Abstract
Hyperglycemia mediates endothelial cell dysfunction through a number of potential mechanisms that could result in the decrease of retinal blood flow early in diabetes. The aim of this study was to explore the role of endothelin receptor A (ETA) in the early decrease of retinal blood flow in diabetic mice. Diabetes was induced by streptozotocin, then ~1 wk later the mice were administered drinking water with or without the ETA receptor antagonist atrasentan (7.5 mg/kg/day) for the following 3 weeks. Non-diabetic age-matched mice with or without atrasentan were included as controls. For each mouse, measurements of retinal vascular diameters and red blood cell (RBC) velocities were obtained via intravital microscopy for the 5–7 feed arterioles (and draining venules) extending out of (and into) the optic disk, and from these values, flow rates and wall shear rates were calculated. Additionally, the number of retinal capillaries was counted by fluorescent immunostaining of platelet-endothelial cell adhesion molecule-1 (PECAM-1). Diabetes induced statistically significant decreases in RBC velocity, flow rate, and wall shear rate, with these alterations partially inhibited by atrasentan. No changes were observed in PECAM-1 expression among groups. The changes induced by diabetes, and the attenuation provided by atrasentan, were greater in the smaller retinal arterioles. In summary, ETA appears to play a role in the early decreases in retinal blood flow in a mouse model of diabetes.
Keywords: diabetic retinopathy, blood flow, endothelin-1
INTRODUCTION
Diabetic retinopathy is the leading cause of blindness in working-aged people in the US. With 10 years duration of type 1 diabetes, the prevalence of retinopathy is ~80%, and this incidence increases to ~95% with 20 years duration (Fong et al., 2004). Hyperglycemia may initiate several pathways that lead to vascular dysfunction, which then could contribute significantly to the subsequent development of retinopathy. A decrease in retinal blood flow is one of the earliest abnormalities observed early in the progression of diabetes, possibly as a result of vascular constriction. Primary interventions directed towards improving flow could hold potential in reversing early metabolic and biochemical alterations, and as such, additional studies delineating the mechanisms and mediators of vasoconstriction are warranted.
Both vascular and extravascular sites in the retina are a rich source of endothelin-1 (ET-1) (Kohner et al., 1995), which plays an important role in retinal vascular autoregulation (Takagi et al., 1996a; Takagi et al., 1996b). The potent vasoconstrictor not only regulates blood flow in normal physiological conditions, but also has been implicated in dysfunctional retinal hemodynamics in diabetic retinopathy (Pang and Yorio 1997), with increases in ET-1 expression possibly contributing to the early diabetic decreases in blood flow (Takagi et al., 1996a).
The vascular actions of ET-1 are mediated by two distinct receptors, ETA and ETB. ET-1 elicits constriction of retinal arterioles predominantly through the activation of vascular smooth muscle ETA receptors (Hein et al., 2009), which supports the postulation that ETA receptor antagonists might be potential therapeutic targets in diabetic retinopathy (Masuzawa et al., 2006; Shaw et al., 2006). However, little in vivo experimentation has been performed to elucidate the mechanisms and extent of ETA contribution to diabetes-induced reductions in retinal blood flow. To address these issues, the present study of streptozotocin (STZ)-induced diabetes in mice explored the use of atrasentan, a selective ETA receptor antagonist, in mediating changes in retinal hemodynamic parameters including vascular diameters, red blood cell velocities, shear rates, and blood flow rates.
MATERIALS AND METHODS
Animals
Nine- to ten-week old C57BL/6 male mice (Jackson Laboratories) were randomly assigned to intraperitoneal (i.p.) injection of STZ (Sigma, St. Louis, MO; 180 mg/kg dissolved in pH 4.5 sodium citrate buffer) or sodium citrate buffer alone, with the STZ injection performed within 15 min of mixing into solution. On day 6 following STZ injection and on the day of experimental measurements, blood glucose levels were checked via a tail vein puncture with a One Touch Ultra Glucometer (Milpitas, CA). Diabetic mice were included in the study if glucose values exceeded 250 mg/dl. After the first week, diabetic mice received subcutaneous insulin injections every other day, up until 48 h prior to the retinal measurements; the dose of insulin was 9–13 U/kg/wk (Humulin N, NPH; Eli Lilly & Co., Indianapolis, IN).
Beginning on day 9 following STZ or vehicle injection, two groups of mice were given 7.5 mg/kg/day atrasentan (provided by Abbott Laboratories Inc, Abbott Park, IL) in distilled drinking water for the final 3 weeks of the protocol, while two other groups of mice were administered distilled water alone. The four groups of mice were as follows: (1) untreated non-diabetic controls, (2) non-diabetic controls administered atrasentan, (3) untreated diabetics, and (4) diabetics administered atrasentan. Mice were housed one per cage, received standard chow, and were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Arteriolar and venular diameter
On the day of the experimental measurements, the mice were prepared as previously described (Lee and Harris 2008; Wright and Harris 2008; Wright et al., 2009). The right femoral vein was cannulated under anesthesia with an i.p. injection of Nembutal sodium solution (pentobarbital; 50 mg/kg) followed 4–5 min later with an i.p. injection of ketamine (50 mg/kg). The eye was dilated with a drop of 1% tropicamide ophthalmic solution (Akron Inc., Buffalo Grove, IL) followed with a drop of 2.5% Gonak™ hypromellose ophthalmic demulcent solution (Akron Inc.) covered by a 5 mm glass coverslip. Using a Nikon Eclipse E600FN microscope with an attached CoolSNAP ES camera (Photometrics, Tucson, AZ), the optic disk was centered in focus for intravital microscopy. A 40–50 μl bolus of 4.5 mg/kg fluorescein isothiocyanate (FITC)–dextran (MW 2,000,000) was injected at a rate of 20 μl/min with a syringe pump (Harvard Apparatus, South Natick, MA), and the vessels in which FITC-dextran appeared first were identified as the arterioles. Two to four min following the bolus injection of FITC–dextran, baseline retinal diameters were recorded (10x objective; fluorescein filter) by sequentially focusing on each of the primary arterioles and venules.
The video was analyzed using NIS Elements Basic Research software (version 2.1–2.3; Nikon Instruments Inc, Melville, NY). Diameter measurements were obtained at three different locations along each vessel within 450 μm of the optic disk, with an average of the three measurements reported. Whenever possible, data were obtained from each of the 5–7 primary arterioles extending from the optic disk, and from each of the 5–7 primary venules draining toward the optic disk.
Red blood cell velocity, microvessel wall shear rate, and blood flow rate
Erythrocytes (RBCs) were fluorescently labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI; Invitrogen Molecular Probes, Eugene, OR) as described previously (Lee and Harris 2008; Wright and Harris 2008). Once the optic disc was visualized via intravital microscopy as described in the preceding paragraphs, labeled RBCs were infused for 2 minutes via syringe pump into the right femoral vein at a rate of 20 μl/min. Images obtained through a rhodamine filter and 10x objective were video-recorded. Using a camera exposure time of 10 ms, DiI-labeled RBCs appeared as fluorescent streaks in the vessels, with the length of the streak proportional to RBC velocity. At least 10 consecutive RBC streaks were averaged per vessel, with the mean velocity Vmean reported in mm/s. Assuming cylindrical geometry, microvessel wall shear rate (SR) was estimated using the Newtonian definition SR = 8(Vmean/D), with D the mean diameter. Vessel flow rate was estimated according to the calculation πVmeanD2/4.
Immunohistochemistry (IHC) of Platelet-Endothelial Cell Adhesion Molecule-1 (PECAM-1, CD31)
On the day of the experiment, mice were euthanized with an overdose of pentobarbital (150–200 mg/kg). The right eye was immediately enucleated and placed in phosphate-buffered 4% paraformaldehyde (FD Neurotechnologies, Inc., Baltimore, MD) for 2.5 hours. The anterior portion of the eye and lens were removed and the eyecup (retina, choroid, and sclera) was placed in 20% sucrose solution at 4°C overnight for cryoprotection (Chen and Nathans, J., 2007; Nishiguchi et al., 2008). The eyecups then were placed in optimal cutting temperature (OCT) compound and cut at 10 μm thickness within 48 hours.
Tissue sections on slides were washed in 0.025% Triton X-100 in phosphate buffered saline (PBS), and then incubated in goat serum for 1.5 hours. After incubation with primary antibody (1:200, purified rat anti-mouse CD31 polyclonal antibody, BD Pharmingen) overnight at 4°C, slides were washed in 0.025% Triton X-100 in PBS followed by incubation with a secondary antibody (1:250, goat anti-rat IgG-Fc fragment-FITC, Jackson Immuno Research Labs, West Grove, PA) for 1 hr in the dark. Slides were washed in PBS and mounted with Vectashield Hardset Mounting medium with DAPI (Vector Laboratories, Inc, Burlinggame, CA). Isotype-matched IgG and omission of the primary antibody served as negative controls. Images were collected using the CoolSNAP ES camera attached to the Nikon Eclipse E600FN microscope described earlier, using a 4x objective with a Nikon fluorescein filter. Capillary density was evaluated by collecting data from 4 cross-sections per retina by counting the number of capillaries per cross-sectional length.
Statistics
Statistical differences between groups were determined via analysis of variance using Student-Newman-Keuls post-hoc corrections (InStat software v 3.05; GraphPad, San Diego, California, USA). Linear regression analyses were used to evaluate the associations between diameters and blood velocity, microvessel wall shear rate, and blood flow rate. Error bars are presented as ± standard error (SE), and statistical significance was set at p<0.05.
RESULTS
Table 1 provides the body weight and plasma glucose values for the four experimental groups. As indicated, STZ induced a 3–4 fold increase in plasma glucose, and caused a decrease in body weight. These changes were unaffected by treatment with atrasentan.
Table 1.
Mean body weights and median plasma glucose values for the four experimental groups, 6 days and 4–5 weeks after injection with either STZ or vehicle alone.
| 6 days after injection | 4–5 weeks after injection | ||||
|---|---|---|---|---|---|
| Group | Number of mice | Weight (g) | Glucose (mg/dl) | Weight (g) | Glucose (mg/dl) |
| Control | 6 | 27.9 ± 0.6 | 160 | 29.7 ± 0.8 | 172 |
| Control + atrasentan | 9 | 27.6 ± 0.6 | 164 | 28.6 ± 0.8 | 176 |
| Diabetic | 9 | 27.4 ± 0.5 | 516 | 20.0 ± 0.5*** | 552 |
| Diabetic + atrasentan | 9 | 26.5 ± 0.5 | 540 | 21.0 ± 0.8*** | 579 |
p<0.001 vs corresponding controls.
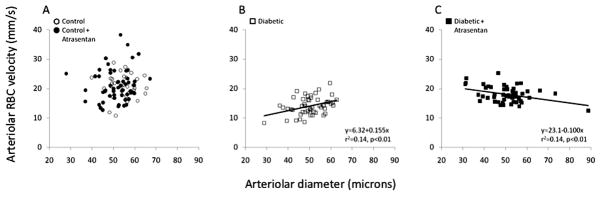
Figure 1 shows the arteriolar diameters and RBC velocities of the four groups, presented as regression analyses. RBC velocities varied from ~10–40 mm/s, with no correlation to arteriolar diameter in the non-diabetic mice (Fig 1A). However, RBC velocity decreased in the untreated diabetic mice, and to a greater extent in the smaller diameter arterioles, as indicated by the statistically significant positive slope shown in Figure 1B. Treatment with atrasentan was able to attenuate the decrease in RBC velocities, especially in the smaller arterioles (as can be seen by comparing Figures 1B and 1C). The velocities from Figure 1 were averaged and are shown in Figure 2 along with the venular averages for all four experimental groups, showing the ~33% decrease induced by diabetes and the partial attenuation of this decrease with atrasentan treatment. As shown in Figure 3, no statistically significant differences were found in the microvessel diameters between groups, although the untreated controls tended to be slightly larger.
Figure 1.
Arteriolar red blood cell (RBC) velocity as a function of diameter in the mouse retina, (A) in non-diabetic mice with and without atrasentan, (B) in untreated diabetic mice, and (C) in atrasentan-treated diabetic mice.
Figure 2.
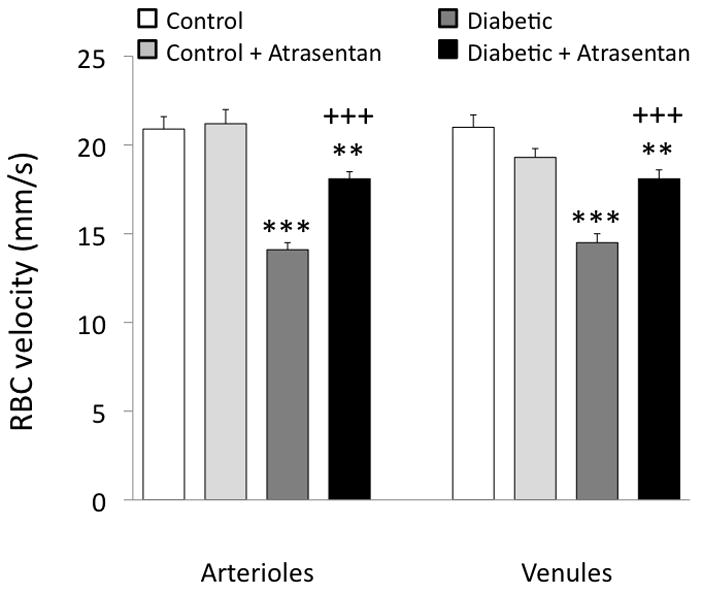
Retinal arteriolar and venular RBC velocity averages in the four groups of mice. **p<0.01 and ***p<0.001 vs controls; +++p<0.001 vs untreated diabetics.
Figure 3.

Retinal arteriolar and venular diameters in the four groups of mice.
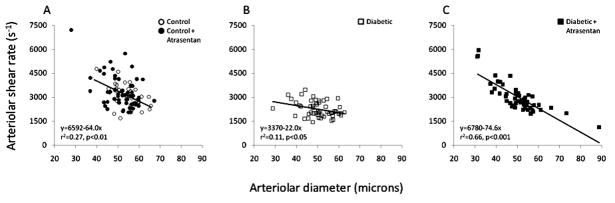
Microvessel wall shear rate varies inversely with diameter as shown in Figure 4A, with values approximately twice as high in the smaller arterioles compared with the larger arterioles. However, this relationship was significantly altered by diabetes, with the slope decreasing almost three-fold (Fig 4B). Atrasentan preserved the relationship between shear rate and diameter as found in controls (Fig 4C). The shear rates from Figure 4 were averaged and are presented in Figure 5, which shows that for both arterioles and venules, there is a significant decrease in untreated diabetics, with no difference between controls and atrasentan-treated diabetics.
Figure 4.
Arteriolar wall shear rate as a function of diameter in the mouse retina, (A) in non-diabetic mice with and without atrasentan, (B) in untreated diabetic mice, and (C) in atrasentan-treated diabetic mice.
Figure 5.
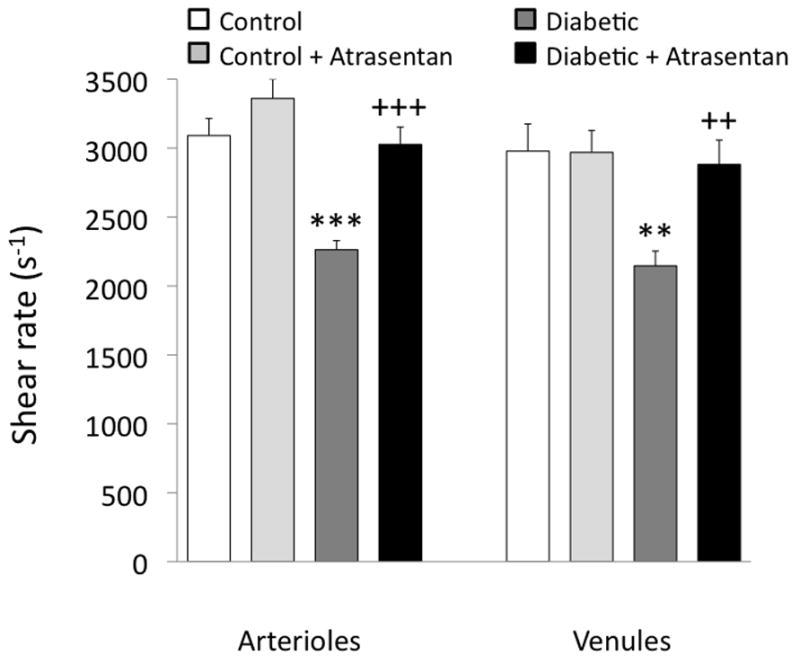
Retinal arteriolar and venular shear rate averages in the four groups of mice. **p<0.01 and ***p<0.001 vs controls; ++p<0.01 and +++p<0.001 vs untreated diabetics.
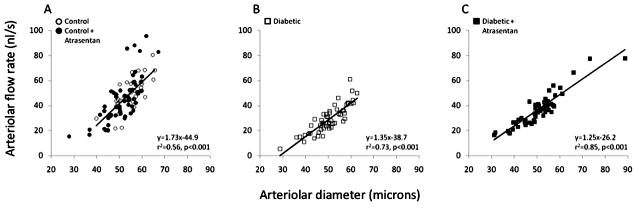
In contrast to shear rate, blood flow rate increases with diameter as shown in Figure 6, with the slope of this relationship tending to decrease slightly in diabetics compared with controls. Average microvessel flow rates decreased by ~40% in diabetics, with this decrease somewhat attenuated by treatment with atrasentan (Figure 7).
Figure 6.
Arteriolar blood flow rate as a function of diameter in the mouse retina, (A) in non-diabetic mice with and without atrasentan, (B) in untreated diabetic mice, and (C) in atrasentan-treated diabetic mice.
Figure 7.
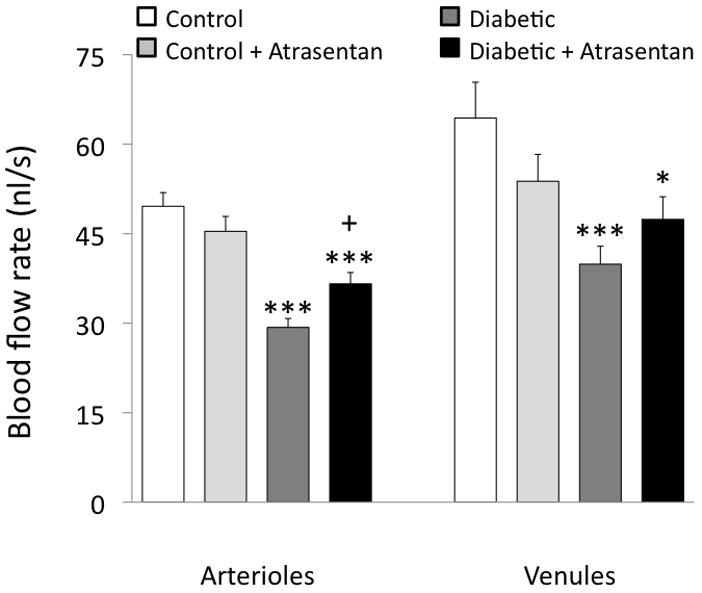
Retinal arteriolar and venular blood flow rate averages in the four groups of mice. *p<0.05 and ***p<0.001 vs controls; +p<0.05 vs untreated diabetics.
Capillary density was estimated by immunostaining cross-sections of retinal tissue for PECAM-1 (Figure 8). As shown in Figure 9, no differences in PECAM-1 staining were seen among the four experimental groups.
Figure 8.
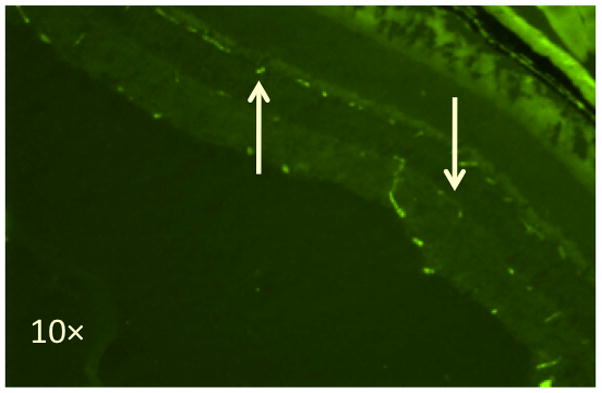
Fluorescent immunostaining of PECAM-1 in the mouse retina. The down arrow points to the inner capillary layer and the up arrow points to the deep capillary layer.
Figure 9.
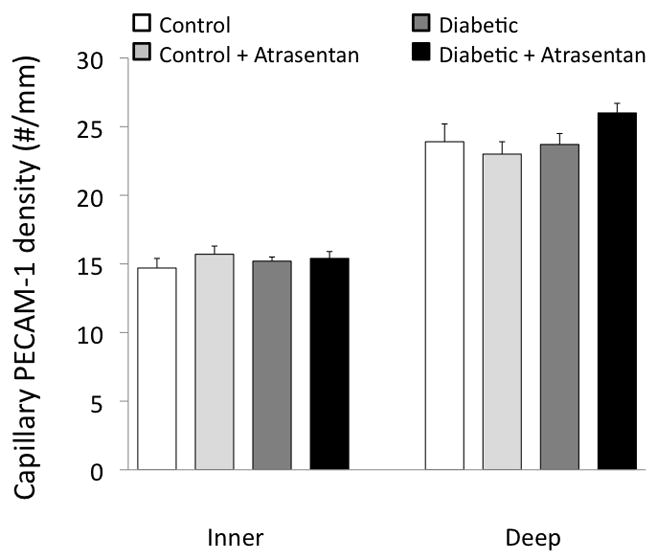
Capillary density (PECAM-1 immunostaining) in the four groups of mice.
DISCUSSION
The current study suggests that endothelin receptor A antagonism with atrasentan can attenuate the decrease in retinal hemodynamics induced in the early stages of diabetes in mice. Among the vascular changes induced by diabetes were decreases in red blood cell velocity, shear rate, and blood flow rate, with each of these decreases at least partially prevented by 3-week atrasentan administration in drinking water. To our knowledge, this is the first report of these vascular effects of ETA receptor antagonism in a mouse model of diabetic retinopathy.
The results are consistent with previous findings in the rat, in which the investigators found that acute administration of the ETA receptor antagonist BQ-123 was able to dose-dependently attenuate the decreases in an index of flow induced by diabetes (Takagi et al., 1996a). Their study also found that the diabetic rat retina was found to have an approximate 2-fold increase in mRNA of preproET-1, and that acute administration of the endothelin converting enzyme inhibitor phosphoramidon normalized the index of flow to values found in non-diabetic controls. The stimulus for the increase in ET-1 in the rat is possibly related to the protein kinase C pathway (Yokota et al., 2003), but whether a similar increase in ET-1 exists in the diabetic mouse retina has yet to be determined.
The changes in retinal hemodynamics induced by diabetes in the current study were more prominent in the smaller feed arterioles extending out of the optic disk, as demonstrated in the regression analyses of arteriolar RBC velocity vs diameter (Fig 1) and arteriolar wall shear rate vs diameter (Fig 4). Moreover, the attenuations of these changes provided by atrasentan were similarly more prominent in the smaller arterioles. These findings could suggest a more dominant role for ET-1 and ETA in the smaller retinal arterioles. Hein et al. have demonstrated immunohistochemical staining of endothelin converting enzyme and ETA receptors in small retinal arterioles of the pig (Hein et al., 2009). In humans, i.v. injections of ET-1 have been found to induce a decrease in retinal blood flow (Polak et al., 2003): since the decrease occurred in the absence of significant constriction of the large retinal arteries, the authors speculated a more dominant effect in the downstream microvessels.
Although ET-1 may play a role in the retinal blood flow decreases induced by diabetes, other vasoconstrictors also are likely involved. We previously have found that 3-week administration of the thromboxane receptor antagonist vapiprost also is able to partially attenuate the STZ-induced decrease in retinal blood flow in the mouse (Wright et al., 2009). The similarity of these two studies may not be surprising given the probable association between ET-1 and thromboxane signaling (Arikawa et al., 2006).
One limitation of the current study is that systemic and intraocular pressures were not measured, with or without atrasentan treatment. However, the diabetes-induced decreases in retinal blood flow and velocity are unlikely affected by changes in retinal perfusion pressure, inasmuch as we have demonstrated negligible changes in either systemic or intraocular pressure in the initial 3–4 weeks following STZ injection in rats and mice (Lee and Harris 2008; Lee et al., 2008; Wright and Harris 2008). A second limitation of the study is the unknown effect on mouse retinal blood flow that may have resulted from the STZ-induced losses in body weight.
The effects of diabetes on retinal vascular shear rates have received little prior attention. The approximate 2-fold decrease in wall shear rate of the smaller feed arterioles in our study could result in a significant decrease in arteriolar production of nitric oxide, given the shear force-mediated pathway of vascular production of the vasodilator. Moreover, decreases in microvascular wall shear have been found to induce increases in endothelial cell adhesion molecules and blood cell adhesion, both of which increase in the diabetic retina (McLeod et al., 1995; Joussen et al., 2001; Joussen et al., 2004).
In the present study, we did not observe a diabetes-induced decrease in capillary density, as quantified by the staining of the endothelial surface molecule PECAM-1. It should be noted that our cross-sectional analysis, while limited to the inner and deep capillary layers, might have included an occasional precapillary arteriole or postcapillary venule. The lack of change in microvascular density is consistent with our previous results (Wright and Harris 2008) in which we found that capillary filling with FITC-dextran is similar between diabetic and non-diabetic mice at the same time point (4 weeks hyperglycemia) as in the present study. This differs from reports in a different animal model, the diabetic rat (Moreno et al., 1995; De la Cruz et al., 1997), using an assay quantifying capillary density as the number of vessels stained with infused horseradish peroxidase (HRP). In their studies, the number of HRP-stained capillaries decreased significantly within the first four weeks of hyperglycemia in the rat and continued to decrease further in the subsequent weeks.
To summarize the current study, three-week drinking water administration of the endothelin receptor A antagonist atrasentan was found to substantially attenuate the microvascular hemodynamic alterations induced by diabetes in the mouse retina.
Acknowledgments
This study was funded by NIH EY017599 (NRH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arikawa E, Cheung C, Sekirov I, Battell ML, Yuen VG, McNeill JH. Effects of endothelin receptor blockade on hypervasoreactivity in streptozotocin-diabetic rats: vessel-specific involvement of thromboxane A2. Can J Physiol Pharmacol. 2006;84:823–33. doi: 10.1139/y06-042. [DOI] [PubMed] [Google Scholar]
- De la Cruz JP, Moreno A, Munoz M, Garcia Campos JM, Sanchez de la Cuesta F. Effect of aspirin plus dipyridamole on the retinal vascular pattern in experimental diabetes mellitus. J Pharmacol Exp Ther. 1997;280:454–9. [PubMed] [Google Scholar]
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R. Retinopathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S84–7. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- Hein TW, Ren Y, Yuan Z, Xu W, Somvanshi S, Nagaoka T, Yoshida A, Kuo L. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci. 2009;50:3329–36. doi: 10.1167/iovs.08-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–52. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44:603–7. doi: 10.2337/diab.44.6.603. [DOI] [PubMed] [Google Scholar]
- Lee S, Harris NR. Losartan and ozagrel reverse retinal arteriolar constriction in non-obese diabetic mice. Microcirculation. 2008;15:379–87. doi: 10.1080/10739680701829802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Morgan GA, Harris NR. Ozagrel reverses streptozotocin-induced constriction of arterioles in rat retina. Microvasc Res. 2008 doi: 10.1016/j.mvr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa K, Goto K, Jesmin S, Maeda S, Miyauchi T, Kaji Y, Oshika T, Hori S. An endothelin type A receptor antagonist reverses upregulated VEGF and ICAM-1 levels in streptozotocin-induced diabetic rat retina. Curr Eye Res. 2006;31:79–89. doi: 10.1080/02713680500478923. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147:642–53. [PMC free article] [PubMed] [Google Scholar]
- Moreno A, De La Cruz JP, Garcia Campos J, Sanchez De La Cuesta F. Prostacyclin-thromboxane balance and retinal vascular pattern in rats with experimentally induced diabetes. Can J Ophthalmol. 1995;30:117–23. [PubMed] [Google Scholar]
- Pang IH, Yorio T. Ocular actions of endothelins. Proc Soc Exp Biol Med. 1997;215:21–34. doi: 10.3181/00379727-215-44110. [DOI] [PubMed] [Google Scholar]
- Polak K, Luksch A, Frank B, Jandrasits K, Polska E, Schmetterer L. Regulation of human retinal blood flow by endothelin-1. Exp Eye Res. 2003;76:633–40. doi: 10.1016/s0014-4835(02)00312-3. [DOI] [PubMed] [Google Scholar]
- Shaw SG, Boden JP, Biecker E, Reichen J, Rothen B. Endothelin antagonism prevents diabetic retinopathy in NOD mice: a potential role of the angiogenic factor adrenomedullin. Exp Biol Med (Maywood) 2006;231:1101–5. [PubMed] [Google Scholar]
- Takagi C, Bursell SE, Lin YW, Takagi H, Duh E, Jiang Z, Clermont AC, King GL. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1. Invest Ophthalmol Vis Sci. 1996a;37:2504–18. [PubMed] [Google Scholar]
- Takagi C, King GL, Takagi H, Lin YW, Clermont AC, Bursell SE. Endothelin-1 action via endothelin receptors is a primary mechanism modulating retinal circulatory response to hyperoxia. Invest Ophthalmol Vis Sci. 1996b;37:2099–109. [PubMed] [Google Scholar]
- Wright WS, Harris NR. Ozagrel attenuates early streptozotocin-induced constriction of arterioles in the mouse retina. Exp Eye Res. 2008;86:528–36. doi: 10.1016/j.exer.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WS, Messina JE, Harris NR. Attenuation of diabetes-induced retinal vasoconstriction by a thromboxane receptor antagonist. Exp Eye Res. 2009;88:106–12. doi: 10.1016/j.exer.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Ma RC, Park JY, Isshiki K, Sotiropoulos KB, Rauniyar RK, Bornfeldt KE, King GL. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes. 2003;52:838–45. doi: 10.2337/diabetes.52.3.838. [DOI] [PubMed] [Google Scholar]