Abstract
Recent years have witnessed a renewed interest in using oscillatory brain electrical activity to understand the neural bases of cognition and emotion. Electrical signals originating from pericranial muscles represent a profound threat to the validity of such research. Recently, McMenamin et al (2010) examined whether independent component analysis (ICA) provides a sensitive and specific means of correcting electromyogenic (EMG) artifacts. This report sparked the accompanying commentary (Olbrich, Jödicke, Sander, Himmerich & Hegerl, in press), and here we revisit the question of how EMG can alter inferences drawn from the EEG and what can be done to minimize its pernicious effects. Accordingly, we briefly summarize salient features of the EMG problem and review recent research investigating the utility of ICA for correcting EMG and other artifacts. We then directly address the key concerns articulated by Olbrich and provide a critique of their efforts at validating ICA. We conclude by identifying key areas for future methodological work and offer some practical recommendations for intelligently addressing EMG artifact.
Recent years have witnessed a renewed interest in using neural oscillations to understand the substrates of mental function and dysfunction (Uhlhaas & Singer, 2010). Electrical activity generated by the pericranial musculature, electromyogenic (EMG) artifact, is one of the most profound threats to the validity of such studies. The danger is intrinsic to the cardinal features of EMG, particularly its high amplitude, broad spectral and anatomical distributions, and sensitivity to psychologically interesting processes. Consequently, even subtle EMG artifact can generate spurious effects and can mask or otherwise alter genuine ones across virtually the entire spectrum of the electroencephalogram (EEG).
A number of tools for EMG correction have been developed, however, the pace of algorithm development and dissemination has outstripped work to rigorously assess the sensitivity and specificity of such tools. Collectively, these issues motivated several recent methodological publications by our group (McMenamin et al., 2010; McMenamin, Shackman, Maxwell, Greischar, & Davidson, 2009; Shackman, 2010; Shackman, McMenamin, Maxwell, Greischar, & Davidson, in press; Shackman et al., 2009). In particular, in McMenamin et al. (2010), we examined the validity of the extended Infomax independent component analysis (ICA) algorithm (Jung et al., 2000a,b; Onton, Westerfield, Townsend, & Makeig, 2006).
It was this report that sparked the accompanying commentary by Olbrich (Olbrich, Jödicke, Sander, Himmerich & Hegerl, 2010). Our response is organized as follows. We begin by briefly summarizing the EMG problem and then outline recent research, including our own, assessing the utility of ICA for correcting artifacts. Next, we directly address the key concerns articulated by Olbrich and critique their efforts at validating ICA. We conclude by identifying key areas for future methodological work and offer some practical recommendations for dealing with EMG artifact.
Nature of the EMG Problem
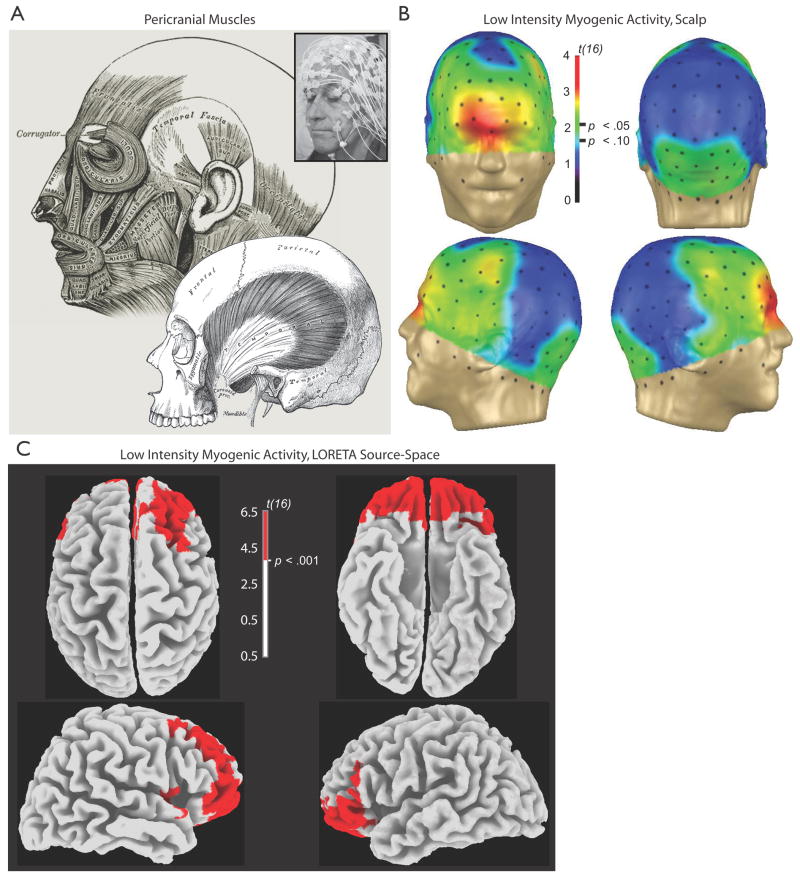
The difficulty encountered when addressing EMG artifact can be attributed to three key factors: a) its spatial and spectral distribution, b) its exquisite sensitivity to a variety of psychologically interesting processes, and c) its lack of stereotypy. The EMG signal has remarkable spatial extent and spectral breadth. Although the EMG power spectrum peaks at relatively high frequencies (~100Hz), it is sufficiently broad to overlap with all EEG frequencies of interest. Goncharova, McFarland, Vaughan, and Wolpaw (2003) report reliable myogenic effects as low as 2 Hz. They also demonstrated that EMG can be detected anywhere on the scalp due to volume conduction of activity generated by muscles across the head, face and neck. Comparably widespread effects were observed by McMenamin (Figure 1).
Figure 1. Myogenic activity.
A. Cranial muscles. Nearly all cephalic electrodes lie atop or adjacent to muscle, making EMG artifact a concern for the entire head—not just anterior electrodes. Key generators of myogenic artifact include the corrugator along the brow, orbicularis oculi around the eyes, frontalis above the brow, masseter on the jaw, the peri-auricular muscles surrounding the ear, and occipitalis at the base of the skull. Lower engraving depicts temporalis. Inset shows the 128-channel EEG array. Engravings adapted from Gray’s Anatomy (Gray, 1918/2000). B. Low intensity myogenic activity on the scalp. Spline-interpolated topographic plots of the thresholded myogenic contrast (Tense minus Relaxed, Eyes Open; n = 17). One-third of the 128-channel array reached threshold (p = .05) and one-half showed trends (p = .10). C. Low intensity myogenic activity increased activity in large regions of the cerebral source-space. Images depict the thresholded myogenic contrast. Note the absence of suprathreshold voxels in primary motor cortex (M1; precentral gyrus). Data were modeled using LORETA, as detailed in McMenamin. Additional images are presented in the Supplement to McMenamin.
These problems are further complicated by the fact that EMG is sensitive to a variety of experimental manipulations. Facial EMG, in particular, is sensitive to numerous cognitive and affective processes, including cognitive load (Cohen et al., 1992; Waterink & van Boxtel, 1994), facial mimicry (Dimberg et al., 2000), vocalization (Brooker & Donald, 1980), and induced emotional states (Borden et al., 1991; Coan and Allen, 2001; Bradley et al., 2001). Such effects are not limited to the face: activity generated by the muscles of the neck, for instance, has been shown to closely track performance motivation (Roesch & Olson, 2007). A consequence of such effects is that changes in neurogenic and myogenic activity are often confounded, allowing muscle activity to masquerade as EEG or fundamentally alter the magnitude or topography of genuine neurogenic effects. Indeed, McMenamin showed that when changes in neurogenic and myogenic activity negatively covaried, alpha-blocking associated with eye-opening was attenuated at locations across the scalp and source-space, including posterior locations far removed from peak myogenic activity. These effects did not reflect an artificially extreme degree of EMG contamination. In fact, the statistical effect-size for the alpha-blocking contrast was three times larger than the myogenic contrast. Together, these observations underscore that even low-intensity myogenic activity represents a serious risk to validity.
Lastly, EMG exhibits a poorly stereotyped response, making removal difficult. EMG arises from the activity of spatially distributed, functionally independent muscle groups, with distinct topographic and spectral signatures. For instance, frontalis activity peaks around 25 Hz, whereas temporalis has a lower peak (~20 Hz) and broad plateau around 40–80 Hz (Goncharova et al., 2003). The spectral composition of myogenic activity can vary with contraction intensity (Goncharova et al., 2003) and fatigue (Chung et al., 2002). This is compounded by the fact that the relative contributions of each muscle can vary substantially across elicitors and individuals (Tassinary et al., 2007). Consistent with this, McMenamin demonstrated that even carefully instructed myogenic activity is characterized by marked individual differences (Table 1). Across participants (n = 17), anywhere from 3% to 67% of the 64 independent components that were extracted were classified as ‘pure’ myogenic activity. Likewise, myogenic components accounted for as little as 1.5% and as much as 78.5% of the variance in EEG activity (see Figures 6–7 in McMenamin). Given such individual differences in the spectral and topographic profile of myogenic activity, canonical spatial or spectral filters or templates of the kind that have been fruitfully applied to the correction of ocular artifact (e.g., Viola et al., 2009) probably are not suitable for correcting typical EMG artifacts.
Table 1.
Individual Differences in Independent Component Classification: Frequencies
| Subject | Unclassifiable Noise | Myo > Neuro | Neurogenic | Myogenic | Low Variance | Ocular | Neuro > Myo | Gross | Sum (Est. Model Order) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 29 | 2 | 8 | 7 | 8 | 3 | 1 | 64 (51) |
| 2 | 11 | 18 | 8 | 4 | 15 | 5 | 1 | 2 | 64 (37) |
| 3 | 12 | 20 | 5 | 5 | 7 | 13 | 2 | 0 | 64 (41) |
| 4 | 4 | 23 | 11 | 3 | 10 | 9 | 3 | 1 | 64 (38) |
| 5 | 8 | 8 | 10 | 17 | 13 | 7 | 1 | 0 | 64 (29) |
| 6 | 16 | 4 | 11 | 8 | 18 | 4 | 3 | 0 | 64 (35) |
| 7 | 22 | 1 | 12 | 2 | 19 | 5 | 0 | 3 | 64 (39) |
| 8 | 22 | 8 | 11 | 4 | 5 | 10 | 1 | 3 | 64 (41) |
| 9 | 14 | 9 | 8 | 8 | 13 | 9 | 2 | 1 | 64 (23) |
| 10 | 3 | 8 | 2 | 37 | 4 | 6 | 3 | 1 | 64 (33) |
| 11 | 14 | 3 | 8 | 21 | 3 | 13 | 2 | 0 | 64 (41) |
| 12 | 15 | 5 | 12 | 15 | 7 | 5 | 3 | 2 | 64 (53) |
| 13 | 16 | 18 | 6 | 10 | 6 | 2 | 4 | 2 | 64 (47) |
| 14 | 13 | 7 | 14 | 7 | 10 | 10 | 2 | 1 | 64 (37) |
| 15 | 3 | 10 | 0 | 43 | 0 | 7 | 0 | 1 | 64 (40) |
| 16 | 1 | 12 | 8 | 10 | 8 | 22 | 2 | 1 | 64 (40) |
| 17 | 10 | 11 | 5 | 21 | 8 | 7 | 1 | 1 | 64 (43) |
| Median | 12 | 9 | 8 | 8 | 8 | 7 | 2 | 1 | 64 (40) |
| Min-Max | 1–22 | 1–29 | 0–14 | 2–43 | 0–19 | 2–22 | 0–4 | 0–3 | 64–64 (23–53) |
Notes: Preliminary inspection of the ICA results indicated that low-variance components were dominated by noise, making them difficult to reliably classify and leading raters to devote an undue amount of time to their consideration. Accordingly, components accounting for <0.2% of scalp variance were categorized as Low Variance, excepting cases where unambiguous classification was possible. Using the protocol detailed in the Supplement to McMenamin, remaining components were classified as Neurogenic, Myogenic, a combination of the two (Neuro > Myo or Myo > Neuro), or artifact (Gross or Ocular). Components that met the minimum variance criterion, but proved impossible to unambiguously classify were considered Noise. Classifications were made by two raters based on inspection of component time-series, power spectra, topography, and where necessary the raw time-series. Interrater reliability was excellent, α=0.98 (see the Supplement to McMenamin). Final classification was by consensus. Post hoc analyses used a Bayesian model order estimation procedure (Beckmann & Smith, 2002, 2004; Rajan & Rayner, 1997) to estimate the minimum number of components, Model Order, necessary to adequately describe our data. Results indicated that the 64-component extraction used by McMenamin was sufficient to avoid underfitting, but moderately overfit most participants.
Validating the use of Infomax ICA for EMG Correction
The Infomax ICA algorithm has rapidly become one of the most prominent techniques for removing EMG and other contaminants from the EEG. Conceptually, ICA-based correction entails three steps. First, ICA is used to perform an unsupervised decomposition of the EEG into temporally independent components (Onton et al., 2006; Onton & Makeig, 2006). Next, components are classified, and those categorized as artifact are discarded (for a detailed classification protocol, see the Supplement to McMenamin). Classification is typically performed manually, although algorithmic techniques have been developed (Shackman et al., 2009). Finally, the ‘artifact-free’ time-series is reconstructed from the remaining components. ICA’s ability to reconstruct the time-series is a key advantage over artifact correction techniques that cannot do so, such as those based on the general linear model (McMenamin et al., 2009). Although ICA shows great promise for correcting EMG and other artifacts (e.g., Jung et al., 2000a,b), attempts to assess its validity have been limited (reviewed in McMenamin and in Shackman et al., 2009). Moreover, few studies have gauged the impact of ICA-based EMG correction on source modeling (‘localization’), an increasingly popular technique for maximizing the anatomical information yielded by the EEG (Pizzagalli, 2007).
Accordingly, McMenamin quantitatively assessed the sensitivity and specificity of ICA on the scalp and in the cerebral source-space using a dataset in which neurogenic and myogenic activity were independently manipulated. Critically, this design allowed us to examine ICA-based EMG correction under conditions in which neurogenic and myogenic activity covaried, as they do in many experimental settings. The degree to which artifact correction produced spurious effects in the absence of artifact was also assessed. Finally, given earlier work suggesting the importance of varying the procedures for discarding non-myogenic artifacts (Shackman et al., 2009), McMenamin tested whether varying these procedures affected the quality of EMG correction1. Importantly, our validation analyses made use of both mean difference and mean equivalence tests (Seaman & Serlin, 1998)—the latter are essential for rigorously demonstrating the sensitivity and specificity of a particular correction technique.
Results revealed that some, but not all, of the correction procedures exhibited adequate sensitivity and specificity on the scalp (see also Shackman et al., 2010). The most sensitive and specific procedure was among the strictest of the nine examined, entailing the rejection of any component showing evidence of EMG, in addition to those reflecting gross or ocular artifacts, noise, or unclassifiable low-variance signals. None of the procedures consistently showed excellent performance on the scalp. That is, a modest number of ‘worst-case’ electrodes evinced under- or over-correction. This residual error on the scalp resulted in poor sensitivity and specificity in the cerebral source-space. This conclusion was not entirely unexpected, given other work highlighting the potential limitations of Infomax ICA (Crespo-Garcia et al., 2008; Fitzgibbon et al., 2007; Wallstrom et al., 2004; Romero et al., 2008; Castellanos & Makarov, 2006; Debener et al., 2008; Hyvärinen, Ramkumar, Parkkonen & Hari, 2010; Klemm, Haueisen & Ivanova, 2009; Lindsen & Bhattacharya, 2010; Vanderperren et al, 2010).
Rebuttal of Olbrich
We next address the three key concerns articulated by Olbrich. Although they raise several conceptually and methodologically important points, none of their specific concerns fundamentally change the implications of our research.
Too Few Components?
Olbrich suggests that the inadequate performance of ICA-based EMG correction reported by McMenamin might reflect the procedures used for extracting independent components, rather than an intrinsic limitation of the Infomax ICA algorithm. In particular, they expressed concern that an insufficient number of components may have been extracted to adequately separate neurogenic and myogenic sources. There are two reasons for rejecting this possibility. First, as noted by McMenamin (their footnote 5), preliminary visual inspection of the 128 components extracted from the native electrode array indicated over-fitting, evidenced by the fission of artifactual signals across components (e.g., ocular and cardiac artifacts loaded onto an excessive number of components; see also Viola et al., 2009). Based on these exploratory analyses, we ultimately elected to use principal components analysis (PCA) to extract 64 components for validation testing. Qualitatively, reducing the number of components attenuated the amount of ‘splitting’ or ‘leakage’ of artifacts across multiple components. Second, using information theoretic procedures, McMenamin demonstrated that extracting 64 components always exceeded the Bayesian estimate of the minimum number required to describe the data (Table 1), providing quantitative evidence against underfitting.
Olbrich also suggested that it might have been more appropriate to compute ICA separately for each condition (i.e., quadrupling the number of components available for separating sources). There are several potential problems with this suggestion, aside from the strong possibility of overfitting the data. First, everything else being equal, reducing the amount of data used to decompose the EEG will tend to degrade the quality of the source separation (Makeig & Onton, in press). Put simply, given fewer exemplars of a particular artifact, ICA will be less able to cleanly separate it from other sources. A second problem with this suggestion is that generating separate ICA-based artifact filters for each condition has the potential to confound errors introduced by artifact correction with differences in neurogenic activity. Artificial correlations between condition and correction errors, like correlations between condition and EMG artifact, pose a direct threat to inferential validity. Fortunately, it is easy to eliminate this artificial threat simply by performing component extraction and classification on the complete dataset for each participant (i.e., all conditions considered simultaneously).
Classification Biases?
Olbrich suggested that our results might reflect problems with component classification, rather than a failing of Infomax ICA. But this concern appears to be entirely theoretical. To our knowledge, a reasonably well-validated algorithm for classifying components simply does not exist. Instead, prior work to develop classification algorithms has largely relied on simple heuristics (e.g., EMG components display more activity >40Hz than <20Hz) or training datasets that were pre-classified by ‘expert’ raters (e.g., Mammone & Morabito, 2008). By contrast, we provided a detailed classification protocol that relied on a comprehensive inspection of each component, including its time-series, spectra, and topography (see the Supplement to McMenamin). This classification protocol proved highly reliable across two independent human raters (αs = .93–.98). Notably, we also systematically examined the influence of 9 different procedures for rejecting myogenic and non-myogenic artifacts (see Footnote 1). Systematic biases in the classification or rejection of components cannot explain the combination of low sensitivity and low specificity in the source-space. Had we been too strict, rejecting all but the most prototypical neurogenic components, we would expect to find excellent sensitivity and poor specificity. Conversely, had we been too liberal, rejecting only the most egregious artifacts, we would expect to find the reverse pattern. The bottom line is that systematic procedural biases cannot readily account for our results, whereas a failure to cleanly separate myogenic and neurogenic sources does.
Confounding Myogenic and Neurogenic Activity?
Finally, Olbrich raise the possibility that our results may reflect the fact that one of our simplifying assumptions, namely that neurogenic activity is consistent across periods of muscle tensing and quiescence, is wrong. The key contrast for testing the sensitivity of ICA-correction technique in McMenamin was comparing the eyes-open, tense condition (OT) after EMG-correction to the uncorrected eyes-open, relaxed condition (OR). Significant differences in this contrast were interpreted as the presence of residual myogenic artifact—an interpretation that hinges on the assumption there is no systematic variation in neurogenic signals during the tensing of facial muscles. Olbrich et al (2010) cite several ways in which this assumption may fail, and we agree that basic neurophysiology dictates that there will be greater neuronal activity in regions innervating the pericranial musculature during the Tense condition. Empirically, however, the observed effects in the Corrected OT-OR contrast are far more consistent with residual artifact.
In particular, McMenamin did not observe effects in the lateral precentral gyrus for the OT-OR contrast (Figure 1), so these regions were not incorporated into the ‘myogenic’ region of interest (ROI) that was relied on for testing the sensitivity of ICA-based EMG correction. Primary motor regions were also not incorporated into the ‘neurogenic’ ROI that were used to test specificity. Thus, neither validation test was likely to have been confounded by differences in neurogenic activity associated with the tensing manipulation2.
A further argument against Olbrich’s interpretation stems from the remarkable similarity between the topography of the corrected and uncorrected myogenic contrasts (OT vs. OR; Figures 1–2 in McMenamin). The distribution of the myogenic contrast is nearly identical before and after ICA-based correction. This implies one of two possibilities. Either ICA-based correction failed to wholly correct the artifact, leaving a substantial residual, or it unmasked a neurogenic signal that was temporally and spatially coincident with EMG. The frontal topography renders the latter suggestion implausible. Collectively, these observations indicate that neural activity associated with muscle tensing had little effect on our conclusions.
Critique of Olbrich’s Simulation
Quantitatively validating any EMG correction tool requires data in which the presence and absence of EMG is definitive or can be reasonably assumed. McMenamin generated such a dataset by instructing participants to tense or relax facial muscles. An alternative approach is to build a synthetic dataset in which the experimenter mathematically ‘injects’ artifact into otherwise artifact-free data. Simple simulations can provide proof-of-principle, but may not be very informative about real-world performance given the substantial variability that is a hallmark of the pericranial EMG (see above and Table 1) and some other kinds of artifact (e.g., Gwin, Gramann, Makeig & Ferris, 2010). A related concern is that the assumptions underlying simple generative models (e.g., the degree of temporal and spatial correlation with neurogenic signals) probably do not characterize real EMG contamination, leading to low external validity (Grouiller et al., 2007; Hoffmann and Falkenstein, 2009). Of course, it is possible to create more complex simulations that can yield a more detailed understanding of the conditions under which a particular correction algorithm is valid (e.g., Delorme, Sejnowski & Makeig, 2007; Fitzgibbon et al., 2007).
But the simulation conducted by Olbrich was not of this kind. Instead, they simulated EMG contamination by superimposing EMG from a single extracranial muscle on the artifact-free EEG at three adjacent channels. The magnitude, spectral characteristics, and topography of simulated artifact was held constant across participants (n = 10). No attempt was made to examine conditions in which neurogenic and myogenic signals covaried. It is not clear what protocol was used for classifying components or even whether rater(s) were blind to the simulation protocol. Analyses were restricted to testing the null, a significant limitation that could have been circumvented using the sorts of equivalence tests employed by McMenamin. This concern is magnified by the strict threshold used for examining mean differences. Given these limitations, it is unlikely that the conclusions drawn by Olbrich from this simulation will accurately predict the real-world performance of ICA-based EMG correction.
Future Challenges
Several factors could plausibly account for the inability of Infomax ICA to fully separate myogenic from neurogenic sources. Testing these hypotheses represents a profitable avenue for future research. First, inadequate separation might reflect the extraction of too many components. Consistent with this possibility, Bayesian estimates of ‘model order,’ the minimum number of components necessary to describe each participant’s EEG, indicated a moderate degree of overfitting that varied substantially across individuals (Table 1). Overfitting could explain poor source separation (Naeem et al., 2009; Ryali et al., 2009). This possibility could be more systematically investigated using information theoretic approaches (Beckmann and Smith, 2004; Calhoun et al., 2001; Li et al., 2007; Moraux and Iannetti, 2009), deflationary approaches (http://www.cis.hut.fi/projects/ica/fastica; Mantini et al., 2008), or stepwise ICA (Hesse and James, 2004) to objectively identify the optimal model order for each participant. Doing so might also facilitate the development of criteria for discarding problematic participants (e.g., those with an unusual number of components).
Second, source modeling was not incorporated into the component classification protocol. Classification was instead based on the visual inspection of time-series, power spectrum, and topography. While this is a conventional approach, it is possible that inspection of component dipoles would have facilitated more accurate classifications, particularly in the case of ‘mixed’ components (e.g., Myogenic dominant). This possibility could be evaluated by modeling dipoles for each independent component3. Those characterized by dipoles at the edge or outside of the brain could, in combination with other criteria, then be classified as artifactual (myogenic or otherwise; Onton and Makeig, 2006, 2009; Milne et al., 2009) and the impact on sensitivity and specificity assessed.
Third, inadequate separation might reflect EMG violating key assumptions of the Infomax algorithm (Bell and Sejnowski, 1995; James and Hesse, 2005; Makeig & Onton, in press). The lengthy blocks of neurogenic and myogenic activation in the present study—and in many studies of emotion (e.g., Coan and Allen, 2001; Davidson et al., 1990; Shackman et al., 2006)—may not adequately satisfy the assumption that component activation is non-Gaussian. Furthermore, if neurogenic and myogenic activity are too closely coupled in the time-domain they would violate the assumption that sources are mutually temporally independent (but cf. Ohla et al., 2009). Future studies should test whether second-order separation algorithms, which do not make such assumptions, yield better separation (Joyce et al., 2004; Romero et al., 2008; Tang et al., 2005). It might also prove fruitful to investigate signal-space projection methods (Nolte and Curio, 1999; Tesche et al., 1995; Uusitalo and Ilmoniemi, 1997) or source separation in the frequency-domain (Anemuller et al., 2003; Hyvärinen et al., 2010; Lee et al., 2008), which may improve the separation of sources with overlapping spectra.
A fourth promising direction is to validate correction techniques using data obtained in the presence and absence of neuromuscular blockade (Whitham et al. 2008, 2007). Doing so would avoid the assumptions necessitated by simulated, scripted, and ad hoc datasets (Shackman et al., 2009).
Recommendations and Conclusions
Although ICA cannot be viewed as a panacea for EMG contamination, careful application is a useful means of rejecting the most dubious results on the scalp. Nevertheless, in cases where myogenic activity is plausible, we cannot recommend the use of source modeling techniques for hypothesis testing. Likewise, the results of McMenamin and others indicate that findings in the upper frequency bands (i.e., beta, gamma) should be interpreted with extreme caution, particularly when they occur in the vicinity of scalp muscles. At minimum, scalp topography plots should be presented (Shackman, 2010; Shackman et al., in press). We recommend that investigators adequately describe the procedures used for classifying and filtering artifactual components and, where relevant, quantitatively assess inter-rater reliability.
There is increasing interest in using scalp-recorded and source-localized EEG to answer fundamental questions about how the mind arises from and interacts with the brain (Makeig et al. 2004; Pizzagalli 2007). The development and careful validation of novel tools for separating myogenic from neurogenic signals will have substantial benefits for this endeavor.
Acknowledgments
We thank Jeff Maxwell, David Bachhuber, and Adam Koppenhaver for their contributions to this research and three anonymous reviewers for critical feedback. The two first authors contributed equally to this report, which was supported by the NIMH (P50-MH069315 and R37/R01-MH43454 to RJD; BWM was supported by T32-HD007151).
Footnotes
Nine procedures for filtering classified components were examined by McMenamin, reflecting the factorial crossing of procedures for discarding myogenic vs. non-myogenic artifacts (see Table 1 and the Supplement to McMenamin). Three procedures for discarding myogenic components were assessed: Minimal-EMG: Myogenic components; Intermediate-EMG: Myogenic and Myogenic > Neurogenic (‘Myogenic-Dominant’ heterogeneous) components; Maximal-EMG: Myogenic, Myogenic > Neurogenic, and Neurogenic > Myogenic (‘Neurogenic-Dominant’ heterogeneous) components. Three procedures for discarding non-neurogenic/non-myogenic (NNNM) components were assessed: Minimal-NNNM: Gross or Ocular components; Intermediate-NNNM: Gross, Ocular or Noise components; Maximal-NNNM: Gross, Ocular, Noise or Low Variance components. Application of the different filtering procedures led to marked differences in the percentage of scalp variance that was discarded (range: 26%–73%; see McMenamin, Figures 6–7).
Similar logic allows us to rule out a significant contribution from between-condition differences in gross arousal—as one might expect if muscle tensing was associated with greater arousal or cognitive workload than quiescence. Olbrich and colleagues (2009) recently used source modeling to show that such differences are associated with altered alpha-band (8–12 Hz) cortical current density in posterior regions of the cingulate, occipital, and temporal cortices. None of these regions contributed to the ROI we used to evaluate the sensitivity of ICA-based artifact correction (i.e., attenuation of myogenic activity). Such regions did overlap with the ROI used for evaluating specificity (i.e., the preservation of neurogenic activity; see Figure 9 in McMenamin), but differences in alpha-band activity associated with tensing could not have influenced the primary test of specificity, which examined the impact of ICA-based correction on the neurogenic contrast (eyes-open/muscles-relaxed vs. eyes-closed/muscles-relaxed) in the absence of EMG artifact.
This could be readily accomplished using the dipfit2 or besafit plug-ins for EEGLAB (http://sccn.ucsd.edu/eeglab/).
Authors acknowledge no conflicts of interest.
References
- Anemuller J, Sejnowski TJ, Makeig S. Complex independent component analysis of frequency-domain electroencephalographic data. Neural Networks. 2003;16:1311–1323. doi: 10.1016/j.neunet.2003.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SA. Probabilistic independent component analysis for functional magnetic resonance imaging. FMRIB Technical Report TR02CB1. 2002 doi: 10.1109/TMI.2003.822821. http://www.fmrib.ox.ac.uk/analysis/techrep/ [DOI] [PubMed]
- Beckmann CF, Smith SA. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Borden JW, Peterson DR, Jackson EA. The Beck anxiety inventory in nonclinical samples: Initial psychometric properties. Journal of Psychopathology and Behavioral Assessment. 1991;13:345–356. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Booker BH, Donald MW. Contribution of the speech musculature to apparent human EEG asymmetries prior to vocalization. Brain and Language. 1980;9:226–245. doi: 10.1016/0093-934x(80)90143-1. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos NP, Makarov VA. Recovering EEG brain signals: Artifact suppression with wavelet enhanced independent component analysis. Journal of Neuroscience Methods. 2006;158:300–312. doi: 10.1016/j.jneumeth.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, Harmon-Jones E. Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology. 2001;38:912–925. doi: 10.1111/1469-8986.3860912. [DOI] [PubMed] [Google Scholar]
- Cohen BH, Davidson RJ, Senulis JA, Saron CD, Weisman DR. Muscle tension patterns during auditory attention. Biological Psychology. 1992;33:133–156. doi: 10.1016/0301-0511(92)90028-s. [DOI] [PubMed] [Google Scholar]
- Chung JW, Kim C, McCall WD., Jr Effect of sustained contraction on motor unit action potentials and EMG power spectrum of human masticatory muscles. Journal of Dental Research. 2002;81:646–649. doi: 10.1177/154405910208100914. [DOI] [PubMed] [Google Scholar]
- Crespo-Garcia M, Atienza M, Cantero JL. Muscle artifact removal from human sleep EEG by using independent component analysis. Annals of Biomedical Engineering. 2008;36:467–475. doi: 10.1007/s10439-008-9442-y. [DOI] [PubMed] [Google Scholar]
- Castellanos NP, Makarov VA. Recovering EEG brain signals: Artifact suppression with wavelet enhanced independent component analysis. Journal of Neuroscience Methods. 2006;158:300–312. doi: 10.1016/j.jneumeth.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. I. Journal of Personality and Social Psychology. 1990;58:330–341. [PubMed] [Google Scholar]
- Debener S, Mullinger KJ, Niazy RK, Bowtell RW. Properties of the ballistocardiogram artefact as revealed by EEG recordings at 1.5, 3 and 7 Tesla. International Journal of Psychophysiology. 2008;67:189–199. doi: 10.1016/j.ijpsycho.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon SP, Powers DM, Pope KJ, Clark CR. Removal of EEG noise and artifact using blind source separation. Journal of Clinical Neurophysiology. 2007;24:232–243. doi: 10.1097/WNP.0b013e3180556926. [DOI] [PubMed] [Google Scholar]
- Goncharova II, McFarland DJ, Vaughan TM, Wolpaw JR. EMG contamination of EEG: Spectral and topographical characteristics. Clinical Neurophysiology. 2003;114:1580–1593. doi: 10.1016/s1388-2457(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Grouiller F, Vercueil L, Krainik A, Segebarth C, Kahane P, David O. A comparative study of different artefact removal algorithms for EEG signals acquired during functional MRI. NeuroImage. 2007;38:124–137. doi: 10.1016/j.neuroimage.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Gray H. Anatomy of the human body. 1918/2000 http://www.bartleby.com/107.
- Gwin JT, Gramann K, Makeig S, Ferris DP. Removal of movement artifact from high-density EEG recorded during walking and running. J Neurophysiol. 2010;103:3526–3534. doi: 10.1152/jn.00105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse CW, James CJ. Stepwise model order estimation in blind source separation applied to ictal EEG. Conf Proc IEEE Eng Med Biol Soc. 2004;2:986–989. doi: 10.1109/IEMBS.2004.1403327. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: A comparison of two prominent methods. PLoS ONE. 2009;3:e3004. doi: 10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen A, Ramkukar P, Parkkonen L, Hari R. Independent components analysis of short-time Fourier transforms for spontaneous EEG/MEG analysis. NeuroImage. 2010;49:257–271. doi: 10.1016/j.neuroimage.2009.08.028. [DOI] [PubMed] [Google Scholar]
- James CJ, Hesse CW. Independent component analysis for biomedical signals. Physiol Meas. 2005;26:R15–R39. doi: 10.1088/0967-3334/26/1/r02. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Gorodnitsky IF, Kutas M. Automatic removal of eye movement and blink artifacts from EEG data using blind component separation. Psychophysiology. 2004;41:313–325. doi: 10.1111/j.1469-8986.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000a;37:163–178. [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000b;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Klemm M, Haueisen J, Ivanova G. Independent component analysis: Comparison of algorithms for the investigation of surface electrical brain activity. Medical and Biological Engineering and Computing. 2009;47:413–423. doi: 10.1007/s11517-009-0452-1. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee TW, Jolesz FA, Yoo SS. Independent vector analysis (IVA): Multivariate approach for fMRI group study. Neuroimage. 2008;40:86–109. doi: 10.1016/j.neuroimage.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping. 2007;28:1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsen JP, Bhattacharya J. Correction of blink artifacts using independent component analysis and empirical mode decomposition. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Makeig S, Onton J. A trial-by-trial pattern approach to event-related EEG analysis: ERP features and EEG dynamics: An ICA perspective. In: Luck S, Kappenman E, editors. Oxford handbook of event-related potential components. (in press) [Google Scholar]
- Mammone N, Morabito FC. Enhanced automatic artifact detection based on independent component analysis and Renyi’s entropy. Neural Netw. 2008;21:1029–1040. doi: 10.1016/j.neunet.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Mantini D, Franciotti R, Romani GL, Pizzella V. Improving MEG source localizations: an automated method for complete artifact removal based on independent component analysis. NeuroImage. 2008;40:160–173. doi: 10.1016/j.neuroimage.2007.11.022. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, Bachhuber DRW, Koppenhaver AM, Greischar LL, et al. Validation of ICA-based myogenic artifact correction for scalp and source-localized EEG. NeuroImage. 2010;49:2416–2432. doi: 10.1016/j.neuroimage.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, Greischar LL, Davidson RJ. Validation of regression-based myogenic correction techniques for scalp and source-localized EEG. Psychophysiology. 2009;46:578–592. doi: 10.1111/j.1469-8986.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraux A, Ianetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- Naeem M, Brunner C, Pfurtscheller G. Dimensionality reduction and channel selection of motor imagery electroencephalographic data. Comput Intell Neurosci. 2009 doi: 10.1155/2009/537504. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G, Curio G. The effect of artifact rejection by signal-space projection on source localization accuracy in MEG measurements. IEEE Trans Biomed Eng. 1999;46:400–408. doi: 10.1109/10.752937. [DOI] [PubMed] [Google Scholar]
- Ohla K, Hudry J, le Coutre J. The cortical chronometry of electrogustatory event-related potentials. Brain Topogr. 2009;22:73–82. doi: 10.1007/s10548-009-0076-7. [DOI] [PubMed] [Google Scholar]
- Olbrich S, Mulert C, Karch S, Trenner M, Leicht G, Pogarell O, Hegerl U. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage. 2009;45:319–322. doi: 10.1016/j.neuroimage.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Olbrich SO, Jödicke J, Sander C, Himmerich H, Hegerl U. ICA-based muscle artefact correction of EEG data: What is muscle and what is brain? Comment on McMenamin et al. NeuroImage. doi: 10.1016/j.neuroimage.2010.04.256. (in press) [DOI] [PubMed] [Google Scholar]
- Onton J, Makeig S. Information-based modeling of event-related brain dynamics. Progress in Brain Research. 2006;159:99–120. doi: 10.1016/S0079-6123(06)59007-7. [DOI] [PubMed] [Google Scholar]
- Onton J, Makeig S. High-frequency broadband modulation of electroencephalographic spectra. Front Hum Neurosci. 2009;3:61. doi: 10.3389/neuro.09.061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onton J, Westerfield M, Townsend J, Makeig S. Imaging human EEG dynamics using independent component analysis. Neuroscience and Biobehavioral Reviews. 2006;30:808–822. doi: 10.1016/j.neubiorev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge University Press; New York, NY, USA: 2007. pp. 56–84. [Google Scholar]
- Rajan JJ, Rayner PJW. Model order selection for the singular value decomposition and the discrete Karhunen-Loeve transform using a Bayesian approach. Vision Image Signal Processing IEEE Proc. 1997;144:116–123. [Google Scholar]
- Roesch MW, Olson CO. Neuronal activity related to anticipated reward in frontal cortex: Does it represent value or reflect motivation? Annals of the New York Academy of Sciences. 2007;1121:431–446. doi: 10.1196/annals.1401.004. [DOI] [PubMed] [Google Scholar]
- Romero S, Mananas MA, Barbanoj MJ. A comparative study of automatic techniques for ocular artifact reduction in spontaneous EEG signals based on clinical target variables: A simulation case. Computers in Biology and Medicine. 2008;38:348–360. doi: 10.1016/j.compbiomed.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ryali S, Glover GH, Chang C, Menon V. Development, validation, and comparison of ICA-based gradient artifact reduction algorithms for simultaneous EEG-spiral in/out echo-planar fMRI recordings. NeuroImage. 2009;48:348–361. doi: 10.1016/j.neuroimage.2009.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MA, Serlin RC. Equivalence confidence intervals for two-group comparisons of means. Psychological Methods. 1998;3:403–411. [Google Scholar]
- Shackman AJ. The potentially deleterious impact of muscle activity on gamma band inferences. Neuropsychopharmacology. 2010;35:847. doi: 10.1038/npp.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. NeuroImage. doi: 10.1016/j.neuroimage.2010.03.037. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Slagter HA, Maxwell JS, Greischar LL, Davidson RJ. Electromyogenic artifacts and electroencephalographic inferences. Brain Topography. 2009;21:7–12. doi: 10.1007/s10548-009-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Tang AC, Liu JY, Sutherland MT. Recovery of correlated neuronal sources from EEG: the good and bad ways of using SOBI. NeuroImage. 2005;28:507–519. doi: 10.1016/j.neuroimage.2005.06.062. [DOI] [PubMed] [Google Scholar]
- Tassinary LG, Cacioppo JT, Vanman EJ. The skeletomotor system: Surface electromyography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. NY: Cambridge University Press; 2007. pp. 267–302. [Google Scholar]
- Tesche CD, Uusitalo MA, Ilmoniemi RJ, Huotilainen M, Kajola M, Salonen O. Signal-space projections of MEG data characterize both distributed and well-localized neuronal sources. Electroencephalogr Clin Neurophysiol. 1995;95:189–200. doi: 10.1016/0013-4694(95)00064-6. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Vanderperren K, De Vos M, Ramautar JR, Novitskiy N, Mennes M, Assecondi S, Vanrumste B, Stiers P, Van den Bergh BR, Wagemans J, Lagae L, Sunaert S, Van Huffel S. Removal of BCG artifacts from EEG recordings inside the MR scanner: a comparison of methodological and validation-related aspects. NeuroImage. 2010;50:920–34. doi: 10.1016/j.neuroimage.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Viola FC, Thorne J, Edmonds B, Schneider T, Eichele T, Debener S. Semi-automatic identification of independent components representing EEG artifact. Clinical Neurophysiology. 2009;120:868–877. doi: 10.1016/j.clinph.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Wallstrom GL, Kass RE, Miller A, Cohn JF, Fox NA. Automatic correction of ocular artifacts in the EEG A comparison of regression-based and component-based methods. International Journal of Psychophysiology. 2004;53:105–119. doi: 10.1016/j.ijpsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Waterink W, van Boxtel A. Facial and jaw-elevator EMG activity in relation to changes in performance level during a sustained information processing task. Biological Psychology. 1994;37:183–198. doi: 10.1016/0301-0511(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Lewis T, Pope KJ, Fitzgibbon SP, Clark CR, Loveless S, et al. Thinking activates EMG in scalp electrical recordings. Clinical Neurophysiology. 2008;119:1166–1175. doi: 10.1016/j.clinph.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, et al. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clinical Neurophysiology. 2007;118:1877–1888. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]