Abstract
One mechanism that may allow older adults to continue to successfully perform certain cognitive tasks is to allocate more resources than their younger counterparts. Most prior studies have not included individuals beyond their 70s. Here, we investigated whether compensatory increases in neural activity previously observed in cognitively high performing young-old adults would continue into old-old age. Event-related potentials were recorded from 72 cognitively high performing subjects, aged 18 to 96 years old, while they participated in a subject-controlled novelty oddball paradigm in which they determined viewing duration of standard, target, and novel visual stimuli. Compared to young and middle-aged subjects, both young-old and old-old subjects exhibited an impairment of preliminary mismatch/match detection operations, indexed by an attenuated anterior N2 component. This may have placed a greater burden on the subsequent controlled decision-making process, indexed by the P3, necessitating the allocation of more resources. The relationship between age and resource allocation, as measured by P3 amplitude, from midlife to very old age (45–96 years old) followed an inverted u-shaped curve (quadratic function). It peaked between the late 60s and early 70s. Thereafter, there was an inverse relationship between age and resource appropriation. This relationship remained significant after controlling for differences in task performance and MMSE. Examining the size of the P3 component across different age groups suggests that although cognitively high performing adults in their early 80s exhibit a reduction in P3 amplitude, they have a relatively well-preserved capacity to appropriate resources. However, by the late 80s, there is a robust decline (relative to young-old adults) in the size of the P3. Our results indicate that when carrying out controlled processing linked to directing attention to salient events, cognitively high performers reach the boundary of their capacity, albeit relatively late in life. This limits their ability to appropriate additional resources as compensatory activity for age-related impairments in earlier visual processing, and suggests that such a mechanism does not tend to “survive” old-old age.
Keywords: cognitive aging, old-old age, compensatory activity, ERPs, P3, resource allocation
1. Introduction
Understanding factors that contribute to successful cognitive aging has become increasingly important as a growing portion of the population is living to very old age (Alzheimer’s Association, 2007; Daffner, 2010). Our research has focused on neural mechanisms that may contribute to different patterns of cognitive aging. In a series of experiments, we have examined electrophysiologic and behavioral responses while cognitively normal adults of different ages participated in a variant of the novelty oddball task in which participants control how long they look at various kinds of visual stimuli (Daffner et al., 2006a; Daffner et al., 2006b; Daffner et al., 2007; Riis et al., 2008). Our emphasis has been on novelty processing because of the notion that increased responsiveness to novelty may not only reflect but also help promote successful cognitive aging (Wilson et al., 2002; Daffner et al., 2006a). In these studies, viewing duration has served as an index of visual attention and exploratory behavior (Berlyne, 1960; Daffner et al., 2000a) and the amplitude of the P3 component has served as an index of the amount of resources allocated to attentional processing (Isreal et al., 1980; Wickens et al., 1983; Sirevaag et al., 1989; Daffner et al., 2000a; Daffner et al., 2003).
Previous investigations using the subject-controlled variant of the novelty oddball task in healthy adults and patients with neurological diseases have yielded valuable information about the relationship between neural activity, as measured by event-related potentials (ERPs), and attentional processing of salient events. For example, across healthy young adults, the amplitude of the novelty P3 response strongly predicted the duration of viewing directed toward novel relative to standard stimuli (Daffner et al., 1998). Within subjects, the novel stimuli that elicited the largest P3 responses were associated with the longest viewing durations (Daffner et al., 1998; Daffner et al., 2000b). Patients with damage to the prefrontal cortex exhibited a marked reduction of both the amplitude of the novelty P3 and the viewing duration of novel stimuli (Daffner et al., 2000a). Across frontal patients and normal controls, the amplitude of the novelty P3 explained a large portion of the variance associated with viewing durations of novel relative to standard stimuli. Damage to the posterior parietal cortex was associated with a substantial reduction of the P3 to both targets and novels (Daffner et al., 2003). However, parietal injury led to a smaller degree of disruption of the processing of novel events, which was circumscribed to the ipsilesional posterior quadrant. Based on this work with patients who had focal brain lesions, we have suggested that the prefrontal cortex may serve as a central node mediating the allocation of attentional resources to novel events, whereas the posterior parietal cortex may provide the neural substrate for updating internal models about the environment to take into account the novel event. Patients with Alzheimer’s disease (AD), including those with mild severity, also generated a very attenuated novelty P3 component and distributed their viewing time evenly between novel and repetitive standard stimuli (Daffner et al., 2001). Across AD subjects, the novelty P3 amplitude accounted for more than half of the variance in viewing duration of novels relative to standards. In both AD patients and frontal patients, the novelty P3 amplitude as well as the viewing duration of novel stimuli inversely correlated with the degree of a patient’s apathy, as measured by caregiver questionnaires.
Our work to date on normal aging has compared cognitively high and cognitively average performers across a large portion of the adult lifespan that has included young (mean age 21.7 years old), middle-aged (mean age 47.8 years old), and what we would now term “young-old” (y-old) (mean age 71.6 years old) adults. Here, we extend our research to include old-old (o-old) adults (80 years and older) who are cognitively high performers. To place the current investigation in context, below we summarize some of the principal results of our earlier studies on aging. We have found that cognitively high performing y-old subjects appropriate more attentional resources, as indexed by the novelty P3 amplitude, than cognitively high performing middle-age and young subjects, and than cognitively average performing y-old subjects. These electrophysiological results occurred in the context of behavioral findings in which cognitively high performing y-old subjects were as engaged by novelty, as indexed by viewing duration, as their cognitively high performing younger counterparts, and more engaged by novelty than cognitively average performing y-old adults. The results provide a strong rationale for suggesting that the increased allocation of resources to novel stimuli observed among cognitively high performing y-old adults does not simply represent less efficient processing, but a successful compensatory mechanism, presumably in response to other age-related changes in neurophysiologic function.
Our findings are in keeping with a recent ERP study by De Sanctis and colleagues (2009), who reported that compared to young subjects and low performing old subjects, high performing old subjects carried out task-switching operations by marked amplification of prefrontal positive electrophysiologic activity, which the authors interpreted as compensatory large-scale recruitment of prefrontal cortical mechanisms. Our findings are also consistent with those results from the functional imaging literature that have shown that old subjects who perform as well as young subjects on source or episodic memory tasks recruit more brain activity than young subjects, and than old subjects who perform worse (Reuter-Lorenz et al., 2000; Cabeza et al., 2002; Rosen et al., 2002; Davis et al., 2008). Thus, one of the mechanisms employed by cognitively high performing older adults to successfully carry out certain mental functions, such as novelty processing, may be the allocation of more resources than their younger counterparts.
Our data also have suggested that over the lifespan, cognitively high performing adults exhibit a very different pattern of age-related changes in novelty P3 amplitude than cognitively average performing adults. In contrast to cognitively high performers, for whom there were no differences in novelty P3 amplitude between young and middle-aged subjects, among cognitively average performers, middle-aged subjects appropriated significantly more resources, as indexed by the P3, than young subjects. Whereas among cognitively high performers there was an augmentation in the size of the P3 between middle-age and y-old age, among cognitively average performers, the size of the P3 decreased substantially from middle-age to y-old age. The hypothesis we currently favor to account for these different patterns of resource appropriation to novel stimuli across the adult lifespan is that cognitively average performing adults may experience age-associated changes in neurophysiologic function years earlier than cognitively high performing adults. At midlife, cognitively average performers still have the capacity to compensate for such a decline by allocating more processing resources (as measured by a larger P3). However, when they reach y-old age and have less capacity, this mechanism may no longer be viable, which is indexed by a decline in P3 amplitude. By contrast, cognitively high performing adults do not need to rely upon such compensatory activity until they reach y-old age, at which time their novelty P3 amplitude becomes larger. We hypothesized that by the time cognitively high performing adults reach o-old age, they may arrive at the limits of their capacity and thus be less able to recruit compensatory activity.1
Almost all of the studies that have addressed the notion of successful aging as being associated with a compensatory augmentation of neural activity have limited their investigation to “older” adults who are only in their 60s and 70s. Thus, very little is known about the fate of this proposed compensatory mechanism in individuals who are in the 9th decade and beyond. We have suggested that age-related compensatory activity observed in the subject-controlled oddball paradigm is particularly dependent on the pool of capacity-limited, controlled processing resources. If so, what should we expect to observe in o-old adults? There is growing evidence that o-old individuals have fewer processing resources than y-old adults (Lindenberger & Baltes, 1997; Singer et al., 2003a; Kemps & Newson, 2006). For example, Kemps and Newson (2006) showed that individuals over 84 years old did significantly worse than those 65 to 84, who, in turn, did significantly worse than young adults 18 to 25 years old on tests of working memory, processing speed, and executive function. Using a mental synthesis paradigm and neuropsychological tests, Newson and colleagues (2003) concluded that the age-related decrements in working memory capacity and executive functioning accelerated after age 85. The Berlin Aging Study (Singer et al., 2003b) also suggested a faster rate of cognitive decline in o-old subjects (mean age 83.0 years) than y-old subjects (mean age 73.8 years), especially on measures of fluid intelligence (e.g., speed of processing, verbal fluency). Rates of cognitive decline were similar for individuals with high and low intellectual reserve (e.g., education) (Lindenberger et al., 1997). Based on findings like these, we predicted that the processing capacity of cognitively high performing o-old subjects might be reduced enough that they would no longer have sufficiently available resources to increase neural activity in response to novel events as a compensatory mechanism. In other words, compared to y-old adults, they would exhibit a marked reduction in their ability to compensate. This would be reflected in a P3 response of cognitively high performing o-old adults that was smaller than that of cognitively high performing y-old adults, and no larger than that of cognitively high performing middle-aged and young adults.
The current paper has adopted the P3 terminology proposed by Friedman and colleagues (2001). The novelty P3 (or P3 to novels) was defined as the P3 response elicited by infrequent events about which the subject received no instruction before the experiment (Friedman et al., 2001). The target P3 (or P3 to targets) was defined as the P3 response evoked by designated events about which the subject had been instructed prior to the experiment and to which the subject was required to generate a specific response (Friedman et al., 2001). Although not emphasized in this study,2 the P3 often has been further divided in terms of the P3a, an anteriorly-distributed P3 component, and the P3b, a posteriorly-distributed P3 component. Novel, non-target deviant, and target events elicit both a P3a and a P3b component, although they tend to do so to varying degrees (Gaeta et al., 2003; Cycowicz & Friedman, 2004; Dien et al., 2004), with the P3 to novel stimuli receiving a much greater contribution from the P3a component, and the P3 to target stimuli receiving a much greater contribution from the P3b component. In keeping with this observation, in the current investigation, the P3 response to novel stimuli at Fz served as an index of the P3a, and the P3 response to target stimuli at Pz served as an index of the P3b (Fjell & Walhovd, 2003).
A particularly advantageous feature of ERPs is their excellent temporal resolution that allows measurement of different information processing operations occurring at the subsecond level. If, as expected, cognitively high performing o-old individuals demonstrate a reduction in P3 amplitude relative to y-old individuals, to what extent is this effect limited to operations indexed by the P3 component? In previous work (Riis et al., 2008), we found that cognitively high performers did not differ from cognitively average performers in terms of ERP correlates of early sensory-perceptual encoding (P1, N1) (Luck, 2005), salience detection (anterior P2) (Riis et al., 2009), or early mismatch-match processing (anterior N2) (Folstein & Van Petten, 2008; Tarbi et al., in press). Rather, cognitively high and average performers differed in the modulation of controlled processing linked to directing attention to salient stimuli and updating internal models about a changing environment, as indexed by the P3 component. We hypothesized that the most prominent differences between o-old and y-old subjects also would be observed for the controlled processing, indexed by the P3 component. An alternative prediction is that among o-old subjects, age-related decline in ERP components would be non-specific. Support for this prediction can be derived from several sources. For example, the common cause hypothesis of aging posits that old age is associated with a similar degree of deterioration across all levels of neurophysiological functioning (Lindenberger & Baltes, 1994; Lindenberger et al., 1997) and thus one would not expect it to be limited to those indexed by the P3 component. Another hypothesis that might lead to the expectation of a decline in amplitude across a large number of ERP components involves limitations of the ability to measure cerebral activity generated by very old brains. Age-related global changes in brain structure in o-old age, such as increased atrophy or CSF space, may markedly reduce the ability to adequately measure the signal derived from cerebral activity, resulting in the apparent diminution of the amplitude of many different ERP components. To investigate whether ERP components other than the P3 would exhibit a similar pattern of age-associated reduction in amplitude, we included measurements of the P1, N1, P2, and N2 components. Finally, since we anticipated a reduction in the compensatory allocation of resources among cognitively high performing o-old adults, we were interested in determining when the most substantial age-related changes begin to occur. This issue was examined by further dividing the o-old group into subjects 80–86 years old and those 87 and older, and by modeling the relationship between age and P3 amplitude, using linear and non-linear regression analyses.
2. Materials and Methods
2.1 Participants
After completing informed consent, participants underwent an evaluation that included a medical, neurological, and psychiatric history, a formal neurological examination, neuropsychological testing, and completion of questionnaires surveying mood and socioeconomic status. Participants had to be in one of four age groups (18–28 years old (young subjects), 45–55 years old (middle-age subjects), 65–79 years old (y-old subjects), 80 and older (o-old subjects)). In subsequent analyses, the o-old group was further divided into subjects 80–86 year olds and those 87 and older. Although this age division was somewhat arbitrary, it ensured at least 10 subjects in the very oldest age group. To be included in the study, participants also had to be English-speaking, have ≥ 12 years of education, a Mini Mental State Exam (MMSE) (Folstein et al., 1975) score ≥ 26, and an estimated IQ on the American Modification of the National Adult Reading Test (AMNART) (Ryan & Paolo, 1992) ≥ 100. Subjects were excluded if they had a history of CNS diseases or major psychiatric disorders based on DSM-IV criteria (American Psychiatric Association, 1994), a history of clinically significant medical diseases, corrected visual acuity worse than 20–40, a history of clinically significant audiological disease, a Geriatric Depression Scale (Yesavage et al., 1981) score of ≥ 10 for the y-old or o-old subjects, or a Beck Depression Inventory (Beck & Steer, 1987) score of ≥ 10 for middle-age or young subjects, or focal abnormalities on neurological examination consistent with a lesion in the CNS.
Subjects completed the AMNART and the Raven’s Progressive Matrices Test to determine estimated IQ scores, and the MMSE to obtain a gross measure of current mental state. IQ estimates from the Raven’s are based on the age of a subject. Young and middle-aged subjects were tested using the Raven’s Standard Progressive Matrices (RSPM) (Raven et al., 1995). Because available norms on the RSPM do not go beyond age 65, older subjects were tested using the Raven’s Colored Progressive Matrices (RCPM) (Raven et al., 1983) which has published norms up to 85 years old. Of note, direct comparisons of the raw scores of the RSPM and RCPM cannot be made because the RCPM was designed as a shorter, easier test (Lezak, 1995).
For subjects 80 years and older, an informant who knew the participant answered questions for the Clinical Dementia Rating (CDR) (Morris, 1993), which provided detailed information about current functional status. All subjects also completed the following six neuropsychological tests: 1) Digit Span subtest of the Wechsler Adult Intelligence Scale-III (WAIS-III) (Wechsler, 1997a); 2) Controlled Oral Word Association Test (COWAT) (Ivnik et al., 1996); 3) Logical Memory II subtest of the Wechsler Memory Scale-III (WMS-III) (Wechsler, 1997b); 4) Visual Retention Test (Youngjohn et al., 1993); 5) Boston Naming Test (Tombaugh & Hubley, 1997); 6) Category (animal) fluency (Spreen & Strauss, 1998). Norms are available for each of these tests across a range of ages, allowing us to compute a percentile score for all subjects on each test. A composite score was determined for each subject by averaging performance (percentile scores) on all six neuropsychological tests. Since performance on our experimental task was particularly dependent upon attention/executive functions, the average percentile score on Digit Span and COWAT was also computed for each subject. For all age groups, except the 87+ group, cognitive status was operationally defined based on performance on the six neuropsychological tests. To meet criteria for a cognitively high performer, subjects had to have a composite percentile score in the top 3rd (≥ 67th percentile). Because there are very limited normative data for adults in their late 80s and 90s, we did not use percentile performance on neuropsychological tests as criteria for defining cognitively high performer in the 87+ age group. It has been estimated that only approximately one third of individuals in this age range are free of mild cognitive impairment or dementia (Unverzagt et al., 2007; Corrada et al., 2008). Thus, participants over the age of 87 were considered to be cognitively high performers if they did not meet criteria for either mild cognitive impairment (Petersen et al., 1999) or clinical dementia based on DSM-IV guidelines (American Psychiatric Association, 1994). Of note, the 87+ subjects were relatively well-matched to the other age groups in terms of high estimated IQ and education. Percentile performance was calculated for these subjects by using norms for the oldest available group.
2.2 Experimental procedures
The experimental procedures used were analogous to the ones described in prior reports (Daffner et al., 2000a; Riis et al., 2008). Two hundred and fifty line drawings, white on black background, were presented in 5 blocks of 50, each at the center of a high-resolution computer monitor. All stimuli subtended a visual angle of approximately 2.75° along their longest dimension. There were three categories of visual stimuli: 1) a repetitive Standard Stimulus (a triangle) − 70% frequency, 2) a Target Stimulus (upside down triangle) – approximately 15% frequency, and 3) Novel Stimuli, randomly drawn from a set of unusual/unfamiliar line drawings (e.g., impossible or fragmented objects) shown only one time each – approximately 15% frequency (Supplementary Fig. S1). Stimuli appeared within a fixation box subtending a visual angle of approximately 3.5° × 3.5° that remained on the screen at all times.
Participants were informed that the experiment involved the study of brain wave activity as they looked at different kinds of drawings. We emphasized that they could view each picture for however long or short they liked. They controlled the viewing duration by pressing a button that led to the erasure of the current stimulus and the onset of a blank screen that lasted between 800 and 1760 ms (mean ~1300 ms), followed by the presentation of the next stimulus. Also, participants were told to respond to the designated target stimulus by pressing a foot pedal (ipsilateral to the button press). Instructions indicated that accuracy was more important than speed. Left and right button press/foot pedal was counterbalanced for all subject groups. Although viewing durations were calculated by subtracting the stimulus onset time from the button press time, all stimuli were displayed for a minimum duration of 600 ms. Subjects also participated in two other conditions not reported here. The order of the conditions was counterbalanced for all subject groups.
2.2.1 ERP recordings
An electrode cap (Electro-Cap International, Eaton, OH, USA) was used to hold to the scalp 35 active tin electrodes whose locations were based on the International 10–20 system. Electrodes were arranged in 5 columns (midline, 2 inner lateral, 2 outer lateral), each with 7 antero-posterior sites (Supplementary Fig. S2). All sites were referenced to the left mastoid, and the impedance between each recording site and the reference was reduced to less than 5K ohms. An electrode was placed beneath the left eye (whose electrical activity was compared to an electrode placed above the left eye) to check for eye blinks and vertical eye movements. Another electrode was placed to the right of the subject’s right eye (referenced to an electrode to the left of the left eye) to check for lateral eye movements. A final electrode was placed over the right mastoid (referenced to the left one) to monitor asymmetrical mastoid activity. (None was identified.) The EEG was amplified by an SA Instrumentation (San Diego, CA, USA) system (model H & W 32BA), using a band filter with negative 3dB cutoffs of 0.01 and 40 Hz, and continuously digitized (200 Hz) by a computer yielding 1280 ms of data from each electrode site, beginning 100 ms before stimulus onset.
2.2.2 Data analysis
A continuous record of the raw EEG was stored on hard disk. Off-line, EEG epochs for each of the stimulus types were averaged separately. Trials with eye movements or amplifier blocking were excluded from data analysis. For all subjects, a blink correction program, using principal component analysis, was employed that computed the impact of the blink on the waveform in each channel (Dale, 1994). The overall P3 in response to each stimulus type was defined as the mean amplitude between 350 and 550 ms. (We also measured the local peak positive amplitude between 350 and 850 ms after stimulus onset, which yielded very similar results, not reported here).
In addition, we examined other components in response to novel, target, and standard stimuli, defined as follows: P1 and posterior N1 were examined over occipital and occipital-parietal sites, which the literature suggests is where the largest effects are found (Luck, 2005; De Sanctis et al., 2007). The P1 component of a subject in response to each visual stimulus type was defined as the mean amplitude of the 20 ms interval centered at the mean local positive peak latency at O1/2 and PO7/8 between 70 and 130 ms after stimulus onset that was calculated for his/her subject group. The N1 component of a subject in response to each visual stimulus type was defined as the mean amplitude of the 20 ms interval centered at the mean local negative peak latency at O1/2 and PO7/8 between 120 and 220 ms after stimulus onset that was calculated for his/her subject group. The P2 component was examined over midline sites. We were particularly interested in the anterior P2, which appears to be an index of stimulus salience (Riis et al., 2009). The P2 component of a subject in response to each visual stimulus type was defined as the mean amplitude of the 40 ms interval centered at the mean local positive peak latency at midline sites between 150 and 250 ms after stimulus onset that was calculated for his/her subject group. The N2 component also was examined over midline sites, as we were especially interested in the size of the anterior N2 component, which has been linked to early mismatch/match detection operations, and indexes when a stimulus presented differs from representations being held in memory (Folstein et al., 2008; Tarbi et al., in press). The N2 component of a subject in response to each visual stimulus type was defined as the mean amplitude of the 40 ms interval centered at the mean local negative peak latency at midline sites between 200 and 350 ms after stimulus onset that was calculated for his/her subject group. All components were measured with respect to the average of the 100 ms prestimulus baseline.
In general, ERP data for each stimulus type were analyzed using analysis of variance (ANOVA), with age group as the between-subjects variables and the 7 midline electrode sites (FPz, Fz, FCz, Cz, CPz, Pz, Oz) as the within-subjects variables. Analyses of scalp distribution focused on determining whether there were antero-posterior differences across subject groups. For the P1 and N1 components, within subject variables included 2 electrode sites (O1/2 and PO 7/8) and 2 hemisphere sites (right and left). In examining scalp site interactions with other variables, raw, not normalized, data were analyzed (Urbach & Kutas, 2002). Analyses that yielded significant interactions between subject group, stimulus type, or electrode site resulted in planned contrasts between the levels of the variable. The Geisser-Greenhouse correction was applied for all repeated measures with greater than 1 degree of freedom. Regression analyses were used to determine the association between age and P3 amplitude.
3. Results
3.1 Participants
Seventy-nine individuals participated in the study. Data from 4 y-old and 3 o-old subjects were excluded because of excessive EEG artifact or equipment malfunction resulting in <25 artifact-free trials of any stimulus category. The characteristics of each group are summarized in Table 1, including the number of subjects, demographic information, the results on more global cognitive measures (e.g., estimated IQ, MMSE), composite percentile scores on the neuropsychological tests, and the pertinent statistical analyses. Middle-aged subjects had more years of education than o-old and than young subjects, many of whom were still in school (Table 1). The groups differed in estimated IQ, based on performance on the AMNART and Ravens. On the AMNART, middle-aged subjects had higher scores than o-old, who, in turn, had higher scores than young subjects. On the Ravens, both o-old and middle-aged subjects had higher estimated IQ scores than either y-old or young subjects. On the MMSE, middle-aged subjects scored higher than o-old subjects. There were no differences across age groups in terms of average percentile performance on the six neuropsychological tests, or average percentile performance on the two tests emphasizing attention/executive function. The o-old group had a significantly higher proportion of subjects with one or more cerebrovascular risk factors, such as hypertension, high cholesterol, heart disease, or diabetes, than any of the other groups (see Table 1).
Table 1.
Subject characteristics (mean (±SD))
| Young (18–30) | Mid-age (45–55) | Young-Old (65–79) | Old-Old (80–96) | Effect of Age (p value) | |
|---|---|---|---|---|---|
| No. of Subjects | 16 | 16 | 13 | 27 | |
| Male/Female | 10/6 | 9/7 | 5/8 | 15/12 | ns |
| Age | 21.5 (1.0) | 50.3 (3.4) | 71.2 (3.1) | 85.5 (5.1) | p < 0.0001 |
| Yrs. of Education | 15.4 (0.6) | 18.9 (4.3) | 16.2 (3.8) | 16.3 (3.8) | p < 0.051 |
| AMNART | 120.6 (4.3) | 125.8 (4.1) | 120.9 (9.6) | 123.5 (3.9) | p < 0.052 |
| Ravens | 116.1 (11.2) | 124.4 (7.0) | 118.3 (45.9) | 126.6 (6.8) | p < 0.013 |
| MMSE | 29.1 (0.7) | 29.4 (1.0) | 29.1 (0.8) | 28.5 (1.3) | p < 0.054 |
| Exec. Function %tile | 72.1 (19.8) | 75.4 (16.1) | 77.4 (17.5) | 77.7 (20.4) | ns |
| Neuropsych. %tile | 71.5 (7.6) | 75.9 (10.3) | 70.6 (10.2) | 75.5 (14.2) | ns |
| Subjects with CVD Risk * | 0/16 | 3/16 | 5/13 | 23/27 | p < 0.00015 |
Mid-age > Old-Old; Mid-age > Young.
Mid-age > Young; Mid-age > Old-Old; Old-Old > Young.
Old-Old > Young-Old; Old-Old > Young; Mid-age > Young; Mid-age > Young-Old.
Mid-age > Old-Old.
Old-Old > Young-Old ≈ Mid-age ≈ Young.
Proportion of subjects with ≥1 cerebrovascular disease risk factor.
3.2 Behavior
3.2.1 Viewing Duration
The behavioral data for each subject group are presented in Table 2. An ANOVA examining mean viewing duration of novel vs. standard stimuli revealed an effect of stimulus type (F(1,68) = 29.16, p < 0.0001) and an interaction between group and stimulus type (F(3,68) = 3.16, p < 0.05). All groups visually explored novel stimuli more than repetitive standard stimuli. The interaction between age and stimulus type was present because the magnitude of the difference between novel and standard stimuli was larger for y-old than o-old subjects (F(1,38) = 6.10, p < 0.05). An ANOVA examining mean viewing duration of novel stimuli vs. target stimuli revealed an interaction between group and stimulus type (F(3,68) = 3.92, p < 0.05). This interaction was due to o-old subjects spending less time looking at novel than target stimuli, whereas all the other groups spent more time looking at novel than target stimuli.
Table 2.
Behavioral data (mean (±SD))
| Viewing Duration to Novels (ms) | Viewing Duration to Standards (ms) | Viewing Duration to Targets (ms) | Target Hit Percentage | Reaction Time to Targets (ms) | |
|---|---|---|---|---|---|
| Young | 2008.5 (1860.5) | 611.9 (276.8) | 2004.3 (977.3) | 96.3 (4.7) | 869.5 (178.4) |
| Mid-Age | 2200.1 (2463.2) | 744.1(633.3) | 1988.4 (950.5) | 96.1 (7.6) | 976.2 (192.6) |
| Young-Old | 4797.7 (5887.7) | 684.3 (283.3) | 1785.3 (566.0) | 96.7 (5.2) | 909.2 (217.8) |
| Old-Old | 1896.5 (2033.7) | 816.9 (255.0) | 2185.1 (926.3) | 93.9 (8.4) | 1085.5 (171.7) |
ms = milliseconds
3.2.2 Target Responses
The groups did not differ in overall target hit accuracy (Table 2). However, there was an effect of group for mean reaction time (RT) (F(3,67) = 6.31, p < 0.001). The o-old group had slower RTs than any other group; middle-aged subjects had slower RTs than young subjects. These results remained significant after controlling for subjects viewing duration of target stimuli.
3.3 Event-related Potentials
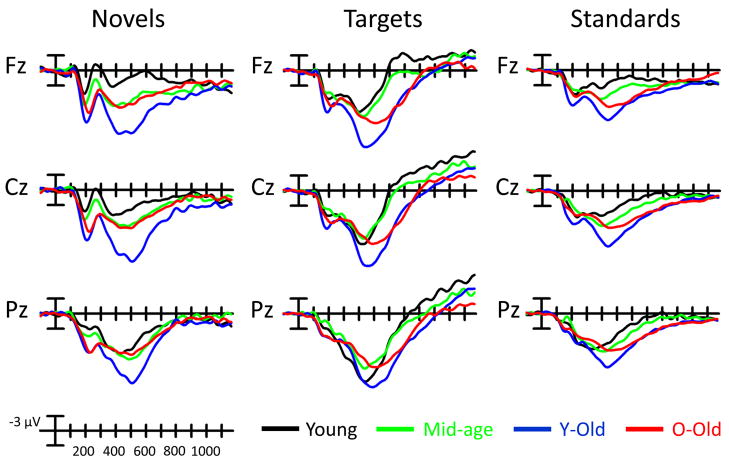
Grand average ERPs at selected midline sites for novel, target, and standard stimuli for all subject groups are illustrated in Fig. 1. (See Supplementary Fig. S3, which presents the grand average ERPs at all 35 scalp electrode sites.) In the sections that follow, we will limit our discussion to the findings most relevant to the objectives of this study.
Figure 1.
Grand average ERP plots in response to novel, target, and standard stimuli at selected midline sites for young, middle-aged, y-old, and o-old age groups.
3.3.1 Overall P3 amplitude and scalp distribution
3.3.1.a Novel stimuli
An age (o-old, y-old, middle-age, young) by site (FPz, Fz, FCz, Cz, CPz, Pz, Oz) ANOVA revealed an effect of age (F(3,68) = 7.76, p < 0.001) and an interaction between age and site (F(18,408) = 4.89, p < 0.0001). Y-old subjects generated a larger P3 response to novel stimuli than any other group. Of particular interest to the objectives of the current study, o-old subjects generated a smaller novelty P3 than y-old subjects (p < 0.01). The interaction between age and site was present because o-old subjects generated a more anteriorly-distributed P3 than all of the other groups. In addition, young subjects generated a less anteriorly-distributed P3 than either y-old subjects (F(6,162) = 7.89, p < 0.001) or middle-aged subjects (F(6,180) = 3.91, p < 0.05), with no difference between the latter two groups. Over central-posterior (Cz-Oz) sites, there were no differences between o-old, middle-aged, or young subjects in the size of the P3 response.
3.3.1.b Target stimuli
An ANOVA revealed an effect of age (F(3,68) = 4.04, p < 0.05) and an interaction between age and site (F(18,408) = 4.63, p < 0.0001). Y-old subjects generated a larger P3 response to target stimuli than the other age groups. There were no overall differences in the size of the target P3 among o-old, middle-aged, and young subjects. The interaction between age and site was due to o-old and y-old subjects having a more anteriorly-distributed target P3 than either middle-aged or young subjects. There were no differences in the scalp distribution of the target P3 between o-old and y-old subjects, or between middle-aged and young subjects.
3.3.1.c P3a and P3b
To address age-related changes of P3a and P3b, the P3 response to novel stimuli at Fz served as an index of P3a and the P3 response to target stimuli at Pz served as an index of P3b (see Introduction). For the P3a response to novel stimuli, there was an effect of age (F(3,68) = 9.82, p < 0.0001). Y-old subjects generated a larger P3a than all the other groups (all p s < 0.01). The young subjects generated a smaller P3a than the other age groups (vs. y-old and o-old, p’s < 0.01; vs. middle-aged, p < 0.05). Middle-aged and o-old subjects did not differ in the size of their P3a response to novel stimuli. The P3b response to target stimuli yielded a marginal effect of age (F(3,68) = 1.99, p = 0.1). Y-old subjects generated a larger P3b than o-old (p < 0.05) and a marginally larger P3b than middle-aged (p < 0.09).
3.3.2 P1, N1, P2, N2 components
To address whether age-related differences observed for the P3 were specific to this ERP component, the amplitudes of the P1, N1, P2, and N2 components were examined. Supplemental Figure S3 includes the O1, O2, PO7, and PO8 electrode sites, where the P1 and N1 components were measured.
There were no age-related changes in P1 amplitude to standard and novel stimuli. For targets, there was an age effect (F(3,68) = 3.62, p < 0.05), which was present because middle-aged subjects generated a smaller P1 than y-old (p < 0.01) and o-old subjects (p < 0.01), especially at the O1/2 sites (group × site interaction, F(3,68) = 2.82, p < 0.05). The pattern of age-related differences in N1 amplitude was the same for all 3 stimulus types, although the effects were only marginal in response to standards and novels. The N1 to standard stimuli yielded a marginal effect of age (F(3,68) = 2.28, p < 0.09) due to o-old subjects generating a smaller N1 than middle-aged (p < 0.05) and young (p = 0.1) subjects. In response to target hits, there was an effect of age (F(3,68) = 3.53, p < 0.05), due to o-old subjects generating a smaller N1 than middle-aged (p < 0.01) and young subjects (p < 0.05). In response to novel stimuli, there was a marginal effect of age (F(3,68) = 2.10, p = 0.1) due to o-old subjects generating a smaller N1 than middle-aged (p < 0.05) and young subjects (p < 0.05). The N1 to all stimulus types did not differ between y-old, middle-aged, and young subjects.
The amplitude of P2 response to standard stimuli did not differ across age groups. An interaction between age and site (F(18,408) = 3.95, p < 0.01) was present because young and middle-aged subjects generated a less anteriorly distributed P2 to standards than the other two age groups. There was an effect of age for the P2 amplitude in response to target hits (F(3,68) = 2.80, p < 0.05), which was due to middle-aged subjects having a smaller P2 than y-old or o-old subjects. The groups did not differ in the scalp distribution of the P2 to target hits. There was a strong effect of age for the P2 amplitude in response to novel stimuli (F(3,68) = 7.10, p < 0.001), with the y-old and o-old subjects generating a larger response than the middle-aged and young subjects. There were no differences in the P2 amplitude between y-old and o-old subjects, or between middle-aged and young subjects. Groups did not differ in the scalp distribution of the P2 to novel stimuli.
The size of the N2 to standard stimuli did not differ across age groups. An interaction between age and site (F(18,408) = 2.88, p < 0.01) was due to young subjects generating a less anteriorly-distributed N2 to standards than y-old and o-old groups. The N2 to target hits did not differ in terms of amplitude or scalp distribution across age groups. There was an effect of age for the amplitude of the N2 to novel stimuli (F(3,68) = 6.22, p < 0.001). This was present because young and middle-aged adults generated a larger N2 to novels than did y-old and o-old adults. There were no differences in the N2 to novel stimuli between young and middle-aged subjects, or between y-old and o-old subjects. The interaction between age and site (F(18,408) = 2.58, p < 0.05) reflected the fact that both young and middle-aged subjects had a much more anteriorly-distributed N2 to novels than o-old subjects, and young subjects had a more anteriorly-distributed N2 than y-old subjects.
3.4 Dividing the o-old into very old and the oldest old
3.4.1 Demographics and Behavior
To better appreciate the age range in which salient declines in the resources appropriated to novel and target stimuli occur, the o-old subjects were divided into two subgroups: an 80–86 year old group (n = 17, mean age (±SD): 82.1 (±1.6) years old), and an 87 and older group (n = 10, 91.2 (±3.7) years old). Demographic and behavioral variables were compared for the 87+ year old, 80–86 year old, and 65–79 year old (y-old), groups. These groups did not differ in terms of years of education or estimated IQ based on AMNART. The 87+ group had lower mean MMSE scores than the other two groups (87+ group: 27.5; 80–86 group: 29.1; y-old group: 29.1, effect of group, F(2,37) = 11.40, p < 0.001). The y-old group had lower mean Ravens Estimated IQ scores than the other two groups (y-old: 118; 80–86 group: 128; 87+ group: 124, effect of group (F (2,35) = 3.92, p < 0.05). A smaller proportion of y-old subjects had 1 or more cerebrovascular risk factors than did the 80–86 group or the 87+ group, with no difference between the latter two groups (y-old group: 5/13; 80–86 group: 13/17; 87+: 10/10, p < 0.01, Kruskal-Wallis Test). A similar pattern was observed for 2+ risk factors (y-old group: 2/13; 80–86 group: 10/17; 87+ group: 8/10, p < 0.01, Kruskal-Wallis Test).
All three of the older age groups viewed novel stimuli longer than standard stimuli (effect of stimulus type, F(1,37) = 12.47, p < 0.01). However, the magnitude of the difference in viewing duration between stimulus types tended to be larger for y-old subjects than either the 80–86 or 87+ year old subjects (age × stimulus type interaction, F(2,37) = 2.80, p = 0.06). Target accuracy did not differ across the three older subject groups. However, 65–79 year olds exhibited faster reaction times than either the 80–86 or the 87+ year olds (effect of group, F(2,36) = 5.37, p < 0.01), with no difference between the latter two groups.
3.4.2 P3 Response to Novel Stimuli and Target Hits
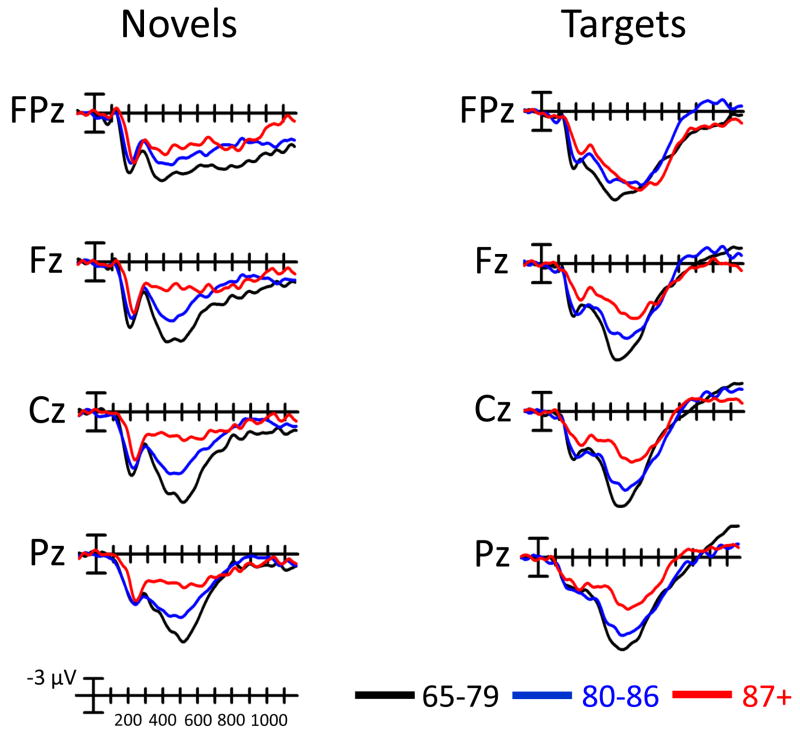
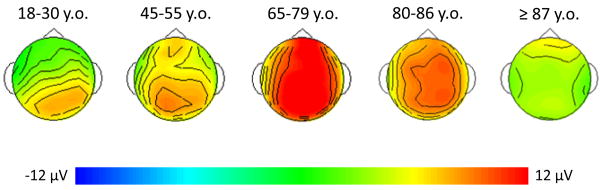
The grand average ERPs in response to novel and target stimuli for the 87+, 80–86, and y-old groups are compared in Fig. 2. Fig. 3 illustrates the novelty P3 surface potential maps (local positive peak around 450 ms) for each of the 5 age groups. An age (87+, 80–86, y-old, middle-age, young) by site (7 midline sites) ANOVA examining P3 to novel stimuli revealed an effect of age (F(4,67) = 8.27, p < 0.0001) and an interaction between age and site (F(24,402) = 4.23, p < 0.0001). The 87+ group generated a smaller novelty P3 than the 80–86 year old group (p < 0.01), who, in turn generated a smaller novelty P3 than the 65–79 group, although the latter was at the p = 0.05 level. The novelty P3 of the 80–86 year old group was larger than that of the young subject group (p < 0.01) but did not differ from that of the middle-age group. The novelty P3 amplitude of the 87+ group did not differ from either the middle-aged or young adult subject groups. The interaction between age and site was present because the 87+ group had a more anteriorly-distributed novelty P3 than all of the other groups. The scalp distribution of the novelty P3 did not differ for 80–86, y-old, and middle-aged subjects. Young subjects had a more posteriorly-distributed novelty P3 than all other groups.
Figure 2.
Grand average ERP plots in response to novel and target stimuli at selected midline sites comparing the 87+, 80–86, and y-old age groups.
Figure 3.
Novelty P3 surface potential maps (local peak ~450 ms) for young, middle-aged, y-old, 80–86, and 87+ age groups (y.o. = year old).
An ANOVA examining the P3 to target hits revealed an effect of age (F(4,67) = 4.48, p < 0.001) and an interaction between age and site (F(24,402) = 4.01, p < 0.0001). The 87+ group generated a smaller target P3 amplitude than the 80–86 year old and y-old groups across all sites; the 87+ group generated a smaller amplitude than middle-aged and young subjects across all sites except FPz. Y-old subjects exhibited a numerically larger target P3 than 80–86 year olds, but this effect was not reliable (p < 0.15). The interaction between age and site was due to the 87+ group having a more anteriorly-distributed P3 than the other groups. There was no difference in target P3 scalp distribution between y-old and 80–86 year old subjects, or between middle-aged and young subjects. Y-old and 80–86 year old subjects generated a more anteriorly-distributed target P3 than either young or middle-aged subjects.
In looking at the P3a alone (P3 to novel stimuli at Fz), there was an effect of age (p < 0.0001). The 87+ group had a smaller P3a than the 80–86 year old group (p <0.05), who in turn had a smaller P3a than the y-old group (p < 0.0001). The 80–86 year old group generated a larger P3a than the young adult group (p < 0.0001). There was no difference in the size of the P3a between the 87+ group and either the middle-aged or young adult groups. Considering the P3b alone (P3 to target hits at Pz), there was an effect of age (F(4,67) = 2.58, p < 0.05). The 87+ group generated a smaller P3b than the 80–86 year old group (p < 0.05) and the y-old group (p < 0.01). The P3b in the latter two groups did not differ. The 87+ group generated a smaller P3b than the young adult group (p < 0.05). There were no differences in the size of the P3 between the 80–86, middle-aged, and young adult groups.
3.4.3 P1, N1, P2, N2 Components across the older subject groups
The size of the P1 and N1 response to standard, target hits, or novel stimuli did not differ across the y-old, 80–86, or 87+ groups. Similarly, these groups did not differ in the size or scalp distribution of the P2 or N2 response to standard, target hits, or novel stimuli.
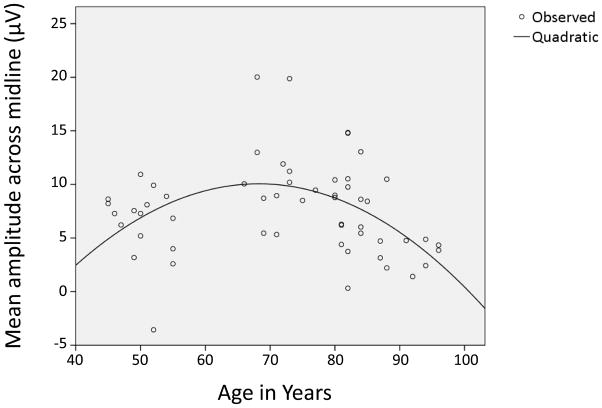
3.5 Statistical Models, Correlations, and Covariance
Examination of Fig. 3 suggested that resources appropriated to novelty processing, as indexed by P3 amplitude, increased between middle-age and y-old age (when it peaked) and then decreased between y-old and o-old age. This pattern of an inverted u-shaped curve was confirmed by a robust non-linear model of the age (45–96) vs. P3 amplitude data using a quadratic function (R = 0.45, R2 = 0.20, p < 0.01) (see Fig. 4).3 Similarly, a quadratic curve could be fitted to the age vs. target P3 amplitude data (R = 0.37, R2 = 0.14, p < 0.05).
Figure 4.
Age (45–96) vs. mean midline novelty P3 amplitude (data fit a quadratic curve, R = 0.45, R2 = 0.2, p < 0.01).
We also examined the pattern of age-related changes in P3 amplitude among cognitively high performers, restricted to older individuals only, ages 65–96, whose data reflected a linear relationship. Age was inversely correlated with mean midline P3 amplitude to novel stimuli (R = −0.55, p < 0.0001). Thus, among subjects 65–96 years old, as individuals got older, P3 amplitude to novel stimuli decreased in size. Similarly, age was inversely correlated with mean midline P3 amplitude to target hits (R = −0.51, p < 0.01). A quadratic formula did not explain more of the variance (R2) than the linear equation, which suggests that among older cognitively high performing subjects, increasing age was associated with a steady rather than an accelerated rate of decline in P3 amplitude.
To help control for the potential impact of differences in task performance on the P3 amplitude in response to novel and target stimuli, several additional analyses were carried out. The correlation between age and P3 amplitude to novel stimuli remained strong after controlling for viewing duration of novel stimuli (R = −0.54, p < 0.0001), the ratio of viewing duration of novels/standards (R = −0.54, p < 0.0001), or MMSE scores (R = −0.43, p < 0.01). Similarly, the correlation between age and P3 amplitude to target hits remained highly significant after controlling for RT to targets (R = −0.49, p < 0.01), target hit rate (R = −0.50, p < 0.01) and MMSE scores (R = −0.41, p < 0.05). (Analogous results were found when employing multivariate regression techniques.) In addition, the ANOVAs used to examine P3 amplitude in response to novel stimuli and target stimuli were carried out again after controlling for the following variables: viewing duration of novel stimuli, the ratio of the viewing duration of novels/viewing duration of standards, RT to target hits, target hit rate, and MMSE score. The P3 results reported in earlier sections “survived” after accounting for these different variables and will not be reiterated here.
3.6 The impact of cerebrovascular risk factors
One of the striking differences in subject characteristics across groups was the age-related increase in the proportion of subjects who had underlying cerebrovascular risk factors. Thus, a potential contribution to the age-associated decline in P3 amplitude among o-old subjects may be cerebrovascular injury. To explore this possibility, the P3 responses to novel and target stimuli of subjects with 0–1 cerebrovascular risk factors were compared to subjects with 2 or more cerebrovascular risk factors among the entire group of o-old subjects (n = 27) and among the 80–86 year old subjects (n = 17). Subject numbers were too small to further divide the 87+ group. Across the entire o-old group, 9/27 subjects had 0–1 risk factors and 18/27 had 2 or more risk factors. Although the mean midline P3 amplitudes to novel and target stimuli were numerically larger for the 0–1 risk factor group than the 2+ risk factor group the effects were not significant (novels: p > 0.34; targets: p > 0.18). Across the 80–86 year old group, 7/17 had 0–1 risk factors and 10/17 had 2 or more risk factors. However, the groups did not differ in the size of midline P3 responses to novel (p > 0.49) and target stimuli (p > 0.85).
4. Discussion
The main purpose of this study was to investigate whether compensatory increases in neural activity observed in cognitively high performing y-old adults would continue into o-old age. The major findings of the study can be summarized as follows. Among cognitively high performers, the relationship between age and resource allocation, as measured by P3 amplitude, from midlife to very old age (45–96 years old) appears to follow an inverted u-shaped curve (quadratic function). It peaks between the late 60s and early 70s. Thereafter, there is an inverse relationship between age and resource appropriation to novel and target stimuli. This relationship remained significant after controlling for differences in task performance and MMSE. Examining the size of the P3 component across different age groups suggests that although cognitively high performing adults in their early 80s exhibit a reduction in P3 amplitude, they have a relatively well-preserved capacity to appropriate resources (i.e., mild changes relative to y-old adults). However, by the late 80s, there is a robust decline in the size of the P3 amplitude to novel and target events. Behaviorally, cognitively high functioning individuals continue to be attracted to novelty, a hallmark of healthy human behavior (Berlyne, 1960; Daffner et al., 2003), at least through their early 90s. However, after age 80 there is an age-related reduction in the magnitude of the difference in viewing duration of novel relative to standard stimuli. Although o-old subjects generated a much smaller, more anteriorly distributed P3 than y-old subjects in response to novel and target stimuli, there were minimal differences between these two groups in ERP correlates of early perceptual encoding, salience detection, or preliminary mismatch/match processing, as indexed by the P1, N1, P2, or N2 components. Compared to young and middle-aged subjects, o-old subjects generated a smaller N1 to the visual stimuli, and both o-old and y-old subjects generated a much smaller anterior N2 to novel stimuli than their younger counterparts. Finally, although there were age-associated differences in the proportion of subjects with cerebrovascular risk factors, the size of the P3 component among old subjects did not appear to be significantly affected by this variable.
4.1 Age-associated capacity limits among cognitively high performing adults
We have argued that the increased allocation of resources among cognitively high performing y-old adults (relative to their younger counterparts) represents a compensatory neural mechanism to counter other age-related declines in neurophysiological function (Riis et al., 2008). We hypothesized that this mechanism would break down in o-old age because such individuals would reach the limits of their capacity, making them less able to recruit additional resources. In the current study, this would manifest as o-old subjects generating a smaller P3 amplitude than y-old subjects, which is what was found.4 The most prominent age-related differences in ERPs among old individuals were observed for the P3 and not earlier components (see below), suggesting that this effect is specific and not attributable to general age-related brain changes, such as global atrophy. The P3 indexes controlled processing involved with directing attention to potentially important stimuli or updating internal models about changes in the environment. Based on the results of neuropsychological and experimental cognitive tests, there is a growing literature suggesting that o-old individuals have fewer controlled processing resources than y-old individuals (Lindenberger et al., 1997; Newson et al., 2003; Singer et al., 2003b; Kemps et al., 2006). However, relatively few investigations have employed methods to more directly measure age-related changes in neural activity in o-old individuals.
Two very recent fMRI studies are pertinent to this issue and have yielded results consistent with our findings. Both investigations compared neural activity in y-old vs. o-old subjects during non-verbal/picture recognition memory tasks. In one study, Beeri and colleagues (2009) compared fMRI activation during picture recognition in y-old (mean age 75.3) vs. o-old (mean age 91.0) subjects. They found that o-old subjects generated less robust activation than y-old subjects, especially in the right hippocampus, parietal and temporal cortices, a finding that remained even after controlling for differences in MMSE, task performance, and degree of cerebral atrophy. In another study, Wang and colleagues (2009) used fMRI to compare the neural correlates of successful recognition memory of y-old (mean age 70.2) and o-old (mean age 87.4) subjects to determine whether the o-old subjects would continue to exhibit the age-related pattern of recruiting larger, more widespread areas than their younger counterparts. O-old subjects performed worse on neuropsychological tests and in terms of overall accuracy and speed on the recognition memory paradigm. Recognition memory performance of the o-old subjects for items studied twice was equivalent to that of y-old subjects for items studied once. Even after matching for memory performance, there was no evidence of greater neural recruitment in the o-old group relative to the y-old group in any cortical area, and there was an age-associated decline in neural activity in the medial parietal cortex. The authors concluded that there may be a plateau in age-related increases in neural recruitment in advanced age. One interpretation offered was that a lack of additional available neural resources limited the capacity to compensate for a decline in cortical efficiency, an idea in keeping with our own formulation.
The neuropsychological and functional imaging studies cited above have not tended to differentiate between cognitively high and cognitively average performing adults. Taken as a whole, our research suggests that cognitively high performers may reach the limits of their capacity at a much older age than cognitively average performers. Review of Supplementary Fig. S4 indicates that the lifespan course for resource appropriation in response to novelty for cognitively high performers is similar to that of cognitively average performers but is “shifted to the right” (i.e., changes occur later). It remains to be determined the extent to which this pattern will be observed for cognitive functions not involved in directing attention to novel stimuli.
Our results suggest that age-related differences in resource allocation, as measured by P3 amplitude, were more robust for the P3a to novel stimuli than for the P3b to target stimuli. This finding is consistent with other reports in the literature (e.g., Fjell and Walhov (2004)). Such results may seem puzzling if one regards decisions about the visual exploration of novel stimuli as not being very demanding. However, the processing of deviant, ambiguous stimuli with no clear predetermined response appears to be particularly dependent on frontal systems (Mesulam, 1986; Goldberg et al., 1994), and may be more demanding than keeping a single target stimulus type in mind and responding whenever it appears. Consistent with this hypothesis, focal damage to the prefrontal cortex results in profound disruption of P3 and behavioral responses to novel stimuli, but largely preserved P3 and behavioral responses to target events (Knight, 1984; Daffner et al., 2003). Given the central role that changes in frontal systems play in many theories of cognitive aging (Raz et al., 1997; West & Schwarb, 2006), it is not surprising that the P3a to novel stimuli may be a particularly sensitive marker.
4.2 Alternative Formulations and Hypotheses
One concern about our formulation is that the differences in P3 amplitude to novel and target stimuli observed between cognitively high performing o-old and y-old subjects may be due to differences in task performance (i.e., viewing duration, target RT) rather than differences in age. Confounding age and performance may be particularly problematic when trying to assess between-group differences in neural activity (Rugg & Morcom, 2005). Our study addressed this issue in several ways. First, we tried to match subject groups in terms of overall neuropsychological functioning by only including cognitively high performers (top third relative to age matched peers). Such a strategy allows the matching of performance across groups to be more generalizable, and not limited to specific experimental conditions (Daselaar & Cabeza, 2005). In addition, we repeated the ANOVAs and correlation analyses after controlling for performance on the experimental task. Such analyses did not alter the findings of our study. Thus, it seems unlikely that the difference in electrophysiological activity across age groups was simply a reflection of differences in performance.
It is important to point out that compared to age-related differences in P3 amplitude, the magnitude of changes in behavioral measures (viewing duration and reaction time) was much smaller. Thus, an alternative explanation for the diminished P3 amplitude observed in o-old subjects is that it is a consequence of very successful cognitive aging that permits these individuals to carry out the task by utilizing fewer resources than y-old subjects. Beeri and colleagues (2009) speculated that lower neural activation of o-old relative to y-old subjects in their fMRI study of recognition memory may be a reflection of their extremely high neural reserve which allowed spared networks to perform more efficiently or be less susceptible to age-related disruption. These researchers point out that the oldest of the old may represent a genetically and pathophysiologically distinct group (Rebeck et al., 1994; Silverman et al., 2008) which sets them apart from their younger counterparts.
Although this is an intriguing hypothesis, there are several reasons why we do not currently favor it. First, our finding of an age-related reduction in P3 amplitude among o-old subjects was not limited to an extraordinary group of individuals who survived into their late 80s and beyond. Rather, we observed a linear decline in the P3 amplitude with increasing age between the late 60s and the mid 90s. It seems unlikely that with each advancing year, those individuals who survive exhibit increasingly more efficient or resilient neural networks. Second, as noted earlier, we have observed an analogous time course of resource allocation in response to novel stimuli among cognitively average performers, but with major shifts occurring earlier in the lifespan, such that the P3 amplitude in response to novelty peaked in middle age and declined in y-old age. It seems improbable that the age-associated decrease in P3 amplitude among cognitively average performers is due to y-old subjects having more efficient or hardy neural networks than their middle-aged counterparts. A more plausible unifying hypothesis about age-related changes in compensatory activity, applicable to both cognitively high and average performing adults, is that the compensatory mechanism of allocating increasing neural resources persists until capacity limits are exceeded (Schneider-Garces et al., 2009).
Our research design constrains the extent to which we can test competing hypotheses about reduced capacity vs. greater efficiency. Future studies using more challenging, parametrically designed tasks with conditions that vary degree of difficulty may be particularly informative. It would allow for a fuller account of age and performance-related differences that manifest at different levels of task demand (Schneider-Garces et al., 2009); (Daffner et al., 2010). The cross-sectional research design used in the current study also limits the inferences that can be made. Although logistically much less feasible, a longitudinal design would permit the measurement of within-subject changes in resource allocation over time. If our hypothesis is correct, we would predict within-subject declines in P3 amplitude among cognitively high performers between their late 60s and early 80s. The alternative hypothesis might lead to the prediction that when tested in their late 60s, cognitively high performing individuals who subsequently survive into their 80s would have generated a smaller P3 amplitude than their cognitively high performing peers who did not survive. Of note, a very implausible explanation would be that within-subject declines in P3 amplitude over the later years of life are the result of age-associated increases in neural efficiency.
4.3 Relationship between age-associated changes in late vs. early information processing stages
Our hypothesis that the most salient age-related differences between cognitively high performing y-old and o-old would be observed for P3 component and not earlier ERPs was confirmed. Although there was a robust difference between y-old and o-old subjects in the amplitude of P3 to novel and target events, a concomitant reduction in P1, N1, P2, and N2 components was not found. These results do not support the notion of relatively uniform age-associated decline across all levels of neurophysiological functioning (Lindenberger et al., 1994; Lindenberger et al., 1997). Rather, they favor a view postulating that with advanced aging, cognitive operations are disproportionately affected (e.g., controlled processing), presumably reflecting the relatively selective disruption of components of the nervous system subserving them. Our findings of the relatively preserved amplitude of ERP components earlier than the P3 also argue against the likelihood that the reduction in P3 amplitude observed in o-old subjects was due to alterations in cerebral structure (e.g., atrophy) that cause a markedly reduced ability to measure brain signal that actually is present.
Although in terms of the size and scalp distribution of ERP components earlier than the P3, y-old and o-old exhibited a similar pattern, both groups generated responses that differed from middle-aged and young subjects. Most pertinent to the current study, compared to their younger counterparts, both y-old and o-old subjects generated a very attenuated anterior N2 response to novel stimuli. We have found a similar pattern of age-related changes in N2 (reduction) and P3 (augmentation) in high performing y-old subjects carrying out n-back working memory tasks (Daffner et al., 2010). Impairment of this preliminary mismatch/match process (Folstein et al., 2008) may undermine the quality of information available for later cognitive operations and thus place a greater burden on the subsequent, controlled decision-making process (indexed by the P3). The idea that higher (controlled processing systems) can be recruited to compensate for the inefficiencies of lower systems is well-established (e.g., Broadbent (1984)) and recently has been emphasized in the cognitive aging literature (e.g., see Reuter-Lorenz and colleagues (2008)). Consistent with this formulation, y-old subjects may compensate for age-related impairments in earlier visual processing, which is manifested as an increase in P3 activity, especially anteriorly. O-old subjects have to contend with at least the same degree of age-associated decline in the preliminary mismatch/match process (indexed by the reduced anterior N2) as y-old subjects. In addition, o-old subjects exhibit a reduction in the size of the N1 to novel stimuli, which may reflect a deterioration of early sensory-perceptual encoding. Our data suggest that in contrast to y-old subjects, o-old subjects may no longer have the capacity to compensate for such decline by allocating additional resources at a later stage in the processing stream.
4.4 Future directions
Our data do not allow us to determine the pathophysiologic changes that underlie age-related differences in the P3 component. We were struck by the increase in cerebrovascular risk factors in the o-old subjects. However, we could not demonstrate a difference in P3 amplitude among our older subjects who had differing numbers of risk factors. Of course, this is a very crude index of actual cerebrovascular disease, as is the absence of a history of clinical strokes or focal abnormalities on neurological examination. The extent to which age-associated electrophysiological changes are due to small vessel ischemia and white matter disease remains to be determined by careful neuroimaging studies done in conjunction with ERPs.
None of our subjects met criteria for dementia or mild cognitive impairment, and all were functioning extremely well compared to age-matched peers. However, high functional status does not rule out the possibility of an underlying neurodegenerative process like AD, which can have a very long pre-clinical phase, and which is increasingly prevalent as individuals get older (Daffner & Scinto, 2000; Nelson et al., 2009; Stern, 2009). Such a process is very likely to reduce information processing capacity and compensatory activity, even in very high performing older adults. Future studies of such individuals that combine ERP measures with imaging or other biomarkers of AD (de Leon et al., 2007; Vemuri et al., 2009) would be particularly informative.
In summary, our results indicate that when carrying out controlled processing linked to directing attention to salient events, cognitively high performers reach the boundary of their capacity relatively late in life. This limits their ability to appropriate additional controlled processing resources as compensatory activity, and suggests that such a mechanism does not tend to “survive” into o-old age. Future studies are needed to confirm whether cognitively average performers follow an analogous time course, but with major milestones occurring much earlier in the lifespan. Additional research also is necessary to determine the extent to which similar patterns will be observed in longitudinal aging studies and for other cognitive operations, and to investigate the pathophysiological mechanisms that contribute to the observed age-associated changes in neurocognitive functioning.
Supplementary Material
Supplementary Fig. S1: Repetitive standard stimulus (70% frequency), target stimulus (15% frequency), and two examples of novel stimuli (15% frequency).
Supplementary Fig. S2: Montage illustrating the location of electrode sites, based on the International 10–20 system, which includes midline, 2 inner lateral, and 2 outer lateral columns, each with 7 antero-posterior sites.
Supplementary Fig. S3: Grand average ERP plots at all 35 electrode sites in response to a) novel, b) target, and c) standard stimuli at all 35 scalp electrode sites for young, middle-aged, y-old, and o-old age groups.
Supplementary Fig. S4: Comparing cognitively high vs. cognitively average performing subjects
S4a: Novelty P3 surface potential maps of cognitively high performing subjects (≥67th percentile on neuropsychological tests relative to age-matched peers) vs. cognitively average performing subjects (33rd–66th percentile on neuropsychological tests relative to age-matched peers), as reported by Riis and colleagues (2008).
S4b: Age vs. mean midline novelty P3 amplitude for cognitively average performing subjects (Riis et al., 2008) and cognitively high performing subjects. Cognitively average performers also exhibited a pattern of an inverted u-shaped curve, (age (18–81) vs. P3 amplitude fitting a quadratic function, R2 = 0.14, R = 0.37, p < 0.05), but which, in contrast to cognitively high performers, peaked in middle-age, not y-old age.
Acknowledgments
This research was funded in part by the National Institute on Aging [R01 AGO17935-07] and by generous support from D. Wimberly and S. Muss. The author would like to thank Dr. R. Sperling for her thoughtful comments, and Katie Gartner for her excellent administrative assistance.
Footnotes
Following a long tradition in experimental psychology and consistent with the formulations of many researchers investigating age-related cognitive changes, we have adopted the term “capacity” to suggest that brains have limited processing resources to carry out a range of mental operations or cognitive tasks. Limited-capacity processing includes updating working memory, directing and sustaining attention to salient or task relevant stimuli, and executing attentional control over task operations. Limitations of processing resources often have been inferred by behavioral measures such as changes in RT or accuracy as a function of task demand (Salthouse, 1988). Processing resources and neural activity can be more directly assessed via measures of cerebral blood flow or metabolic activity, or, as was done in the current study, by measuring ERPs such as P3 amplitude (Daffner et al., 2010). Compensatory neural activity reflects the utilization of processing resources to counter other age-related declines in neurophysiologic functioning (e.g., that may occur at earlier information processing stages). Closely linked to information processing capacity and compensatory activity is the concept of “cognitive reserve” (Stern, 2009), which includes the ability to utilize alternative cognitive strategies or neural networks in response to cerebral decline or injury.
There are several reasons why we have elected not to emphasize the labeling of the P3 response in terms of P3a and P3b components. First, one of the most commonly observed age-related changes in the P3 response to novel and target stimuli is an anterior shift in scalp distribution (Friedman et al., 1993; Fabiani & Friedman, 1995). There are several hypotheses regarding the source of this age-related change. We favor the view that this age-associated change reflects increased recruitment of anterior processors to carry out the task. Alternative explanations for the age-related anterior shift in P3 include a failure of older subjects to habituate an orienting response (indexed by the anterior P3a) to repeated presentations of novel or target events, or a reduced ability of older subjects to maintain a mental template of the target which, when presented, is more surprising or unexpected and thus also elicits an anteriorly-distributed P3a response. The current study was not designed to address the mechanisms that underlie the age-related shift in P3 scalp distribution. Second, we employed a subject-controlled novelty oddball task. We have shown in young adult subjects that decisions about how long to explore novel stimuli appear to elicit a larger P3b component than do novel stimuli in the traditional oddball paradigm, where they are understood to be task-irrelevant (Chong et al., 2008).
In examining the novelty P3 data for cognitively average performing subjects (33rd–66th percentile on neuropsyhological tests relative to age-matched peers), we found a similar pattern of an inverted u-shaped curve (see Supplementary Fig. S4), but which peaked in middle-age, not y-old age (age (18–81) vs. P3 amplitude to novel stimuli, R = 0.37, R2 = 0.14, p < 0.05).
Of note, the pattern of age-related changes in resource allocation, as indexed by the P3, was observed not only for novels and target hits, but also standards. (As with the other stimulus types, an ANOVA of the midline P3 response to standards demonstrated an effect of age (F(1,68) = 8.61, p < 0.0001) and an interaction between age and site (F(18,408) = 5.44, p < 0.0001), with y-old subjects generating the largest response and o-old subjects the most anteriorly distributed response (see Fig. 1 and Supplementary Fig. S3).) As we have suggested previously (Daffner et al., 2006a; Riis et al., 2008), these results indicate that the older groups may differ from the younger ones in what has been termed overall cognitive set or neural state in response to the demands of the task (Rugg et al., 2005). Perhaps, this reflects a uniform compensatory mechanism to cope with age-related physiological decline. A potential cost of responding to all stimulus types (both salient and repetitive) by appropriating additional neural activity is the more rapid depletion of resources in o-old individuals who are hypothesized to have reduced information processing capacity.
Disclosure Statement
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Association; 2007. [Accessed on August 31, 2009]. http://www.alz.org/national/documents/Report_2007FactsAndFigures.pdf Last updated 2007. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory: Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Beeri MS, Lee H, Cheng H, Wollman D, Silverman JM, Prohovnik I. Memory activation in healthy nonagenarians. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2009.02.022. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyne D. Conflict, Arousal and Curiosity. New York, NY: McGraw-Hill; 1960. [Google Scholar]
- Broadbent DE. Performance and its measurement. Br J Clin Pharmacol. 1984;18(Suppl 1):5S–11S. doi: 10.1111/j.1365-2125.1984.tb02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Chong H, Riis JL, McGinnis SM, Williams DM, Holcomb PJ, Daffner KR. To ignore or explore: top-down modulation of novelty processing. J Cogn Neurosci. 2008;20:120–134. doi: 10.1162/jocn.2008.20003. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D. The old switcheroo: when target environmental sounds elicit a novelty P3. Clinical Neurophysiology. 2004;115:1359–1367. doi: 10.1016/j.clinph.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Daffner K, Scinto LF. Early Diagnosis of Alzheimer’s Disease: An Introduction. In: Scinto LF, Daffner K, editors. Early Diagnosis of Alzheimer’s Disease. Totowa: Humana Press, Inc; 2000. pp. 1–28. [Google Scholar]
- Daffner KR. Promoting Successful Cognitive Aging: A Comprehensive Review. J Alzheimers Dis. 2010;19:1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Chong H, Riis J, Rentz DM, Wolk DA, Budson AE, et al. Cognitive status impacts age-related changes in attention to novel and target events in normal adults. Neuropsychology. 2007;21:291–300. doi: 10.1037/0894-4105.21.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Acar D, Calvo V, Faust R, et al. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000a;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Calvo V, Faust R, West WC, et al. The influence of stimulus deviance on electrophysiologic and behavioral responses to novel events. Journal of Cognitive Neuroscience. 2000b;12:393–406. doi: 10.1162/089892900562219. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Cohen LG, Kennedy BP, West WC, et al. Regulation of attention to novel stimuli by frontal lobes: an event-related potential study. NeuroReport. 1998;9:787–791. doi: 10.1097/00001756-199803300-00004. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Rentz DM, Scinto LFM, Faust R, Budson AE, Holcomb PJ. Pathophysiology underlying diminished attention to novel events in patients with early AD. Neurology. 2001;56:1377–1383. doi: 10.1212/wnl.56.10.1377. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, et al. Increased responsiveness to novelty is associated with successful cognitive aging. Journal of Cognitive Neuroscience. 2006a;18:1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, et al. Age-related differences in attention to novelty among cognitively high performing adults. Biological Psychology. 2006b;72:67–77. doi: 10.1016/j.biopsycho.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LFM, Weitzman AM, Faust R, Rentz DM, Budson AE, et al. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. Journal of Cognitive Neuroscience. 2003;15:294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- Daffner K, Chong H, Sun X, Tarbi E, Riis JL, McGinnis SM, et al. Mechanisms underlying age and performance related differences in working memory. J Cogn Neurosci. doi: 10.1162/jocn.2010.21540. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Dissertation Abstracts International 55–07B. 1994. Source localization and spatial discriminant analysis of event-related potentials: linear approaches (brain cortical surface) p. 2559. [Google Scholar]
- Daselaar SM, Cabeza R. Age-Related Changes in Hemispheric Organization. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. Oxford University Press; 2005. pp. 325–353. [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The Posterior Anterior Shift in Aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer’s disease. Annals of the New York Academy of Sciences. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Gomez-Ramirez M, Sehatpour P, Wylie GR, Foxe JJ. Preserved executive function in high-performing elderly is driven by large-scale recruitment of prefrontal cortical mechanisms. Hum Brain Mapp. 2009;30:4198–4214. doi: 10.1002/hbm.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis P, Katz R, Wylie GR, Sehatpour P, Alexopoulos GS, Foxe JJ. Enhanced and bilateralized visual sensory processing in the ventral stream may be a feature of normal aging. Neurobiology of Aging. 2007;10:1576–1586. doi: 10.1016/j.neurobiolaging.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. On the topography of P3a and P3b across the adult lifespan--a factor-analytic study using orthogonal procrustes rotation. Brain Topography. 2003;15:153–164. doi: 10.1023/a:1022654116566. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Life-span changes in P3a. Psychophysiology. 2004;41:575–583. doi: 10.1111/j.1469-8986.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30:383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Gaeta H, Friedman D, Hunt G. Stimulus characteristics and task category dissociate the anterior and posterior aspects of the novelty P3. Psychophysiology. 2003;40:198–208. doi: 10.1111/1469-8986.00022. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Podell K, Lovell M. Lateralization of Frontal Lobe Functions and Cognitive Novelty. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:371–378. doi: 10.1176/jnp.6.4.371. [DOI] [PubMed] [Google Scholar]
- Isreal JB, Wickens CD, Chesney GL, Donchin E. The event-related brain potential as an index of display-monitoring workload. Human Factors. 1980;22:211–224. doi: 10.1177/001872088002200210. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- Kemps EB, Newson RS. Comparison of adult age differences in verbal and visuo-spatial memory: The importance of pure parallel and validated measures. Journal of Clinical and Experimental Neuropsychology. 2006;3:341–356. doi: 10.1080/13803390490918228. [DOI] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalography and Clinical Neurophysiology. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychology and Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychol Aging. 1997;12:410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge: The MIT Press; 2005. [Google Scholar]
- Mesulam MM. Frontal cortex and behavior. Annals of Neurology. 1986;19:320–325. doi: 10.1002/ana.410190403. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating Scale (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newson RS, Kemps EB, Luszez MA. Cognitive Mechanisms Underlying Decrements in Mental Synthesis in Older Adults. Aging Neuropsychology and Cognition. 2003;10:28–43. [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment. Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford, UK: Oxford Psychologists Press; 1995. [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s Progressive Matrices and Vocabulary Scales. London: H. K. Lewis & Co. LTD; 1983. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Perls TT, West HL, Sodhi P, Lipsitz LA, Hyman BT. Reduced apolipoprotein epsilon 4 allele frequency in the oldest old Alzheimer’s patients and cognitively normal individuals. Neurology. 1994;44:1513–1516. doi: 10.1212/wnl.44.8.1513. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, McGinnnis S, Tarbi E, Sun X, Holcomb PJ, et al. Age-related changes in early novelty processing as measured by ERPs. Biological Psychology. 2009;82:33–44. doi: 10.1016/j.biopsycho.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, et al. Compensatory neural activity distinguishes different patterns of normal cognitive aging. NeuroImage. 2008;39:441–454. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O’Hara R, Race EA, Desmond JE, Glover GH, et al. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Morcom AM. The Relationship Between Brain Activity, Cognitive Performance, and Aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging:Linking Cognitive and Cerebral Aging. Oxford University Press; 2005. pp. 132–154. [Google Scholar]
- Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist. 1992;6:53–62. [Google Scholar]
- Salthouse TA. Resource-Reduction Interpretations of Cognitive Aging. Developmental Review. 1988:238–272. [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, et al. Span, CRUNCH, and Beyond: Working Memory Capacity and the Aging Brain. J Cogn Neurosci. 2009:655–669. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JM, Schnaider-Beeri M, Grossman HT, Schmeidler J, Wang JY, Lally RC. A phenotype for genetic studies of successful cognitive aging. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:167–173. doi: 10.1002/ajmg.b.30483. [DOI] [PubMed] [Google Scholar]
- Singer T, Lindenberger U, Baltes PB. Plasticity of memory for new learning in very old age: a story of major loss? Psychol Aging. 2003a;18:306–317. doi: 10.1037/0882-7974.18.2.306. [DOI] [PubMed] [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE) Psychology and Aging. 2003b;18:318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Sirevaag EJ, Kramer AF, Coles MG, Donchin E. Resource reciprocity: an event-related brain potentials analysis. Acta Psychologica. 1989;70:77–97. doi: 10.1016/0001-6918(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbi E, Sun X, Holcomb PJ, Daffner KR. Surprise? Early visual processing of novelty is not modulated by attention. Psychophysiology. doi: 10.1111/j.1469-8986.2010.01129.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Hubley AM. The 60-item Boston Naming test: norms for cognitively intact adults aged 25 to 88 years. Journal of Clinical and Experimental Neuropsychology. 1997;19:922–932. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Sujuan G, Lane KA, Callahan C, Ogunniyi A, Baiyewu O, et al. Mild cognitive dysfunction: an epidemiological perspective with an emphasis on African Americans. J Geriatr Psychiatry Neurol. 2007;20:215–226. doi: 10.1177/0891988707308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39:791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Kruggel F, Rugg MD. Effects of advanced aging on the neural correlates of successful recognition memory. Neuropsychologia. 2009;47:1352–1361. doi: 10.1016/j.neuropsychologia.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Administration and Scoring Manual. 3. San Antonio, TX: The Psychological Corporation; 1997a. Wechsler Adult Intelligence Scale. WAIS-III. [Google Scholar]
- Wechsler D. Administration and Scoring Manual. 3. San Antonio, TX: The Psychological Corporation; 1997b. Wechsler Memory Scale. WMS-III. [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Wickens C, Kramer A, Vanasse L, Donchin E. Performance of Concurrent Tasks: A Psychophysiological Analysis of the Reciprocity of Information-Processing Resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Rose TL, Lapp D. Validity of the Geriatric Depression Scale in Subjects with Senile Dementia. Palo Alto, CA: Veterans Administration Medical Clinic; 1981. [Google Scholar]
- Youngjohn JR, Larrabee GJ, Crook TH. New adult- and education-correction norms for the Benton Visual Retention Test. The Clinical Neuropsychologist. 1993;7:155–160. doi: 10.1080/13854049308401517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1: Repetitive standard stimulus (70% frequency), target stimulus (15% frequency), and two examples of novel stimuli (15% frequency).
Supplementary Fig. S2: Montage illustrating the location of electrode sites, based on the International 10–20 system, which includes midline, 2 inner lateral, and 2 outer lateral columns, each with 7 antero-posterior sites.
Supplementary Fig. S3: Grand average ERP plots at all 35 electrode sites in response to a) novel, b) target, and c) standard stimuli at all 35 scalp electrode sites for young, middle-aged, y-old, and o-old age groups.
Supplementary Fig. S4: Comparing cognitively high vs. cognitively average performing subjects
S4a: Novelty P3 surface potential maps of cognitively high performing subjects (≥67th percentile on neuropsychological tests relative to age-matched peers) vs. cognitively average performing subjects (33rd–66th percentile on neuropsychological tests relative to age-matched peers), as reported by Riis and colleagues (2008).
S4b: Age vs. mean midline novelty P3 amplitude for cognitively average performing subjects (Riis et al., 2008) and cognitively high performing subjects. Cognitively average performers also exhibited a pattern of an inverted u-shaped curve, (age (18–81) vs. P3 amplitude fitting a quadratic function, R2 = 0.14, R = 0.37, p < 0.05), but which, in contrast to cognitively high performers, peaked in middle-age, not y-old age.