Abstract
Background
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) expressing Panton-Valentine Leukocidin (PVL) cause severe skin and soft tissue infections (SSTI), necrotizing pneumonia and other invasive infections. PVL toxin has been implicated as a virulence factor and antibody to a component of this toxin is under investigation as a vaccine candidate. The role of PVL in pathogenesis remains controversial and it is unknown if human serum antibody to PVL modulates infection.
Methods
We determined antibody levels to PVL in sera from children aged 0-18 years presenting with PCR-confirmed PVL-positive MRSA SSTI with or without prior MRSA infection or SSTI, PVL-positive MRSA invasive infections, PVL-negative MRSA infections and uninfected controls. We also measured antibody-mediated neutralization of PVL-induced lysis of human polymorphonuclear cells.
Results
Antibody to PVL was present in healthy children reaching adult levels by 4-6 years with a nadir at 4-6 months likely due to loss of maternal antibody. Children with a primary PVL-positive MRSA infection had moderate levels of antibody to PVL that increased following infection. Children with prior MRSA or SSTI infections had high levels of antibody to PVL at the onset of infection. There was no increase in antibody to PVL in this populations’ sera after the onset of infection. Sera from children with PVL-positive MRSA SSTIs, particularly those with prior MRSA or SSTI, and convalescent sera from children with invasive PVL-positive MRSA infection, potently inhibited PVL-induced lysis of PMNs.
Conclusions
Neutralizing antibody to PVL does not protect children against primary or recurrent CA-MRSA SSTI.
Keywords: Staphylococcus aureus, MRSA, Panton-Valentine leukocidin, antibody
In the last decade, infection with community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has increased in both adults and children [1-5]. CA-MRSA causes skin and soft tissue infection (SSTI) and, less commonly, necrotizing pneumonia and other invasive infections in otherwise healthy patients [2, 3, 6, 7]. In the United States, S. aureus type USA 300 accounts for the majority of CA-MRSA, and along with other CA-MRSA types, characteristically produces Panton-Valentine leukocidin (PVL) [6, 8-12]. While purified PVL, composed of proteins LukS and LukF, has clear pore-forming cytotoxic activity on human polymorphonuclear cells (PMNs), its role in pathogenesis remains controversial. Animal studies have indicated a pathogenic role [13-15], no effect [16-18] or even an anti-virulence role [19, 20]. The LukS component, within a multivalent vaccine, is under phase 1 clinical trials in humans [21]. We evaluated the levels of antibody to PVL in uninfected children and in children with MRSA SSTI and invasive infections to determine if cytotoxic-neutralizing antibodies to PVL were associated with resistance to infection.
Methods
Study oversight
The Institutional Review Board, Children’s Hospital Boston approved this study.
Study design and subjects
Uninfected controls were 152 children between 0 and 18 years attending a pre-operative clinic between September and December 2008 (fig 1). Serum was obtained prospectively from excess blood drawn for complete blood counts. Sera were collected within 48 hours of the blood draw and stored at −20°C. Children were excluded if there was concurrent infection or a history of immunodeficiency upon chart review.
Figure 1.
Antibody levels to PVL components LukF and LukS. Points represent IgG antibody (ng/mL) to LukF and LukS and lines the median value for each group. A and B: Age related levels of IgG antibody to LukF (A) and LukS (B) in 152 controls. Logistic regression, controlling for age and age-age interaction (age2), detected a non-linear (j-shaped) relationship between age and antibody level. C and D: Antibody levels in children with PVL-positive MRSA infections in the serum sample collected closest to the onset of infection from 52 children with PVL-positive and 14 children with PVL-negative MRSA infection and 152 controls. Median values and interquartile ranges (IQR) for levels of antibody to LukF among children with PVL-positive and PVL-negative MRSA infections and controls were 2,427 ng/mL (1,131-11,846), 1,114 ng/mL (267-2,399, and 477 ng/mL (106-2,205) respectively; and to LukS, 1,762 ng/mL (501-9,140), 1,538 ng/mL (357-5,925), and 693 ng/mL (135-2,797) respectively. Logistic regression controlling for age and day of illness was used to compare PVL-positive to PVL-negative patients and age was used to control the comparison between PVL-positive or PVL-negative patients to controls.
MRSA-infected subjects were identified from October 2008 to September 2009 from microbiology reports. Data obtained from chart reviews and an administered questionnaire (inpatients only) included 1) demographics 2) culture source 3) diagnosis 4) onset of illness 5) history of MRSA infection or SSTI (hereafter “prior MRSA/SSTI”) 6) household contact with MRSA infection or SSTI 7) immunodeficiency 8) intravenous immunoglobulin (IVIgG) administration. Subjects were excluded if they had immunodeficiency or a history of IVIgG administration.
Bacterial Strains
S. aureus strain LAC, belonging to sequence type USA300, was provided by M. Otto, NIH, Bethesda, MD.
PVL gene detection
S. aureus DNA was extracted and the lukS/lukF-PVL genes were amplified with previously described primers and protocols [22, 23].
Recombinant PVL purification
The lukF and lukS genes were cloned separately into a maltose-binding fusion vector (pMAL-c2x), as described elsewhere [20]. Recombinant proteins were purified according to the manufacturer’s instructions.
PVL antibody quantification
Antibody concentrations to LukF and LukS in pharmaceutical 10% IVIgG were determined by ELISA using known total IgG as a standard. Wells coated with antibody to human IgG-γ chain or recombinant LukF or LukS were incubated with known amounts of human IgG followed by rabbit antibody to human IgG-whole molecule. The ODs from wells coated with antibody to human IgG--γ chain generated a standard curve for the amount of IgG bound. The standard curve was used to quantify antibody to LukF and LukS in IVIgG. IVIgG was the standard for all subsequent ELISAs to quantify antibody present in patients’ sera.
Preparation of human PMNs
Informed consent was obtained under a Partner’s Healthcare Institutional Review Board approved protocol. Blood was drawn from healthy adult volunteers, and PMNs isolated using a previously described method [24].
Measuring inhibition of PMN lysis by PVL antibody
Functionality of antibody to PVL was measured by its ability to inhibit PVL-induced lysis of human PMNs, which was quantified by release of lactate dehydrogenase (LDH) using an in vitro assay kit. We incubated 1×106 fresh human PMNs, 25 ng each of recombinant LukF and LukS proteins and human sera. A positive control included PMNs, recombinant proteins and PBS. A negative control included PMNs, serum at its highest concentration and PBS. Reactions were incubated at room temperature for 1 hour. After centrifugation of samples, 30 μl of supernatant was used to measure LDH activity according to the manufacturer’s instructions. Percent LDH release was calculated by dividing the OD of the sample by the OD of the positive control. The “75% inhibition titer” was defined as the lowest serum dilution resulting in ≥75% inhibition of PVL-induced lysis of PMN.
Measuring antibody to S. aureus surface proteins
To determine a potential relationship between antibody to PVL and antibody to S. aureus surface proteins, we measured antibody to whole cells of S. aureus strain LAC by ELISA and, from serial dilutions of patient sera, calculated the half maximal effective concentration (EC50) for binding. We compared EC50s between MRSA infected groups and controls, and we looked for correlations between EC50s, day of illness and LukF and LukS antibody levels.
Statistics
Trends in antibody levels to PVL and differences in antibody levels between patient groups were determined using logistic regression controlling for various factors (figures 1-4 legends). Differences in 75% inhibition titers were analyzed using a Wilcoxon test with t approximation comparing selected group pairs. Differences in EC50 were compared using a Kruskal-Wallis test. Analyses were performed using SAS statistical software version 9.1. Correlations between 75% titer and PVL antibody levels, EC50 and day of illness and EC50 and PVL antibody levels were determined by correlation analysis and Spearman r-value using Prism4 statistical software. Two-sided p-values <= 0.05 were considered statistically significant.
Figure 4.
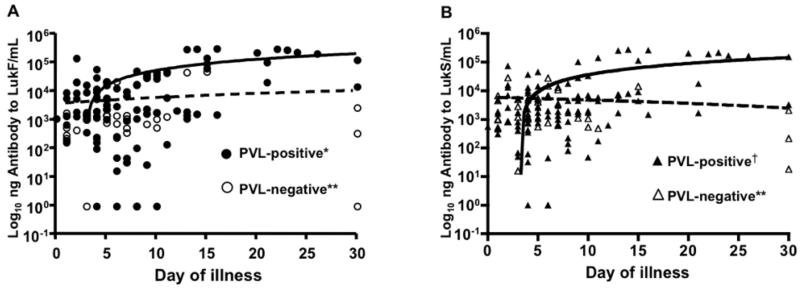
Antibody to PVL over time in children with PVL-positive MRSA infection with or without prior MRSA or SSTI. Points represent IgG antibody to LukF. (A) and LukS (B) in children with PVL-positive MRSA infection including 16 serum samples from 15 children with prior MRSA or SSTI and 84 serum samples from 33 children with no prior MRSA or SSTI, plotted by day of illness. Each patient had between one and nine serum samples obtained on different days of illness. Statistical analysis was performed by logistic regression controlling for patient age and for multiple samples. Significant increases in antibody levels over time were found for children with no prior MRSA/SSTI (¶¶ p=0.005 and †† p= 0.058 for upward trend in increase in antibody level by day of illness) but not for children with a prior MRSA/SSTI (¶ p>0.05 for upward trend in increase in antibody level by day of illness).
Results
Study cohort characteristics
Healthy controls were 60% male and 69% white. Demographic characteristics of MRSA infected children are shown in table 1. There were no statistical differences in sex, age or race distribution between children with PVL-positive or PVL-negative MRSA infections. Children with PVL-negative S. aureus infections were more likely to have had a healthcare-associated exposure (p<0.001) and an invasive infection (p=0.003), a finding consistent with the established epidemiology of CA-MRSA and hospital-acquired MRSA infections.
Table 1. Characteristics of children with methicillin resistant Staphylococcus aureus infection.
| Total N=66 (%) |
PVL-positive N=52 (%) |
PVL- negative N=14 (%) |
P- valuesa |
|
|---|---|---|---|---|
| Sex | ||||
| Male | 38 (57.58) | 28 (53.88) | 10 (71.43) | 0.362 |
| Race | 0.301 | |||
| White | 37 (56.06) | 32 (61.54) | 5 (35.71) | |
| Black | 9 (13.64) | 6 (11.54) | 3 (21.43) | |
| Hispanic | 8 (12.12) | 6 (11.54) | 2 (14.29) | |
| Asian | 2 (3.03) | 1 (1.92) | 1 (7.14) | |
| Other/unknown | 10 (15.15) | 7 (13.46) | 3 (21.43) | |
| Age Group | 0.794 | |||
| 0-2 months | 9 (13.64) | 7 (13.46) | 2 (14.29) | |
| 3-5 months | 1 (1.52) | 1 (1.92) | 0 (0.00) | |
| 6-11 months | 8 (12.12) | 7 (13.46) | 1 (7.14) | |
| 12-23 months | 14 (21.21) | 12 (23.08) | 2 (14.29) | |
| 2-3 years | 9 (13.64) | 6 (11.54) | 3 (21.43) | |
| 4-6 years | 4 (6.06) | 2 (3.03) | 2 (14.29) | |
| 7-9 years | 2 (3.03) | 1 (1.92) | 1 (7.14) | |
| 10-12 years | 5 (7.58) | 4 (7.69) | 1 (7.14) | |
| 13-15 years | 8 (12.12) | 7 (13.46) | 1 (7.14) | |
| 16-18 years | 6 (9.09) | 5 (9.62) | 1 (7.14) | |
| Age mean (range) | 5.25 (.04- 18) |
4.83 (.04-17) |
5.36 (.04- 18) |
|
| Prior MRSA or SSTI | 0.143 | |||
| Yes | 23 (34.85) | 16 (30.77) | 7 (50.00) | |
| Unknown | 5 (7.58) | 3 (5.77) | 2 (14.29) | |
| MRSA or SSTI contact | 0.281 | |||
| Yes | 17 (25.76) | 15 (28.85) | 2 (14.29) | |
| Unknown | 25 (37.88) | 17 (32.69) | 8 (57.14) | |
|
Healthcare-associated
exposure † |
19 (28.79) | 6 (11.54) | 13 (92.86) | <0.001 |
| Disease type | 0.003 | |||
| SSTI | 43 (63.15) | 39 (75.0) | 24 (28.57) | |
| Invasive‡ | 23 (34.85) | 13 (25.00) | 10 (71.43) |
p-value calculated using Fisher’s exact test.
Includes hospitalization or surgery within 1 year, dialysis, indwelling catheter or residence in a long-term care facility.
Invasive infections include osteomyelitis (PVL-positive 1, PVL-negative 1), bacteremia (PVL-positive 3, PVL-negative 2), pneumonia (PVL-positive 4, PVL-negative 2), pleural effusion (PVL-negative 1), pyelonephritis (PVL-negative 1), and septic arthritis (PVL-negative 1).
Quantification of antibody to LukF and LukS in human sera
The lot of IVIgG used as a standard for each assay contained 32,200 ng/mL and 18,200 ng/mL of antibody to LukF and LukS, respectively. Standard curves obtained from the IVIgG were reproducible with mean slopes of 9.31 (95% CI=8.47-10.16) and 8.83 (95% C.I.=8.06-9.61) for antibody to LukF and LukS, respectively. Values of r2 ranged from 0.94 to 1.00 for all standard curves.
Panton-Valentine leukocidin antibody in uninfected controls
Antibody to LukF and LukS proteins in healthy controls ranged from non-detectable to 16,556 and 40,980 ng/mL, respectively. Age-associated trends in antibody levels to both LukF and LukS were non-linear but significant (p<0.001), with higher levels in children aged 0-2 months, lower levels in infants aged 3-11 months and increasing levels in children aged 1-6 years (Figs 1A-B).
Antibodies to Panton-Valentine leukocidin in MRSA-infected children and uninfected controls
Antibody levels to LukF and LukS proteins in serum samples collected closest to the onset of infection from children with PVL-positive or PVL-negative MRSA infections and controls are shown in Figs. 1C-1D. After controlling for patient age and day of illness, antibody levels to LukF were significantly higher (p<0.001) in sera from patients with PVL-positive MRSA infections compared to controls (Fig. 1C). There were no significant differences between antibody levels to LukF in these initial sera from children with PVL-positive MRSA and from those with PVL-negative MRSA infections (p=0.082) or between antibody levels to LukF in sera from children with PVL-negative MRSA infections and uninfected controls (p=0.212, Fig. 1C).
Similar to the findings with the LukF protein, antibody levels to LukS were significantly higher (P<0.001) in sera from children with PVL-positive MRSA infections compared with uninfected controls after controlling for patient age and day of illness (Fig 1D). Again, as with the antibody levels to LukF, the antibody levels to LukS were not different between sera from children with PVL-positive and PVL-negative infections (p=0.623). In contrast to LukF antibody levels, there were slightly higher (p=0.046) antibody levels to LukS in sera from children with PVL-negative MRSA infections compared to controls (Fig. 1D).
On univariate analysis, only a prior MRSA/SSTI was associated with significantly elevated levels of antibody to both LukF (p=0.036) and LukS (p=0.006, Table 2). Other potential predictors of antibody levels, including day of illness, a household contact with MRSA infection or SSTI, PVL-status of the infecting strain and MRSA disease category (invasive or SSTI), were not independently associated with elevated levels of antibody to LukF or LukS. On multivariate analysis (controlling for all potential predictors except a household MRSA/SSTI contact) only a prior MRSA/SSTI remained significantly associated with elevated levels of LukF and LukF (p=0.011 and 0.002, respectively). Patient age was also associated with higher antibody levels to LukF (p=0.052) and LukS (p=0.021) on multivariate analysis.
Table 2. Potential predictors of levels of antibody to LukF and LukS.
| Antibody to LukF | Antibody to LukS | |||||
|---|---|---|---|---|---|---|
| Mean antibody to LukF (ng/mL) (SEM)a |
Mean antibody to LukS (ng/mL) (SEM) |
|||||
| Predictor | Univariate p-value† |
Multivariate p-value‡ |
Univariate p-value† |
Multivariate p-value‡ |
||
| Age | - | 0.064 | 0.052 | - | 0.013 | 0.021 |
| Day of Illness | - | 0.700 | 0.472 | - | 0.937 | 0.409 |
|
Prior MRSA or
SSTI |
0.036 | 0.011 | 0.006 | 0.002 | ||
| Yes | 25,598 (9,050) |
13,426 (4,465) |
||||
| No | 12,415 (7,908) |
10,242 (6,639) |
||||
| Unknown | 28,254 (25,050) |
35,076 (28,597) |
||||
|
MRSA/SSTI
Contact |
0.295 | - | 0.222 | - | ||
| Yes | 17,238 (9,367) |
8,613 (4,495) |
||||
| No | 17,723 (12,463) |
15,128 (10,455) |
||||
| Unknown | 19,335 (7,669) |
14,556 (6,430) |
||||
|
Disease
Category |
0.229 | 0.534 | ||||
| SSTI | 12,444 (3,839) |
6,736 (1,950) |
0.829 | 0.687 | ||
| Invasive | 28,986 (15,025) |
25,379 (12,500) |
||||
| PVL | 0.058 | 0.074 | 0.515 | 0.236 | ||
| Positive | 21,562 (7,264) |
15,642 (5,794) |
||||
| Negative | 5,753 (3,520) |
4,285 (1,944) |
||||
Standard error of the mean
p-value calculated using logistic regression with single variable.
p-value calculated using logistic regression controlling for age, day of illness, prior MRSA or SSTI, disease category and PVL status of isolate
Antibody levels to PVL proteins among children with MRSA infections
As it is likely that many S. aureus infections, particularly invasive ones, are preceded by a period of colonization and/or clinically-unapparent infection, which could potentially induce antibody to S. aureus antigens prior to symptoms of infection, we analyzed antibody levels to LukF and LukS in serum samples obtained closest to the onset of infection from PVL-positive MRSA infected children with SSTI, and compared these with samples from children with and without prior MRSA/SSTI. We also compared these two groups to those with invasive MRSA infections. After controlling for age and day of illness, we found no significant differences in antibody levels to LukF or LukS between children with prior MRSA/SSTI and children with invasive infections (Fig. 2) or between children with no prior MRSA/SSTI and children with invasive infections (Fig. 2). However, children with PVL-positive MRSA SSTI with prior MRSA/SSTI had significantly higher levels of antibody to LukF and LukS than children presenting with MRSA SSTI without a history of prior MRSA/SSTI (p=0.001 for both comparisons, Fig. 2).
Figure 2.
Children with PVL-positive MRSA infection and prior MRSA or SSTI had higher levels of antibody to PVL than children with no prior MRSA or SSTI. Points represent levels of IgG antibody to LukF and LukS, and lines represent the median antibody levels, in children with PVL-positive MRSA infection, including 15 children with prior MRSA/SSTI, 24 patients with no prior MRSA /SSTI and 13 patients with invasive infection. Medians and IQR for levels of antibody to LukF among children with prior MRSA/SSTI, no prior MRSA/SSTI and invasive infection were 9,587 ng/mL (2,378-22,537), 1,433 ng/mL (319-5,546) and 3,039 ng/mL (1,077-41,296) respectively; and to LukS 6,867 ng/mL (1,085-15,587), 1,020 ng/mL (370-2,675) and 2,553 ng/mL (446-36,665) respectively. There was no difference in levels of antibody to PVL in children with PVL-positive invasive MRSA infection in serum samples collected closest to the onset of infection compared to either if the other two groups. Statistical analysis was performed by logistic regression controlling for patient age and day of illness.
Changes in antibody levels to PVL proteins as a consequence of MRSA infection
To determine if an antibody response to PVL proteins was a consequence of infection with PVL-producing MRSA, we measured antibody to LukF and LukS in all available serum samples. The antibody levels to LukF and LukS in children with PVL-positive MRSA infection increased when plotted by day of illness (Fig. 3A-3B, p<0.001 and p=0.001, respectively), whereas antibody levels to LukF and LukS from children with PVL-negative MRSA infections did not increase significantly. When comparing the changes in levels of antibody to the PVL proteins between children with PVL-positive or PVL-negative MRSA, the differences between the slopes were also significantly different (p=0.004 and 0.001, respectively, Figs. 3A-3B).
Figure 3.
Antibody to PVL over time in children with PVL-positive or PVL-negative MRSA infection. Points represent IgG antibody to LukF (A) and LukS (B) in 105 serum samples from 51 children with PVL-positive MRSA and 33 serum samples from 14 children with PVL-negative MRSA infection, plotted by day of illness. Each patient had between one and nine serum samples obtained on different days of illness. Statistical analysis was performed by logistic regression controlling for patient age and multiple samples. Significant increases in antibody levels over time were found for children with PVL-positive MRSA (* p<0.001 for upward trend in individual slope and p=0.004 for comparison with the slope of the line for antibody levels in children with PVL-negative MRSA; † p=0.001 for upward trend in individual slope and for the comparison with the slope of the line for antibody levels in children with PVL-negative MRSA). No significant increase in antibody to LukF or LukS was detected in children with PVL-negative MRSA (** p>0.05 for upward trend in antibody level over time).
Antibody levels to both LukF and LukS increased when plotted by day of illness among children with PVL-positive MRSA infection and no prior MRSA/SSTI (Figs. 4A-4B, p=0.005 and 0.058, respectively) but did not increase in children with a prior MRSA/SSTI. Thus, an initial PVL-positive MRSA infection produced a robust antibody response to PVL but a subsequent infection did not further boost antibody levels.
Neutralizing activity of antibody to PVL in MRSA-infected subjects and controls
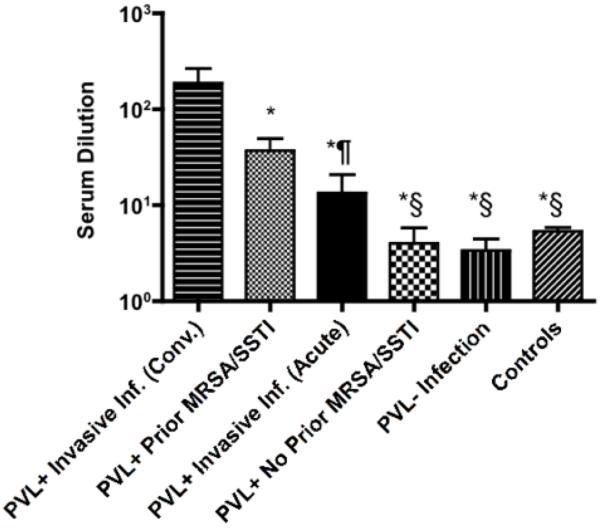
The highest 75% inhibition titers were detected in patients convalescing from PVL-positive, invasive MRSA infection (sera obtained after day 10 of illness) and those with a PVL-positive SSTI who had a prior MRSA/SSTI (Fig. 5). Sera from both of these groups had significantly higher 75% inhibition titers compared to sera from all other groups, including acute-phase sera (obtained before day 10 of illness) from patients with PVL-positive invasive MRSA infection, PVL-positive MRSA SSTI and no prior MRSA/SSTI, PVL-negative MRSA infections and controls. The ability of sera to inhibit PVL-induced PMN lysis significantly correlated with the overall level of antibody to LukF and LukS (r=0.79, p<0.001 and r=0.70, p<0.001, respectively), indicating that most of the antibody to PVL in serum had cytotoxin-neutralizing activity.
Figure 5.
Neutralizing antibody responses to PVL. Bars represent mean value of serum dilution required to inhibit ≥75% of PV-induced cytotoxicity and error bars the standard errors of the mean. Patients include 13 children with PVL-positive MRSA SSTI with prior MRSA/SSTI, 9 children with PVL-positive MRSA SSTI without prior MRSA/SSTI, 10 children with convalescent invasive PVL-positive MRSA infection (sera obtained after day 10 of illness), 9 children with acute invasive PVL-positive MRSA infection (sera obtained before day 10 of illness), 8 children with PVL-negative MRSA infection and 21 controls. Statistical analysis was performed by a Wilcoxon two-sample test with t approximation. * p<0.05 when compared to convalescent invasive PVL-positive MRSA infection group, § p<0.05 when compared to PVL-positive MRSA SSTI with prior MRSA/SSTI group ¶ p=0.055 when compared to PVL-positive MRSA SSTI with prior MRSA/SSTI group.
Antibody to S. aureus surface proteins in MRSA-infected subjects and controls
Antibody to S. aureus surface proteins in the sera obtained closest to the onset of infection from 64 MRSA infected patients and 17 controls generated EC50 levels ranging from 193-44,400. There was no significant difference in EC50s between patients with PVL-positive MRSA SSTIs with and without prior MRSA/SSTI, PVL-positive invasive MRSA, PVL-negative SSTI and invasive MRSA (data not shown). There was also no correlation between EC50 and LukF or LukS antibody levels or between EC50 and day of illness. Thus, the levels of naturally acquired antibody to S. aureus surface proteins is not markedly affected by MRSA infection.
Discussion
We found that antibody to PVL is naturally acquired in childhood in an age-related fashion typical of that for antibodies to other bacterial antigens [25-27]. Higher levels of antibody were present in neonates likely due to transplacental acquisition of IgG, with subsequent decreases temporally associated with loss of maternal antibody. Antibody levels rose with age, reaching a plateau by approximately 4-6 years of age. Notably, children infected with MRSA strains containing the lukF and lukS genes, especially those with invasive infection, developed a significant cytotoxin-neutralizing antibody response to PVL. Of critical importance, children with CA-MRSA SSTI with a prior MRSA/SSTI had significantly higher cytotoxic-neutralizing antibody levels to PVL compared to children presenting with their first MRSA SSTI.
Overall, it appears that high levels of functional antibody to PVL are present in serum samples from children diagnosed with a MRSA SSTI and prior MRSA/SSTI, indicating that this antibody has, at a minimum, no role in preventing recurring SSTI. Antibody to PVL might actually promote susceptibility to CA-MRSA infection if it neutralizes the pro-inflammatory activities of PVL, as has been demonstrated in experimental animal infections [20]. Of additional importance, phase 1 clinical trials injecting humans with the LukS component of PVL have commenced [21]. Our results suggest this immunogen may contribute little to protective immunity and might potentially increase susceptibility to infection.
Three recent studies have addressed PVL-antibody responses in humans. Verkaik et al. [28] found increased titers to LukS but not LukF in patients with a PVL-positive SSTI. Brown et al. [29] determined the antibody response to PVL in children presenting with PVL-positive SSTI, osteomyelitis and pneumonia, reporting that children with invasive infection had significant increases in antibody to LukF and LukS. The authors noted that antibody levels to PVL in patients with invasive infections correlated with the day of illness, but did not control for this variable when comparing antibody levels in children with invasive disease to SSTI. Our data show that after controlling for day of illness, age, a history of prior infection and PVL status of the infecting MRSA isolate, the initial values of antibody to PVL in children with SSTI and invasive disease were similar.
Croze et al. [30] reported antibody levels to PVL in paired serum samples from patients with PVL-negative and PVL-positive SSTI and invasive infections. They found a greater increase in PVL antibody levels in patients with PVL-positive infections than PVL-negative infections and that patients with SSTI and invasive infection had similar increases in antibody levels to PVL. Interestingly, one study patient with recurrent furunculosis had an elevated antibody to PVL level on presentation, which did not rise over time. Our data are consistent with these findings, in that antibody levels to PVL in sera from patients with SSTI or invasive disease were similar in the initial serum samples. However, our cohort included many more patients with recurrent infections and when controlling for other factors, only a history of recurrent disease was associated with elevated levels of antibody to PVL.
To assess the rate of recurrence in our population, we reviewed records at Children’s Hospital Boston from 2005-2009 and found that 12% of children with MRSA SSTI had a prior MRSA SSTI. Of all MRSA SSTI isolates obtained in this time period, 24% were from children who had or who would subsequently develop recurrent MRSA SSTI (CRH, unpublished observation).
The research design and analysis present limitations. First, our control cohort was derived from children undergoing pre-operative evaluations for surgery. Whereas we excluded children with concurrent infection through chart reviews, a complete infectious history from these patients was not available. Therefore, some of the 152 control children may have had MRSA colonization or infection. Given that MRSA nasal colonization prevalence in Boston is less than 1% in children [31], the impact on our findings of missing such information would likely be minimal. Second, we only included children in this study if a concurrent blood sample was available. It is possible that children who had blood drawn were different than those who were treated for MRSA infection without further laboratory evaluations. Despite this potential limitation, the sera we compared were from children with MRSA SSTI with or without prior MRSA/SSTI on whom blood was drawn and therefore who likely represent subjects with comparable disease severities. Third, in our definition of children with prior MRSA/SSTI we included children with a history of SSTI without further microbiological data. Given the high recurrence rates of CA-MRSA, frequently associated with the same strain [32-34], we believe that it was appropriate to assume that children with PVL-positive MRSA SSTI and a history of SSTI likely had PVL-positive MRSA in the past. Including children with a prior SSTI not due to a PVL-producing S. aureus would likely have reduced the overall levels of antibody to PVL in this group. Therefore, this possibility does not negate the major conclusion of this study that cytotoxic-neutralizing antibody to PVL is elevated in children with MRSA SSTI and recurrent SSTI and thus antibody to PVL is not associated with protection from infection. Finally, antibodies measured to the component parts of PVL protein may differ from antibodies that bind to the protein after oligomerization. However, given the close correlation we detected between cytotoxic-neutralization capability against functional PVL and the concentration of antibody measured in the sera to the individual LukF and LukS proteins, we would presume that antibodies binding to oligomerized PVL are present in amounts proportional to those for the individual LukF and LukS proteins.
In summary, we found that acquired antibody responses to PVL in children are typical of those associated with responses to other bacterial antigens, with higher levels in neonates compared to children 6 months -4 years old likely due to transplacental transfer of maternal IgG. Children with a primary PVL-positive MRSA infection mounted a robust antibody response to PVL compared to children with PVL-negative MRSA infections. A history of prior MRSA/SSTI was strongly associated with elevated levels of cytotoxin-neutralizing antibodies to PVL in the serum sample collected closest to the onset of subsequent MRSA SSTI. Overall it appears that high functional levels of antibody to PVL in children with prior infection do not provide protection against recurrent PVL-positive MRSA SSTI, and inclusion of the LukS component of PVL in experimental vaccines under development for S. aureus infection may provide little benefit and might have some potential detrimental effects in the context of enhancing susceptibility to infection.
Summary.
Antibody responses to the Panton-Valentine leukocidin measured in healthy children and those with skin and soft tissue MRSA infections indicated natural antibody is acquired by age 4-6, increases with infection and is not associated with protection from recurrent infection.
Acknowledgments
We thank M. Otto for providing the S. aureus LAC strain. We thank Dionne Graham, Jing Zhou and Leslie Kalish from the Clinical Research Program, Children’s Hospital Boston, for reviewing the statistical analysis of our data. This research was supported by NIH grant AI046706 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Potential Conflicts of Interest: Christina Hermos: None; Pauline Yoong: None; Gerald Pier: Dr. Pier reports having received consulting income from Boehringer Ingelheim, Cangene Corp, Selecta Biosciences, AMGEN and Astra-Zenica and licensing income for inventions from GlaxoSmithKlein, Alopexx Pharmaceuticals and Sanofi Aventis.
References
- 1.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 3.Gorak EJ, Yamada SM, Brown JD. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis. 1999;29:797–800. doi: 10.1086/520437. [DOI] [PubMed] [Google Scholar]
- 4.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 5.Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 7.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 8.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 9.Kilic A, Li H, Stratton CW, Tang YW. Antimicrobial susceptibility patterns and staphylococcal cassette chromosome mec types of, as well as Panton-Valentine leukocidin occurrence among, methicillin-resistant Staphylococcus aureus isolates from children and adults in middle Tennessee. J Clin Microbiol. 2006;44:4436–4440. doi: 10.1128/JCM.01546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diep BA, Stone GG, Basuino L, et al. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2008;197:1523–1530. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 11.Chambers HF. Community-associated MRSA--resistance and virulence converge. N Engl J Med. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 12.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 13.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 14.Tseng CW, Kyme P, Low J, et al. Staphylococcus aureus Panton-Valentine leukocidin contributes to inflammation and muscle tissue injury. PLoS One. 2009;4:e6387. doi: 10.1371/journal.pone.0006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diep BA, Chan L, Tattevin P, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 17.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 18.Villaruz AE, Bubeck Wardenburg J, Khan BA, et al. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J Infect Dis. 2009;200:724–734. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A. 2010;107:2241–2246. doi: 10.1073/pnas.0910344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. http://www.clinicaltrials.gov/ct2/show/NCT01011335?term=NABI&rank=5.
- 22.Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42:4947–4955. doi: 10.1128/JCM.42.11.4947-4955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClure JA, Conly JM, Lau V, et al. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J Clin Microbiol. 2006;44:1141–1144. doi: 10.1128/JCM.44.3.1141-1144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated Staphylococcal Poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2005;73:6752–6762. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julander IG, Granstrom M, Hedstrom SA, Mollby R. The role of antibodies against alpha-toxin and teichoic acid in the diagnosis of staphylococcal infections. Infection. 1983;11:77–83. doi: 10.1007/BF01641071. [DOI] [PubMed] [Google Scholar]
- 26.Holmlund E, Quiambao B, Ollgren J, Nohynek H, Kayhty H. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine. 2006;24:57–65. doi: 10.1016/j.vaccine.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 27.Bonventre PF, Linnemann C, Weckbach LS, et al. Antibody responses to toxic-shock-syndrome (TSS) toxin by patients with TSS and by healthy staphylococcal carriers. J Infect Dis. 1984;150:662–666. doi: 10.1093/infdis/150.5.662. [DOI] [PubMed] [Google Scholar]
- 28.Verkaik NJ, Dauwalder O, Antri K, et al. Immunogenicity of toxins during Staphylococcus aureus infection. Clin Infect Dis. 2010;50:61–68. doi: 10.1086/648673. [DOI] [PubMed] [Google Scholar]
- 29.Brown EL, Bowden MG, Bryson RS, et al. Pediatric antibody response to community-acquired Staphylococcus aureus infection is directed to Panton-Valentine leukocidin. Clin Vaccine Immunol. 2009;16:139–141. doi: 10.1128/CVI.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croze M, Dauwalder O, Dumitrescu O, et al. Serum antibodies against Panton-Valentine leukocidin in a normal population and during Staphylococcus aureus infection. Clin Microbiol Infect. 2009;15:144–148. doi: 10.1111/j.1469-0691.2008.02650.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee GM, Huang SS, Rifas-Shiman SL, et al. Epidemiology and risk factors for Staphylococcus aureus colonization in children in the post-PCV7 era. BMC Infect Dis. 2009;9:110. doi: 10.1186/1471-2334-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang SS, Diekema DJ, Warren DK, et al. Strain-relatedness of methicillin-resistant Staphylococcus aureus isolates recovered from patients with repeated infection. Clin Infect Dis. 2008;46:1241–1247. doi: 10.1086/529381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller LG, Quan C, Shay A, et al. A prospective investigation of outcomes after hospital discharge for endemic, community-acquired methicillin-resistant and -susceptible Staphylococcus aureus skin infection. Clin Infect Dis. 2007;44:483–492. doi: 10.1086/511041. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen DM, Mascola L, Brancoft E. Recurring methicillin-resistant Staphylococcus aureus infections in a football team. Emerg Infect Dis. 2005;11:526–532. doi: 10.3201/eid1104.041094. [DOI] [PMC free article] [PubMed] [Google Scholar]