolute t-values for this contrast. The sensitivity of MVPA varies with the number of voxels included in the analysis (e.g., Spiridon and Kanwisher, 2002). Thus, we defined regions of interest (ROIs) comprising the 2, 4, 8, 16, 32, 64, 128, 256, and 512 peak-activated voxels. These voxels were not required to be spatially contiguous. Finally, we used MVPA to measure the distinctiveness of stimulus-evoked activation patterns within each ROI (see Multi-voxel pattern analysis). To maintain independence between voxel selection and pattern classification, we used different runs to define masks and to measure neural distinctiveness. Definition of the overall VVC region was based on anatomical scans and therefore independent from other analysis.
To test the generality of results from the regions described above, we also defined a set of ROIs for each of the four stimulus categories comprising the 2 through 512 voxels that showed the strongest responses to that category. Finally, we also analyzed responses across the entire anatomically defined VVC region.
Searchlight analysis
We used a multivariate searchlight approach to map age differences in distributed object codes across the brain (Kriegeskorte et al., 2006). For each voxel in the brain, we measured the distinctiveness of visual activation patterns within a 10-mm-radius sphere centered on that voxel (see Multi-voxel pattern analysis). Thus, the value at each voxel describes the degree to which patterns of activation in the local neighborhood of that voxel differentiate among different stimuli. In this way, we derived a whole-brain map of category distinctiveness for each participant. To permit inter-subject comparisons, these maps were spatially normalized using high-resolution T1-weighted images from each participant. These normalized maps were then entered into a second-level analysis to compare neural distinctiveness among younger and older adults. Random-effects t-maps were thresholded at p < .001 (uncorrected for multiple comparisons) with an extent threshold of 20 contiguous voxels (Buckner et al., 2000; Cabeza et al., 2002; Huettel et al., 2001; Park et al., 2003). All activation coordinates are reported in MNI space.
Global correlation analysis
Our region-of-interest and searchlight analyses provide information about local differences in category distinctiveness between younger and older adults. However, these techniques are uninformative with respect to possible age differences in the distribution of category representation across the brain. To assess age differences in the global distribution of category coding, we measured the relationship between neural distinctiveness in older and younger groups across all voxels in the brain. Unlike the ROI and searchlight methods described above, this analysis takes into account the distinctiveness scores from all brain voxels simultaneously. We first computed whole-brain maps of neural distinctiveness for each subject (see Searchlight analysis) and averaged these maps separately for younger and older participants. Next, we correlated the average distinctiveness scores among young participants with the average distinctiveness scores among older participants across all voxels. This technique resembles the Brinley plot (Brinley, 1965), long a staple of cognitive aging research.
Results
Region-of-interest analysis
Younger and older adults showed no differences in the size of the anatomically defined ventral visual cortex ROI (t(23) = .31, p=.77).
Neural distinctiveness scores were analyzed using a mixed ANCOVA with a between-subjects factor of age (young, old) and a within-subjects covariate of mask size (2 to 512 voxels). Mask size was transformed using the logarithm to the base 2. Visual inspection of the data suggested a quadratic relationship between mask size and distinctiveness (Figure 1A); therefore, the second-order effect of mask size was included in the model.
Figure 1.
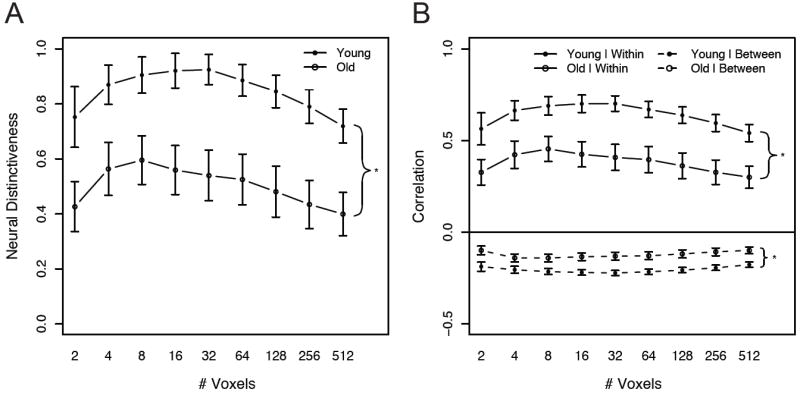
Region-of-interest analysis of age differences in the distinctiveness of neural activation patterns in ventral visual cortex. Panel A: Older adults showed significantly lower neural distinctiveness than younger adults. Panel B: Older adults showed significantly lower within-category correlations (solid lines) and significantly higher between-category correlations (dotted lines) than younger adults. Error bars denote the standard error of the mean. Asterisks indicate significant effects of age group.
Critically, the distinctiveness of distributed category representations was significantly diminished in older adults (F(1, 23) = 11.3, p = .0027; Figure 1A). In other words, activation patterns within peak object-sensitive regions of ventral visual cortex discriminated among visual categories less sensitively in older adults than in younger adults. Pairwise t-tests showed that age differences in neural distinctiveness were significant at each mask size (ts(23) >= 2.26, ps <= .034).
Neural distinctiveness scores also showed a strong quadratic main effect of mask size: VVC activation patterns for different stimuli were more distinctive at moderate mask sizes and less distinctive at very small and very large mask sizes (F(1, 23) = 18.7, p < .001; Figure 1A). Distinctiveness is likely relatively low at small mask sizes because patterns across small numbers of voxels are too variable to reliably distinguish among stimuli. On the other hand, distinctiveness scores decrease at large mask sizes because large masks include many voxels that are uninformative about stimulus conditions. Thus, neural distinctiveness is maximized at intermediate mask sizes.
Because the index of neural distinctiveness used here combines information from correlations within and between stimulus categories, age differences in distinctiveness could be driven by differences in within-category correlations, between-category correlations, or both. Thus, we examined the effects of aging on within- and between-category correlations separately. Correlation values were submitted to a mixed ANCOVA including factors of age group, log-transformed mask size, and the square of transformed mask size, as described above.
Both within- and between-category correlations showed robust effects of age group (Figure 1B). Within-category correlations were reduced in older adults (F(1, 23) = 11.1, p = .0029). In contrast, older adults showed increased (i.e. less negative) correlations between categories (F(1, 23) = 12.0, p = .0021). Thus, age differences in neural distinctiveness stem from both decreased within-category reliability and increased between-category similarity in older adults1.
The preceding analysis focused on patterns of activation within regions of VVC that responded strongly to all object categories. Haxby and colleagues (2001) showed that regions of VVC that activate preferentially to one stimulus category (e.g. faces) can also decode responses to other categories (e.g. houses). Thus, we also examined age differences in neural distinctiveness within regions of VVC that responded maximally to each stimulus category. For each of the four stimulus categories, we identified the voxels within the VVC that showed the strongest response to that category, compared to scrambled images. Neural distinctiveness was significantly reduced in older adults across the voxels most sensitive to faces (F(1, 23) = 15.0, p < .001), houses (F(1, 23) = 14.6, p < .001), pseudo-words (F(1, 23) = 12.5, p = .0017), and chairs (F(1, 23) = 7.1, p = .014). Finally, age differences in neural distinctiveness persisted when the entire anatomical VVC ROI was considered (t(23) = 3.23, p = .0037). In sum, age differences in neural distinctiveness are robust across a wide range of voxel selection methods.
Computational accounts of cognitive aging suggest that neural representations of stimuli may be relatively sparse in younger adults and relatively distributed in older adults. In other words, older adults may use may use more neural resources to encode a particular stimulus than younger adults (Li et al., 2001; see Li and Sikström, 2002, Figure 2). Thus, older adults may be able to compensate for increased neural noise by distributing stimulus representations across more processing nodes. If this is the case, age differences in category distinctiveness should be largest for small masks (at which MVPA is most sensitive to sparse representations) and should diminish for larger masks (at which MVPA is sensitive to both sparse and distributed representations). In contrast to this prediction, age differences in neural distinctiveness within the VVC did not vary with mask size. Interactions between age and the quadratic effect of mask size failed to approach significance (Fs < 1; Figure 1). Similarly, age differences in within- and between-category correlations did not interact with mask size (Fs < 1). Thus, we found no evidence that older adults can increase the distinctiveness of neural representations by distributing category representations across larger numbers of voxels within the VVC.
Figure 2.
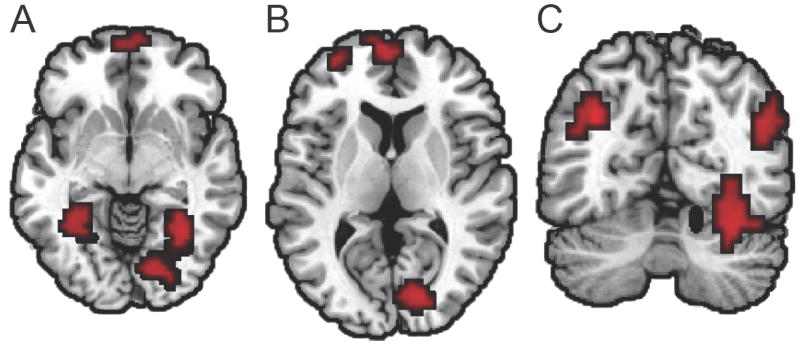
Whole-brain searchlight analysis of age differences in neural distinctiveness. Regions showing significantly higher neural distinctiveness scores for younger compared to older adults are highlighted in red and include bilateral ventral visual cortex (Panel A; z = -6), right striate and left and medial prefrontal cortex (Panel B; z = 8), and bilateral inferior parietal cortex (Panel C; y = -64). No regions showed significantly higher distinctiveness scores for older adults. All coordinates are given in MNI space.
Prior studies have reported increased inter-trial variability of the hemodynamic response function in older adults (D’Esposito et al., 1999; Huettel et al., 2001). This variability has been hypothesized to reflect age differences in neuro-vascular coupling (D’Esposito et al., 2003). In other words, increased variability of the BOLD signal in older adults may stem from vascular rather than neural changes. Such non-neural changes in BOLD variability could have biased our results: perhaps age differences in neural distinctiveness are driven solely by age differences in trial-by-trial variability of neuro-vascular coupling. To investigate this possibility, we measured BOLD variability in each subject. We quantified BOLD variability as the average mean-square error (derived from the General Linear Model, implemented in SPM5) within a control brain region not activated by our task, the posterior cingulate cortex (results for this analysis were qualitatively similar when BOLD variance was measured in the VVC instead). We then repeated the analyses described above while statistically controlling for individual differences in BOLD variability. Regardless of the criteria used to define regions of interest within the VVC, age differences in neural distinctiveness remained significant after controlling for BOLD variability (all categories vs. baseline: F(1, 22) = 8.50, p = .008; faces vs. baseline: F(1, 22) = 11.79; p < .0024; houses vs. baseline: F(1, 22) = 11.40, p < .0027; pseudo-words vs. baseline: F(1, 22) = 9.52, p < .0054; chairs vs. baseline: F(1, 22) = 4.72, p = .041). High BOLD variability was also associated with reduced neural distinctiveness (Fs(1, 22) >= 9.28, ps <= .006). In summary, both age and BOLD variability had significant effects on neural distinctiveness. Critically, effects of age on distinctiveness survived correction for BOLD variability, suggesting that the age differences reported above cannot be explained solely by differences in neuro-vascular coupling.
Searchlight analysis
Whole-brain analysis confirmed age differences in neural distinctiveness within the ventral visual pathway: younger adults showed higher category selectivity than older adults in bilateral VVC (Figure 2A; Table 1). Age differences in category distinctiveness were not restricted to the ventral visual stream. Older adults also showed decreased distinctiveness in early visual cortex, including right striate cortex and extending into extrastriate cortex (Figure 2B; Table 1). We also observed age differences beyond the visual cortex. Older adults showed decreased selectivity of category coding in bilateral inferior parietal cortex (Figure 2C; Table 1) and in left and medial prefrontal regions (Figure 2A, 2B; Table 1)2. Overall, neural distinctiveness was highest in visual areas (particularly the VVC); distinctiveness scores were reduced in parietal and frontal regions (Table 1).
Table 1.
Age differences in neural distinctiveness.
| Anatomical location | Number of voxels | MNI coordinates | Neural distinctiveness | Peak t-score | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | Younger adults | Older adults | |||
| R. Visual Cortex | 289 | 15 | -79 | 15 | .58 | .23 | 5.20 |
| L. VVC | 40 | -30 | -56 | -10 | .78 | .39 | 3.94 |
| R. VVC | 289 | 34 | -56 | -5 | .74 | .34 | 4.54 |
| R. Inferior Parietal Cortex | 66 | -34 | -60 | 35 | .34 | .11 | 5.70 |
| L. Inferior Parietal Cortex | 32 | 49 | -64 | 30 | .30 | .05 | 4.41 |
| L. Prefrontal Cortex | 136 | -30 | 53 | 10 | .19 | .03 | 4.09 |
| M. Prefrontal Cortex | 136 | -11 | 53 | 30 | .25 | .05 | 5.39 |
Prior reports have suggested that older adults are able to compensate for impaired processing in visual cortex using frontal and parietal mechanisms (Park and Reuter-Lorenz, 2009). If frontal circuits can indeed counteract age-related changes in visual processing, then older adults should exhibit higher neural distinctiveness than younger adults in some brain regions outside the visual cortex. However, our data did not support this proposition: no regions showed higher distinctiveness for older adults than younger adults.
Searchlight analysis revealed age-related decline in neural distinctiveness in several distinct brain regions. Age differences in these regions may stem from a common cause; alternatively, different mechanisms may explain age changes in different regions. To explore these possibilities, we assessed correlations in neural distinctiveness among the brain regions showing an overall age difference in distinctiveness. For each subject, we assessed average neural distinctiveness within four groups of brain regions: early visual (right striate cortex), late visual (bilateral VVC), parietal (bilateral inferior parietal), and prefrontal (medial and lateral PFC). Neural distinctiveness scores were averaged for each subject within 10 mm of the peak activation for each ROI (peak coordinates are reported above). Scatter-plots for all pairs of ROIs are displayed in Figure 3; correlation coefficients are presented in Tables 2 and 3. Correlations were estimated separately for younger and older adults. In younger adults, neural distinctiveness in early and late visual regions was significantly correlated (r(11) = .84, p < .001). No other correlations were significant (ps > .05). Similarly, distinctiveness in early and late visual areas was also significantly correlated in older adults (r(10) = .86, p < .001). Older adults also showed significant correlations in distinctiveness between early visual and parietal ROIs (r(10) = .80, p = .0016) and between late visual and parietal ROIs (r(10) = .87, p < .001). No other correlations were significant among the older adults. In sum, neural distinctiveness in early and late visual areas was highly correlated for both younger and older adults. Correlations between other pairs of regions were not significant or were inconsistent across age groups.
Figure 3.
Correlations of neural distinctiveness scores across regions. Correlations between right striate and bilateral ventral visual regions were significant in both younger adults (filled circles; solid lines) and in older adults (open circles; dotted lines). Correlation coefficients are provided in Tables 2 and 3.
Table 2.
Correlations between neural distinctiveness scores across ROIs. Younger adults only.
| Early visual | Late visual | Parietal | Prefrontal | |
|---|---|---|---|---|
| Early visual | . |
r = .84* p < .001 |
r = .46 p = .11 |
r = .007 p = .98 |
| Late visual | . | . |
r = .54 p = .058 |
r = .37 p = .22 |
| Parietal | . | . | . |
r = .26 p = .40 |
| Prefrontal | . | . | . | . |
Table 3.
Correlations between neural distinctiveness scores across ROIs. Older adults only.
| Early visual | Late visual | Parietal | Prefrontal | |
|---|---|---|---|---|
| Early visual | . |
r = .86* p < .001 |
r = .80* p = .0016 |
r = .42 p = .18 |
| Late visual | . | . |
r = .87* p < .001 |
r = .27 p = .39 |
| Parietal | . | . | . |
r = .18 p = .58 |
| Prefrontal | . | . | . | . |
Global correlation analysis
Region-of-interest and searchlight analyses focused on local differences in neural distinctiveness. However, aging may also affect the global distribution of category distinctiveness across the brain. We assessed age differences in the spatial distribution of category coding using a global correlation analysis, including all voxels in the brain.
Results from this global correlation analysis are presented in Figure 4. Each point in this scatter-plot describes the neural distinctiveness in the local neighborhood of a single voxel for younger adults (horizontal axis) versus older adults (vertical axis). This analysis revealed a highly significant linear relationship between age groups (r = .929, p < .001; Figure 4): voxels with high distinctiveness among younger participants tended to show high distinctiveness in older participants as well. Thus, the neural substrates of category representation were highly similar across age groups. Importantly, however, the slope of the best-fit line was significantly less than one (99.9% confidence interval of β: .515 to .523; Figure 3). Thus, any given voxel showed nearly double the distinctiveness in younger adults as in older adults. In sum, older adults encoded object category using the same neural resources but with uniformly lower distinctiveness than their younger counterparts.
Figure 4.
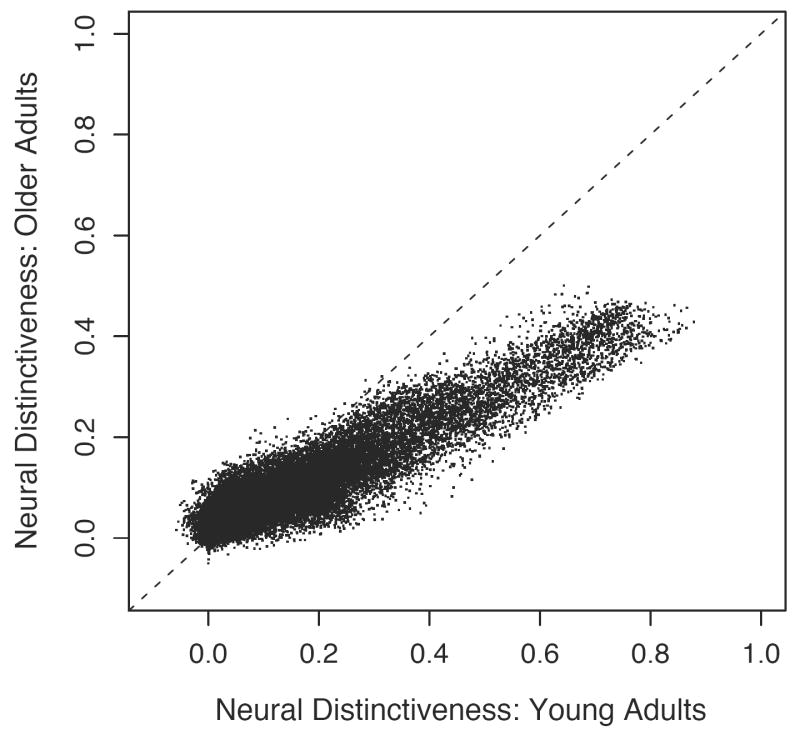
Global correlation between neural distinctiveness scores in younger and older adults. Each point describes the neural distinctiveness in the local neighborhood of a single voxel among younger adults (horizontal axis) and older adults (vertical axis). Both groups used the same neural resources to represent visual stimuli (r = .929), but the distinctiveness of any given voxel in older adults was reduced by almost 50% compared to younger adults (β = .519).
Discussion
Computational models of cognitive aging posit that neural representations become less distinctive in old age (Li et al., 2001). The present study explored age differences in the distinctiveness of distributed visual representations, applying multi-voxel pattern analysis (MVPA) to an earlier study of the aging visual system (Park et al., 2004). In agreement with univariate studies of the aging visual system, we showed that multi-voxel activation patterns in the ventral visual cortex (VVC) evoked by different stimulus categories were less distinctive among older adults. Critically, this age-related dedifferentiation was not restricted to the VVC; older adults also exhibited decreased neural distinctiveness in early visual cortex, inferior parietal cortex, and medial and lateral prefrontal cortex. Finally, results from multiple analyses provided no support for the notion that older adults compensate for decreased selectivity in perceptual brain regions by increasing selectivity in other regions.
We first tested the hypothesis that fMRI activation patterns elicited by different stimulus categories would become less distinctive in old age. Multiple analyses confirmed age differences in neural distinctiveness. Region-of-interest analysis using subject-specific anatomical masks of the ventral visual cortex (VVC) showed that activation patterns in object-sensitive regions of VVC are less distinctive among older adults (Figure 1A). These age differences in neural distinctiveness reflected changes in both within- and between-category correlations (Figure 1B). Older adults showed significantly lower correlations within categories across runs. In other words, fine-grained spatial activation patterns for a given category are less consistent across time in old age. Furthermore, older adults showed significantly higher (i.e. less negative) correlations between categories. Thus, differences between categories were less pronounced in older adults. Finally, we showed that age differences in neural distinctiveness were not specific to a particular choice of ROI within the VVC: distinctiveness was uniformly and significantly reduced in older adults across all ROIs tested, including the entire anatomical ROI.
Second, we conducted a whole-brain analysis of age differences in neural distinctiveness. A multivariate searchlight procedure (Kriegeskorte et al., 2006) confirmed age-related differences in the ventral visual stream (Figure 2A). This analysis also revealed age differences in early visual cortex and inferior parietal cortex, as well as medial and lateral prefrontal regions (Figure 2B, 2C). Critically, no brain regions showed higher distinctiveness in older adults compared to younger adults. Correlations among regions revealed strong relationships between neural distinctiveness scores in early and late visual regions in both age groups. On the other hand, distinctiveness scores in visual regions were uncorrelated with scores in frontal regions (Figure 3; Tables 1 and 2). These results suggest that a common mechanism may explain age-related declines in both early and late visual areas, while an independent mechanism may explain declines in frontal regions. Impaired coding of simple visual features like orientation and spatial frequency in early visual cortex may impact the coding of object category in the VVC. Consistent with this speculation, single-unit recording studies of visual representation indeed show that visual features are encoded less selectively in V1 and V2 in senescent macaques (Schmolesky et al., 2000; Wang et al., 2005). Alternatively, correlations between early and late visual areas may reflect a general disruption of visual attention in older adults (Madden, 2007). Finally, we assessed the ability of older adults to compensate for reduced neural distinctiveness in visual cortex by increasing selectivity in other brain regions. Prior studies suggest that older adults may compensate for altered visual processing by engaging additional neural circuits (Grady et al., 1994; Madden et al., 2004). In contrast to this view, our results suggest that older adults do not compensate for decreased neural distinctiveness in the visual cortex by increasing distinctiveness in other regions. First, age differences in distributed category coding did not vary with the number of voxels analyzed, suggesting that older adults did not compensate for noisy ventral visual responses by engaging more processing nodes within the VVC (Figure 1). Second, neural distinctiveness scores were higher for younger adults than for older adults across several brain regions (Figure 2), but no regions showed higher distinctiveness for older adults than for younger adults. Finally, a global correlation analysis revealed that aging affects the distinctiveness but not the spatial distribution of category coding. In other words, older and younger adults use the same brain regions to encode visual categories, but neural distinctiveness is uniformly decreased by about 50% throughout the aging brain (Figure 4). In sum, we found no evidence that older adults can increase the distinctiveness of visual representations by engaging additional processing resources within the VVC, by recruiting brain regions outside the visual cortex, or by altering the spatial distribution of category coding across the brain.
Our results are broadly consistent with studies of the aging visual system in non-human animals. Leventhal and colleagues (Leventhal et al., 2003; Schmolesky et al., 2000) found that single neurons in early visual cortex showed weaker stimulus preferences in senescent macaques compared to young controls. Similar results have been reported in cats (Hua et al., 2006) and rats (Wang et al., 2006). In other words, single-cell responses to different visual stimuli are more similar in older animals. The present study confirms and extends these results. We report an analogous effect in humans: our results show that responses to different visual stimuli are less distinctive in older adults than in younger adults. Furthermore, while single-cell studies of the aging visual system have focused on local changes in visual activity, our results reveal age differences in distributed representations as well.
While animal studies have focused on age differences in visual responses in early visual (Schmolesky et al., 2000) and dorsal-stream regions (Yang et al., 2008), we report age differences in category representation in the ventral visual cortex. However, our observation of age-related deficits in early visual cortex suggests that age differences in ventral visual activity may stem from altered processing of simple features in primary visual cortex. Indeed, we found that neural distinctiveness scores in early visual cortex were strongly predictive of distinctiveness in the ventral visual cortex. Aging is also associated with impaired communication within the visual cortex (Wang et al., 2005), providing further support for the notion that the ventral visual stream receives degraded inputs from early visual cortex in aging humans. The present study does not directly test this hypothesis; future research should continue to investigate the relationship between age-related changes in early and late visual processing.
Our results also dovetail with research on aging and neural complexity. According to Tononi and colleagues (1998; 1994), complex neural systems are characterized by both functional integration and functional segregation. Our findings of reduced within-category correlations in older adults may reflect declines in functional integration within neural networks that process visual objects; on the other hand, enhanced between-category correlations in older adults may reflect impaired functional segregation. Thus, our results are compatible with the view that neural complexity is reduced in older adults. In this regard, our findings agree with computational modeling work by Li and Sikström (2002), who linked age-related declines in neural distinctiveness to reduced computational complexity. Future studies should use explicit measures of neural complexity (Tononi et al., 1994) to assess age differences in functional integration and segregation in visual cortex.
Future studies should also test the generality of our results across different tasks and experimental designs. Participants did not make overt responses in the present study; our results do not exclude the possibility that older adults can compensate for reduced neural distinctiveness in the context of a task that requires them to respond to visual stimuli. Future studies should measure age differences in neural distinctiveness in the context of a demanding task and relate distinctiveness measures to behavioral indices of compensation. Forthcoming work from our lab shows that neural distinctiveness is indeed associated with a range of behavioral tests in older adults (Park et al., unpublished data). The present study also used a block design, which does not permit analysis of individual trials or different stages within a trial. Future studies should extend this work to event-related designs to reveal the temporal evolution of age differences in neural distinctiveness.
Previous research has documented age-related increases in the variability of the hemodynamic response function across trials in early vision and motor regions (D’Esposito et al., 1999; Huettel et al., 2001). To the extent that these increases in response variability are attributable to non-neural processes (e.g. altered neuro-vascular coupling), they might artificially depress measures of neural distinctiveness in older adults. As recommended by D’Esposito and colleagues (2003), we took several steps to minimize the effects of age differences in BOLD variability on our results. First, our analysis focused on interactions between age group and experimental conditions, avoiding confounds due to age differences in overall response magnitude. Second, we used β values and not the usual t-statistic to assess responses to experimental stimuli; because β values are not scaled by model error, they may be less susceptible to individual differences in BOLD variability (Rypma and D’Esposito, 2000). Third, reasoning that averaging across time would reduce signal variability, we averaged BOLD responses both (1) across trials within a block and (2) across blocks within a run before submitting data to MVPA.
In addition to these methodological precautions, several features of our data also suggest that the age differences we report here cannot be explained solely in terms of non-neural age differences. First, in a previous report of these data, we found that average t-values in the VVC did not differ significantly between age groups (Park et al., 2004). In fact, t-values were non-significantly higher in older adults. This observation argues against an age difference in signal-to-noise ratio (SNR) in our data: if older adults have reduced SNR, they should also have lower t-values. However, this is not the case in this analysis. Second, in the present report, we showed that age differences in neural distinctiveness remained significant after statistically controlling for individual differences in BOLD variability.
Age differences in neuro-vascular coupling may also have influenced our analysis of inter-regional correlations in neural distinctiveness (Figure 3). Specifically, positive correlations in distinctiveness scores between brain regions may reflect global changes in BOLD variability (D’Esposito et al., 2003). However, two features of our data are inconsistent with this view. First, correlations between posterior and anterior regions were generally small and non-significant (Tables 2 and 3), arguing against a global explanation of individual differences in distinctiveness. Second, if correlations were driven by age differences in neuro-vascular coupling, then these correlations should vanish when only considering younger participants, who were assumed to have healthy vascular function. However, we found significant positive correlations between regions within younger adults as well as older adults.
In summary, while age groups may differ in both neural and non-neural components of the BOLD signal, we argue that non-neural differences cannot adequately explain our finding of reduced neural distinctiveness in older adults. Future studies should continue to investigate the relationship between BOLD response properties and MVPA, and should replicate the present results using non-hemodynamic measurements like EEG and MEG.
In conclusion, we show for the first time that the distinctiveness of distributed patterns of neural activation declines in old age. We observed age differences in neural distinctiveness in early and late visual cortex, as well as in parietal and prefrontal regions. Moreover, our results provided no support for the notion that older adults can increase the distinctiveness of neural representations. Our results lend novel support to computational models of cognitive aging and have important implications for the understanding of compensatory mechanisms in older adults. Finally, our results highlight the value of multivariate pattern analysis to the study of representational change in the aging brain.
Acknowledgments
This research was supported by an NDSEG fellowship (to J.C.) and National Institute on Aging Grant R37AG006265, Neuroimaging of Dedifferentiation and Memory Across the Lifespan (to D.C.P.). We thank Jérôme Prado, Rebecca Compton, Katherine Sledge Moore, and two anonymous reviewers for their helpful comments on an earlier version of this manuscript.
Footnotes
Following the recommendation of an anonymous reviewer, we repeated this analysis after randomly permuting category labels for each run and each subject. In this analysis, we found no difference between age groups. Thus, age differences in neural distinctiveness are specific to non-arbitrary category labels.
Analysis of ventral visual responses showed that age differences in neural distinctiveness did not vary with the number of voxels included in the analysis, suggesting that older adults did not increase the distinctiveness of neural representations by recruiting additional neural resources (see Region-of-interest analysis, above). When we repeated this analysis using the parietal and frontal regions identified by the multivariate searchlight analysis, we found an analogous result: age differences in neural distinctiveness did not decrease as more voxels were included in the analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Brinley JF. Cognitive sets, speed, and accuracy of performance in the elderly. In: Welford AT, Birren JE, editors. Behavior, aging, and the nervous system. Thomas; Springfield, IL: 1965. pp. 114–149. [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. Journal of Cognitive Neuroscience. 2000;2(12 Suppl):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson N, Locantore J, McIntosh A. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Venkatraman V, Tan JC, Gutchess A, Sutton B, Hebrank A, Leshikar E, Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. Journal of Cognitive Neuroscience. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell L, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Zarahn E, Aguirre GK, Rypma B. The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. NeuroImage. 1999;10:6–14. doi: 10.1006/nimg.1999.0444. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice H, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neuroscience and Biobehavioral Reviews. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg M. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cerebral Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. The Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458:632–635. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J, Gobbini I, Furey M, Ishai A, Schouten J, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nature Reviews Neuroscience. 2006;7:523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiology of Aging. 2006;27:155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Li S, Lindenberger U, Sikström S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes P. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Li S-C, Sikström S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neuroscience and Biobehavioral Reviews. 2002;26:795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychology and Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Madden D. Aging and visual attention. Current Directions in Psychological Science. 2007;16:70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Provenzale JM, Huettel SA. Age-related changes in neural activity during visual target detection measured by fMRI. Cerebral Cortex. 2004;14:143–155. doi: 10.1093/cercor/bhg113. [DOI] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: Multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Noll DC, Taylor SF. Working memory for complex scenes: age differences in frontal and hippocampal activations. Journal of Cognitive Neuroscience. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Payer D, Marshuetz C, Sutton B, Hebrank A, Welsh RC, Park DC. Decreased neural specialization in old adults on a working memory task. Neuroreport. 2006;17:487–491. doi: 10.1097/01.wnr.0000209005.40481.31. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: Reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature Neuroscience. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Serences J, Ester E, Vogel E, Awh E. Stimulus-specific delay activity in human primary visual cortex. Psychological Science. 2009;20:207–214. doi: 10.1111/j.1467-9280.2009.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiridon M, Kanwisher N. How distributed is visual category information in human occipito-temporal cortex? An fMRI study. Neuron. 2002;35:1157–1165. doi: 10.1016/s0896-6273(02)00877-2. [DOI] [PubMed] [Google Scholar]
- Tononi G. Complexity and coherency: integrating information in the brain. Trends in Cognitive Sciences. 1998;2:474–484. doi: 10.1016/s1364-6613(98)01259-5. [DOI] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M, Erickson K, Chaddock L, Prakash R, Colcombe S, Morris K, Doerksen S, Hu L, McAuley E, Kramer A. Dedifferentiation in the visual cortex: An fMRI investigation of individual differences in older adults. Brain Research. 2008;1244:121–131. doi: 10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Wang H, Xie X, Li X, Chen B, Zhou Y. Functional degradation of visual cortical cells in aged rats. Brain Research. 2006;1122:93–98. doi: 10.1016/j.brainres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Ma Y, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cerebral Cortex. 2005;15:403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Williams M, Dang S, Kanwisher N. Only some spatial patterns of fMRI response are read out in task performance. Nature Neuroscience. 2007;10:685–686. doi: 10.1038/nn1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang J, Liang Z, Li G, Wang Y, Ma Y, Zhou Y, Leventhal A. Aging affects the neural representation of speed in macaque area MT. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn221. [DOI] [PMC free article] [PubMed] [Google Scholar]



