Abstract
Motivation and executive function are both necessary for the completion of goal-directed behavior. Research investigating the manner in which these processes interact is beginning to emerge and has implicated middle frontal gyrus (MFG) as a site of interaction for relevant neural mechanisms. However, this research has focused on state motivation, and it has not examined functional lateralization. The present study examined the impact of trait levels of approach and avoidance motivation on neural processes associated with executive function. Functional magnetic resonance imaging was conducted while participants performed a color-word Stroop task. Analyses identified brain regions in which trait approach and avoidance motivation (measured by questionnaires) moderated activation associated with executive control. Approach was hypothesized to be associated with left-lateralized MFG activation, whereas avoidance was hypothesized to be associated with right-lateralized MFG activation. Results supported both hypotheses. Present findings implicate areas of middle frontal gyrus in top-down control to guide behavior in accordance with motivational goals.
Keywords: Approach, Avoidance, Motivation, Executive Function, Laterality, fMRI
Motivation and executive function are both considered necessary for the completion of goal-directed activities (Pochon et al., 2002), though the manner in which they combine to guide behavior remains a matter of debate (Pessoa, 2009). Typically, theories of brain function relate orbitofrontal cortex (OFC) to motivation, especially with the representation of the value of rewards and punishments (O'Doherty, 2004). Yet motivation is important for the maintenance of goal-directed behavior over time (Dickinson & Balleine, 1994), which suggests the involvement of dorsolateral prefrontal cortex (DLPFC, part of the middle frontal gyrus), a brain region more commonly associated with executive function and working memory. The manner in which regions typically associated with executive function might be influenced by motivational factors remains unclear. One aim of the present study was to identify interactive effects of motivation and executive function on goal-directed behavior.
Neural Correlates of Motivation and Executive Function
Motivation has been conceptualized as selecting goals based on their predicted value (e.g., reward or punishment), initiating behavior to achieve goals, and maintaining goal-directed action (Campbell & Pritchard, 1976; Jones, 1955; Lindsley, 1957). One of the most basic distinctions made in the motivation literature is in regard to approach and avoidance, which research suggests are mediated by two separable systems (Elliot & Thrash, 2002; Lang, Bradley, & Cuthbert, 1998). Specifically, the approach motivational system mediates responses to potential positive outcomes (rewards), whereas the avoidance motivational system mediates responses to potential negative outcomes (punishments). Executive function, in contrast, comprises processes by which goal-directed action is carried out, such as maintaining salient information in working memory and inhibiting non-goal-related responses (Miyake et al., 2000). Research on the interactive contributions of motivation and executive function is beginning to emerge, including studies that investigate the effects of state motivation on neural processes associated with tasks involving executive function (e.g., Pochon et al., 2002; Taylor et al., 2004). This research has consistently implicated areas of prefrontal cortex (PFC) in the integration of motivation and executive function processes (Gilbert & Fiez, 2004; Krawczyk, Gazzaley, & D'Esposito, 2007; Locke & Braver, 2008; Rowe, Eckstein, Braver, & Owen, 2008; Szatkowska, Bogorodzki, Wolak, Marchewka, & Szeszkowski, 2008). For example, Pochon et al. (2002) examined the relation between reward processing, a facet of motivation, and performance on a working memory task. Results revealed that two areas of PFC, specifically left middle frontal gyrus (MFG) and inferior frontal gyrus (IFG), were activated by both working memory demands and increasing levels of reward. Taylor et al. (2004) conducted a similar study that examined the interaction between state motivation and working memory by manipulating motivation in terms of both reward and punishment. Consistent with the findings of Pochon et al., motivational processes interacted with working memory load, evidenced by changes in right MFG and IFG and left MFG, such that activation increased with both working memory load and reward magnitude. Hence, at least two studies provide some preliminary evidence that MFG and IFG are involved in the neural integration of executive function and motivational processes, when the degree of motivational state (e.g., potential for high reward) is manipulated.
Also relevant to understanding the neural mechanisms involved in integrating motivational and executive processes is the long line of research that suggests that PFC is lateralized with respect to emotional valence/motivational direction, with right PFC associated with unpleasant emotion and avoidance motivation and left PFC associated with pleasant emotion and approach motivation (for reviews see Heller, 1993, Davidson & Irwin, 1999, and Tomarken & Keener, 1998). Evidence for this distinction is provided by a variety of research findings using a number of different methodologies including neuropsychological testing (e.g., Flor-Henry, 1976), lesion patients (e.g., Gainotti, 1972), and electroencephalography (EEG, e.g., Davidson, Ekman, Saron, Senulis, & Friesen, 1990). This pattern of motivation-related lateralization in PFC is consistent with specialized functions of the two hemispheres. For example, the right hemisphere has been associated with spatial attention and other processes important for monitoring the environment for potential threat (for review see Nitschke, Heller, & Miller, 2000), a key aspect of avoidance motivation (Elliot & Thrash, 2002). Since neither Pochon et al. (2002) nor Taylor et al. (2004) directly tested lateralization of findings, which we have argued is essential for examining lateralized influences on motivational processes (e.g., Herrington et al., 2005, 2010), those studies cannot provide insight into this issue.
Complicating the issue, research on PFC asymmetry has often confounded emotional valence and motivational direction by using manipulations that are pleasantly valenced and approach-related (or unpleasantly valenced and avoidance-related). Harmon-Jones and colleagues (for review see Harmon-Jones, Gable, & Peterson, 2010) have attempted to disentangle emotional valence and motivational direction using EEG. Their work suggests that lateralization of activity is driven by motivational direction, rather than emotional valence. However, this work has not identified specific regions of PFC involved in approach and avoidance motivation (Tomarken & Zald, 2009; Towers et al., 2008), as traditional low-density EEG provides only coarse spatial localization. Given that this coarse localization has the potential to average out important heterogeneity in PFC activity, it remains possible that both emotional valence and motivational direction are associated with asymmetry in PFC. Therefore, one goal of the present study is to provide better spatial localization within PFC of areas instantiating motivation-related processes by using functional magnetic resonance imaging (fMRI), a method that provides better spatial resolution than traditional low-density EEG for locating specific areas of activity.
Although PFC asymmetries for motivational processes have regularly been observed in EEG and other methodologies, they have been elusive in studies employing fMRI. Herrington et al. (2005) were the first using fMRI to demonstrate leftward lateralization of activity in DLPFC in the context of pleasant emotional information. As discussed in Herrington et al. (2010), one reason that lateralization findings are uncommon in fMRI may be that asymmetries of activity are rarely tested directly in the fMRI literature. Relevant to the present study, a recent fMRI study (Berkman & Lieberman, 2010) directly tested lateralization with regard to motivational processes and found leftward lateralization of activation for approach behavior and rightward lateralization for avoidance behavior in superior, lateral PFC. Additionally, leftward lateralization was found to correlate with a measure of the Behavioral Activation System, which has been associated with approach motivation (Elliot & Thrash, 2002; Spielberg et al., 2010). Although appropriate tests for lateralization were conducted in this study, activation was averaged across fairly large areas of the brain (all of Superior Frontal Gyrus, MFG, and IFG, at and above MNI z = 2), which did not allow for more precise localization. Thus, in addition to investigating the interactive effects of motivation and executive function on goal-directed behavior, the present study directly examined hemispheric lateralization in relation to these processes in a voxel-wise manner using an approach similar to Herrington et al. (2005; 2010).
Present Study
As described above, previous research has typically studied state motivation that is generated by experimental contingencies. The present study extends this research by examining the impact of trait, rather than state, motivation on neural processes associated with executive function. Trait motivation has been conceptualized as a longstanding dispositional tendency to be sensitive to the presence or absence of potential positive or negative outcomes and to energize behavior to approach positive outcomes or avoid negative outcomes (Elliot, 2006). Such motivational tendencies have been hypothesized to influence problem-solving, coping styles, and personality (e.g., Elliot & McGregor, 2001; Elliot & Thrash, 2002; Gable, Reis, & Elliot, 2003). Understanding the neural instantiation of such tendencies and how they interact with executive function can therefore provide insights into individual differences in goal-directed behavior.
The present study tested the hypothesis that activity in regions of prefrontal cortex typically associated with maintaining goal-directed behavior is modulated by individual differences in trait motivation. To test this hypothesis, brain areas implicated in integrating neural processes associated with motivation and executive function were examined with fMRI. As reviewed above, both Pochon et al. (2002) and Taylor et al. (2004) examined motivational effects on working memory. The present study sought to extend that work by examining motivational influences on executive function through the use of a color-word Stroop (1935) task. This task is well established in the fMRI literature (for review see Banich, 2009) and was utilized because it recruits executive control on a number of levels (Liu, Banich, Jacobson, & Tanabe, 2006): (a) directing attention to a less automatic process (i.e., color identification over word reading), (b) selecting between distinct semantic concepts (e.g., choosing the concept of red over the concept of blue), and (c) selecting between different behavioral responses (e.g., choosing the response linked to red over the response linked to blue). Based on the findings of Pochon et al. and Taylor et al., trait motivation was hypothesized to moderate brain activation in MFG and IFG. Results were also expected to vary as a function of brain lateralization: approach temperament was hypothesized to moderate activation in left MFG and IFG, and avoidance temperament was hypothesized to moderate activation in right MFG and IFG.
Method
Participants
Participants were recruited from a larger pool of undergraduates, who completed a series of questionnaires as partial fulfillment of enrollment in an introductory psychology course. The questionnaires included the Penn State Worry Questionnaire (PSWQ, Meyer, Miller, Metzger, & Borkovec, 1990; Molina & Borkovec, 1994) as a measure of anxious apprehension, and portions of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson, Clark, et al., 1995; Watson, Weber, et al., 1995), which contains measures of anxious arousal and anhedonic depression. Participants were contacted if they scored above the 80th percentile on one of the three psychopathology dimensions and below the 50th percentile on the other two dimensions (creating three “pure” groups), if they scored above the 80th percentile on all three psychopathology dimensions (creating a “comorbid” group), or below the 50th percentile on all three psychopathology dimensions (creating a “control” group). Group membership was ignored in data analyses for the present study except when testing whether group membership was a confounding effect1. Participants were then screened for claustrophobia, left-handedness, history of serious brain injury, abnormal hearing or vision, metal in their body, pregnancy, or non-native English.
A total of 107 participants completed the laboratory protocol. Participants were not used if (a) they moved more than 3.3 mm relative to the volume used for registration (the middle volume of the time series) or more than 2 mm relative to the previous volume (one participant exceeded this criterion only during the last block of words; therefore, this block was not used), (b) if they committed errors on 15% or more of the trials, (c) if they exhibited reaction times greater than 3 standard deviations from the mean, or (d) if their scans exhibited apparent signal loss due to magnetic susceptibility in areas of interest. This left 87 participants (37 female, mean age = 19.07). One participant's scans exhibited scanner artifact throughout the time series. Independent components analysis, as implemented in MELODIC (Beckmann & Smith, 2004), was used to isolate and remove this artifact. After removal, no artifact was apparent.
Questionnaires
To measure approach and avoidance temperament, three questionnaires were administered that have been previously associated with these constructs (Elliot & Thrash, 2002; Spielberg et al., under review): the Behavioral Activation and Behavioral Inhibition Scales (Carver & White, 1994), the Extraversion and Neuroticism sub-scales of the NEO-Five Factor Inventory (Costa & McRae, 1992), and the Positive and Negative Temperament sub-scales of the General Temperament Survey (Watson & Clark, 1993). These scales were used as indicators in confirmatory factor analysis using AMOS. Based on previous research (Elliot & Thrash, 2002; Spielberg et al., 2010), two latent factors were modeled, with Behavioral Activation, Extraversion, and Positive Temperament used as indicators for approach temperament and Behavioral Inhibition, Neuroticism, and Negative Temperament used as indicators for avoidance temperament. Maximum likelihood estimation was used, and the two latent factors were allowed to co-vary freely. Factor scores were extracted with the regression method to use as measures of approach and avoidance temperament.
Stimuli and Experimental Design
Participants completed two tasks, a color-word Stroop and an emotion-word Stroop (duration of each task = 12 min 20 sec) in fMRI and EEG sessions (findings from the emotion-word Stroop and EEG sessions are not presented here). The order of presentation of the two tasks and the two sessions was counterbalanced across participants. In the color-word Stroop task, blocks of color-congruent or color-incongruent words alternated with blocks of neutral words. Additional neutral trials were intermixed 50:50 in congruent and incongruent blocks to prevent the development of word-reading strategies. This type of blocked-design color-word Stroop task has been shown to effectively elicit Stroop interference (Banich et al., 2000b; Milham & Banich, 2005; Milham, Banich, Claus, & Cohen, 2003). The order of presentation of blocks in the present investigation was counterbalanced for each participant. In addition to the word blocks, there were four fixation blocks (one at the beginning, one at the end, and two in the middle of the session) and five rest blocks (one at the beginning, one at the end, and one between each word block). In the fixation condition a fixation cross intensified in place of word presentation, and in the rest condition the subject was instructed to rest and keep their eyes open while the screen was blank.
The task consisted of 256 trials presented in 16 blocks (four congruent, four incongruent, and eight neutral) of 16 trials each, with a variable ITI (2000 +/- 225 ms) between trial onsets. A trial began with presentation of a word for 1500 ms, followed by a fixation cross for an average of 500 ms. Each trial consisted of one word presented in one of four ink colors (red, yellow, green, blue), each color occurring equally often with each word type. The task consisted of congruent trials in which the word named the ink color in which it was printed (e.g., the word “RED” printed in red ink), incongruent trials in which the word named a color incongruent with the ink color in which it was printed (e.g., “GREEN” in red ink), and neutral trials in which the word was unrelated to color (e.g., “LOT” in red ink). Neutral words were matched with color words for word frequency and length. Each word (visual angle 6 – 16 degrees) was centered on a black background and projected. Participants responded to the color of the ink with their index and middle fingers using a four-button response box (James Long Company) under each hand.
fMRI Data Collection
The fMRI data were 370 three-dimensional (3D) images acquired using a Siemens gradient-echo echo-planar imaging sequence (TR 2000 ms, TE 25 ms, flip angle 80°, FOV = 220 cm) on a Siemens Allegra 3T scanner. Each image consisted of 38 oblique axial slices (slice thickness 3 mm, 0.3mm gap, in-plane resolution 3.4375 × 3.4375 mm) acquired parallel to the anterior and posterior commissures. After the fMRI acquisition, a 160-slice MPRAGE structural sequence was acquired (spatial resolution 1 mm × 1 mm × 1 mm) and used to warp the participant's functional data into standard space.
fMRI Data Reduction and Preprocessing
Image processing and statistical analysis was implemented primarily using FEAT v5.98 (FMRI Expert Analysis Tool, FMRIB's Software Library, http://www.fmrib.ox.ac.uk/analysis/research/feat/), part of the FSL analysis package (http://www.fmrib.ox.ac.uk/fsl). The first three time points (fMRI volumes) of the data set corresponding to each task for each subject were discarded to allow the MR signal to reach a steady state. Functional data for each participant were motion-corrected using FMRIB's linear registration tool, MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2003), intensity-normalized, temporally filtered with a nonlinear high-pass filter, and spatially smoothed using a 3D Gaussian kernel (FWHM = 5 mm). Temporal low-pass filtering was carried out using AFNI's 3dDespike tool (http://afni.nimh.nih.gov/).
fMRI Data Processing
Regression analyses were performed on the processed functional time series of each participant using FILM, FMRIB's Improved Linear Model with autocorrelation correction (Woolrich, Ripley, Brady, & Smith, 2001). Four explanatory variables, one for each word type block (congruent, incongruent, neutral) and one modeling the rest condition, were included in the regression model (fixation was left unmodeled). These explanatory variables modeled all trials in the blocks, irrespective of whether they were correct or incorrect (i.e., trials were still modeled if they were incorrect). For each explanatory variable, the vector of assigned weights corresponding to word type was convolved with a gamma function to better approximate the temporal course of the blood-oxygen-dependent (BOLD) hemodynamic response function. Each explanatory variable yielded a per-voxel effect-size parameter estimate (β) map representing the magnitude of activation associated with that explanatory variable. In order to create comparisons of interest, β values for the relevant parameters were contrasted. The comparison of interest was the incongruent vs. congruent block contrast (IvC). For each subject, these functional activation maps, as well as the corresponding structural MRI map, were warped into a common stereotaxic space (the MNI152 template, standard with FSL v4.1) using FMRIB's Non-Linear Image Registration Tool, FNIRT (Andersson, Jenkinson, & Smith, 2007).
Group inferential statistical analyses were carried out using FLAME (FMRIB's Local Analysis of Mixed Effects). Brain activation captured in the IvC contrast was entered as a dependent variable into multiple regression analysis with approach and avoidance temperament scores as predictor variables. This regression analysis produced two beta maps, one corresponding to the unique variance associated with approach temperament (with the shared variance associated with avoidance removed) and one corresponding to the unique variance associated with avoidance temperament (with the shared variance associated with avoidance removed). T-tests were conducted on the βs for approach and avoidance temperament and then converted to z-scores to determine the significance of the βs. A frontal gray-matter mask was used to limit the number of voxels under consideration. A second mask was used for lateralization analyses that contained only left hemisphere frontal gray matter.
Monte Carlo simulations via AFNI's AlphaSim program were used to estimate the overall significance level (probability of a false detection) for thresholding the 3D functional z-map image (Ward, 2000). These simulations were conducted for several individual voxel z-threshold values, providing the appropriate cluster size giving an overall two-tailed family-wise error rate of 0.05. A threshold z-value of 2.3263 and minimum cluster size of 83 voxels was used. For lateralization analyses, a threshold z-value of 2.3263 and a minimum cluster size of 49 voxels was used (this cluster size threshold is lower because only the left hemisphere was examined for lateralization analyses).
The within-subjects findings for the present study are in line with previous research (e.g., Liu et al., 2006). Specifically, activation was observed in several areas, including bilateral MFG, IFG, precuneus, parietal lobule, and anterior cingulate cortex. Therefore, findings for the main effect of IvC will not be discussed in detail. However, for regions associated with approach or avoidance temperament, the amount of overlap with areas in which there is a positive main effect for IvC (greater activity during incongruent than during congruent trials) will be listed.
Lateralization was tested using a locally written Matlab program. This program conducted a repeated-measures homogeneity of slopes ANCOVA, with hemisphere as the repeated measure, approach and avoidance temperament scores as continuous predictors, and fMRI activation for the IvC contrast as the dependent variable. This ANCOVA was conducted on a per-voxel basis, and the resultant β map was thresholded in the manner described above.
In order to foster comparability of areas between hemispheres, the functional and structural images for each participant were flipped along the x axis in native space (rather than MNI standard space) and registered to the MNI template. In lateralization analyses, the left-hemisphere functional data for the un-flipped images was compared to the right-hemisphere functional data for the flipped images2.
Reaction Time Analyses
Mean reaction time (RT) was calculated for the congruent and incongruent trials for each participant, and an interference score was calculated by subtracting the congruent RT from the incongruent RT and dividing this difference by the sum of the two RTs (to account for overall mean differences in RT). RT interference was entered as a dependent variable in a regression analysis with approach and avoidance temperament entered simultaneously as predictors.
To assess the potential effect of neural activity related to motivational temperaments on behavioral performance, a score for each ROI identified in the earlier analysis in which approach temperament and avoidance temperament predicted fMRI activation (i.e., not the lateralization analysis in which hemisphere was a factor) was created by averaging β values across voxels in each ROI, for each participant. ROI scores were then correlated with RT interference and error interference (created by subtracting errors for congruent trials from errors for incongruent trials). Spearman rank-order correlations were used for error analyses.
Results
Confirmatory Factor Analysis
The two-factor model of scales contributing to approach and avoidance scores was successfully estimated and was associated with a χ2 value of 13.46, with 8 degrees of freedom. The comparative fit index value (Bentler, 1990) was 0.985, and the root mean square error of approximation value (Brown & Cudeck, 1993) was 0.089, indicating that the model provided a good fit to the data. All measurement weights were significant at p < 0.001, and the standardized estimates are provided in Table 1.
Table 1. Self-Report Indicators for Approach and Avoidance Temperament.
| Indicator Variable | Standardized Coefficient Value |
|---|---|
| Approach Temperament | |
| BAS | 0.705 |
| NEO-FFI Extraversion | 0.970 |
| GTS Positive Temperament | 0.773 |
| Avoidance Temperament | |
| BIS | 0.777 |
| NEO-FFI Neuroticism | 0.983 |
| GTS Negative Temperament | 0.886 |
Note. BAS = Behavioral Activation Scale (Carver & White, 1994). NEO-FFI = NEO-Five Factor Inventory (Costa & McRae, 1992). GTS = General Temperament Survey (Watson & Clark, 1993). BIS = Behavioral Inhibition Scale (Carver & White, 1994).
Behavioral Analysis
In order to confirm the presence of a Stroop interference effect, a paired t-test comparing RT for incongruent trials (mean = 820ms, SD = 149ms) to RT for congruent trials (mean = 631ms, SD = 92ms) was conducted. Incongruent trials had longer RTs than congruent trials (t(86) = 16.2, p < 0.001). Additionally, a paired t-test comparing number of errors for incongruent trials (mean = 2.9, SD = 2.7) to number of errors for congruent trials (mean = 0.7, SD = 1.0) was conducted. Incongruent trials produced more errors than did congruent trials (t(86) = 7.9, p < 0.001). To examine effects of motivation on behavioral interference, a regression was conducted with RT interference score as the dependent variable and approach and avoidance temperament scores entered simultaneously as predictors. In combination, they controlled 7% of the variance in RT interference (p = .05). Approach and avoidance each contributed unique variance to Stroop interference, ΔR2 = .06, p = 0.02, and ΔR2 = .05, p = 0.03, respectively, indicating that trait motivation was positively related to the ability to inhibit dominant responses on the Stroop3.
Moderation of Neural Activation by Approach and Avoidance Temperament
Table 2 lists brain regions where activation related to IvC was moderated by trait approach or avoidance temperament. In line with present hypotheses, activation in two regions in left superior PFC increased as approach temperament increased, as illustrated in Figure 1a and 1b and Figure 2a. The first region was located in Superior Frontal Gyrus (SFG, BA 8) and MFG (BA 9), in what is considered anterior DLPFC. The second region was also located in MFG (BA 9) but more posterior to the first region, in mid DLPFC. Importantly, these findings are consistent with the hypothesis that neural activity in the left hemisphere is associated with approach motivation. Unexpectedly, an area in medial-posterior Orbitofrontal Cortex (OFC, BA 11) exhibited the opposite relationship with approach temperament, with activation decreasing as approach temperament increased. This area is pictured in Figure 1c.
Table 2. Brain Areas Moderated by Approach and Avoidance Temperament.
| Location | |||||||
|---|---|---|---|---|---|---|---|
| Region | Cluster Size | Direction of Relationship | Mean z-value | X | Y | Z | % Overlap |
| Approach Temperament | |||||||
| L MFG/SFG (BA 8/9) | 97 | Positive | 2.61 | -18 | 46 | 36 | 36.08 |
| L MFG (9) | 92 | Positive | 2.64 | -36 | 30 | 46 | 35.87 |
| L OFC (BA 11) | 107 | Negative | -2.91 | -18 | 10 | -26 | 7.48 |
| Avoidance Temperament | |||||||
| R MFG (BA 9/8/6) | 100 | Positive | 2.65 | 36 | 12 | 44 | 12.00 |
| L MFG/SFG (BA 8/9) | 99 | Positive | 2.61 | -8 | 42 | 36 | 53.54 |
Note. L = left. R = right. SFG = Superior Frontal Gyrus. MFG = Middle Frontal Gyrus. OFC = Orbitofrontal Cortex. BA = Brodmann's Area. Location = coordinates are for the maximum z-value and are for MNI152 space, with the x axis moving from left to right. % Overlap = the percentage of overlap between that cluster and areas exhibiting a positive main effect of IvC.
Figure 1.
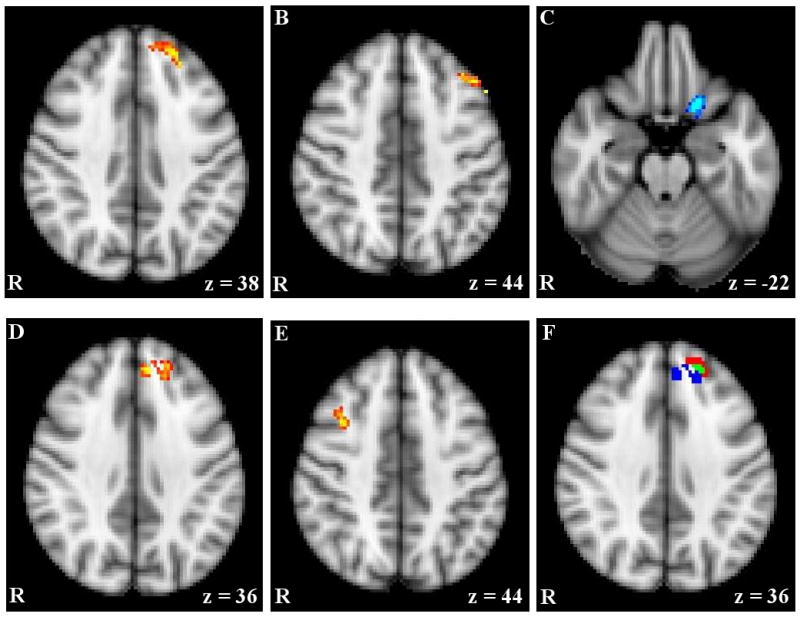
fMRI activation moderated by approach and avoidance temperament. Notes. A & B) Activation for Incongruent – Congruent contrast (IvC) correlating positively with approach temperament. C) Activation for IvC correlating negatively with approach temperament. D & E) Activation for IvC correlating positively with avoidance temperament. F) Overlap between activation correlating with approach and avoidance temperament; red = activation associated with approach; blue = activation associated with avoidance; green = overlap in activation. R = right. z = MNI 152 z coordinate.
Figure 2.

3-D rendering of fMRI activation moderated by approach and avoidance temperament (MRIcron, www.cabiatl.com/mricro). Notes. A) Activation for Incongruent – Congruent contrast (IvC) correlating positively with approach temperament or positively with avoidance temperament; green = activation associated with approach; red = activation associated with avoidance; yellow = overlap in activation. B) Activation for IvC correlating positively with avoidance temperament.
Results for avoidance motivation were also consistent with hypotheses regarding brain activation and lateralization. Specifically, activation in a right MFG area (BA 9/8/6), located in posterior DLPFC, increased as avoidance temperament increased, suggesting that the right hemisphere is particularly relevant for avoidance motivation. Unexpectedly, activation in an area in left SFG and MFG (BA 8/9) also increased as avoidance temperament increased. This area had an overlap of 18 voxels with the left MFG/SFG area associated with approach temperament described (i.e., 18% of the ROI associated with avoidance, 19% of the ROI associated with approach) and, thus, may represent a brain region that tracks trait motivation more generally. The areas associated with avoidance motivation are presented in Figure 1d and 1e and Figure 2a and 2b, and the overlap in approach and avoidance areas is presented in Figure 1f and Figure 2a.
Correlations between Brain Activation and Behavior
In order to explore the potential influence of the brain areas associated with motivation on successful behavioral performance, an average β for each cluster, for each participant, was calculated and correlated with RT and error interference. These correlations are presented in Table 3. The left medial-posterior OFC cluster (associated with approach temperament) was positively correlated with RT interference, suggesting that activation in this area reflects processing that distracts from the current goal. Similarly, the more posterior left MFG cluster (associated with approach temperament) exhibited a positive correlation with error interference. This area may be associated with some aspect of approach-related processing (e.g., desire to perform the task quickly), which led to an increase in errors. No reliable associations emerged between avoidance-related regions and behavioral indices.
Table 3. Correlations between Regions of Interest and Behavior.
| Region | RT | p-value | Errors | p-value |
|---|---|---|---|---|
| Approach Temperament | ||||
| L MFG/SFG (BA 8/9) | 0.026 | 0.814 | 0.081 | 0.455 |
| L MFG (9) | -0.055 | 0.614 | 0.228 | 0.034 |
| L OFC (BA 11) | 0.236 | 0.028 | -0.042 | 0.701 |
| Avoidance Temperament | ||||
| R MFG (BA 9/8/6) | 0.100 | 0.357 | 0.172 | 0.112 |
| L MFG/SFG (BA 8/9) | 0.089 | 0.411 | 0.059 | 0.588 |
Note. L = left. R = right. SFG = Superior Frontal Gyrus. MFG = Middle Frontal Gyrus. OFC = Orbitofrontal Cortex. IFG = Inferior Frontal Gyrus. BA = Brodmann's Area. RT = incongruent vs. congruent reaction time interference. Errors = incongruent vs. congruent error interference. Correlations for RT are Pearson product-moment correlations. Correlations for Errors are Spearman rank-order correlations.
Lateralization Analyses
Given work indicating that motivation influences laterality of activation in frontal regions, analyses were carried out to examine whether the activity in regions associated with each temperament type (approach, avoidance) were asymmetric across the hemispheres. Five clusters emerged, listed in Table 4, in which moderation of brain activation by approach temperament was lateralized. Three of these clusters overlapped with the three clusters identified as being associated with approach temperament (see Table 2), indicating that these effects are lateralized. Two additional clusters emerged, one located in SFG (BA 6) and one in medial OFC (BA 11). In order to explore the nature of the relationship between these areas and approach temperament, the average β for each cluster, for each hemisphere, for each participant, was calculated and correlated with approach temperament scores (holding avoidance temperament constant). These partial correlations are presented in Table 4.
Table 4. Brain Areas in Which Effects Are Lateralized.
| Location | Left Hem | Right Hem | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Cluster Size | Mean z-value | X | Y | Z | Partial r | P | Partial r | P |
| Approach Temperament | |||||||||
| SFG (BA 8/9)* | 61 | 2.64 | -8 | 54 | 38 | 0.189 | 0.081 | -0.206 | 0.057 |
| MFG/IFG (9/45)* | 299 | 2.65 | -48 | 36 | 30 | 0.346 | 0.001 | -0.230 | 0.033 |
| OFC (BA 11)* | 70 | 2.71 | -20 | 10 | -26 | -0.252 | 0.019 | 0.281 | 0.009 |
| SFG (BA 6) | 116 | 2.87 | -44 | 2 | 60 | 0.241 | 0.025 | -0.186 | 0.086 |
| OFC (BA 11) | 56 | 2.63 | -2 | 32 | -24 | 0.204 | 0.060 | -0.125 | 0.253 |
| Avoidance Temperament | |||||||||
| MFG (BA 9/8/6)* | 104 | 2.80 | -36 | 8 | 50 | -0.148 | 0.174 | 0.311 | 0.004 |
Note. * These clusters overlapped with clusters listed in Table 2. SFG = Superior Frontal Gyrus. MFG = Middle Frontal Gyrus. IFG = Inferior Frontal Gyrus. OFC = Orbitofrontal Cortex. BA = Brodmann's Area. Hem = Hemisphere. Location = coordinates are for the maximum z-value and are for MNI152 space, with the x axis moving from left to right
One cluster emerged, listed in Table 4, in which moderation of brain activation by avoidance temperament was lateralized. This cluster overlapped with the right MFG cluster associated with avoidance temperament listed in Table 2, indicating that this effect was lateralized. In order to explore the nature of the relationship between these areas and avoidance temperament, an average β for each cluster, for each hemisphere, for each participant, was calculated and correlated with avoidance temperament scores (holding approach temperament constant). These partial correlations are presented in Table 4. In sum, the regions that are associated with temperament exhibited significantly lateralized activation.
Discussion
This study is one of the first to examine the moderation of neural activation related to executive function by trait motivation. The findings have important implications for understanding the interactive contributions of motivation and executive function for goal-directed behavior. As hypothesized, approach temperament moderated activation to the contrast of incongruent vs. congruent blocks (IvC) in two regions of left MFG (a relatively anterior region in BA 8 and 9 and a relatively posterior area in BA 9 only), and avoidance temperament moderated activation for the same contrast in one region of right MFG (BA 9/8/6), all of which were lateralized effects.
Trait Motivation and Left Anterior MFG
The moderation of neural activation related to the IvC contrast by both approach and avoidance temperament in the more anterior MFG area (BA 8/9) is consistent with research implicating this region in executive function, particularly processes associated with inhibiting a competing response. For example, using a Go-NoGo task, several studies found increased activation in this area when a response set changes and a relatively automatic behavior must be inhibited (Horn, Dolan, Elliott, Deakin, & Woodruff, 2003; Kaladjian et al., 2009; Liddle, Kiehl, & Smith, 2001). Extending this research, a number of studies indicate that this area is recruited when conflicting information requires the inhibition of a competing response using tasks such as the flanker, color-word Stroop, and Wisconsin Card Sorting (WCST) tasks (Fan, Flombaum, McCandliss, Thomas, & Posner, 2003; Konishi, Jimura, Asari, & Miyashita, 2003). Two studies specifically implicate this area in responding to conflicting emotional information (Ferstl, Rinck, & Von Cramon, 2005; Schirmer, Zysset, Kotz, & von Cramon, 2004), raising the possibility that this area responds preferentially to affectively valenced information.
The more anterior area of left MFG has also been associated with the anticipation and receipt of motivationally salient stimuli. Abler, Walter, Erk, Kammerer, and Spitzer (2006) and Ramnani and Miall (2003) found that activation in this area tracked the perceived probability of a rewarding outcome, with higher activation associated with greater likelihood of reward. Holsen et al. (2005) found activation in this area in fasting participants who were viewing images of food. This activation decreased after participants had eaten, indicating that activation tracked the current motivational salience of stimuli. Four studies have also implicated this area of MFG in responding to the receipt of monetary rewards (Bickel, Pitcock, Yi, & Angtuaco, 2009; Cox, Andrade, & Johnsrude, 2005; Goldstein et al, 2007; Pochon et al., 2002). Importantly, Pochon et al. (2002) found that this area responded to both the receipt of rewards and working memory load, suggesting that this area is involved with the integration of motivational and executive function processes. In addition to the anticipation and receipt of reward, one study implicated this area of left MFG in punishment anticipation (Knutson, Adams, Fong, & Hommer, 2001), and another study found activation in this area of MFG in response to receiving monetary punishments (Dillon et al., 2008), suggesting that this area is not specific to one type of potential outcome but rather responds to motivational salience in general.
Overall, these studies suggest that the more anterior area of left MFG identified in the present study is involved in both inhibiting a competing response and anticipating and responding to motivationally salient stimuli. It has been unclear why this region is involved in these apparently different processes. One hypothesis that resolves this confusion is that this region implements a motivational set that biases processing in other brain areas, in a top-down manner, to respond in a goal-congruent fashion. Theoretically, a region involved in implementing a motivational set should become engaged when goal-relevant information is received, in order to evaluate the success of current goal-attainment strategies and adjust behavior to increase the likelihood of goal attainment. Therefore, a region implementing a motivational set will become more active when goal-incongruent behavior must be inhibited and/or when encountering environmental cues that predict future outcomes.
Present results support the proposal developed above that the more anterior MFG region is involved in implementing a motivational set. Specifically, approach and avoidance temperament can be thought of as dispositions to engage motivational sets, approaching positive outcomes and avoiding negative outcomes, respectively. Therefore, increased levels of approach or avoidance temperament should be associated with increased activity in areas involved in implementing a motivational set. Given that both approach and avoidance were associated with the more anterior MFG area in the present study, it is possible that this area is involved in implementing a motivational set without being specific to certain types of goals (although independently, since they were entered into the regression simultaneously). The overlap between approach and avoidance is supported by research linking this MFG region to both reward (e.g., Abler et al., 2006) and punishment (e.g., Knutson et al., 2001). However, activation in this area was lateralized only for approach temperament, indicating that, even if this area is recruited by increased motivation generally, it is recruited in a different fashion or to a different degree by each temperament (specifically, more bilaterally for avoidance). Alternatively, the overlap may reflect a lack of spatial resolution to distinguish nearby areas.
Approach Motivation and Left MFG
Approach temperament was associated with another, more posterior, area of MFG, not moderated by avoidance temperament. This effect was lateralized. Similar to the more anterior MFG area discussed above, the more posterior area of MFG may be involved in imposing a motivational set, although one specific to reward outcomes. Specifically, this more posterior MFG region has been associated with the anticipation (Abler et al., 2006; Dreher, Kohn, & Berman, 2006; Ramnani & Miall, 2003) and receipt (Cox et al., 2005; Landmann et al., 2007) of monetary reward. This more posterior MFG area has also been associated with responding to pleasant stimuli, such as pleasant pictures (Canli, Desmond, Zhao, Glover, & Gabrieli, 1998; Dolcos, LaBar, & Cabeza 2004). Using a direct test of lateralization similar to that employed in the present study, Herrington et al. (2005) found left-lateralized activation in this area in response to pleasant words. Interestingly, Knutson, Bhanji, Cooney, Atlas, and Gotlib (2008) found that, relative to healthy controls, individuals with depression exhibited decreased activation in this area when anticipating monetary rewards. This finding is consistent with research suggesting that depression is associated with decreased levels of approach temperament (e.g., Spielberg et al., 2010). The results of the present study suggest that decreased activation in this more posterior MFG area contributes to depression by decreasing the ability to bias behavior towards potential rewarding goals.
Also consistent with present findings, the more posterior MFG area has been associated with inhibition of a competing response in studies using various paradigms, (e.g., Kaladjian et al., 2009; Liddle et al., 2001; also observed in a meta-analysis of Go/NoGo, Buchsbaum, Greer, Chang, & Berman, 2005; Milham et al., 2003; Nakao et al., 2005; Konishi et al., 2003). Researchers have also observed activation in this area prior to successful performance in the Go/NoGo task (Garavan, Ross, Murphy, Roche, & Stein, 2002) and during working memory processes involved in the organization of upcoming action (Volle et al., 2005). Taken together with the results of the present study, research suggests that the more posterior MFG area is involved with implementing a motivational set in order to attain potential rewarding goals.
Although both MFG areas related to approach temperament appear to be involved in implementing a motivational set, there may be contributions to this process that are specific to each area. One potential area of specificity is suggested by the association between the more posterior MFG area and organization of upcoming goal-directed behavior (Volle et al., 2005). The more anterior MFG area did not evidence a similar association in this study. One model of approach/avoidance motivation (Scholer & Higgins, 2008) distinguishes between approach/avoidance at the level of the goal (whether the goal is desirable and should be approached, or undesirable and should be avoided) and at the level of the strategy used to obtain a goal (whether approach- or avoidance-related means are used to obtain a goal). Since processes related to the organization of upcoming goal-directed behavior are at the level of the strategy, it may be that the more posterior MFG region is more strongly linked to the level of the strategy than the more anterior MFG region.
Avoidance Motivation and Right MFG
As hypothesized, avoidance temperament moderated activation to IvC in right MFG (BA 9/8/6), and this effect was lateralized. This area has been associated with functions similar to the more posterior MFG area associated with approach, except that it has been linked to anticipation of potential negative rather that positive outcomes. Specifically, two studies have linked activation in this area to the threat of shock (Butler et al., 2007; Dalton, Kalin, Grist, & Davidson, 2005). Cooper and Knutson (2008) suggested that this area responds not to threat specifically but rather to the uncertainty of the outcome. They found that activity in this area reflected the level of uncertainty of both pleasant and unpleasant potential outcomes. Therefore, this area may be involved in monitoring uncertainty about aspects of the environment that may be detrimental to achieving goals. This area has also been associated with the receipt of negative outcomes, including monetary punishments (Bickel et al., 2009; Dillon et al., 2008) and painful thermal stimuli (Derbyshire, Jones, Gyulai, Clark, & Townsend, 1997). Interestingly, Keedwell, Andrew, Williams, Brammer, and Phillips (2005) observed more activation in this area during a sad mood induction in depressed individuals than in healthy controls. This finding is consistent with an association between depression and greater levels of avoidance temperament (Spielberg et al., 2010).
This right MFG area has also been implicated in inhibition of a dominant response in various paradigms (e.g., Buchsbaum et al., 2005; Kaladjian et al., 2009; Konishi et al., 2003; Lee, Dolan, & Critchley, 2008; Liddle et al., 2001; Ullsperger & Von Cramon, 2001). In addition, it has been associated with working memory in a number of studies using variants of the n-back task (Owen, McMillan, Laird, & Bullmore, 2005), including one employing emotional stimuli (Rama et al., 2001). Similar to the study conducted by Volle et al. (2005), two studies (Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000; Rowe & Passingham, 2001) attempted to tease apart activation during a working memory task. These studies suggest that this area is associated with organization of action using information maintained in working memory, rather than the maintenance of information itself. In summary, this right MFG area appears to be involved in implementing a motivational set. The association between this area and organization of upcoming goal-directed behavior (Rowe et al., 2000; Rowe & Passingham, 2001) suggests that, like the more posterior left MFG area associated with approach temperament, this area is linked to the level of the strategy used to obtain a goal (Scholer & Higgins, 2008).
Approach Motivation and Left OFC
Unexpectedly, approach temperament moderated activation in a region of left OFC such that greater approach temperament was associated with less recruitment of this OFC area. Upon first examination, this finding is inconsistent with the hypothesis that approach motivation/pleasant emotion should be associated with increased activation in left hemisphere. However, recent research suggests an opposite pattern of lateralization in OFC. In a meta-analysis of neuroimaging studies examining the experience of emotion, pleasant emotion was associated with right OFC activation, whereas unpleasant emotion was associated with left OFC activation, in an area overlapping that found in the present study (Wager et al., 2008). Thus, the finding of opposing patterns of lateralization in MFG and OFC for approach temperament is consistent with previous research. Generally, OFC is hypothesized to be involved in maintaining the current and expected motivational value of stimuli (O'Doherty & Dolan, 2006).
In the context of the present study, one possible explanation for the opposing relationships with approach temperament observed in MFG and OFC is that, as part of implementing a motivational set, MFG inhibits activation in areas that provide competing information, in this case an area providing information about the expected unpleasant value associated with the harder task level, which could, for example, be due to the increased chance of making a mistake. This hypothesis is supported by the present finding that activation in this OFC area was positively correlated with reaction-time interference, indicating that suppression of activity in this area may lead to better task performance. Recent research has examined connections between DLPFC and OFC (e.g., Szatkowska et al., 2008), although the direction of influence is a matter of debate. Future research should examine connections between these areas under different task circumstances (e.g., different levels of reward or punishment) to determine whether top-down biasing from MFG is affecting activation in OFC.
Strengths and Limitations
The present study has several strengths, including the use of a relatively large sample size for the fMRI literature and direct tests for effects of laterality. Further, it extends the literature on motivation by examining trait approach and avoidance motivation, as opposed to manipulations of state motivation, and carefully measured approach and avoidance temperament by estimating latent factors from multiple indices. As with any study, however, there are several limitations that must be considered when interpreting the results. First, the present study used only self-report measures of approach and avoidance temperament. Future research should add behavioral measures, such as the level of reward and punishment responsiveness in monetary learning tasks (e.g., Moustafa, Cohen, Sherman, & Frank, 2008). Second, no explicit (e.g., monetary) rewards or punishments were given in the present task. Future research should examine how trait motivation drives or interacts with state motivation to influence executive function. Third, the present study employed a task that does not recruit all aspects of executive function. Future research should examine the interaction of trait motivation and other aspects of executive function, such as shifting and updating (Miyake et al., 2000).
In spite of these limitations, the present study adds to the existing literature by identifying several areas in MFG that appear to be involved in implementing motivational sets to guide behavior in a goal-congruent manner. In accordance with hypotheses, approach and avoidance temperament were associated with lateralized activation in left and right MFG, respectively. Another area of MFG may be involved with both approach and avoidance temperament. Research reviewed indicates that these MFG areas have been associated with inhibiting goal-incongruent behavior, organizing upcoming behavior in accordance with goals, and anticipating and responding to motivationally salient stimuli.
Research Highlights.
Interaction of motivation and executive function instantiated in MFG.
Trait approach motivation associated with left lateralized MFG activation.
Trait avoidance motivation associated with right lateralized MFG activation.
Acknowledgments
This work was supported by the National Institute of Drug Abuse (R21 DA14111) and the National Institute of Mental Health (R01 MH61358, T32 MH19554, P50 MH079485). The authors wish to thank Naomi Sadeh for her helpful comments.
Footnotes
For all fMRI analyses performed, a second analysis was conducted with psychopathology group entered as a between-subject factor. These analyses tested whether the relationship between temperament score and brain activation (and hemisphere in the laterality analysis) differed by psychopathology group. No findings differed by psychopathology group, indicating that the results of the present study are not driven by sample selection. As well, correlations between temperament and brain activation from clusters associated with approach or avoidance (i.e., those in Table 2) remained significant even when the variance associated with depression and anxiety was partialled out. Additionally, psychopathology group did not significantly predict brain activation from these clusters, even without partialling out the variance associated with approach and avoidance temperament. Thus, present findings are not confounded with depression and anxiety.
The reverse comparison was also used, with unflipped right hemisphere data and flipped left hemisphere data, which produced extremely similar results. As well, all lateralization comparisons were reanalyzed using a symmetrical template (ICBM 152 2009a Nonlinear Symmetric T1 Atlas, Fonov, Evans, McKinstry, Almli, & Collins, 2009), which also produced extremely similar results. In addition, fMRI analyses conducted using FEAT were rerun using FSL's outlier de-weighting (Woolrich, 2008) procedure to test whether findings were driven by outliers. Findings were virtually identical, indicating that findings were not due to outliers.
Additional analyses were conducted to rule out the possibility that associations between trait motivation and brain activation were driven by a mutual association with overt behavior. Specifically, reaction time and error interference were entered as covariates of no interest into the general linear model with approach and avoidance predicting brain activation. All results remained significant, indicating that the association between trait motivation and brain activation is not due to a shared relationship with performance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey M. Spielberg, University of Illinois at Urbana-Champaign
Gregory A. Miller, University of Illinois at Urbana-Champaign
Anna S. Engels, Pennsylvania State University and University of Illinois at Urbana-Champaign
John D. Herrington, Children's Hospital of Philadelphia
Bradley P. Sutton, University of Illinois at Urbana-Champaign
Marie T. Banich, University of Colorado at Boulder
Wendy Heller, University of Illinois at Urbana-Champaign.
References
- Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. Journal of psychiatric research. 2007;41:511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimization (Tech Rep TR07JA1) Oxford, United Kingdom: University of Oxford, FMRIB Centre; 2007. [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek, et al. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Smith S. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience. 2010;22:1970–1979. doi: 10.1162/jocn.2009.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: Fictive and real money gains and losses. Journal of Neuroscience. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Buchsbaum BR, Greer S, Chang W, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Pan H, Tuescher O, Engelien A, Goldstein M, Epstein, et al. Human fear-related motor neurocircuitry. Neuroscience. 2007;150:1–7. doi: 10.1016/j.neuroscience.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Campbell JP, Pritchard RD. Motivation theory in industrial and organizational psychology. In: Dunnette MD, editor. Handbook of industrial and organizational psychology. Chicago: Rand McNally; 1976. [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. NeuroReport. 1998;9:3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Cooper JC, Knutson B. Valence and salience contribute to nucleus accumbens activation. NeuroImage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO personality inventory (NEOPI-R and Five Factor Inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Cox SM, Andrade A, Johnsrude IS. Learning to like: a role for human orbitofrontal cortex in conditioned reward. Journal of Neuroscience. 2005;25:2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davidson RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005;17:969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology. Journal of Personality and Social Psychology. 1990;58:330–341. [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Animal Learning and Behavior. 1994;22:1–18. [Google Scholar]
- Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2007;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Kohn P, Berman KF. Neural coding of distinct statistical properties of reward information in humans. Cerebral Cortex. 2006;16:561–573. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- Elliot AJ. The hierarchical model of approach-avoidance motivation. Motivation and Emotion. 2006;30:111–116. [Google Scholar]
- Elliot AJ, McGregor HA. A 2 × 2 achievement goal framework. Journal of Personality and Social Psychology. 2001;80:501–519. doi: 10.1037/0022-3514.80.3.501. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: Approach and avoidance temperaments and goals. Journal of Personality and Social Psychology. 2002;82:804–818. doi: 10.1037//0022-3514.82.5.804. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Rinck M, Cramon DY. Emotional and temporal aspects of situation model processing during text comprehension: An event-related fMRI study. Journal of Cognitive Neuroscience. 2005;17:724–739. doi: 10.1162/0898929053747658. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Lateralized temporal-limbic dysfunction and psychopathology. Annals of the New York Academy of Sciences. 1976;280:777–795. doi: 10.1111/j.1749-6632.1976.tb25541.x. [DOI] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- Gable SL, Reis HT, Elliot AJ. Evidence for bivariate systems: An empirical test of appetition and aversion across domains. Journal of Research in Personality. 2003;37:349–372. [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociableexecutive functions in the dynamic control of behavior: Inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Emotional behavior and hemispheric side of the lesion. Cortex. 1972;8:41–55. doi: 10.1016/s0010-9452(72)80026-1. [DOI] [PubMed] [Google Scholar]
- Gilbert AM, Fiez JA. Integrating rewards and cognition in the frontal cortex. Cogn Affect Behav Neurosci. 2004;4:540–552. doi: 10.3758/cabn.4.4.540. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7:476–489. [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, Fisher JE, Stewart JL, Banich MT, et al. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Heller W, Mohanty A, Engels AS, Banich MT, Webb AG, et al. Localization of asymmetric brain function in emotion and depression. Psychophysiology. 2010;47:442–454. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, et al. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–676. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones MR. Nebraska symposium on motivation. Vol. 3. Lincoln, Nebraska: University of Nebraska Press; 1955. [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Nazarian B, Roth M, Anton JL, et al. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disorders. 2009;11:530–538. doi: 10.1111/j.1399-5618.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biological Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:159–163. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Jimura K, Asari T, Miyashita Y. Transient activation of superior prefrontal cortex during inhibition of cognitive set. Journal of Neuroscience. 2003;23:7776–7782. doi: 10.1523/JNEUROSCI.23-21-07776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk DC, Gazzaley A, D'Esposito M. Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Research. 2007;1141:168–177. doi: 10.1016/j.brainres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Landmann C, Dehaene S, Pappata S, Jobert A, Bottlaender M, Roumenov D, et al. Dynamics of prefrontal and cingulate activity during a reward-based logical deduction task. Cerebral Cortex. 2007;17:749–759. doi: 10.1093/cercor/bhk028. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lee TW, Dolan RJ, Critchley HD. Controlling emotional expression: Behavioral and neural correlates of nonimitative emotional responses. Cerebral Cortex. 2008;18:104–113. doi: 10.1093/cercor/bhm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DB. Psychophysiology and motivation. In: Jones MR, editor. Nebraska symposium on motivation. Vol. 5. Lincoln, Nebraska: University of Nebraska Press; 1957. [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: Response and non-response related aspects of attentional selection as ascertained by fMRI. Cerebral Cortex. 2006;16:827–834. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behavior Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Molina S, Borkovec TD. The Penn State Worry Questionnaire: Psychometric properties and associated characteristics. In: Davey GCL, Tallis F, editors. Worrying: Perspectives on theory, assessment, and treatment. Chichester, England: Wiley; 1994. pp. 265–283. [Google Scholar]
- Moustafa AA, Cohen MX, Sherman SJ, Frank MJ. A role for dopamine in temporal decision making and reward maximization in parkinsonism. Journal of Neuroscience. 2008;28:12294–12304. doi: 10.1523/JNEUROSCI.3116-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato, et al. A functional MRI comparison of patients with obsessive–compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Research: Neuroimaging. 2005;139:101–114. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Miller GA. Anxiety, stress, and cortical brain function. In: Borod JC, editor. The Neuropsychology of Emotion. Oxford, United Kingdom: Oxford University Press; 2000. pp. 298–319. [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dolan RJ. The role of human orbitofrontal cortex in reward prediction and behavioral choice: Insights from neuroimaging. In: Zald DH, Rauch SL, editors. The orbitofrontal cortex. Oxford, United Kingdom: Oxford University Press; 2006. pp. 265–284. [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in Cognitive Sciences. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, et al. The neural system that bridges reward and cognition in humans: An fMRI study. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P, Martinkauppi S, Linnankoski I, Koivisto J, Aronen HJ, Carlson S. Working memory of identification of emotional vocal expressions: An fMRI study. NeuroImage. 2001;13:1090–1101. doi: 10.1006/nimg.2001.0777. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Miall RC. Instructed delay activity in the human prefrontal cortex is modulated by monetary reward expectation. Cerebral Cortex. 2003;13:318–327. doi: 10.1093/cercor/13.3.318. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philosophical Transactions: Biological Sciences. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Eckstein D, Braver T, Owen AM. How does reward expectation influence cognition in the human brain? Journal of Cognitive Neuroscience. 2008;20:1980–1992. doi: 10.1162/jocn.2008.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Passingham RE. Working memory for location and time: Activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14:77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RSJ, Passingham RE. The prefrontal cortex: Response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Zysset S, Kotz SA, Yves von Cramon D. Gender differences in the activation of inferior frontal cortex during emotional speech perception. NeuroImage. 2004;21:1114–1123. doi: 10.1016/j.neuroimage.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Heller W, Silton RL, Stewart JL, Miller GA. Approach and avoidance profiles distinguish dimensions of anxiety and depression. 2010 Manuscript submitted for publication. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General. 1935;18:643–662. [Google Scholar]
- Szatkowska I, Bogorodzki P, Wolak T, Marchewka A, Szeszkowski W. The effect of motivation on working memory: An fMRI and SEM study. Neurobiology of Learning and Memory. 2008;90:475–478. doi: 10.1016/j.nlm.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Luan Phan K, Fitzgerald KD, Gehring WJ. A functional neuroimaging study of motivation and executive function. NeuroImage. 2004;21:1045–1054. doi: 10.1016/j.neuroimage.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Keener AD. Frontal brain asymmetry and depression: A self-regulatory perspective. Cognition and Emotion. 1998;12:387–420. [Google Scholar]
- Tomarken AJ, Zald DH. Conceptual, methodological, and empirical ambiguities in the linkage between anger and approach: Comment of Carver and Harmon-Jones (2009) Psychological Bulletin. 2009;135:209–214. doi: 10.1037/a0014735. [DOI] [PubMed] [Google Scholar]
- Towers DN, Engels AS, Stewart JL, Spielberg JM, Miller GA, Heller W. Choosing sides: Happily approaching left frontal cortex in psychopathology and emotion. Paper presented in symposium, Patients are a virtue: What studies of clinical and neurological patients reveal about physiological mechanisms of emotion, presented at the annual meeting of the Society for Psychophysiological Research; Austin. 2008. Oct, [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Volle E, Pochon JB, Lehericy S, Pillon B, Dubois B, Levy R. Specific cerebral networks for maintenance and response organization within working memory as evidenced by the double delay/double response paradigm. Cerebral Cortex. 2005;15:1064–1074. doi: 10.1093/cercor/bhh207. [DOI] [PubMed] [Google Scholar]
- Wager TD, Feldman Barrett L, Bliss-Moreau E, Lindquist KA, Duncan S, Kober H, et al. The neuroimaging of emotion. In: Lewis M, Haviland-Jones JM, Feldman Barrett L, editors. Handbook of emotions. third. New York: Guilford Press; 2008. pp. 272–290. [Google Scholar]
- Ward DB. Simultaneous inference for FMRI data. [July 27, 2006];2000 http://afni.nimh.nih.gov./pub/dist/doc/manual/AlphaSim.pdf.
- Watson D, Clark LA. Behavioral disinhibition versus constraint: A dispositional perspective. In: Wegner DM, Pennebaker JW, editors. Handbook of mental control. New York, NY: Prentice Hall; 1993. pp. 506–527. [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]


