Abstract
The last decade has seen considerable discussion regarding a theoretical account of medial prefrontal cortex (mPFC) function with particular focus on the anterior cingulate cortex. The proposed theories have included conflict detection, error likelihood prediction, volatility monitoring, and several distinct theories of error detection. Arguments for and against particular theories often treat mPFC as functionally homogeneous, or at least nearly so, despite some evidence for distinct functional subregions. Here we used functional magnetic resonance imaging (fMRI) to simultaneously contrast multiple effects of error, conflict, and task-switching that have been individually construed in support of various theories. We found overlapping yet functionally distinct subregions of mPFC, with activations related to dominant error, conflict, and task-switching effects successively found along a rostral-ventral to caudal-dorsal gradient within medial prefrontal cortex. Activations in the rostral cingulate zone (RCZ) were strongly correlated with the unexpectedness of outcomes suggesting a role in outcome prediction and preparing control systems to deal with anticipated outcomes. The results as a whole support a resolution of some ongoing debates in that distinct theories may each pertain to corresponding distinct yet overlapping subregions of mPFC.
Keywords: ACC, conflict, error, task-switching, prefrontal cortex, fMRI
Introduction
Since the inception of neuroimaging, there has been great interest in understanding the role of medial prefrontal cortex (mPFC) in cognition. Much of this interest has been generated due to ubiquitous mPFC activation found in a variety of paradigms. For example, mPFC is sensitive to errors (Gehring et al., 1993), conflict (Botvinick et al., 2001), attention (Posner and Petersen, 1990), task-switching (Bush et al., 2002; Shima and Tanji, 1998; Wager et al., 2004), pain (Talbot et al., 1991), reward (Rushworth et al., 2004), and volition (Nachev et al., 2005) among other functions.
Several attempts have been made to try to distill these varied functions into a parsimonious computational framework. One prominent theory posits that mPFC acts as a conflict monitor, detecting co-activation between incompatible responses and calling for control processes to resolve discrepancies (Botvinick et al., 2001). Another theory involves mPFC directly in selection-for-action, biasing for relevant stimulus-response associations and against irrelevant stimulus-response associations (Petersen et al., 1989; Posner and Petersen, 1990), but see (Botvinick et al., 1999). Other theories highlight predictive roles of mPFC as either signaling prediction errors (Alexander and Brown, In Press; Holroyd and Coles, 2002; Holroyd et al., 2005) or predicting error-likelihood (Brown and Braver, 2005, 2007). Although each theory can capture an impressive amount of data, no single theory has thus far been able to successfully account for the varied mPFC literature.
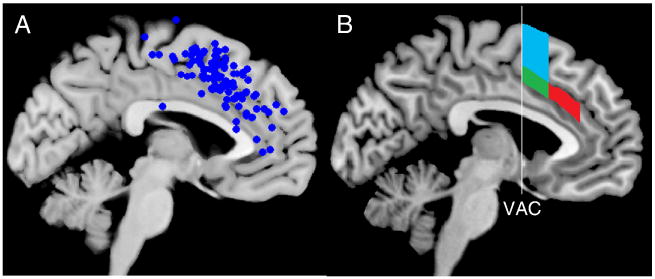
One issue that plagues many theories of mPFC function is a rather loose definition of the cortical area within mPFC that is being modeled (Rushworth et al., 2004). Figure 1a illustrates a plot of 110 activation peaks from 43 response conflict studies that report activation in the mPFC (Nee et al., 2007). Although there is some clustering, the activations cover a large portion of cortex, likely spanning several different functional regions (see also Ridderinkhof et al., 2004). Indeed, anatomical and functional work has suggested several divisions of mPFC (Beckmann et al., 2009; Braver et al., 2001; Fan et al., 2008; Kiehl et al., 2000; Lutcke and Frahm, 2008; Picard and Strick, 1996, 2001; Vogt et al., 1995). One distinction is between the anterior cingulate (ACC) and medial frontal gyrus. The ACC can be divided by a vertical line through the anterior commissure (VAC) into a rostral cingulate zone (RCZ) involved in cognitive functions and a caudal cingulate zone (CCZ) more strongly involved in motoric functions. Similarly, portions of the medial frontal gyrus have been divided into the motoric supplementary motor area (SMA) and the more cognitive pre-SMA. Moreover, it has been suggested that the RCZ can be further divided into anterior (RCZa) and posterior (RCZp) zones (Picard and Strick, 1996). The activation peaks in Figure 1a range across each of these five zones, potentially reflecting several distinct, but related functions (Figure 1b).
Figure 1.
A) Plot of 110 peaks from 43 studies examining response conflict (adapted from (Nee et al., 2007). Clustering is evident in RCZp, as well as the pre-SMA. B) Zones corresponding to RCZa (red), RCZp (green), and the pre-SMA (cyan) as described by Picard and Strick (2001). VAC – vertical anterior commissure line.
A challenge for understanding mPFC function is to identify precisely where particular cognitive phenomena originate. Some research suggests that conflict and errors activate the same areas within mPFC (van Veen et al., 2004) and have led to models that consider errors a special case of conflict (Botvinick et al., 2001; Yeung et al., 2004). However, other work has suggested that errors and conflict involve distinct mPFC regions (Braver et al., 2001; Kiehl et al., 2000; Menon et al., 2001; Ridderinkhof et al., 2004). Moreover, there have been some suggestions that task-switching and conflict produce more dorsal activations than those related to reward and error processes (Ridderinkhof et al., 2004; Rushworth et al., 2004; Ullsperger and von Cramon, 2001). However, no study has directly compared conflict, errors, and task-switching within the same subjects. Given the anatomical variability of the mPFC (Vogt et al., 1995) such within-subjects comparisons are critical for ascertaining the functional properties of mPFC.
There is reason to expect both commonalities and distinctions in mPFC activations to conflict, task-switching, and error processing. On the one hand, all three functions are related to difficulties in selection and hence the monitoring and/or prediction of these difficulties. On the other hand, selection can occur at different levels: conflict relates to difficulties in response selection, task-switching involves selecting amongst task-sets, and errors require both of these in addition to general task re-engagement. Hence, different forms of selection may each relate to different sub-portions of the mPFC. A further distinction may arise between conflict and errors on one side and task-switching on the other. The former require strengthening of the current task representation whereas the latter requires strengthening of a new task representation. However, a different account could group both task-switches and errors as a need to change behavior, whereas correct responses in the face of conflict do not. Understanding which signals are evident in mPFC and which are absent is paramount to proper theoretical and computational account of these functions.
Here, we examine activations related to errors, conflict, and task-switching within mPFC. We demonstrate that although each of these phenomena produce widespread activation across mPFC, different portions of mPFC can be distinguished that respond preferentially to different task-related manipulations. We suggest that various theories of mPFC function may pertain to different portions of mPFC and that these theories may be best thought of as complementary rather than competitive.
Materials and Methods
Subjects
Twenty-nine subjects (17 female; mean age 23.9 years old) were recruited from the Bloomington area to participate in this study. One subject failed to complete the study and another was discarded due to poor image quality, leaving 28 subjects for behavioral analyses and 27 subjects for fMRI analyses. Subjects were compensated $25 an hour for their participation in addition to a performance-based bonus.
Task
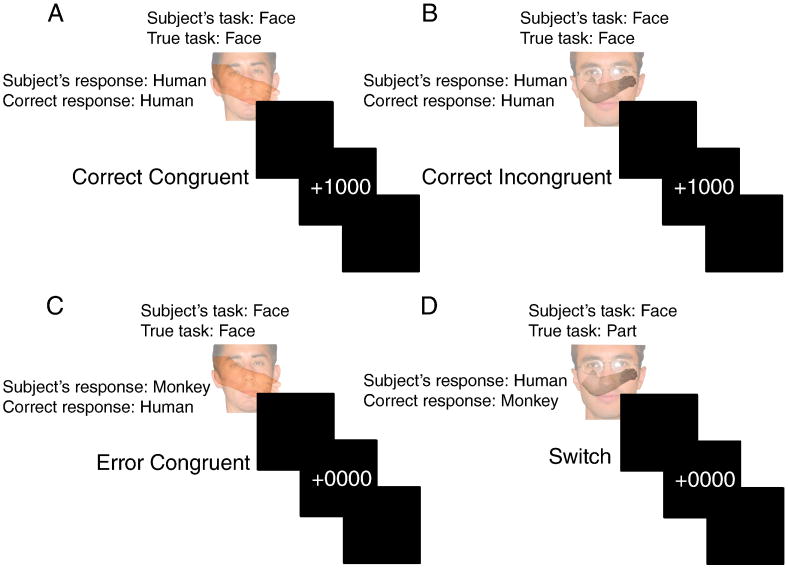
The task was designed in order to compare conflict, task-switching, and errors (Figure 2). On each trial, subjects were presented with an overlaid face and body part (i.e. a limb). Faces and body parts could either be human or monkey. In the face task, subjects responded to the species of the face; in the body part task, subjects responded to the species of the body part. Responses were made with the left or right index finger of each hand. Species-to-response mappings were counter-balanced across subjects. Hence, conflict1 could be manipulated by having both the face and body part indicate the same response (congruent; no conflict) or different responses (incongruent; conflict). Having two well-matched tasks allowed us to examine effects of switching between tasks. Errors were expected to naturally occur as is often the case in conflict and task-switching situations.
Figure 2.
Depiction of the task design. Subject's were presented with a face overlaid with a body part (limb) and responded to the species of the relevant task (face or part). The relevant task was not explicitly cued and had to be derived from feedback. A) The face and body part are of the same species (human) producing a Congruent trial. The subject responds appropriately and receives positive feedback (+1000). B) The face and body part are of different species and the subject is on the correct task (face) and responds appropriately (human). C) The subject responds inappropriately on a Congruent trial. D) The subject is on the face task but the task has changed to part, unbeknownst to the subject. Upon receiving negative feedback the subject now must switch tasks.
Feedback representing the presence or absence of monetary reward indicated whether the subject was correct (+1000) or incorrect (+0000). The task (face task or body part task) was never explicitly cued and had to be deduced from feedback. The task changed without warning every 5-15 trials. Subjects might tend to anticipate a higher probability of switching as more trials elapse without a switch. To counter this possibility, beginning with the fifth trial without a switch, we set the probability of switching to 0.2 on each trial given that a switch had not yet occurred. This renders the task switch unpredictable as a function of the number of trials since the last switch. The run length on the same task then follows an exponential distribution, which has a constant hazard function of probability 0.2. If 15 trials occurred without a switch, then the task switched on the following trial. During a given trial, each stimulus appeared for 1500 ms, followed by a 500 ms blank interval, followed by feedback for 1000 ms. The inter-trial interval was randomly jittered between 1000 and 7000 ms (Dale, 1999). Subjects performed 5 runs of 82 trials each.
On congruent trials, both the face and the body part indicated the same response, e.g. human or monkey. On incongruent trials, the face and the body part indicated different responses. Congruent and incongruent trials were randomly interspersed in equal proportions. Notably, only on incongruent trials could the subject discover that the relevant task had switched since it is on these trials where the incorrect task could lead to an erroneous response. Hence, task-switches could only follow negative feedback after an incongruent trial. A trial was classified as a task-switch only if the subject was correct on the first incongruent trial following a potential switch. Otherwise, the trial was considered an error. Hence, congruent trials could lead to correct responses or errors. Incongruent trials could lead to correct responses, task-switches, or errors.
Using reduced-reward as a cue for switching has produced robust mPFC activation in previous work (Bush et al., 2002; Shima and Tanji, 1998; Williams et al., 2004). As a result of our design to probe implicitly-cued switches, the task-switch trials shared strong commonalities with both conflict and error trials. Task-switches followed incongruent stimuli that elicit conflict. Task-switches were made in response to reduced reward that is the same kind of feedback given during error trials. Hence, our design allowed us to test whether the mPFC responds differentially to task-switching over-and-above conflict, and differentially to errors over-and-above reduced reward.
Image Acquisition and Preprocessing
Images were acquired on a 3T Siemens Trio. Stimuli were presented to the subject via a projector at the rear of the scanner, reflected off a mirror mounted to the headcoil. Experimental tasks were presented using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA).
Functional T2*-weighted images were acquired using an EPI sequence with 33 contiguous slices and 3.44 × 3.44 × 3.75 mm voxels (TR = 2000 ms; echo time = 25 ms; flip angle = 70; field of view = 220). Phase and magnitude images were collected to estimate the magnetic inhomogeneity. T1-weighted MPRAGE images were collected for normalization.
Functional data were spike-corrected to reduce the impact of artifacts using AFNI's 3dDespike (http://afni.nimh.nih.gov/afni). Subsequent processing and analyses were done using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). Functional images were corrected for differences in slice timing using sinc-interpolation (Oppenheim et al., 1999) and head movement using least-squares approach and a 6 parameter rigid body spatial transformation. Images were corrected for distortion and movement-by-susceptibility artifacts using the FieldMap toolbox implemented in SPM5 (Andersson et al., 2001). Structural data were coregistered to the functional data and segmented into gray and white-matter probability maps (Ashburner and Friston, 1997). These segmented images were used to calculate spatial normalization parameters to the MNI template, which were subsequently applied to the functional data. 8-mm full-width/half-maximum isotropic Gaussian smoothing was applied to all functional images prior to analysis using SPM5. All analyses included a temporal high-pass filter (128 s), and each image was scaled to have a global mean intensity of 100.
Image Analysis
Analyses were conducted using the general linear model implemented in SPM5. Due to the close temporal proximity of the stimulus and feedback, two separate models were created: one to assess conflict effects and another to assess task-switching and error effects. Contrasts were created to match events of non-interest and isolate processes of interest. For subjects demonstrating greater than 3 mm of motion across a session or greater than 0.5 mm of motion between TRs, 24 motion regressors were included to capture linear, quadratic, differential, and squared differential residual motion variance (Lund et al., 2005).
To assess effects of conflict, predictors were time-locked to the onset of the stimulus and convolved with a canonical hemodynamic response function (HRF) implemented in SPM5 that took into account lag in the hemodynamic response and assumed a peak approximately 5 seconds after event onset. Separate predictors were included for correct incongruent and congruent trials. Error and switch trials were modeled separately. Conflict was assessed by contrasting correct incongruent and congruent trials.
To assess effects of task-switching and errors, predictors were time-locked to feedback and convolved with a canonical HRF. Separate predictors were included for task-switch, congruent errors, incongruent errors, correct congruent, and correct incongruent trials. Since task-switches could only occur to feedback following an incongruent trial, task-switching was assessed by contrasting task-switch trials with correct incongruent trials, thereby subtracting out effects of conflict. Error-related activation was assessed by contrasting error trials with correct trials. Task-switches and errors were also directly compared. Follow-up analyses separately examined accuracy (error or correct) × congruency interactions.
To establish the networks involved in conflict, task-switching, and errors, we performed whole-brain analyses on the contrasts described above. Whole-brain analyses were thresholded at p < 0.001 uncorrected at the voxel-level with a 100 voxel minimum cluster extent, producing a cluster-corrected threshold of p < 0.05. This provided an equivalent t-statistic height threshold for all contrasts whereas other corrections can produce variable t-statistic thresholds. Notably, mPFC activations surpassed height-corrected thresholds as well, so the observed activations were not a result of our threshold being too lenient.
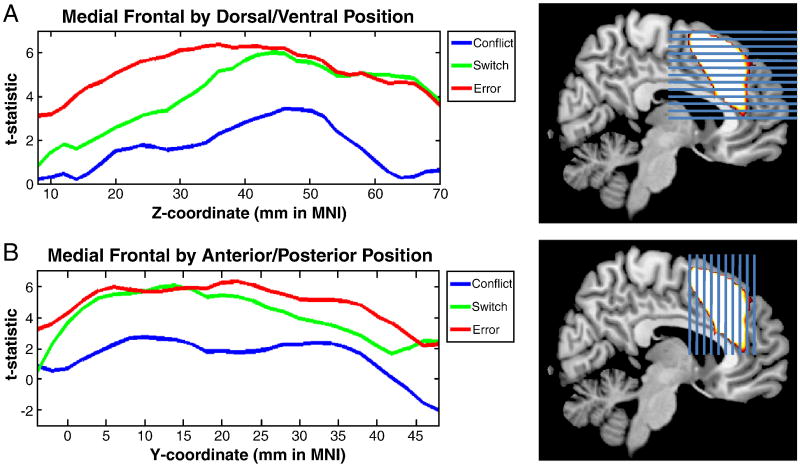
Subsequent analyses sought to compare neural responses to conflict, task-switching, and errors within the mPFC. To provide an mPFC region of interest (ROI) to compare conflict, task-switching, and errors, a separate contrast was defined that collapsed across correct incongruent, task-switch, and error trials, subtracting out correct congruent trials as a baseline, also height thresholded at p < 0.001 uncorrected using 100 voxel cluster extent to control for multiple comparisons. Once again, a lenient threshold was used in order to create a large exploratory ROI, but activations also surpassed more stringent corrected thresholds. Subsequent analyses focused only on voxels within this ROI. To examine heterogeneity within the mPFC, separate analyses collapsed across all significant voxels within the ROI in the z-plane and the y-plane. The former allowed the assessment of conflict, task-switching, and error contributions by dorsal/ventral position in mPFC whereas the latter provided assessments by anterior/posterior position in mPFC.
To better quantify the patterns suggested by this analysis, spherical ROIs (radius = 5 mm) were placed in the mPFC according to demarcations suggested by Picard and Strick (2001). To provide unbiased ROIs for these analyses, we first averaged together the coordinates of studies reporting activation in RCZa according to Picard and Strick (2001) to derive a candidate peak for RCZa (Barch et al., 2000; Botvinick et al., 1999; Carter et al., 2000; Casey et al., 2000; Herrmann et al., 2001; Rubia et al., 2001). We did the same for RCZp (Menon et al., 2001; Rubia et al., 2001). Mean peaks were converted from Talairach space to MNI space. Next, these peaks were plotted on a mean anatomical image created from the subjects in this study. The peaks were then adjusted so that the RCZp peak was anterior to the VAC and the RCZa peak was posterior to the genu of the corpus callosum within cingulate cortex. Spheres for the pre-SMA and CG were placed so that they would not overlap with the spheres in RCZa and RCZp and also followed the anatomical boundaries described by Picard and Strick (2001). The resultant ROIs were centered as follows: cingulate gyrus (MNI center 0, 38, 10), RCZa (MNI center 0, 28, 32), RCZp (MNI center 0, 10, 46), and pre-SMA (MNI center 0, 8, 58). These ROIs were used to examine condition × region interactions, brain-behavioral correlations, and hemodynamic time-courses.
To ensure that the results were not unduly affected by the placement of our ROIs, a second analysis examined the entire functional ROI described above. The functional ROI was carved into 4 separate zones:(10≤ z ≤ 18), (20 ≤ z ≤ 38), (40 ≤ z ≤ 52), and (54 ≤ z ≤ 70). Boundaries were defined according to visual inspection of overlap with anatomical boundaries. These zones contained the spherical ROIs described above, with the inferior most one containing CG, the next containing RCZa, the next RCZp, and the dorsal most containing the pre-SMA. Although these zones blurred across anatomical boundaries due to the inclusion of the entire functional ROI, they provided a reasonable assessment of the robustness of the spherical ROI based results. These results did not differ appreciably from the spherical ROI approach and are described in the supplemental data.
Results
Behavioral Results
The behavioral data demonstrated anticipated effects of conflict in error-rates and reaction times (RTs) and task-switching in RTs. All RT analyses were performed on correct trials only. In terms of conflict, subjects made more errors on incongruent (19.6%) than congruent (5.2%) trials (t(27) = 5.70, p < 0.001). Subjects were also slower on incongruent trials (815.7ms) compared to congruent trials (745.0 ms) demonstrating a significant conflict effect (t(27) = 7.25, p < 0.001). Trials following task-switches did not differ in accuracy compared to task-repeat trials (97.1% vs. 96.7%, t(27) = 1.09, p > 0.25). However, RTs were greater following task-switch trials (799.9 ms) compared to task-repeat trials (772.6 ms) indicating a significant switch cost (t(27) = 3.07, p < 0.01). There was no congruency × switch interaction (i.e. the switch-cost was the same regardless of whether task-switches were followed by congruent or incongruent trials; t(27) = 0.54, p > 0.6), and conflict and switch costs were uncorrelated (r = 0.06, p > 0.75) suggesting that effects of conflict and task-switching were independent. Finally, subjects were slower following error trials (799.8 ms) compared to correct trials (776.3 ms) demonstrating typical post-error slowing t(27) = 2.47, p < 0.05).
Imaging Results
Whole-Brain Contrasts
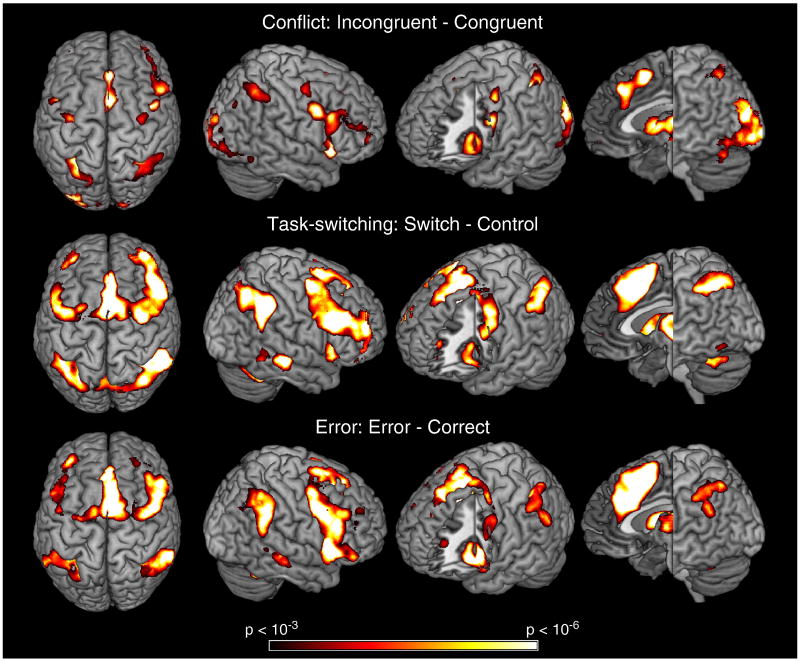
First, we assessed neural effects of conflict, task-switching, and error processing using whole-brain contrasts. The conflict contrast (correct incongruent – correct congruent) produced 2 peaks of activation within mPFC both localized in RCZ (Figure 3). The first peak was located dorsal and just posterior to the genu of the corpus callosum focused in the paracingulate gyrus in RCZa. The second was more posterior and dorsal, just anterior to the VAC placing it in RCZp. Activations also proceeded more dorsally into the pre-SMA. The switch contrast (task-switch – correct incongruent) overlapped entirely with the conflict contrast and included greater extension in to the pre-SMA. The error contrast (error – correct) was similar to the switch contrast, but with greater extension more ventrally into the cingulate gyrus. These results are summarized in Figure 3 and Table 1.
Figure 3.
Whole-brain results from each contrast of interest. Each contrast produces a similar pattern of lateral frontal, medial frontal, and lateral posterior parietal activations. Switch-related activations (middle) produced activations in more dorsal portions of mPFC that were not found in the Conflict contrast (top). Error-related activations in mPFC (bottom) extended more inferiorly and anteriorly.
Table 1. Contrast results in mPFC.
| Peaks within mPFC | |||||
|---|---|---|---|---|---|
| Contrast | X | Y | Z | t-statistic | location |
| Inc-Con | 6 | 10 | 52 | 8.3 | RCZp/pre-SMA |
| 4 | 34 | 38 | 7.41 | RCZa | |
| 10 | 30 | 24 | 5.82 | Cingulate sulcus | |
| Switch-Control | 2 | 14 | 42 | 11.72 | RCZp |
| -2 | 8 | 58 | 9.8 | pre-SMA | |
| 8 | 24 | 36 | 9.44 | RCZa | |
| Error-Correct | 8 | 16 | 46 | 11.91 | RCZp |
| 4 | 22 | 32 | 10.25 | RCZa | |
| -8 | 16 | 40 | 10.02 | RCZp | |
| Switch-Conflict | -2 | 8 | 60 | 10.1 | pre-SMA |
| 14 | 10 | 64 | 8.32 | pre-SMA | |
| -2 | 14 | 42 | 7 | RCZp | |
| Error-Switch | -8 | 52 | 14 | 6.45 | rACC/mFG |
| -2 | 32 | 10 | 5.93 | CG | |
| -4 | 12 | 36 | 5.4 | Cingulate sulcus | |
T-statistics (d.f. = 26) for each contrast.
CG = cingulate gyrus
mFG = medial frontal gyrus
rACC = rostral anterior cingulate cortex
RCZa = anterior rostral cingulate zone
RCZp = posterior rostral cingulate zone
Pre-SMA = pre-supplementary motor area
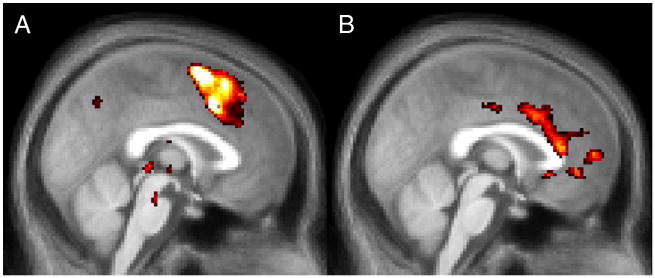
To examine neural differences between conflict, task-switching and error processing, we directly compared contrasts isolating these effects (Figure 4; Table 1). Task-switching produced significantly greater activation in the pre-SMA than conflict, as well as stronger activation in RCZp. Errors produced stronger activation than task-switching in the cingulate gyrus, just adjacent to the corpus callosum. No other significant differences were found in the mPFC2. Taken together, the whole-brain results suggested that task-switching was more dominant in dorsal and posterior portions of mPFC and errors were more dominant ventral and anterior portions.
Figure 4.
A) Contrast of Task-Switching and Conflict contrasts. Differences were found throughout much of the mPFC and strongest in dorsal aspects including RCZp and the pre-SMA. B) Contrast of Error and Task-Switch trials. Differences were largely restricted to the cingulate gyrus adjacent to the corpus callosum in Brodmann's Area 24.
Regions of Interest Analyses
To provide a more detailed picture of the results described above, we examined the strength of conflict, task-switching, and errors as a function of dorsal/ventral position in mPFC and anterior/posterior position in mPFC (see Methods). These analyses provide a continuous map of functional variation. As can be seen in Figure 5, conflict effects were weaker than both task-switching and error effects throughout the mPFC. In ventral and anterior portions of the ROI including both the cingulate gyrus and the dorsal anterior cingulate, error effects were stronger than task-switching effects. However, in the pre-SMA in the dorsal and posterior portions of the ROI, both errors and task-switching effects were of equal strength.
Figure 5.
Activations by position in the mPFC. First, an ROI was defined that combined Conflict, Task-Switching, and Error-related activations. Next, this ROI was sliced horizontally through the z-plane (A, left) and vertically through the y-plane (B, left). Within each slice, the average t-statistic was computed separately for Conflict (blue), Switching (green), and Error (red) contrasts. In ventral and anterior regions of the mPFC ROI, Error-related activation dominated. In dorsal and posterior regions, Errors and Switching were equivalent. Conflict-related activation was less strong than both Switching and Error-related activation throughout.
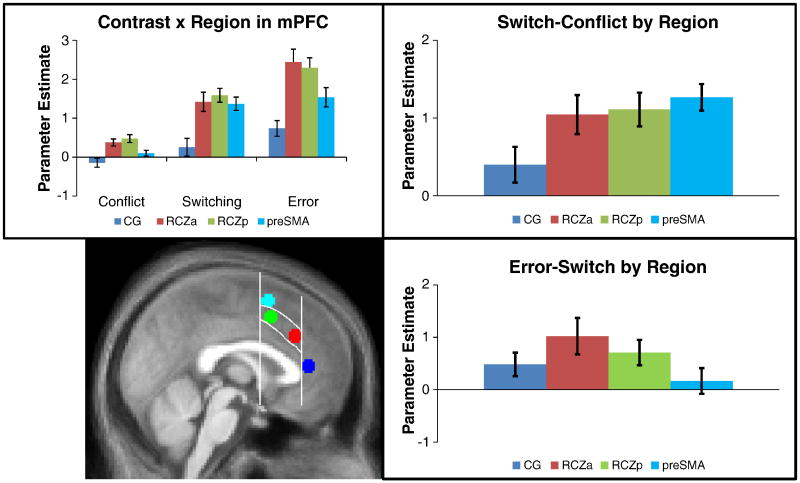
To better quantify these patterns, we placed spherical ROIs in 4 different regions in the mPFC (see Methods; Figure 6). The first was placed in the cingulate gyrus (CG, blue), just superior and anterior to the genu of the corpus callosum. The second was placed more posterior and dorsal in RCZa (red). The third was placed in RCZp (green) and the fourth in the pre-SMA dorsal to RCZp (cyan). These ROIs follow the demarcations of Picard and Strick (2001). To formally examine functional heterogeneity within the mPFC, we tested the contrast (conflict, task-switch, error) by region (CG, RCZa, RCZp, pre-SMA) interaction. This interaction was significant (F(6,156) = 4.00, p < 0.001).
Figure 6.
Bottom Left : Spherical ROIs were placed into 4 sub-regions based upon the averaged anatomical image of the subjects in this study and demarcations suggest by Picard and Strick (2001). Vertical lines are drawn through the tip of the genu of the corpus callosum and through the anterior commisure, with the curved lines outlining RCZ between these landmarks. Spheres were placed in the cingulate gyrus anterior to the genu (blue), RCZa (red), RCZp (green), and pre-SMA (cyan) dorsal to RCZ and anterior to the anterior commisure. Top left: Conflict, Switching, and Error contrasts as a function of region. Top right: A comparison of Switching and Conflict contrasts by region. Bottom right: A direct contrast of Error and Switch trials (see Methods).
In order to quantify the nature of the contrast × region interaction, we conducted follow-up tests which examined regional effects for each contrast of interest. These tests are summarized in Table 2. First, examination of conflict effects revealed a significant effect of region (F(3,78) = 9.06, p < 0.001). Conflict effects were significant in both RCZa and RCZp and strongest in RCZp. No significant effects of conflict were found in CG or pre-SMA.
Table 2. Region of interest analyses.
| Region | ||||
|---|---|---|---|---|
| Contrast | CG | RCZa | RCZp | preSMA |
| Inc - Con (Conflict) | -1.26 (n.s.) | 4.21 (**) | 4.80 (***) | 1.42 (n.s.) |
| Switch - Control (Switch) | 1.13 (n.s.) | 5.73 (***) | 8.87 (***) | 8.04 (***) |
| Error - Correct (Error) | 3.69 (*) | 7.39 (***) | 8.95 (***) | 6.21 (***) |
| Switch - Conflict | 1.73 (n.s.) | 4.14 (**) | 5.10 (***) | 7.41 (***) |
| Error - Conflict | 4.34 (**) | 5.88 (***) | 6.45 (***) | 5.33 (***) |
| Error - Switch | 2.65 (+) | 2.94 (*) | 2.51 (+) | 0.81 (n.s.) |
T-statistics (d.f. = 26) for each contrast within regions of interest.
CG = cingulate gyrus
RCZa = anterior rostral cingulate zone
RCZp = posterior rostral cingulate zone
preSMA = pre-supplementary motor area
n.s. – not significant (p > 0.05)
p < 0.05
p < 0.01
p < 0.001
p < 0.0001
Switching-related activation also varied by region (F(3,78) = 11.74, p < 0.001). Task-switching effects were significant in RCZa, RCZp, and pre-SMA, but not CG. Task-switching effects were strongest in RCZp and pre-SMA. Compared to conflict, task-switching elicited stronger activation throughout the mPFC and these differences were greater in more dorsal regions of mPFC. Confirming these patterns, the region × contrast (task-switch, conflict) interaction was significant (F(3,78) = 3.78, p < 0.05), reflecting increasing switch > conflict effects in more dorsal aspects of mPFC. These results suggest that task-switching effects are strongest in RCZp and pre-SMA.
The error contrast was strong in all mPFC regions and strongest in RCZa and RCZp, also demonstrating regional variability (F(3,78) = 15.39, p < 0.001). Error-related activation was stronger than conflict-related activation throughout mPFC and varied by region, showing the strongest differences in RCZ (region × contrast (error, conflict) interaction: F(3,78) = 5.27, p < 0.01). Contrasting errors and task-switching, the region × condition (error, task-switch) interaction was also significant (F(3,78) = 2.80, p < 0.05). Whereas errors showed greater activation than task-switching in CG, RCZa, and RCZp, there was no difference in the pre-SMA.
In summary, our data revealed widespread heterogeneity within the mPFC with significant region × contrast interactions when considering all contrasts together, as well as each pair of contrasts (i.e. conflict vs task-switching, conflict vs. errors, task-switching vs. errors). Conflict effects were most strongly associated with activation in RCZ. Switch-related activation was greater than conflict-related activation throughout the mPFC and largest in the pre-SMA. Similar to conflict, error-related activation was strongest in RCZ. Unlike conflict, however, there was significant error-related activation in CG, whereas task-switching did not activate this region significantly and conflict showed a trend in the opposite direction. To examine the robustness of these effects we repeated the above analyses by dividing the entire functional mPFC ROI into 4 zones (see Methods). This analysis produced similar results demonstrating that these effects are robust to minor variations in ROI definition (Figure S1).
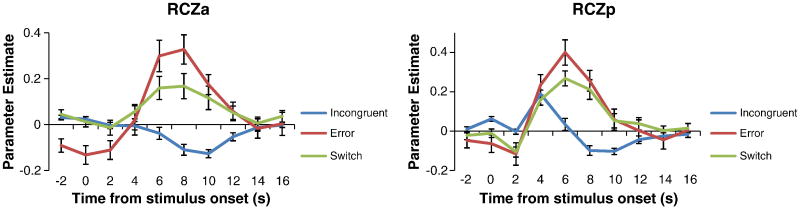
Time-Course Distinctions in RCZ
The previous analyses were derived from parameter estimates assuming a canonical HRF shape. Such analyses can often miss more subtle qualitative differences between regions. In order to examine such differences in more detail, additional analyses examined the time-courses of activations. A striking pattern emerged that differentiated RCZa and RCZp. Although incongruent trials produced strong activations in RCZp, the hemodynamic response showed strong deactivation in RCZa. This pattern was in stark contrast to responses to switches and errors that produced strong activations in both RCZa and RCZp (Figure 7, see also Figure S2). To quantify this difference, we performed a region (RCZa, RCZp) × condition (incongruent, switch, error) × time ANOVA. Confirming visual impressions, this analysis revealed a significant region × condition × time interaction (F(18,468) = 1.69, p < 0.05). These effects were unlikely to be due to vasculature differences between the regions since there was no main effect of region (F(1,26) = 1.306, p > 0.25) nor a region × time interaction (F(2,52) = 1.166, p > 0.3). Instead, the 3-way interaction suggests a functional differentiation between RCZa and RCZp (see Supplemental Data; Figure S2 for additional time-course analyses).
Figure 7.
Finite impulse responses (FIRs) demonstrating the time-courses of the hemodynamic response in RCZa and RCZp. Whereas hemodynamic responses to errors and task-switches were similar in the two regions, incongruent trials produced deactivations in RCZa and activations in RCZp.
Brain-Behavior Relationships
To confirm that neural activations were related to behavioral effects, we correlated behavioral indices of conflict, task-switching, and errors with activation in CG, RCZa, RCZp, and pre-SMA across subjects. These results are summarized in Table 3. Two comparisons achieved significance as assessed by corrected thresholds: a positive relationship between conflict and RCZa activation and a negative relationship between errors and RCZa activation. Due to the confirmatory nature of these tests, we also report uncorrected results.
Table 3. Brain-behavior correlations within regions of interest.
| CG | RCZa | RCZp | preSMA | |||||
|---|---|---|---|---|---|---|---|---|
| Pearson | Robust | Pearson | Robust | Pearson | Robust | Pearson | Robust | |
| r | t-stat | r | t-stat | r | t-stat | r | t-stat | |
| Conflict | -0.41* | -1.87 | 0.57** | 4.54** | 0.37† | 3.39** | -0.12 | -0.19 |
| Task-Switching | -0.15 | -0.48 | -0.18 | -0.81 | 0.09 | 0.37 | -0.37† | -2.04† |
| Errors | -0.52* | -2.68* | -0.60** | -3.26** | -0.43* | -2.00† | 0.02 | -0.09 |
Pearson correlations and robust regressions for each contrast-behavioral index relationship within regions of interest.
p < 0.06
p < 0.05
p < 0.05 corrected (Holm-Bonferroni)
Behavioral conflict effects (incongruent – congruent RT) showed a significant correlation with conflict-related activation (incongruent – congruent activation) in RCZa and a borderline sub-threshold correlation with RCZp. These correlations were significant at corrected levels using robust regression to lessen the impact of outliers. Conflict was negatively correlated with conflict-related activation in CG, but this correlation did not survive robust regression suggesting it may be driven by outliers. Hence, regions showing significant conflict-related activation also showed significant correlations with behavioral conflict.
Behavioral error rates (% errors) were significantly correlated with error-related activation (error – correct activation) in RCZa. Sub-threshold correlations were also found in CG and RCZp, but not pre-SMA. By contrast, behavioral switch costs (task-switch – task-repeat RT) were not correlated with switch-related activation in CG, RCZa, or RCZp, but there was a borderline sub-threshold correlation in pre-SMA.
In accordance with the univariate analyses, activations in RCZ demonstrated strong relationships with behavior with regards to both conflict and errors. Consistent with a ventral/anterior dominance of errors, activations in CG correlated only with errors. Consistent with a dorsal/posterior dominance of task-switching, activations in pre-SMA demonstrated a borderline correlation with task-switching, but no other correlations. Hence, the correlational data confirmed that the patterns uncovered in the univariate analyses were not epiphenomenal, but instead were closely tied to behavior.
Conflict Monitoring and Prediction in RCZ
Our correlational analyses revealed positive correlations between conflict and RCZ activation, but the reverse pattern for errors. Interactions between congruency and accuracy have been previously documented, yet their origin is unclear. Whereas correct responses to incongruent stimuli produce stronger activations than correct responses to congruent stimuli, the opposite has been found of errors (Scheffers and Coles, 2000; Yeung et al., 2004). That is, congruent errors produce greater activation than incongruent errors. Yeung and colleagues (2004) demonstrated that such a congruency × accuracy interaction is predicted by the conflict monitoring model. However, models positing that the ACC responds to deviations from expected outcomes also predict such a pattern (Alexander and Brown, In Press; Holroyd and Coles, 2002; Holroyd et al., 2005). To our knowledge, congruency × accuracy interactions have been documented in EEG, but not in fMRI. So, we began by confirming their presence in these data and then used activations on task-switch trials to adjudicate between conflict monitoring and outcome prediction models.
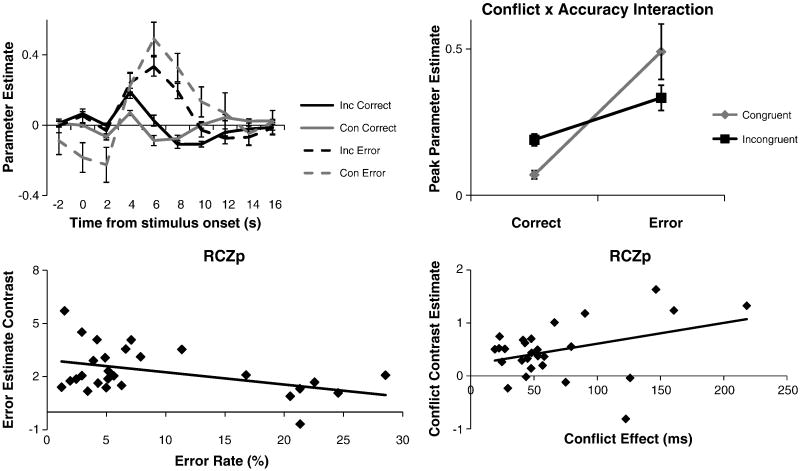
Time-course peaks were examined for correct congruent, correct incongruent, incorrect congruent, and incorrect incongruent trials (Figure 8). One subject was excluded due to having no errors on congruent trials. We focused on RCZp since all conditions produced activation in RCZp. We performed a 2 × 2 ANOVA with factors of congruency (congruent, incongruent) and accuracy (correct, error) on the peaks of activation. Interestingly, there was no main effect of congruency (F(1,25) = 0.20, p > 0.6), but there was a main effect of accuracy (F(1,25) = 10.24, p < 0.01). The lack of a main effect of congruency was belied by a congruency × accuracy interaction (F(1,25) = 19.28, p < 0.001). As depicted in Figure 8, in contrast to the previously documented effect of conflict on correct trials (correct incongruent > correct congruent, t(25) = 4.68, p < 0.001), congruent errors produced marginally greater activation than incongruent errors (t(25) = 1.92, p = 0.066).
Figure 8.
Congruency × Accuracy interactions in RCZp. Top Left: Time courses of activation in RCZp for correct congruent, correct incongruent, incorrect congruent, and incorrect incongruent trials. Top Right: RCZp demonstrated a conflict (incongruent, congruent) × error (correct, error) interaction. Bottom Left: Error rates measured behaviorally correlated negatively with the Error neural contrast. Bottom Right: RT measurements of conflict correlated positively with the Conflict neural contrast.
One way to discriminate between conflict monitoring and outcome prediction models is to examine task-switch trials. Prior to feedback, task-switch and correct incongruent trials are identical for the subject: in both cases subjects resolve conflict from a stimulus deemed irrelevant by the task they are currently performing. Hence, both should generate the same amount of response conflict. However, task-switch and correct incongruent trials diverge at the time of feedback. On task-switch trials, negative feedback signals to the subject that the task they were performing was not the correct task and that they need to switch tasks. On correct incongruent trials, positive feedback signals that the subject was correct and to continue to perform the task as they had been. Since task-switch trials occur somewhat infrequently relative to correct incongruent trials, negative feedback indicating a switch is more surprising than positive feedback indicating a repeat. Therefore, from the conflict model, task-switch and correct incongruent trials should show similar ACC activation, but by the outcome prediction model, task-switch trials should show greater activation than correct incongruent trials. As previously reported, switch-related activation was stronger than conflict-related activation, supporting the outcome prediction model.
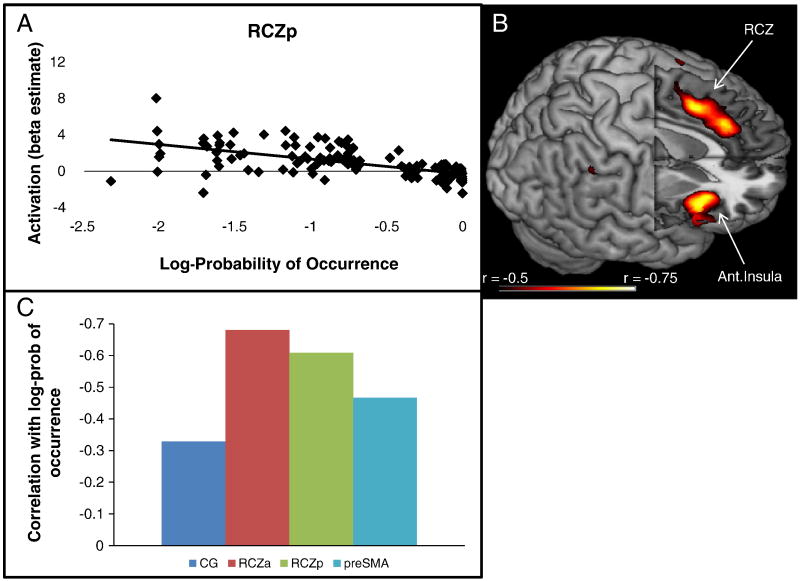
Recent work suggests that the mPFC responds generally to unexpectedness regardless of whether an outcome is desirable or not (Jessup et al., 2010; Oliveira et al., 2007). To further examine this idea, we probed activation in RCZp during feedback as a function of outcome probability. For each subject, we calculated the probability of an outcome (correct, error, or task-switch) given a stimulus type (congruent or incongruent). As depicted in Figure 9, outcome log-probability was a highly reliable predictor of activation in RCZp (r = -0.609, p < 0.001). That is, activation in RCZp increased as a function of the rarity of an outcome. In order to explore whether this pattern was unique to RCZp, or a property of other regions as well, we repeated the test on the whole-brain using a strict threshold to restrict this somewhat post-hoc analysis (p < 0.01, FWE corrected). Outcome log-probability was a significant predictor of activation in several frontal and parietal regions. As depicted in Figure 9, there were two distinct activation peaks within medial PFC which localized to RCZp and RCZa. Hence, RCZ appears to be strongly related to violations of expected outcomes. We also compared the correlations between activation and outcome log probability in the four medial PFC regions of Figure 6 using Steiger's Z statistic. Confirming the whole-brain patterns, correlations were significantly stronger in RCZa than CG (z = 5.15, p < 0.0001) and pre-SMA (z = 3.02, p < 0.01). Correlations in RCZp were also stronger than those in CG (z = 3.08, p < 0.01) and pre-SMA (z = 2.12, p < 0.05). RCZp and RCZa did not differ from each other (z = 1.41, p > 0.15), nor did CG differ from pre-SMA (z = 1.39, p> 0.15). Hence, activation in RCZ appeared to be preferentially associated with unexpectedness (Jessup et al., 2010).
Figure 9.
A) Activation in RCZp as a function of the log-probability of outcomes (correct | congruent; error | congruent; correct | incongruent; error | incongruent; switch | incongruent). B) whole-brain exploratory correlations with log-probability of outcomes demonstrated foci in RCZa, RCZp, and the insula. C) Correlations between activations in CG, RCZa, RCZp, and preSMA and log-probability of outcomes.
Discussion
At first glance, the broadly overlapping activation clusters produced by conflict, task-switching, and errors lend themselves to the idea that there is a great deal of functional homogeneity within the mPFC. However, more detailed analysis produced a varied picture, highlighting the heterogeneity inherent in the mPFC. We now discuss each investigated zone in more detail.
Cingulate Gyrus
The cingulate gyrus activation we found was preferential to errors. This activation was located just rostral and dorsal to the genu of the corpus callosum in a region often referred to as the rostral ACC3 (rACC; Bush et al., 2000). The rACC is generally thought to be affective in nature (Bush et al., 2000; Phan et al., 2002; Wager et al., 2009). For example, a meta-analysis of studies involving emotion demonstrated that the rACC responds to negative valence (Wager et al., 2009). These results are in contrast to meta-analyses of conflict (Nee et al., 2007; Ridderinkhof et al., 2004) and switching (Wager et al., 2004) that demonstrate activations in more dorsal aspects of mPFC, such as RCZ (see also Ridderinkhof et al., 2004). Studies directly comparing emotional (Whalen et al., 1998) and cognitive (Bush et al., 1998) versions of the Stroop task reveal a similar dichotomy with rACC involved in the emotional Stroop and RCZ involved in cognitive Stroop (Bush et al., 2000). In the present study, rACC activation was strongly related to error trials, but not task-switching or conflict. This suggests a role in negative affect related to slips in performance that is distinct from control functions involved in conflict and task-switching (but see Magno et al., 2006). Although more ventral aspects of the cingulate are often also involved in negative valence, such as pre-genual and sub-callosal regions (Wager et al., 2009), we did not find those areas here. These results suggest heterogeneity in the affective portions of the cingulate and that the rACC can be disentangled from more ventral portions of the cingulate (Beckmann et al., 2009).
Interestingly, although reduced reward characterized both task-switch and error trials, the rACC was selectively responsive to errors. One distinction between error and task-switch trials is fault. On error trials the subject could have made a correct response, but failed to. On task-switch trials, the task was switched unbeknownst to the subject, so the subject could not have been correct, barring clairvoyance. Hence, although reduced reward is generally negative, only when that negativity was coupled with fault did the rACC become active. The sensitivity to fault in rACC may suggest a role in assigning blame in order to correct erroneous slips in performance (Walton et al., 2004). This function may be accomplished via connections with the mesencephalic dopamine system (Holroyd and Coles, 2002; Holroyd et al., 2005; Williams and Goldman-Rakic, 1998). Consistent with these ideas, rACC demonstrates phasic responses to errors when punishment reinforces correct responding, a pattern which is mirrored in the nucleus accumbens, but dramatically different from tonic signals generated in RCZ (Simoes-Franklin et al., 2009). Hence, whereas RCZ may be important in ongoing control, rACC may instead assign blame during errors.
RCZ
RCZ demonstrated strong effects of conflict, task-switching, errors, and unexpectedness. Despite responsiveness to all manipulations, important distinctions were identified. Whereas errors and task-switching produced robust activation in RCZa and RCZp, incongruent trials demonstrated deactivations in RCZa and activations in RCZp. This interesting interaction suggests functional differentiations between RCZa and RCZp. Other work has also suggested functional differences between RCZa and RCZp. Braver and colleagues documented a dissociation with error effects more strongly localized in RCZa and effects of response inhibition more strongly localized to RCZp (Braver et al., 2001). Other work comparing error and conflict-related activation has also demonstrated a focus in RCZa for error effects with a contrasting posterior focus of conflict effects (Garavan et al., 2003; Kiehl et al., 2000; Menon et al., 2001). Finally, meta-analyses of conflict (Nee et al., 2007; Ridderinkhof et al., 2004) and switching (Wager et al., 2004) have suggested the center of mass for these functions lies in RCZp. Taken together, these data suggest that RCZa and RCZp can be functionally distinguished. Regional distinctions between error and conflict effects call into question models that cast both error monitoring and conflict effects as resulting from a single underlying conflict detection mechanism (Yeung et al., 2004). Hence, revision may be necessary.
How can we understand the functional roles of RCZa and RCZp? Some authors have suggested that the two regions correspond to two competing theories of mPFC function (Picard and Strick, 2001). On the one hand, RCZa may be involved in evaluative (Botvinick et al., 2001) or predictive functions (Brown and Braver, 2005, 2007; Rushworth et al., 2004) and on the other hand, RCZp may be involved in response selection (Picard and Strick, 2001; Swick and Turken, 2002; Turken and Swick, 1999). That is, whereas RCZa is involved in detecting situations that may require increased cognitive control (Botvinick et al., 1999), RCZp may be involved in selecting appropriate responses where uncertainty or conflict may make such selection difficult. In support of this idea, response conflict, which calls for increased demands on response selection, preferentially activates RCZp (Nee et al., 2007; Ridderinkhof et al., 2004). However, studies examining conflict × context interactions have nearly universally converged on RCZa (Botvinick et al., 1999; Brown and Braver, 2005, 2007; Carter et al., 2000; Casey et al., 2000). For example, compared to congruent trials, RCZa activation is high on incongruent trials when incongruent trials are unlikely, but this pattern is reduced or sometimes even reversed if incongruent trials are likely (Carter et al., 2000; Casey et al., 2000). Brown and Braver (2005) compared situations where errors were likely versus situations where errors were unlikely. When controlling for conflict, correct responses in high error-likelihood trials produced enhanced activation in RCZa compared to low error-likelihood trials, but this pattern was reversed for errors. These results highlight a predictive role of RCZa.
Interestingly, although conflict effects were present in RCZa when assessed by the contrast of correct incongruent – correct congruent, the hemodynamic response revealed deactivations for both of these trial types. By contrast, task-switches and errors produced activation in RCZa. This pattern is consistent with a prediction error signal (Jessup et al., 2010; Oliveira et al., 2007). Since accuracies were generally high, positive feedback was expected after incongruent trials. When these expectancies were met, there was a deactivation in RCZa. However, when this expectancy was violated and subject's received negative feedback either due to a switch or an error, RCZa became activated. By contrast, all conditions led to activation in RCZp. Hence, whereas RCZa may code for prediction errors, RCZp may represent increased demands on response selection.
Notably, activations in RCZa and RCZp are likely to be interdependent. To the degree with which predictions in RCZa are met, response selection demands on RCZp may be reduced. That is, predictions may proactively reduce control demands. However, if predictions are violated, selection demands would be expected to increase, causing the need for reactive control (Braver et al., 2007). Consistent with this possibility, we found an inverse relationship between outcome probability and activation in both RCZa and RCZp that may reflect this interdependence.
Pre-SMA
In our data, the pre-SMA was strongly activated during task-switch trials -- this was the only region in which error-related activation was not stronger than switching-related activation. Moreover, switching-related activation in the pre-SMA was marginally correlated with behavioral switch costs. Previous work has suggested that the pre-SMA is involved in the selection of action sets in both humans and monkeys (Rushworth et al., 2004; Shima et al., 1996). Selection of action sets is naturally needed in response to a task-switch as new stimulus-response mappings must be engaged. Following an error, a similar engagement may need to occur if the error is a result of inattention and improper task engagement. Indeed, some research has suggested that task sets decay over time (Altmann and Gray, 2002) and errors may signal the need for task set re-engagement. Therefore, common and equivalent activation in the pre-SMA for task-switching and errors may be due to the selection of action sets. Other research has highlighted a role of the pre-SMA in self-generated action (Nachev et al., 2005; Passingham et al., 2009) and monitoring intention (Lau et al., 2004). Both of these processes would be considered components of the ability to select action sets.
Another possibility is that activation in the pre-SMA may reflect processes related to post-error slowing. On both task-switches and errors, subjects received negative feedback that led to slowed responses on the following trial. Moreover, the amount of slowing following switch-feedback (27.3 ms) was similar in magnitude to the amount of slowing following error-feedback (23.5 ms). Although it is somewhat difficult to distinguish between these accounts it should be noted that post-error slowing did not correlate with error-related activation in the pre-SMA (r = -0.13). Moreover, the regional interactions between task-switches and errors demonstrate that these processes differ in important ways. Hence, although we cannot rule out the idea that post-error slowing may underlie some commonalities between task-switch and error-related activation, it is clear that neural differences exist between task-switches and errors.
Other Considerations
It should be noted that we have yet to consider a large amount of work done in other species regarding the functional role of the mPFC. Although there are many studies that are quite relevant to the matter at hand, how to draw homologues between human mPFC and monkey mPFC is up for debate. In particular, some authors have tentatively suggested that human RCZ can be associated with cingulate motor areas of the monkey (Picard and Strick, 1996, 2001), but these associations have been speculative. Other work has suggested that monkeys do not have a homologue to RCZ based on the observation that monkeys do not have a dorsal area 32 (Cole et al., 2009; Vogt et al., 1995). Functionally, whereas a hallmark of human RCZ is responsiveness to conflict, ACC cells in the monkey do not demonstrate sensitivity to conflict (Ito et al., 2003; Nakamura et al., 2005). These conflict effects are instead found more dorsally in monkey mPFC (Stuphorn et al., 2000). Monkey mPFC cells respond preferentially to a variety of combinations of visual, motor, and outcome signals (Matsumoto et al., 2003), especially the anticipation of outcomes (Amador et al., 2000; Shidara and Richmond, 2002) as well as discrepancies between actual and expected outcomes (Alexander and Brown, In Press; Amador et al., 2000; Matsumoto et al., 2007), similar to our findings here in human RCZ, especially RCZa. Together, these results suggest that a great deal of caution must be exercised when comparing mPFC in humans and monkeys. With this in mind, we have chosen to draw mainly from human work. However, it should be noted that using reduced reward as a cue for task-switching has been shown to effectively recruit mPFC activity in both humans (Bush et al., 2002; Williams et al., 2004) and monkeys (Shima and Tanji, 1998).
There is also a great deal of pertinent neuropsychological work, which we consider only briefly here. This literature has been somewhat varied with mPFC lesions sometimes causing performance decrements related to conflict (Cohen et al., 1999; di Pellegrino et al., 2007; Ochsner et al., 2001; Stuss et al., 2001; Swick and Turken, 2002; Turken and Swick, 1999) and error correction (Modirrousta and Fellows, 2008; Swick and Turken, 2002), but sometimes demonstrating no impairment (Critchley et al., 2003; Fellows and Farah, 2005; Janer and Pardo, 1991; Swick and Jovanovic, 2002; Swick and Turken, 2002). Even when lesion locations appear to be matched between samples, impairments are sometimes found (e.g. Stuss et al., 2001), but sometimes not (e.g. Fellows and Farah, 2005). Hence, although valuable insights can be gained from neuropsychological work, further examination is necessary to resolve apparent discrepancies.
Limitations
Task-switching is studied most conventionally with response to a cue which likely differs somewhat from reward based task-switching (Bush et al., 2002). However, reward-related switching also has a rich tradition owing to the Wisconsin Card Sorting Task and other related paradigms (Grant and Berg, 1948; Monchi et al., 2001). Although reward-related switching entails potentially more complex processing than cue-driven switches, we chose to use reward-related switching in order to provide a close approximation of both conflict (incongruency) and error (negative feedback) processes. Hence, although a cue-related switch might have afforded more precision, it may not have served as an ideal control for the comparisons we examined. Nevertheless, comparisons of cue-driven switches, conflict, and errors would also be desirable.
Errors may not all be uniform and different types of errors may have dissociable implications for the medial prefrontal cortex. In particular, there is a distinction between errors that are immediately recognized by the subject and errors that require feedback to detect. For instance, if the subject forgot what task to perform, they may not know whether or not an error had occurred until signaled by feedback. Our design could not tease apart conscious and unconscious errors. Moreover, close timing of error commission and feedback made separation of these events difficult. It seems reasonable to assume, however, that subjects were aware of many of their errors upon commission. Even so, hemodynamic responses to errors peaked 2 seconds (1 TR) later than responses to correct trials suggesting that error processing continued until feedback. However, this hemodynamic response may be a mixture of earlier peaking conscious errors and later peaking unconscious errors, as well as a combination of error commissions and feedback processing (Holroyd et al., 2004; Luu et al., 2003). Future investigations that vary the timing of these events will be an important avenue of further investigation.
Towards a Unified Model of mPFC Function
The previous discussion suggests that various regions of mPFC perform related, but distinguishable functions. We have suggested that the pre-SMA may be involved in the initiation or selection of action sets. RCZp may operate at a different level, helping select amongst competing responses. RCZa may form and evaluate predictions based upon contextual information that anticipates selection difficulty and/or error-likelihood. Finally, rACC may provide a basis for assigning blame when predictions go awry and errors are committed. In this taxonomy, the functions of different zones of mPFC are highly inter-related. This high degree of inter-relatedness may explain why mPFC activation is often widespread and varied across studies since different manipulations are likely to favor each of these functions differentially.
Although this model is speculative, it forms a basis for thinking about modeling the mPFC in a different light. Several competing models have been formed to explain different sets of mPFC data, many of which may reflect different portions of mPFC and different functions. By our account, many of these models are in fact related to different aspects of mPFC and need not be mutually exclusive. Instead, piecing together the varied models of mPFC function may provide us a more complete picture of the role of mPFC in cognition.
Supplementary Material
Acknowledgments
This research was supported in part by AFOSR FA9550-07-1-0454 (JB), R03 DA023462 (JB), R01 DA026457 (JB), P50 MH-62196 (SK) and the Indiana METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc. The authors thank E. Dinh for help with data collection.
Footnotes
We recognize that there are several theories that operationalize the difference between incongruent and congruent trials that do not invoke the notion of conflict. However, the conflict account is arguably the most prominent such theory. So, we use “conflict” as a convention and convenient label for present purposes although we discuss other potential mechanisms in the Discussion.
Note, errors and conflict were not directly compared in these analyses. But see following analyses demonstrating differences between these contrasts throughout mPFC.
Rostral ACC (rACC) should not be confused with the rostral cingulate zone (RCZ). The former indicates portions of the ACC that lay anterior to the genu of the corpus callosum and extending inferiorly, whereas the latter indicates portions of the ACC posterior and dorsal to the corpus callosum. RCZ is considered part of the dorsal ACC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander W, Brown J. Computational models of performance monitoring and cognitive control. Topics in Cognitive Science. doi: 10.1111/j.1756-8765.2010.01085.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann EM, Gray WD. Forgetting to remember: the functional relationship of decay and interference. Psychol Sci. 2002;13:27–33. doi: 10.1111/1467-9280.00405. [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol. 2000;84:2166–2170. doi: 10.1152/jn.2000.84.4.2166. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K. Modeling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Multimodal image coregistration and partitioning--a unified framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Sabb FW, Noll DC. Anterior cingulate and the monitoriing of response conflict: evidence from an fMRI study of overt verb generation. J Cogn Neurosci. 2000;12:298–309. doi: 10.1162/089892900562110. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in working memory. Oxford University Press; New York: 2007. pp. 76–106. [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn Affect Behav Neurosci. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H. Impairments of attention after cingulotomy. Neurology. 1999;53:819–824. doi: 10.1212/wnl.53.4.819. [DOI] [PubMed] [Google Scholar]
- Cole MW, Yeung N, Freiwald WA, Botvinick M. Cingulate cortex: diverging data from humans and monkeys. Trends Neurosci. 2009;32:566–574. doi: 10.1016/j.tins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Ciaramelli E, Ladavas E. The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci. 2007;19:275–286. doi: 10.1162/jocn.2007.19.2.275. [DOI] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Rotte M, Grubich C, Ebert AD, Schiltz K, Munte TF, Heinze HJ. Control of semantic interference in episodic memory retrieval is associated with an anterior cingulate-prefrontal activation pattern. Hum Brain Mapp. 2001;13:94–103. doi: 10.1002/hbm.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat Neurosci. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, Coles MG, Cohen JD. A mechanism for error detection in speeded response time tasks. J Exp Psychol Gen. 2005;134:163–191. doi: 10.1037/0096-3445.134.2.163. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Janer KW, Pardo JV. Deficits in selective attention following bilateral anterior cingulotomy. Journal of Cognitive Neuroscience. 1991;3:231–241. doi: 10.1162/jocn.1991.3.3.231. [DOI] [PubMed] [Google Scholar]
- Jessup RK, Busemeyer JR, Brown JW. Error effects in anterior cingulate cortex reverse when error likelihood is high. J Neurosci. 2010;30:3467–3472. doi: 10.1523/JNEUROSCI.4130-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Lund TE, Norgaard MD, Rostrup E, Rowe JB, Paulson OB. Motion or activity: their role in intra- and inter-subject variation in fMRI. Neuroimage. 2005;26:960–964. doi: 10.1016/j.neuroimage.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Lutcke H, Frahm J. Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high-resolution fMRI. Cereb Cortex. 2008;18:508–515. doi: 10.1093/cercor/bhm090. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Magno E, Foxe JJ, Molholm S, Robertson IH, Garavan H. The anterior cingulate and error avoidance. J Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Suzuki W, Tanaka K. Neuronal correlates of goal-based motor selection in the prefrontal cortex. Science. 2003;301:229–232. doi: 10.1126/science.1084204. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Abe H, Tanaka K. Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci. 2007;10:647–656. doi: 10.1038/nn1890. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Medial prefrontal cortex plays a critical and selective role in ‘feeling of knowing’ meta-memory judgments. Neuropsychologia. 2008;46:2958–2965. doi: 10.1016/j.neuropsychologia.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 2005;93:884–908. doi: 10.1152/jn.00305.2004. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Kosslyn SM, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, Rauch SL. Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychologia. 2001;39:219–230. doi: 10.1016/s0028-3932(00)00114-7. [DOI] [PubMed] [Google Scholar]
- Oliveira FT, McDonald JJ, Goodman D. Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. J Cogn Neurosci. 2007;19:1994–2004. doi: 10.1162/jocn.2007.19.12.1994. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW, Buck JR. Discrete-time signal processing. 2nd. Prentice Hall; Upper Saddle River, N.J.: 1999. [Google Scholar]
- Passingham RE, Bengtsson SL, Lau HC. Medial frontal cortex: from self-generated action to reflection on one's own performance. Trends Cogn Sci. 2009 doi: 10.1016/j.tics.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the processing of single words. Journal of Cognitive Neuroscience. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG. Performance monitoring in a confusing world: error-related brain activity, judgments of response accuracy, and types of errors. J Exp Psychol Hum Percept Perform. 2000;26:141–151. doi: 10.1037//0096-1523.26.1.141. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002;296:1709–1711. doi: 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Shima K, Mushiake H, Saito N, Tanji J. Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci U S A. 1996;93:8694–8698. doi: 10.1073/pnas.93.16.8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Simoes-Franklin C, Hester R, Shpaner M, Foxe JJ, Garavan H. Executive function and error detection: The effect of motivation on cingulate and ventral striatum activity. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39:771–786. doi: 10.1016/s0028-3932(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Swick D, Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–1253. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci U S A. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Turken AU, Swick D. Response selection in the human anterior cingulate cortex. Nat Neurosci. 1999;2:920–924. doi: 10.1038/13224. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van Veen V, Holroyd CB, Cohen JD, Stenger VA, Carter CS. Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn. 2004;56:267–276. doi: 10.1016/j.bandc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. 2004;7:1259–1265. doi: 10.1038/nn1339. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.