Abstract
Due to the high solubility of oxygen in perfluorocarbons (PFCs), these compounds have been explored for improved cell and tissue oxygenation. The goal of this study is to investigate the effects of a PFC emulsion on cellular growth and function in a tissue engineered construct. A perfluorotributylamine (PFTBA) emulsion was co-encapsulated at 10 vol% with mouse βTC-tet insulinoma cells in calcium alginate beads and cultured under normoxic and severely hypoxic conditions. The number of metabolically active cells and the induced insulin secretion rate were measured over time for up to 16 days. Results showed no significant effect of PFTBA relative to the PFTBA-free control. The alginate-PFC-cell system was also modeled mathematically, and simulations tracked the number of viable cells over time under the same conditions used experimentally. Simulations revealed only a small, likely experimentally undetectable difference in cell density between the PFC-containing and PFC-free control beads. It is concluded that PFTBA up to 10 vol% has no significant effect on the growth and function of encapsulated βTC-tet cells under normoxic and hypoxic conditions.
Keywords: Perfluorotributylamine, βTC-tet, encapsulated cells, normoxia, hypoxia
1.0 Introduction
A major challenge in developing and implementing tissue engineered substitutes is ensuring sufficient oxygenation of cells within constructs, as oxygen is a significant parameter affecting cell viability and function (Gross et al. 2007b; Papas et al. 2000; Papas et al. 1999). This is especially true for substitutes which rely on diffusion for the transport of dissolved oxygen (DO). Therefore, a means of enhancing oxygen delivery by increasing the solubility and/or diffusivity of DO within a construct might prove beneficial and desirable.
The high solubility of oxygen in perfluorocarbons (PFCs) has led investigators to explore their use in the form of a non-aqueous phase or an emulsion, as a blood substitute, in organ preservation, and in tissue engineered devices. It is reasonable to expect a positive effect in systems where the PFC phase is convectively transported between an oxygenator and a culture or tissue where oxygen is delivered and used. This has been confirmed experimentally, as in the study of Radisic et al. who demonstrated that adding the PFC emulsion, Oxygent, to medium perfusing a parallel plate cardiac bioreactor increased the DO concentration throughout the bioreactor and consequently supported a greater density of cardiomyocytes (Radisic et al. 2005). In their system, the DO concentration in the medium entering the bioreactor was kept constant at 160 Torr using a gas exchanger, thereby replenishing the PFC emulsion with oxygen after each bioreactor pass. Further studies with the same system showed improved properties of cardiac constructs cultured in PFC-supplemented medium (Radisic et al. 2006).
In systems where PFCs are not convectively transported, enhanced oxygenation can be achieved by the delivery of DO from the PFC to an aqueous phase in contact, and/or by the increased effective diffusivity realized when PFCs are dispersed as an emulsion in an aqueous phase. An example of the former is the two-layer method (TLM) of pancreas preservation, in which the organ is suspended between University of Wisconsin (UW) solution and oxygenated PFC. The PFC layer serves as an oxygen reservoir, while the UW solution provides other nutrients, including adenosine to promote ATP synthesis in the tissue (Matsumoto 2005). With non-human pancreata, there exist reports of significantly improved islet yield and in vitro function when islets were isolated from organs preserved in the TLM relative to organs preserved in UW solution alone (Atias et al. 2008; Matsumoto et al. 2002). Improved islet yields for pancreata preserved by the TLM versus the UW solution alone have also been reported for human tissues (Lakey et al. 2002; Ricordi et al. 2003; Tsujimura et al. 2004). Other groups have observed contrary results, however, in which the TLM did not improve islet isolation or transplantation outcomes. For example, Caballero-Corbalan et al. compared the outcome of 200 human islet isolations performed after storage in either the UW solution only or by the TLM over short (<6 hours) or prolonged (up to 18 hours) cold ischemic time. They observed no significant improvement in the islet yield, purity, or function with the TLM (Caballero-Corbalan et al. 2007). Similarly, Kin et al. reported no beneficial effects of the TLM on human islet isolation and transplantation (Kin et al. 2006). Papas et al. offered a mechanistic explanation of these outcomes, in that PFCs do indeed improve oxygenation but only in a thin peripheral layer of tissue, while oxygen is not delivered to inner tissue domains (Papas et al. 2005). For this, convective oxygen transport through the native organ vasculature may be necessary.
PFC addition to culture media has also produced mixed results. With rat islets in culture, Zekorn et al. observed a marked improvement in islet insulin secretory function when the culture medium was supplemented with PFCs (Zekorn et al. 1991). On the other hand, Bergert et al. (Bergert et al. 2005) did not observe similar results. In the latter study, the effects of PFCs on islet viability and function were characterized by measuring cell death, apoptosis, mRNA levels of insulin, insulin content, and stimulated insulin secretion. These extensive measurements indicated that the addition of PFC failed to provide any advantage over conventional protocols for islet culturing (Bergert et al. 2005).
In tissue engineering, there is a gaining interest to incorporate PFCs in hydrogels to improve the oxygenation of encapsulated cells. Khattak et al. reported that encapsulating a PFC emulsion along with human HepG2 hepatomas in calcium alginate hydrogels increased cellular growth and metabolic activity over a 10-day period (Chin et al. 2008; Khattak et al. 2007). With islets, mathematical simulations indicated that cell oxygenation was improved when a PFC emulsion was incorporated at a 70% PFC concentration in the encapsulating material, or when islets were dispersed into smaller aggregates, in both spherical microcapsules and planar slabs (Johnson et al. 2009). It is generally accepted that in these systems PFCs increase oxygenation by enhancing dissolved oxygen effective diffusivity through the matrix, not by serving as an oxygen reservoir, as the PFCs have only limited capacity to supply oxygen and they do not become reoxygenated. However, it remains unclear whether the increase in effective diffusivity is sufficient to produce consistent, statistically significant, and experimentally measurable positive effects, especially in applications with encapsulated insulin-secreting cells, which constitute a commonly used architecture for a pancreatic tissue substitute (Sambanis 2007). Furthermore, in the design of such systems, it would be important for the PFC to be incorporated at a concentration that does not compromise the mechanical integrity and immunoprotective properties of the encapsulating matrix.
In this study, we addressed this question by investigating experimentally the effect of a PFC emulsion, perfluorotributylamine (PFTBA), on the viability, metabolic activity, and insulin secretory function of mouse βTC-tet insulinoma cells encapsulated in calcium alginate beads and cultured under normoxic and hypoxic conditions. We limited the PFTBA concentration to 10 vol% to ensure that the bead properties would not be compromised, as the alginate/PFC beads were unstable when prepared with higher PFC concentrations. Furthermore, we constructed a mathematical model of the alginate-PFC-cell system and compared simulations with the experimental results. It is concluded that PFTBA at 10 vol% in calcium alginate causes an increase in the oxygenation and density of cells in the beads under both normoxic and hypoxic conditions, which, however, is likely too small to be detectable experimentally. The implications of these findings in the development of pancreatic substitutes based on encapsulated cells are discussed.
2.0 Materials and Methods
2.1 Cell culture and Cell Encapsulation
Murine insulinoma βTC-tet cells (Efrat et al. 1995) were obtained from the laboratory of Dr. Shimon Efrat, Albert Einstein College of Medicine, Bronx, NY. Cells were cultured as monolayers in T-175 flasks, in a 37°C, 5% CO2 humidified incubator. Culture medium consisted of Dulbecco's Modified Eagle's Medium (DMEM, Sigma Chemical Co., St. Louis MO) with 25 mM glucose, supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and L-glutamine to a final concentration of 6 mM, and it was changed every 2-3 days. Cell monolayers that reached 80% to 90% confluency were split by treatment with 0.25% trypsin-EDTA (Sigma); passage number increased by one at each splitting. Cells of passage number 36-48 were used in this study.
For encapsulation, cells were harvested by trypsin-EDTA and encapsulated at a density of 3.5×107 cells/ml in 2% w/v low viscosity, high mannuronic content alginate (product LVM, NovaMatrix, Drammen, Norway) containing the appropriate concentration of PFTBA emulsion (0 or 10 vol%). Using an electrostatic droplet generator (Nisco Engineering Inc., Zurich, Switzerland), cells were encapsulated according to the procedure of Stabler et al. (Stabler et al. 2001). Certain alginate bead preparations were coated with poly-L-lysine (PLL) (MW = 19,200; Sigma, St. Louis, MO) and a final alginate layer to form alginate/PLL/alginate (APA) beads. The protocol used to coat the beads was as described by Sun (Sun 1988) except that the beads were not exposed to sodium citrate at the final step. Studies were performed with two bead sizes, 500 ± 150 μm and 1000 ± 200 μm in diameter.
The PFTBA emulsion was prepared following a protocol adapted from Joseph et al. (Joseph et al. 1985). An amount of 600 μl of Tyrode's salt solution (Sigma) was added to 95 mg of egg yolk lecithin (~99% purity, Sigma) and sonicated twice for 15 seconds at 300 W, with a one minute interval between each sonication. An amount of 400 μl of PFTBA (Sigma) was then added to the mixture and sonicated the same way for ten times The emulsion was filtered through a 0.8 μm membrane filter (Millipore, Billerica MA) and added to alginate at a 1:10 ratio. For one of the control groups, 1000 μl of Tyrode's salt solution was added to 95 mg of egg yolk lecithin and sonicated twelve times. This solution was added to alginate, also at a 1:10 ratio.
2.2 Culturing of Encapsulated Cells
Alginate-encapsulated βTC-tet cells were cultured in a 37°C, 5% CO2 incubator for 1 day before experimentation to allow for recovery from the encapsulation procedure. The coating procedure was carried out a day after encapsulation. For normoxic experiments, encapsulated cells were placed into T-75 flasks and cultured on a rocking platform in a 37°C, 5% CO2 incubator for 16 days. Number of metabolically active cells and insulin secretory function were assessed on Days 1, 2, 4, 8, 12, and 16. For hypoxic experiments, T-flasks or multiwell plates were placed in a Plexiglas chamber with inlet and outlet ports, which was placed on a rocking platform in a 37°C incubator. A gas mixture of 5% CO2 balanced with N2 was continuously supplied through the chamber, lowering the oxygen to a severely hypoxic level of approximately 2% air saturation, as measured by an oxygen polarographic sensor (Ingold Messtechnik, Urdorf, Switzerland). For experiments under long-term hypoxia, encapsulated cells in T-75 flasks were cultured in the chamber for 13 days. Number of metabolically active cells and cell viability were assessed on Days 1, 4, 7, 10, and 13. For short-term hypoxic experiments, encapsulated cells were transferred to a 6-well plate and cultured in the chamber for 3 hours. Insulin secretion tests were then carried out, also in the chamber. In all studies, cells were fed by completely replacing the medium with fresh every 2-3 days.
To measure the rates of glucose consumption of encapsulated cells under normoxia, 0.5 ml of beads were cultured in 2.5 ml of fully supplemented medium in T-12.5 flasks for 7 days. Medium samples of 1.0 ml volume were collected daily and replaced with fresh medium. Rates of glucose consumption were evaluated by measuring the concentrations in samples.
2.3 Insulin Secretion Measurements
A bead volume of 0.1 ml was removed from the main culture and loaded in a well of a 6-well plate, which was placed on a rocking platform in a 37°C, 5% CO2 incubator, or, for hypoxia studies, in the chamber. The beads were exposed to 5.0 ml of basal medium (glucose-free, non-supplemented DMEM) for 60 min, then switched to 3.2 ml of stimulating medium (16 mM glucose, fully supplemented DMEM) for 30 min. Medium samples withdrawn at the start and end of each stimulation period were stored at -80°C for later assay of insulin by radio140 immunoassay. The beads used in these insulin secretion experiments were not returned to the main culture. The 30 min secretion period was sufficient to capture the stimulated secretory response (Cheng et al. 2006). Secretion rates were calculated in pmol insulin per hour and per 108 cells.
2.4 Assays
Numbers of metabolically active encapsulated cells were measured using the metabolic indicator alamarBlue™ (Invitrogen Corp, Carlsbad, CA). A bead volume of 0.1 ml was placed in a well of a 12-well plate and mixed with 1.0 ml of medium and 0.1 ml of alamarBlue™ reagent. Plates were positioned on a rocking platform in a 37°C, 5% CO2 incubator. After 1.5 hours (3 hours for hypoxia experiments), 0.1 ml medium sample was withdrawn from each well, and its absorbance measured using a Spectra Max Gemini Plate Reader (Molecular Devices, Sunnyvale, CA) with an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Relative intensity units (RIUs) were normalized to the RIUs of the Day 1 samples measured under the same conditions. Cell viability was determined using Trypan Blue assay (Sigma), where 0.1 ml of alginate beads were dissolved in 0.2 ml of 2% sodium citrate solution (Sigma). A volume of 0.1 ml of the resulting suspension was added to 0.2 ml of Trypan Blue reagent and loaded on a hemocytometer for cell counting.
Insulin concentration in collected samples was assayed by rat insulin radioimmunoassay (Linco Research, St Charles, MI), using a CobraTM II Series Auto-Gamma Counter (Packard Instruments). Glucose concentrations were determined by the Glucose Trinder Assay (Diagnostic Inc., Oxford, CT), with absorbance measured at 505 nm wavelength using a SpectraMax Plus Plate Reader (Molecular Devices, Sunnyvale, CA).
2.5 Statistical Analysis
Statistical significance of differences between experimental groups on a certain day, and between nested groups on different days, was evaluated using General Linear Model, ANOVA. Statistical significance was defined as p<0.05.
3.0 Results and Discussion
3.1 PFTBA Effects on Encapsulated βTC-tet Cells under Normoxic Conditions
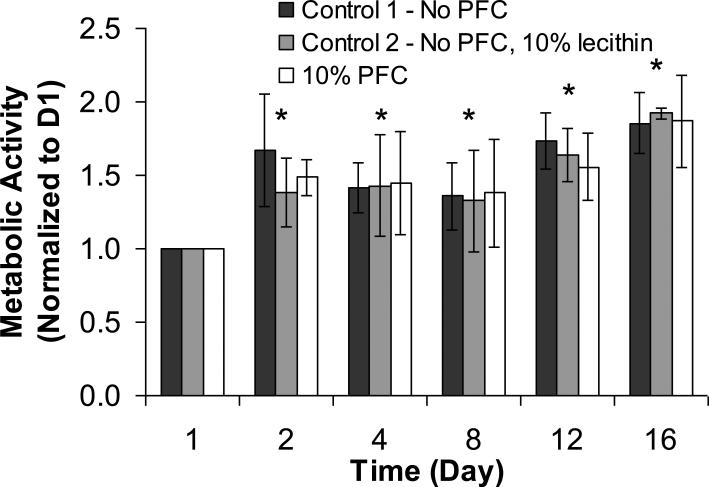
Cell metabolic activity in APA beads with no PFTBA (Control 1), 10 vol% lecithin solution (Control 2), and 10 vol% PFTBA emulsion (Experiment) was measured by alamarBlue™ over a period of 16 days. The second control group was included to ensure that lecithin, the emulsifier used to make the PFTBA emulsion, did not have an effect on cells by itself, and that any changes observed in the PFC group were solely due to the presence of PFTBA. The droplet size of the PFTBA emulsion in the alginate beads was measured microscopically and remained at 1-2 μm throughout the experimental period, indicating that the emulsion was stable. The integrity of APA beads was also maintained throughout this period as the PLL layer reduced the diffusion of calcium ions out of the alginate matrix and had an overall stabilizing effect on the beads (Benson et al. 1997; Simpson et al. 2003). Results with 1000 μm APA beads are shown in Figure 1. All data were normalized to the measurement on Day 1, which was set equal to 1. There was a gradual increase in the number of metabolically active cells over time for all groups. This trend is compatible with earlier findings with βTC insulinomas encapsulated in high mannuronic alginate beads (Stabler et al. 2001). At any particular time point, there were no statistical differences in metabolic activity among the different groups of beads.
Figure 1.
Metabolic activity of encapsulated βTC-tet cells under normoxic conditions. The number of metabolically active cells was measured as a function of time by alamarBlue™ and normalized to Day 1. Measurements were performed with calcium alginate/ poly-L-lysine/ alginate (APA) beads of 1000 μm average diameter with no PFTBA, APA beads with 10 vol% lecithin, and APA beads with 10 vol% PFTBA (n = 3 each). The initial density of the encapsulated cells was 3.5×107 cells/ml. * p<0.05 when compared to Day 1.
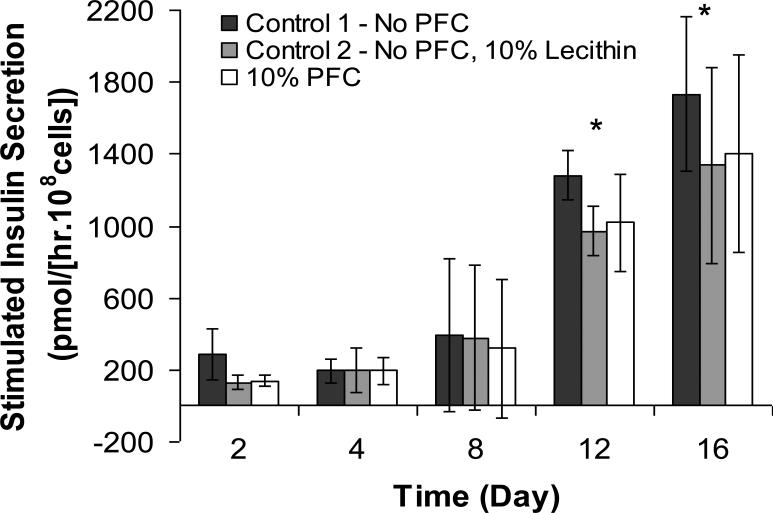
Data on the insulin secretion rate (ISR) measured under 16 mM glucose stimulation are shown in Figure 2. There was a general increase in ISR with time up to Day 16 for all groups. As with the alamarBlue™ measurements, there were no statistical differences in ISR among the different bead groups at any time point. However, the temporal increase in insulin secretion was higher than the increase in metabolic activity shown in Figure 1, suggesting an increase in the insulin secretion rate per cell over time. Although the precise reason for this is unclear, it is possible that it was caused by an increasing hypoxic environment within the bead due to cell growth, which resulted in higher lactate production and locally more acidic conditions over time; an acidic pH has been shown to cause elevated insulin release from βTC3 cells, an earlier variant of the βTC-tet insulinoma line (Simpson et al. 2000).
Figure 2.
Stimulated insulin secretion rate (ISR) of βTC-tet cells under normoxic conditions. ISR was measured over 30 min of stimulation by 16 mM glucose, normalized to the initial number of encapsulated βTC-tet cells, and expressed on a per unit time basis. Measurements were carried out with 1000 μm average diameter APA beads with no PFTBA, APA beads with 10 vol% lecithin, and APA beads with 10 vol% PFTBA (n = 3 each). Each glucose stimulation episode followed 1 hour of exposure to basal, 0 mM glucose, medium. * p<0.05 when compared to Day 1.
It should be noted that similar results were obtained with βTC-tet cells encapsulated in calcium alginate beads without PLL coating of both 500 and 1000 μm beads (results not shown). In these experiments, too, there were no differences in metabolic activity or insulin secretion rates between PFTBA-containing beads and PFTBA-free controls (control 1) at all time points. However, the integrity of these beads started to become compromised towards the end of the 16-day experiment due to the absence of the stabilizing PLL layer.
The effect of PFTBA on the glucose consumption rate (GCR) was also studied with βTC-tet cells encapsulated in 1000 μm average diameter calcium alginate beads with no PFTBA (control 1) and 10 vol% PFTBA emulsion. The GCR in both bead groups increased by approximately 60% over seven days in culture due to cell growth. However, the presence of PFTBA had no significant effect on the GCR of the cells over that time period (data not shown).
3.2 PFTBA Effects on Encapsulated βTC-tet Cells under Hypoxic Conditions
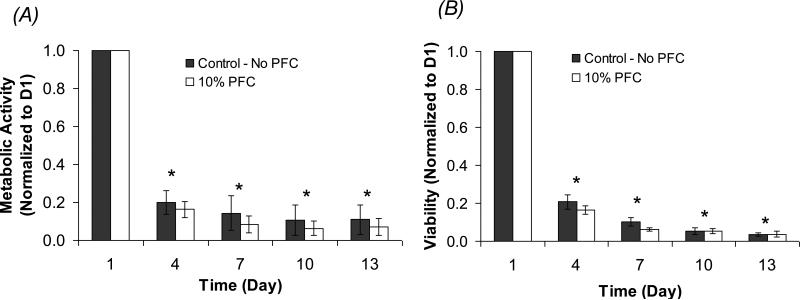
Figure 3A shows the temporal profile of the cell metabolic activity, measured by alamarBlue™ and normalized to Day 1, for βTC-tet cells in 500 μm alginate-only beads with no PFTBA (Control) and 10 vol% PFTBA emulsion, under severely hypoxic conditions (~2% air saturation, or 0.004 mM). The percent viability of the cells in beads was also measured by Trypan Blue and is reported in Figure 3B. A smaller bead size was chosen for this hypoxic study to ensure that the only oxygen-limiting factor was the external condition, not diffusion limitation within the beads.
Figure 3.
Metabolic activity and % viability of encapsulated βTC-tet cells under severely hypoxic conditions (2% air saturation, or 0.004 mM). The number of metabolically active cells (A) and the % viability (B) were measured by alamarBlue™ and Trypan Blue, respectively, as a function of time and normalized to Day 1. Measurements were performed with calcium alginate beads of 500 μm average diameter with no PFTBA and 10 vol% PFTBA (n = 5 each). * p<0.05 when compared to Day 1.
Results showed that both the metabolic activity and the cell viability for the two groups of beads declined significantly from Day 1 to Day 4, to a lesser extent from Day 4 to Day 7, and stayed at a very low level until the end of the experiment on Day 13. As with the normoxic experiment, there were no statistical differences in cell metabolic activity and viability between the two bead groups at any time point. Due to the significant cell death, ISR measurements were not performed with these hypoxic cultures.
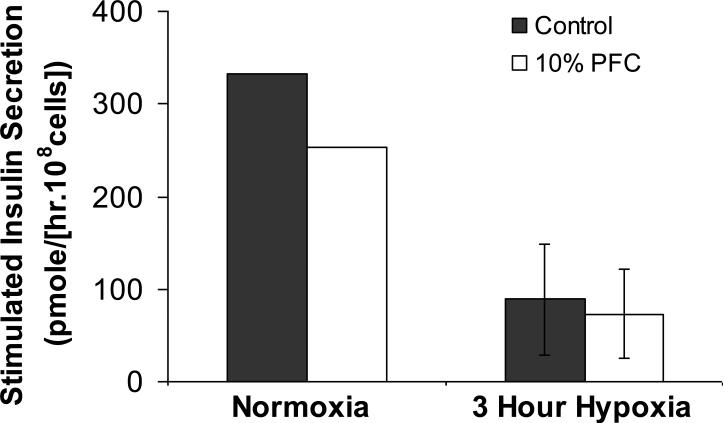
Experiments were also carried out under short-term hypoxia to investigate whether the PFTBA phase had a significant effect on the encapsulated cells under these conditions. For this, cells in 500 μm alginate-only beads with no PFTBA (control) and 10 vol% PFTBA emulsion were cultured under hypoxia for 3 hours, then subjected to an insulin secretion test, also under hypoxia. Results on ISR measured over 30 min under 16 mM glucose stimulation are shown in Figure 4. The insulin secretory function of the encapsulated cells was compromised under the hypoxic conditions implemented; however, the presence of 10% PFTBA did not have an effect on the induced insulin secretion relative to the control.
Figure 4.
Stimulated insulin secretion rate (ISR) of βTC-tet cells under severely hypoxic conditions. The encapsulated cells were cultured for 3 hours under hypoxic conditions, then subjected to a secretion test. The secretion episode consisted of cells exposed for 1 hour to basal, 0 mM glucose medium, followed by a 30 min of stimulation in 16 mM glucose medium, all under hypoxia. The ISR over the stimulation period was measured and is reported in the figure. The ISR under normoxic conditions is included for reference. Beads were of 500 μm average diameter.
4.0 Mathematical Simulations
The mathematical model used to simulate the system of encapsulated cells with a perfluorocarbon emulsion was an extension of the previously published model of Gross et al (Gross et al. 2007a). The assumptions incorporated in the model are listed below.
DO is the only nutrient that limits cell proliferation (i.e., all other essential nutrients are in excess throughout the construct).
Spatial constraint is the only other factor besides DO concentration that limits the rate and extent of cell proliferation within the construct.
As cells cannot adhere to the alginate matrix, cells do not migrate within the matrix; hence any remodeling in cell distribution is due entirely to cellular growth and death at each locale.
The PFC emulsion is homogeneously distributed throughout the contiguous alginate matrix.
Equilibration of dissolved oxygen between the aqueous and PFC phases at each locale is instantaneous.
The values of the model parameters, including the effective diffusivity of DO through the encapsulated system, remain constant over the time period of the simulations.
The concentration gradient within the aqueous phase is the only driving force for oxygen diffusion.
There is no external boundary layer effect when modeling the beads placed in well mixed medium, as in this study.
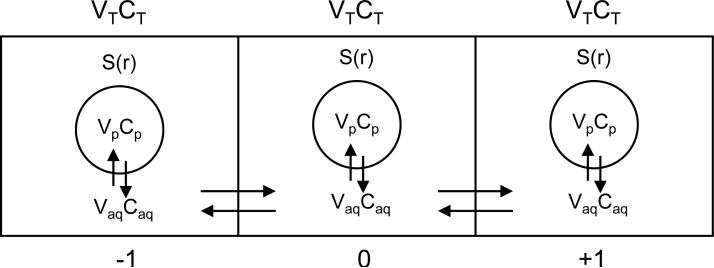
Figure 5 is a schematic of the configuration that is considered. Volume elements along a bead radius each consist of an aqueous and a PFC phase, with cells present in the aqueous phase only and DO in both phases. Diffusion of oxygen occurs between compartments, and the aqueous and PFC phases in each compartment are always in equilibrium (assumption 5 above). The model equations describing DO diffusion for spherical geometry are as follows
| (1) |
| (2) |
| (3) |
where t is time and r is radial position in the construct; CT(r,t), Caq(r,t), and Cp(r,t) are the concentrations of DO in the total volume (VT), the volume of the aqueous phase (Vaq), and the volume of the PFC phase (Vp), respectively, as functions of radial position and time; Deff is the effective oxygen diffusivity through the alginate/PFC matrix; VT, Vaq, and Vp are the total, aqueous, and PFC volumes; S(r,t) is the rate of oxygen consumption per unit volume of aqueous phase as a function of radial position and time, which is based on a Monod model with kinetic parameters vmax (maximum oxygen consumption rate) and Km (Monod model parameter); and X(r,t) is the cell density as a function of radial position and time.
Figure 5.
Configuration considered to solve for the DO concentration profile. In solving for the total DO concentration (CT, amount of DO in aqueous plus PFC phases divided by the compartment volume) at compartment ‘0’, diffusional transport occurs by the DO concentration differences in the aqueous phase (Caq) between compartment ‘0’ and the neighboring compartments (‘-1’ & ‘+1’). Additionally, the DO concentrations in the PFC and aqueous phases, CP and Caq, respectively, are always in equilibrium according to the partition coefficient. Parameter S(r) is the oxygen consumption rate by the cells per unit volume of aqueous phase, as described in Equation (3).
The effective diffusivity was calculated using the equation given by Radisic et al. (Radisic et al. 2005), describing Deff in a two phase system.
| (4) |
| (5) |
| (6) |
In the above equations, Daq and Dp are the diffusivities of oxygen through the aqueous calcium alginate phase and the PFC phase, respectively; K is the partition coefficient of oxygen in PFC vs. the aqueous phase; and ϕ is the volume fraction of the PFC emulsion (Vp/VT).
The equations used for determining the growth and death of cells radially through the alginate matrix over time are the same as reported in Gross et al (Gross et al. 2007a) and are listed below.
| (7) |
| (8) |
(Monod's model for the specific growth rate μg with kinetic parameters μg,max and Kg, accounting for spatial constraints)
| (9) |
(modified Monod's model for the specific death rate μd maximizing the cell death rate at Caq=0 and minimizing it at Caq>>Kd)
In the above equations, μg and μd are the specific cell growth and death rates, respectively; μg,max and μd,max are the maximum specific growth and death rates, respectively; μd,min is the minimum death rate, which prevails under an abundance of oxygen; Kg and Kd are Monod model parameters; and Xmax in the maximum cell density that can be accommodated in the construct. The ratio X/Xmax is thus the fractional maximum occupancy at a particular locale in the construct.
Initial and boundary conditions
Equations (1)-(8) were solved with the following initial and boundary conditions.
| (10) |
(the initial concentration of oxygen throughout the spherical construct is constant and known)
| (11) |
(the cells are initially distributed homogeneously throughout the construct at a known density)
| (12) |
(symmetry condition for oxygen concentration at the center of the sphere) To simulate encapsulated cells in an infinite volume of medium, Equation 13 was used:
| (13) |
(the concentration of DO at the surface of the bead is equal to the DO concentration in the surrounding medium, Cb, which is known and assumed constant). This approximates beads cultured in T-flasks, in a large volume of medium on a rocking platform.
The appropriate initial and boundary conditions, and baseline parameter values listed in Table 1 were used. The ratio of the diffusion coefficient of DO in the PFC to the aqueous phase is 3.46. With this value and the partition coefficient being equal to 16, the effective diffusivity in the composite alginate/10% PFC material was calculated from equations (4), (5) and (6) to be 1.8 cm2/day, compared to 1.4 cm2/day for the alginate-only material. Model equations were solved using finite differences. From Caq(r,t), the average aqueous intrabead DO concentration, or AIDO, was calculated as follows:
Table I.
Baseline parameter values used in simulating the temporal profiles of cell density and dissolved oxygen concentration in the system of βTC-tet cells encapsulated in spherical calcium alginate beads with and without a perfluorocarbon emulsion.
| Parameter | Value | Reference | |
|---|---|---|---|
| Diffusivity of oxygen in cell-containing alginate | D aq | 1.4 cm2/day | Tziampazis and Sambanis (1995), Mehmetoglu et al. (1996), Stabler (2004) |
| Diffusivity of oxygen in PFTBA | D p | 4.85 cm2/day | Tham et al. (1973) |
| Partition coefficient of oxygen between PFTBA and water | K | 16 | Lowe et al. (1998) |
| Maximum oxygen consumption | v max | 2.88 mmole/(day × 109 cells) | Tziampazis and Sambanis (1995) |
| Monod parameter - oxygen | K m | 0.01 mM | Tziampazis and Sambanis (1995) |
| Maximum specific growth rate | μ g,max | 0.04 day-1 | Simpson et al. (2005) and sensitivity analysis |
| Maximum specific death rate | μ d,max | 1.4 day-1 | Graeber et al. (1996) |
| Minimum specific death rate | μ d,min | 0.00273 day-1 | Tziampazis et al. (unpublished) |
| Monod parameter - growth | K g | 0.01 mM | Tziampazis et al. (unpublished) |
| Monod parameter - death | K d | 0.001 mM | Tziampazis et al. (unpublished) |
| Maximum cell density | X max | 9 × 108 cells/ml | Calculated based on a 10 μm diameter cell |
| 8.1 × 108 cells/ml (With PFC) |
4.1 Simulations under Normoxic Conditions
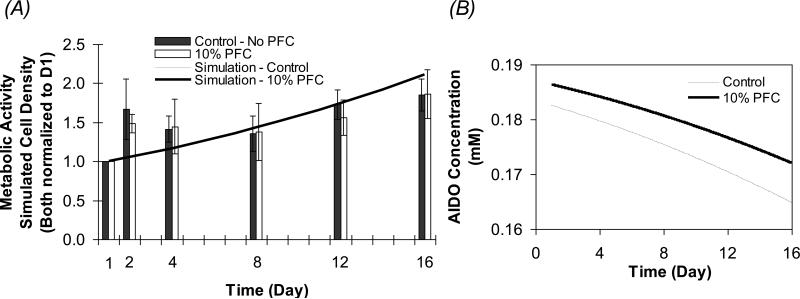
The model equations were solved to calculate the concentration of oxygen, Caq and cell density in the aqueous phase, Xaq as functions of radial position and time for beads of 1000 μm in diameter. Figures 6A and B show the time profiles of the cell density, normalized to Day 1, along with experimental data on cell metabolic activity from Figure 1, and AIDO concentration with no PFC (Control) and 10% PFC over 16 days in culture. The cell growth predicted by the model is in good agreement with the experimental results for the APA beads. Due to the increased effective diffusivity in the composite alginate/PFC phase, the AIDO concentration for the PFC-containing beads started at a higher value of 0.186 mM, when compared to 0.182 mM for the control beads (Figure 6B). As the cell density, and hence the oxygen consumption, increased within the beads over time, the AIDO declined accordingly. The decrease in AIDO, however, was smaller in the PFC-containing beads relative to the control, and on Day 16 the AIDO concentrations were calculated to be 0.172 and 0.165 mM for the PFC and control beads, respectively. Although the presence of PFC improved the DO effective diffusivity and the AIDO concentration in the beads, this supported only a slightly higher cell density relative to the PFC-free control. The maximum difference in simulated cell densities was on Day 16, where the control and PFC beads had cell densities of 7.75 and 7.79×107 cells/ml, respectively. This difference is small, making it essentially impossible to detect experimentally.
Figure 6.
Simulated temporal profiles under normoxic conditions (0.2 mM). The model equations were solved for 1000 μm average diameter beads to calculate the cell density (A) and the average intrabead DO concentration (AIDO) (B) in the aqueous phase in beads containing no PFC and 10% PFC. Simulated values are compared to the experimental data shown in Figure 1 obtained from APA beads under similar conditions. In (A), the scale of the graph makes the simulations for the 10% PFC and control beads essentially coincide.
These findings are different from those reported by Khattak et al. (Khattak et al. 2007) and by Chin et al. (Chin et al. 2008), who observed a statistical increase in the density of HepG2 cells encapsulated in calcium alginate containing 10% perfluorooctylbromide (PFOB) relative to PFC-free controls. The reason for this difference is not clear. The oxygen solubility is higher in PFOB relative to PFTBA, with the partition coefficients being 17.7 and 16 respectively. This, however, results only in a minimal difference in the effective diffusivities calculated by equations (4), (5) and (6), with values being the same within two significant figures, so the difference in solubility cannot explain the difference in results. A second possibility is the lower maximum oxygen consumption rate (vmax) of βTC-tet relative to HepG2 cells. Indeed, reported vmax values for HepG2 cells of 5.44 (Chin et al. 2008) and 17 mmol/(109 cells·day) (Mishra and Starly 2009) are significantly higher than the 2.88 mmol/(109 cells·day) reported for βTC3 cells (Tziampazis and Sambanis 1995), resulting in a higher oxygen demand in the alginate matrix. A third possibility would be the higher growth rate of HepG2 cells both as monolayers and in beads, which may also result in a higher oxygen demand in the beads over time.
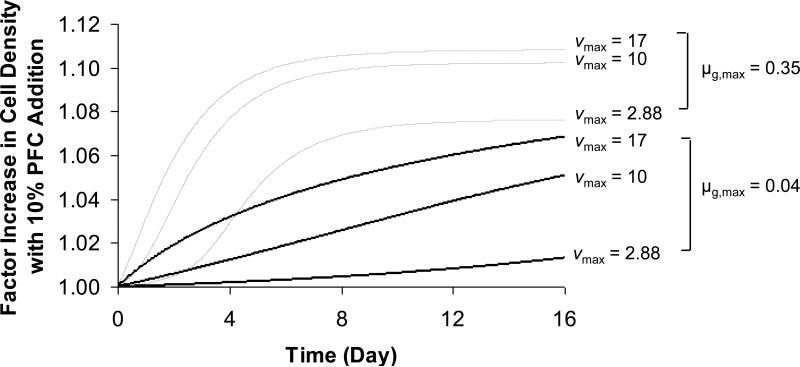
We therefore investigated the sensitivity of the model solutions to the values of vmax and μg,max. Simulations were carried out with vmax equal to the value used in this study and the two values for HepG2 cells reported above; for μg,max, model solutions were calculated with the value used in this study and with a higher value of 0.35 day-1, which corresponds to a much faster growing cell type with a doubling time of 2 days (Gross et al. 2007a). The rest of the model parameters were kept as in Table 1, including the maximum permissible cell density of 9×108 cells/ml in the alginate matrix. Results are plotted as factor increase in cell density in the PFC-containing beads relative to PFC-free control vs. time and are shown in Figure 7. As expected, cells with a higher μg,max arrive at their steady state cell density sooner, and the PFC effect increases with vmax, or as the oxygen demand within the alginate matrix increases. However, the cell density supported by the presence of PFC is predicted to be no more than 11% higher than the control, which may be detectable experimentally but does not improve the performance of the system in a substantive way.
Figure 7.
Simulated factor increase in cell density vs. time resulting from the incorporation of 10% PFC relative to PFC-free control for 3 different oxygen consumption rates, vmax (mmol/(109 cells·day)) and 2 different growth rates, μg,max (day-1). Remaining model parameters are the same as those reported in Table 1.
4.2 Simulations under Hypoxic Conditions
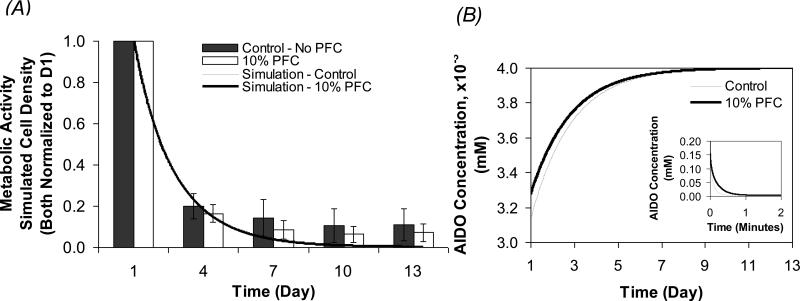
Simulations on the effect of PFC on encapsulated cells under severely hypoxic conditions were also carried out for 500 μm beads. Figure 8A shows the time profiles of the cell density, normalized to Day 1, along with experimental data from Figure 3A on cell metabolic activity, under 0.004 mM external DO over 13 days in culture. Figure 8B shows the time profiles of the AIDO concentration in beads with no PFC (control) and 10% PFC; the inset in this figure shows the same AIDO profiles under higher resolution for the initial 2 minutes.
Figure 8.
Simulated temporal profiles under hypoxic conditions (0.004 mM). Model solutions for cell density (A) and average intrabead DO concentration (AIDO) (B) in the aqueous phase of 500 μm average diameter beads containing no PFC and 10% PFC. Simulated values are compared to the experimental data obtained from calcium alginate beads under similar conditions and reported in Figure 3. In (A), the scale of the graph makes the simulations for the 10% PFC and control beads essentially coincide. The inset in (B) shows the change in AIDO concentration for the initial 2 min.
Model-predicted cell densities for both groups of beads decreased drastically within the first 4 days, which was also reflected in the increase AIDO concentration towards the external DO due to cell death. The simulated cell density was generally in good agreement with the experimental results. However, the model appears to underestimate the number of metabolically active cells on Days 7, 10, and 13. This could be due to the beads being temporarily removed from their hypoxic environment at every experimental time point from Day 4 onwards, which is not accounted for in the model.
As with the normoxia simulations, the AIDO concentration in the PFC-containing beads started at a higher value of 0.033 mM, when compared to 0.031 mM in the control beads (Figure 8B). This difference was not significant enough to change the rate of cell death under hypoxia. At Day 4, simulations showed only a slightly higher cell density of 5.96×106 cells/ml in the PFC-containing relative to 5.77×106 cells/ml for the control. At steady state, the presence of PFC sustained a cell density that is only 3.7% higher than the control beads under these conditions. The model also shows that the capacity of PFC to provide oxygen to the aqueous phase is minimal, as observed by the decrease in the AIDO concentration within the first 2 minutes after exposure to hypoxia (Figure 8B inset). This lack of significant difference in the calculated cell density between the two groups of beads demonstrates the limited capacity of PFC to provide long-term oxygen support to the construct under the conditions examined.
PFCs are effective as oxygen carriers when convectively transported from a domain of high oxygen, where they become oxygenated, to a domain of low oxygen, where they deliver the oxygen they contain. This is supported by the fact that oxygen is not chemically bound to the PFC carrier and that the thermodynamics of oxygen absorption by PFC follow a linear Henry law profile rather than a sigmoidal profile, as is the case for hemoglobin (Lowe et al. 1998). Our study shows, however, that in a stationary system, such as an encapsulated cell construct, the increase in construct oxygenation due to the addition of 10% PFC has a minimal effect on the viable cell number and function when cultured in a high or low oxygen environment. A system in which the incorporation of PFC could potentially have an added benefit in maintaining viable cells is when cells or tissues are surrounded by a large volume of PFC. This is, however, not feasible with encapsulated cells where the PFC emulsion is dispersed, as having a high concentration of PFC emulsion will compromise the integrity of the beads and possibly result in cytotoxic effects from the emulsifier. Furthermore, the cell density that can be accommodated in beads will be reduced due to the reduction of the volume of the aqueous phase.
5.0 Conclusions
In summary, the addition of PFTBA emulsion of 10 vol% to encapsulated systems of βTC-tet cells in calcium alginate beads did not have a statistically significant benefit to cell growth and metabolic activity, or to the induced insulin secretion from the cells. These results were obtained under normoxic conditions with APA and alginate-only beads, as well as under long- and short-term severely hypoxic conditions with alginate-only beads. This finding was consistent with the results of mathematical simulations that noted only a small, likely experimentally undetectable, increase in cell density in PFC-containing beads. Simulations also indicated an increased positive effect of PFC on cells with a higher oxygen consumption and growth rates, but this was also modest in magnitude.
ACKNOWLEDGEMENTS
This work was supported by grants from the ERC Program of the National Science Foundation under Award Number EEC-9731643, the NIH (DK47858, DK73991, DK76801) and by a GAANN Fellowship from the U.S. Department of Education (for F. Goh) through the Center for Drug Discovery, Development and Delivery (CD4). This financial support is gratefully acknowledged.
Abbreviations
- AIDO
average intrabead dissolved oxygen
- APA
alginate/PLL/alginate
- DMEM
Dulbecco's Modified Eagle's Medium
- DO
dissolved oxygen
- GCR
glucose consumption rate
- ISR
insulin secretion rate
- PFC
perfluorocarbon
- PFTBA
perfluorotributylamine
- PLL
poly-L-lysine
- RIU
relative intensity units
- TLM
two layer method
- UW
University of Wisconsin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atias S, Mizrahi SS, Shaco-Levy R, Yussim A. Preservation of pancreatic tissue morphology, viability and energy metabolism during extended cold storage in two-layer oxygenated University of Wisconsin/perfluorocarbon solution. Isr Med Assoc J. 2008;10(4):273–6. [PubMed] [Google Scholar]
- Benson JP, Papas KK, Constantinidis I, Sambanis A. Towards the development of a bioartificial pancreas: effects of poly-L-lysine on alginate beads with BTC3 cells. Cell Transplant. 1997;6(4):395–402. doi: 10.1177/096368979700600406. [DOI] [PubMed] [Google Scholar]
- Bergert H, Knoch KP, Meisterfeld R, Jager M, Ouwendijk J, Kersting S, Saeger HD, Solimena M. Effect of oxygenated perfluorocarbons on isolated rat pancreatic islets in culture. Cell Transplant. 2005;14(7):441–8. doi: 10.3727/000000005783982873. [DOI] [PubMed] [Google Scholar]
- Caballero-Corbalan J, Eich T, Lundgren T, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Korsgren O, others No beneficial effect of two-layer storage compared with UW-storage on human islet isolation and transplantation. Transplantation. 2007;84(7):864–9. doi: 10.1097/01.tp.0000284584.60600.ab. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Constantinidis I, Sambanis A. Insulin secretion dynamics of free and alginate-encapsulated insulinoma cells. Cytotechnology. 2006;51(3):159–70. doi: 10.1007/s10616-006-9025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, Khattak SF, Bhatia SR, Roberts SC. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnol Prog. 2008;24(2):358–66. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]
- Efrat S, Fusco-DeMane D, Lemberg H, al Emran O, Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci U S A. 1995;92(8):3576–80. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JD, Constantinidis I, Sambanis A. Modeling of encapsulated cell systems. J Theor Biol. 2007a;244(3):500–10. doi: 10.1016/j.jtbi.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JD, Long RC, Jr., Constantinidis I, Sambanis A. Monitoring of dissolved oxygen and cellular bioenergetics within a pancreatic substitute. Biotechnol Bioeng. 2007b;98(1):261–70. doi: 10.1002/bit.21421. [DOI] [PubMed] [Google Scholar]
- Johnson AS, Fisher RJ, Weir GC, Colton CK. Oxygen consumption and diffusion in assemblages of respiring spheres: Performance enhancement of a bioartificial pancreas. Chemical Engineering Science. 2009;64(22):4470–4487. [Google Scholar]
- Joseph PM, Fishman JE, Mukherji B, Sloviter HA. In vivo 19F NMR imaging of the cardiovascular system. J Comput Assist Tomogr. 1985;9(6):1012–9. doi: 10.1097/00004728-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Khattak SF, Chin KS, Bhatia SR, Roberts SC. Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnol Bioeng. 2007;96(1):156–66. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- Kin T, Mirbolooki M, Salehi P, Tsukada M, O'Gorman D, Imes S, Ryan EA, Shapiro AM, Lakey JR. Islet isolation and transplantation outcomes of pancreas preserved with University of Wisconsin solution versus two-layer method using preoxygenated perfluorocarbon. Transplantation. 2006;82(10):1286–90. doi: 10.1097/01.tp.0000244347.61060.af. [DOI] [PubMed] [Google Scholar]
- Lakey JR, Tsujimura T, Shapiro AM, Kuroda Y. Preservation of the human pancreas before islet isolation using a two-layer (UW solution-perfluorochemical) cold storage method. Transplantation. 2002;74(12):1809–11. doi: 10.1097/00007890-200212270-00031. [DOI] [PubMed] [Google Scholar]
- Lowe KC, Davey MR, Power JB. Perfluorochemicals: their applications and benefits to cell culture. Trends Biotechnol. 1998;16(6):272–7. doi: 10.1016/s0167-7799(98)01205-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto S. Clinical application of perfluorocarbons for organ preservation. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):75–82. doi: 10.1081/bio-200046698. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Qualley SA, Goel S, Hagman DK, Sweet IR, Poitout V, Strong DM, Robertson RP, Reems JA. Effect of the two-layer (University of Wisconsin solution-perfluorochemical plus O2) method of pancreas preservation on human islet isolation, as assessed by the Edmonton Isolation Protocol. Transplantation. 2002;74(10):1414–9. doi: 10.1097/00007890-200211270-00013. [DOI] [PubMed] [Google Scholar]
- Mishra A, Starly B. Real time in vitro measurement of oxygen uptake rates for HEPG2 liver cells encapsulated in alginate matrices. Microfluidics and Nanofluidics. 2009;6(3):373–381. [Google Scholar]
- Papas KK, Hering BJ, Guenther L, Rappel MJ, Colton CK, Avgoustiniatos ES. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;37(8):3501–4. doi: 10.1016/j.transproceed.2005.09.085. [DOI] [PubMed] [Google Scholar]
- Papas KK, Long RC, Jr., Constantinidis I, Sambanis A. Effects of short-term hypoxia on a transformed cell-based bioartificial pancreatic construct. Cell Transplant. 2000;9(3):415–22. doi: 10.1177/096368970000900312. [DOI] [PubMed] [Google Scholar]
- Papas KK, Long RC, Jr., Sambanis A, Constantinidis I. Development of a bioartificial pancreas: II. Effects of oxygen on long-term entrapped betaTC3 cell cultures. Biotechnol Bioeng. 1999;66(4):231–7. [PubMed] [Google Scholar]
- Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288(3):H1278–89. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12(8):2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Fraker C, Szust J, Al-Abdullah I, Poggioli R, Kirlew T, Khan A, Alejandro R. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75(9):1524–7. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- Sambanis A. Bioartificial Pancreas. In: Lanza R, Langer R, Vacanti J, editors. Principles of Tissue Engineering. 3rd ed Academic Press; Boston: 2007. pp. 619–630. [Google Scholar]
- Simpson NE, Bennett LK, Papas KK, Sambanis A, Constantinidis I. Effects of pH on murine insulinoma betaTC3 cells. Biochem Biophys Res Commun. 2000;273(3):937–41. doi: 10.1006/bbrc.2000.3022. [DOI] [PubMed] [Google Scholar]
- Simpson NE, Grant SC, Blackband SJ, Constantinidis I. NMR properties of alginate microbeads. Biomaterials. 2003;24(27):4941–8. doi: 10.1016/s0142-9612(03)00418-6. [DOI] [PubMed] [Google Scholar]
- Stabler C, Wilks K, Sambanis A, Constantinidis I. The effects of alginate composition on encapsulated betaTC3 cells. Biomaterials. 2001;22(11):1301–10. doi: 10.1016/s0142-9612(00)00282-9. [DOI] [PubMed] [Google Scholar]
- Sun AM. Microencapsulation of pancreatic islet cells: a bioartificial endocrine pancreas. Methods Enzymol. 1988;137:575–80. doi: 10.1016/0076-6879(88)37053-9. [DOI] [PubMed] [Google Scholar]
- Tsujimura T, Kuroda Y, Avila JG, Kin T, Oberholzer J, Shapiro AM, Lakey JR. Influence of pancreas preservation on human islet isolation outcomes: impact of the two-layer method. Transplantation. 2004;78(1):96–100. doi: 10.1097/01.tp.0000133515.37892.d5. [DOI] [PubMed] [Google Scholar]
- Tziampazis E, Sambanis A. Tissue engineering of a bioartificial pancreas: modeling the cell environment and device function. Biotechnol Prog. 1995;11(2):115–26. doi: 10.1021/bp00032a001. [DOI] [PubMed] [Google Scholar]
- Zekorn T, Siebers U, Bretzel RG, Heller S, Meder U, Ruttkay H, Zimmermann U, Federlin K. Impact of the perfluorochemical FC43 on function of isolated islets: a preliminary report. Horm Metab Res. 1991;23(6):302–3. doi: 10.1055/s-2007-1003682. [DOI] [PubMed] [Google Scholar]