Abstract
Object
This study was designed to investigate the efficacy of delayed thymosin β4 (TB4) treatment of traumatic brain injury (TBI) in rats.
Methods
Young adult male Wistar rats were divided into the following groups: 1) Sham group (6 rats); 2) TBI + Saline group (9 rats); 3) and TBI + Tβ4 group (10 rats). TBI was induced by controlled cortical impact over the left parietal cortex. Thymosin β4 (6 mg/kg) or saline was administered intraperitoneally starting at Day 1 and then every 3 days for an additional 4 doses. Neurological function was assessed using a modified neurological severity score (mNSS), footfault and Morris water maze tests. Animals were killed 35 days after injury, and brain sections stained for immunohistochemistry to assess angiogenesis, neurogenesis, and oligodendrogenesis after Tβ4 treatment.
Results
Compared to the saline treatment, delayed Tβ4 treatment did not affect lesion volume but significantly reduced hippocampal cell loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, increased oligodendrogenesis in the CA3 region, and significantly improved sensorimotor functional recovery and spatial learning.
Conclusions
These data for the first time demonstrate that delayed administration of Tβ4 significantly improves histological and functional outcomes in rats with TBI, indicating that Tβ4 has considerable therapeutic potential for patients with TBI.
Keywords: angiogenesis, neurogenesis, oligodendrogenesis, rat, thymosin beta4, traumatic brain injury
Traumatic brain injury (TBI) is the leading cause of death and disability in young people.3 Neurologic impairment is caused by both immediate brain tissue disruption (primary injury) and postinjury cellular and molecular events (secondary injury) that worsen the primary neurologic insult. Ischemia, edema, and inflammation may cause secondary injury. The most prevalent and debilitating features in survivors of TBI are cognitive deficits and motor dysfunctions.14 Despite advances in basic research as well as improved neurological intensive care in recent years, no effective pharmacological therapy for TBI is available that would promote functional recovery after TBI.34,44 Several TBI clinical trials targeting a single pathophysiologic pathway have failed; thus, it is likely that successful therapy may require targeting multiple injury pathways.34 There is also an urgent need for efficient therapy to improve posttraumatic morbidity and mortality.
Thymosin β4 is a polypeptide of 43-amino acids that was first isolated from bovine thymus tissue and subsequently found to be present in all mammalian species studied.31 The major intracellular function of Tβ4 is G-actin-sequestration,62 which is necessary for cell motility and organogenesis.13 Recent studies have demonstrated that Tβ4 is a multifunctional peptide. It inhibits inflammation and apoptosis,42 and promotes tissue repair in skin,37,38 cornea,18,20 and heart.7,42,43 Thymosin β4 is an essential paracrine factor of endothelial progenitor cells5,22,26 which mediate cardioprotection,50 and it promotes angiogenesis after ischemic injury.46,48 Safety, tolerability and efficacy of Tβ4 are being evaluated in clinical patients with acute myocardial infarction13 and other diseases.18,20,47
Thymosin β4 is ubiquitously distributed in mammalian tissues including the nervous system. Its presence in the nervous system may play a role in many cellular processes including mobility, axonal path-finding, neurite formation, proliferation and neuronal survival.40,61 Our recent study demonstrated that Tβ4 improves neurological functional recovery in mice with experimental autoimmune encephalomyelitis.63 However, the efficacy of Tβ4 treatment for TBI has not been studied. In the present study, we hypothesize that Tβ4 is a potential treatment that reduces cell loss, promotes angiogenesis, neurogenesis, and oligodendrogenesis, and improves functional recovery in rats with TBI.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System.
TBI Model
A controlled cortical impact (CCI) model of TBI in the rat was utilized for the present study.16,30 Young adult male Wistar rats (327.6 ± 18.1 g) were anesthetized with chloral hydrate (350 mg/kg body weight) administered intraperitoneally. The rectal temperature was maintained at 37°C using a feedback-regulated water-heating pad. The rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The contralateral craniotomy allowed for movement of cortical tissue laterally. The dura mater was kept intact over the cortex. Injury was delivered by impacting the left (ipsilateral) cortex with a pneumatic piston containing a 6-mm-diameter tip at a rate of 4 m/second and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
Experimental Groups and Treatment
Young adult male Wistar rats were divided into 3 groups: 1) sham group (6 rats); 2) TBI + saline group (9 rats); and 3) TBI + Tβ4 group (10 rats). TBI was induced by controlled cortical impact over the left parietal cortex. Sham rats underwent surgery without injury. Thymosin β4 (RegeneRx Biopharmaceuticals Inc, Rockville, MD) dissolved in saline at a dose of 6 mg/kg was administered intraperitoneally starting at Day 1 after injury and then it was administered every 3 days for 4 additional doses. The dose of Tβ4 was selected based on our previous study.27,67 Animals in the saline-treated group received an equal volume of saline. For labeling proliferating cells, 5-bromo-2′-deoxyuridine(BrdU, 100 mg/kg; Sigma, St. Louis, MO) was injected intraperitoneally into rats daily for 10 days, starting 1 day after TBI. All rats were killed 35 days after TBI or surgery.
Evaluation of neurological outcome
All functional tests were performed by investigators who were blinded to the treatment status.
Morris Water Maze Test
To detect spatial learning impairments, a recent version of the Morris water maze test was used.10 The procedure was modified from previous versions15,32,33,54 and has been found to be useful for chronic spatial memory assessment in rodents with brain injury.10,27 All animals were tested during the last five days (that is, from 31 to 35 days after TBI or surgery) before being killed. The swimming pool was located in a large room, where there were many clues external to the maze (for example, pictures on the walls, lamps and a camera on the ceiling); these were visible from the pool and presumably used by the rats for spatial orientation. The position of the cues remained unchanged throughout the experiment. Data collection was automated by the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA). For data collection, the blue pool (1.8 m in diameter) was subdivided into four equal quadrants formed by imaging lines. At the start of a trial, the rat was placed at one of four fixed starting points, randomly facing toward a wall (designated North, South, East and West) and allowed to swim for 90 seconds or until it found the platform. If the animal found the platform, it was allowed to remain on it for 10 seconds. If the animal failed to find the platform within 90 seconds, it was placed on the platform for 10 seconds. Throughout the test period the platform was located in the northeast (NE) quadrant 2 cm below water in a randomly changing position, including locations against the wall, toward the middle of the pool, or off-center but always within the target quadrant. If the animal was unable to find the platform within 90 seconds, the trial was terminated and a maximum score of 90 seconds was assigned. If the animal reached the platform within 90 seconds, the percentage of time traveled within the NE (correct) quadrant was calculated relative to the total amount of time spent swimming before reaching the platform and employed for statistical analysis. The advantage of this version of the water maze is that each trial takes on the key characteristics of a probe trial because the platform is not in a fixed location within the target quadrant.45
Footfault Test
To evaluate sensorimotor function, the footfault test was carried out before TBI and at 1, 4, 7, 14, 21, 28 and 35 days after TBI or surgery. The rats were allowed to walk on a grid.64 With each weight-bearing step, a paw might fall or slip between the wires and if this occurred, it was recorded as a footfault.1,2 A total of 50 steps were recorded for each right forelimb and hindlimb.
The mNSS test
Neurological functional measurement was performed using the mNSS score test.8 The test was carried out on all rats preinjury and on Days 1, 4, 7, 14, 21, 28, and 35 after TBI. The mNSS is a composite of the motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), and reflex tests and has been used in previous studies.28 In this TBI model, injury in the left hemispheric cortex of rats causes sensory and motor functional deficiency with elevated scores on motor, sensory, and beam balance tests in the early phase after injury (Day 1 after injury). Absent reflexes and abnormal movements can be measured in rats with severe injury. Slow recovery in asymmetry deficiency as reflected by beam balance test results has been reported in unilateral brain injuries including TBI28 and ischemia.8 This test is suitable for evaluating long-term neurological function after unilateral brain injury.
Tissue Preparation and Measurement of Lesion Volume
At Day 35 after TBI, rats were anesthetized intraperitoneally with chloral hydrate, and they were perfused transcardially first with saline solution, followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Their brains were removed and post-fixed in 4% paraformaldehyde for 2 days at room temperature. The brain tissue was cut into 7 equally spaced 1-mm coronal blocks and processed for paraffin sectioning. A series of adjacent 6-μm-thick sections were cut from each block in the coronal plane and stained with H&E. For lesion volume measurement, the 7 brain sections were traced using a microcomputer imaging device (MCID, Imaging Research), as previously described.9 The indirect lesion area was calculated (i.e., the intact area of the ipsilateral hemisphere is subtracted from the area of the contralateral hemisphere),55 and the lesion volume presented as a volume percentage of the lesion compared with the contralateral hemisphere. H&E sections from Blocks E and F containing hippocampus were used to acquire images of the dentate gyrus and CA3 regions at a magnification of 20. To evaluate the cell loss after TBI, we counted the number of cells per millimeter in the dentate gyrus and CA3 regions.
Immunohistochemistry Analysis
To examine the effect of Tβ4 on cell proliferation, oligodendrocyte progenitor cells (OPCs), mature oligodendrocytes, and angiogenesis, coronal sections were histochemically stained with mouse anti-BrdU,27 neuron-glia antigen 2 (NG2), 2′3′ cyclic nucleotide 3′ phosphodiesterase (CNPase), and rabbit anti-human von Willebrand factor (vWF),27 respectively. For BrdU detection, 6-μm paraffin-embedded coronal sections were deparaffinized and rehydrated. Antigen retrieval was performed by boiling sections in 10-mM citrate buffer (pH 6.0) for 10 minutes.8 After washing with PBS, sections were incubated with 0.3 % H2O2 in PBS for 10 minutes, blocked with 1% BSA containing 0.3 % Triton-X 100 for 1 hour at room temperature, and incubated with mouse anti-BrdU (1:200; Dako, Carpinteria, CA) or anti- NG2 (1:100, Chemicon, Temecula, CA, USA) or anti-CNPase (1:100, Chemicon, Temecula, CA, USA) at 4°C overnight. After washing, sections were incubated with biotinylated anti-mouse antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 minutes. After washing, sections were incubated with an avidin-biotin-peroxidase system (ABC kit, Vector Laboratories, Inc.). Diaminobenzidine (Sigma, St. Louis, MO) was then used as a sensitive chromogen for light microscopy. Sections were counterstained with hematoxylin.
To identify vascular structure, brain sections were deparaffinized and then incubated with 0.4% Pepsin solution at 37°C for 1 hour. After washing, the sections were blocked with 1% BSA at room temperature for 1 hour, and then incubated with rabbit anti-human vWF (1:200; DakoCytomation, Carpinteria, CA) at 4°C overnight. After washing, sections were incubated with biotinylated anti-rabbit antibody (1:200; Vector Laboratories, Inc.) at room temperature for 30 minutes. The subsequent procedures were the same as for BrdU staining.
The BrdU-positive, NG2-positive and CNPase-positive cells, and vWF-stained vascular structures in the dentate gyrus, CA3, and cortex of the ipsilateral hemispheres were examined at a magnification of 20 or 40 and counted.
Immunofluorescent Staining
Newly generated neurons were identified by double labeling for BrdU and NeuN. After dehydration, tissue sections were boiled in 10 mM citric acid buffer (pH 6) for 10 minutes. After washing with PBS, sections were incubated in 2.4 N HCl at 37°C for 20 minutes. Sections were then incubated with 1% BSA containing 0.3% Triton-X-100 in PBS, followed by incubation with mouse anti-NeuN antibody (1:200; Chemicon, Temecula, CA) at 4°C overnight. FITC-conjugated anti-mouse antibody (1:400; Jackson ImmunoResearch, West Grove, PA) was added to sections at room temperature for 2 hours. Sections were then incubated with rat anti-BrdU antibody (1:200; Dako, Glostrup, Denmark) at 4°C overnight, followed by incubation with Cy3-conjugated anti-rat antibody (1:400; Jackson ImmunoResearch) at room temperature for 2 hours. Each of the steps was followed by three 5-minute rinses in PBS. Tissue sections were mounted with Vectashield mounting medium (Vector Laboratories). Images were collected with fluorescent microscopy and merged. The NeuN/BrdU-colabeled cells in the dentate gyrus and the cortex were counted at a magnification of 40.
Double immunostaining for BrdU and NG2 was performed to demonstrate the proliferation of OPCs. Double immunostaining CNPase and BrdU was used to identify the differentiation of OPCs.63
Cell Counting and Quantitation
For quantitative measurements of BrdU+, NG2+, NeuN+, CNPase+, NG2+/BrdU+, NeuN+/BrdU+, and CNPase+/BrdU+ cells, we used 5 slides from each brain, with each slide containing 5 fields of view in the lesion boundary zone from the epicenter of the injury cavity (bregma −3.3 mm), 3 fields of view in the ipsilateral CA3 and 9 fields of view in the ipsilateral dentate gyrus in the same section. The fields were digitized under the light microscope (Nikon, Eclipse 80i, Melville, NY) at a magnification of either 200 or 400 using a CoolSNAP color camera (Photometrics, Tucson, AZ) interfaced with a MetaMorph image analysis system (Molecular Devices, Downingtown, PA). The immunopositive cells were calculated and divided by the measured areas, and were presented as numbers per square millimeter. Cell counts were performed by observers blinded to the individual treatment status of the animals. All counting was performed on a computer monitor to improve visualization and in one focal plane to avoid oversampling.65 To evaluate whether intraperitoneally administered TB4 reduces neuronal damage after TBI, the number of cells was counted in the dentate. Although H&E staining is not neuron-specific, the morphological characteristics of neuronal cells in the dentate gyrus and CA3 region aid in counting them. Counts were averaged and normalized by measuring the linear distance (in mm) of the dentate gyrus and CA3 for each section. Although it is just an estimate of the cell number, this method permits a meaningful comparison of differences between groups. For cell proliferation, the total number of BrdU+ cells was counted in the lesion boundary zone, CA3 and dentate gyrus. The cells with BrdU (brown stained) that clearly localized to the nucleus (hematoxylin stained) were counted as BrdU+ cells. For analysis of neurogenesis, additional sections used in the above studies were used to evaluate neurogenesis in the dentate gyrus and the cortex by calculating the density of BrdU-labeled cells and BrdU/NeuN-colabeled cells.40 We mainly focused on the ipsilateral dentate gyrus and its subregions, including the subgranular zone (SGZ), granular cell layer (GCL), and the molecular layer. The number of BrdU+ cells (red stained) and NeuN/BrdU-colabeled cells (yellow after merge) were counted in the dentate gyrus and the lesion boundary zone. The percentage of NeuN/BrdU-colabeled cells over the total number of BrdU+ cells in the corresponding regions (dentate gyrus or cortex) was estimated and used as a parameter to evaluate neurogenesis.59 Similarly, the number of BrdU+ cells (red stained) colabeled with NG2 or CNPase (green stained) was counted to demonstrate OPC proliferation and differentiation, respectively.63
Statistical Analysis
All data are presented as the means ± SDs. Data were analyzed using ANOVA for repeated measurements of functional tests (spatial performance and sensorimotor function). For lesion volume, cell counting, cell proliferation, NG2+ cells, CNPase+ cells, and vWF-stained vascular density, a one-way ANOVA followed by post hoc Student-Newman-Keuls tests was used to compare the difference between the Tβ4-treated, saline-treated and sham groups. Statistical significance was set at p < 0.05.
Results
Delayed Tβ4 treatment Does Not Change Body Weight in Rats That Sustained TBI
The mean body weight (g) of the rats in the 3 treatment groups was 330.8 ± 21.8 (sham), 329.3 ± 18.2 (saline) and 323.4 ± 15.9 (Tβ4) before sham surgery and TBI. The rats lost 4–6% of body weight by Day 1 after TBI, and body weight returned to preinjury level by Day 7, and gradually increased during the 35-day study period. There was no significant difference in body weight among the 3 groups.
Delayed Tβ4 Treatment Does Not Reduce Lesion Volume in Rats That Sustained TBI
Rats were killed at 35 days post-TBI for histological measurements. Delayed Tβ4 treatment (24 hours postinjury) did not reduce lesion volume after TBI. For TBI rats treated with saline and Tβ4, the lesion volume was 14.2 ± 3.9% and 15.7 ± 3.6%, respectively.
Treatment with Tβ4 Improves Spatial Learning
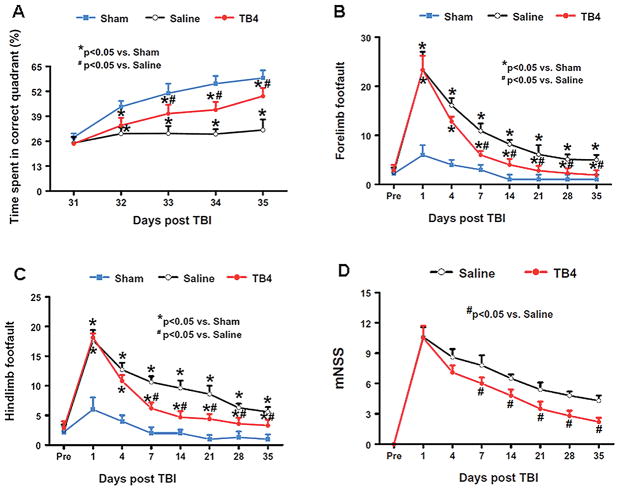
To detect spatial learning deficits, the water maze protocol was used. The time spent in the correct quadrant (that is, northeast) by sham rats increased significantly from Days 32–35 after surgery, as compared to time spent in the correct quadrant at Day 31 (p < 0.05). The saline-treated rats were impaired relative to sham-operated rats (p < 0.05) at Days 32–35 after TBI. The Tβ4-treated rats with TBI showed significant improvement at Day 33 (p = 0.011), Day 34 (p = 0.003), and Day 35 (p = 0.001) when compared with the saline-treated rats (Fig. 1A).
Fig. 1.
The effect of Tβ4 on functional recovery after TBI. A: Effect of Tβ4 on spatial learning function 31–35 days after TBI. TBI significantly impairs spatial learning at Days 32–35 compared to sham controls. Delayed treatment with Tβ4 improves spatial learning performance measured by a recent version of the water maze test at Days 33–35 compared with the saline group. B: Effect of Tβ4 on sensorimotor function (forelimb footfault) after TBI. TBI significantly impairs sensorimotor function at Days 1–35 compared with sham controls. Delayed Tβ4 treatment significantly reduces forelimb foot faults at Days 7–35 compared with the saline-treated group. C: Effect of Tβ4 on sensorimotor function (hindlimb footfault) after TBI. TBI significantly impairs sensorimotor function at Days 1–35 compared with sham controls. Delayed Tβ4 treatment significantly reduces hindlimb foot faults at Days 7–35 compared with the saline-treated group. D: Line graph showing the functional improvement detected on the mNSS. Treatment with Tβ4 significantly lowers mNSS scores at Days 7–35 compared with the saline group. Data represent mean ± SD. There were 6, 9, and 10 rats in the sham, saline, and Tβ4 groups, respectively. Pre = preinjury level.
Treatment With Tβ4 Reduces the Incidence of Foot Fault
The incidence of forelimb footfaults during baseline (preoperatively) was about 4–5% (Fig. 1B). TBI significantly increased the occurrence of right forelimb footfaults contralateral to the TBI at 1 to 35 days postinjury compared with the preinjury baseline and sham controls (p < 0.05). Treatment with Tβ4 significantly reduced the number of contralateral forelimb footfaults at 7 to 35 days after TBI compared with treatment with saline (that is, for Days 7, 14, 21, 28 and 35, p = 0.002, 0.011, 0.027, 0.023, and 0.035, respectively).
Similar foot fault results were found for the contralateral hindlimb (Fig. 1C). As compared to preinjury baseline and sham controls, TBI significantly increased the incidence of contralateral hindlimb footfaults at 1 to 35 days post-injury (that is, for Days 1, 4, 7, 14, 21, 28 and 35, p = 0.007, 0.003, 0.014, 0.006, 0.001, 0.022, and 0.036, respectively). Treatment with Tβ4 significantly reduced the number of contralateral hindlimb footfaults at 7 to 28 days after TBI compared to treatment with saline (i.e., for Days 7, 14, 21, 28, and 35, p = 0.011, 0.005, 0.020, 0.008, and 0.032, respectively).
Treatment With Tβ4 Reduces the mNSS
Figure 1D shows that there is no significant difference in the mNSS score between the saline- and Tβ4-treated groups at Days 1 and 4 post-TBI. However, significantly improved scores were measured at Days 7–35 after TBI in the Tβ4-treated group compared with the saline-treated group (that is, for Days 7, 14, 21, 28, and 35, p = 0.011, 0.023, 0.009, 0.017, and 0.005, respectively).
Treatment With Tβ4 Reduces Cell Loss in the CA3 and Dentate Gyrus
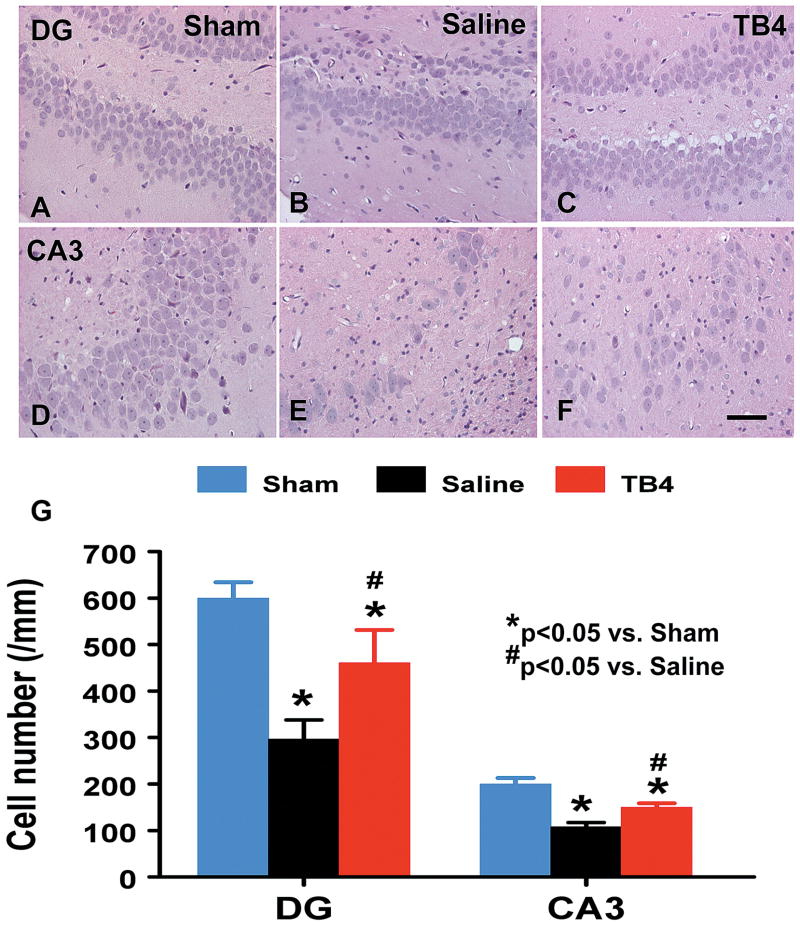
When examined at 35 days post TBI (Fig. 2), the neuron counts in the ipsilateral CA3 (p = 0.001) and dentate gyrus (p = 0.002) had significantly decreased after TBI (Fig. 2B and 2E) compared to sham controls (Fig. 2A and 2D). As compared to saline controls, Tβ4 treatment significantly increased the neuron counts in the CA3 (p = 0.033, Fig. 2C) and dentate gyrus (p = 0.007, Fig. 2F).
Fig. 2.
The effect of TB4 on cell number in the ipsilateral dentate gyrus and CA3 region at 35 days after TBI. H&E staining: A-F. Delayed treatment with TB4 (C, F) significantly reduces cell loss as compared with the saline-treated group (B, E) (p < 0.05). The cell number in the dentate gyrus and CA3 region is shown in (G). Scale bar = 25μm (F, applicable to A–F). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Saline group. N (rats/group) = 6 (Sham); 9 (Saline); and 10 (TB4).
Treatment With Tβ4 Promotes Angiogenesis in the Injured Cortex and Hippocampus
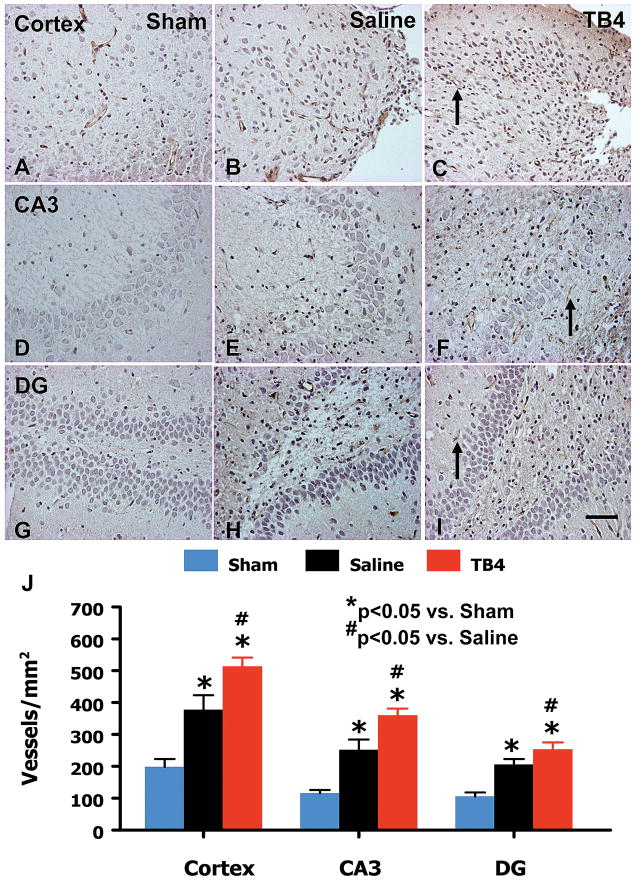
Von Willebrand factor-staining has been used to identify vascular structure in the brain after TBI.29 TBI alone significantly increased vascular density in the cortex (p = 0.013), CA3 (p = 0.005), and dentate gyrus (p = 0.004) of the ipsilateral hemisphere compared with sham controls. Treatment with Tβ4 significantly increased the vascular density in the cortex (p = 0.013), CA3 (p = 0.022), and dentate gyrus (p = 0.002) compared with saline treatment (Fig. 3).
Fig. 3.
The effect of TB4 on vWF-staining vascular structure in the injured cortex, ipsilateral dentate gyrus, and CA3 region 35 days after TBI. TBI alone (B, E, and H) significantly increases the vascular density in these regions compared to sham controls (p < 0.05). TB4 treatment (C, F, and I; arrow as example showing vWF-staining vascular structure) further enhances angiogenesis after TBI compared to the saline-treated groups (p < 0.05). The density of vWF-stained vasculature is shown in (J). Scale bar = 50 μm (I, applicable to A-I). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Saline group. N (rats/group) = 6 (Sham); 9 (Saline); and 10 (TB4).
Treatment With Tβ4 Promotes Cell Proliferation in the Injured Cortex and Hippocampus
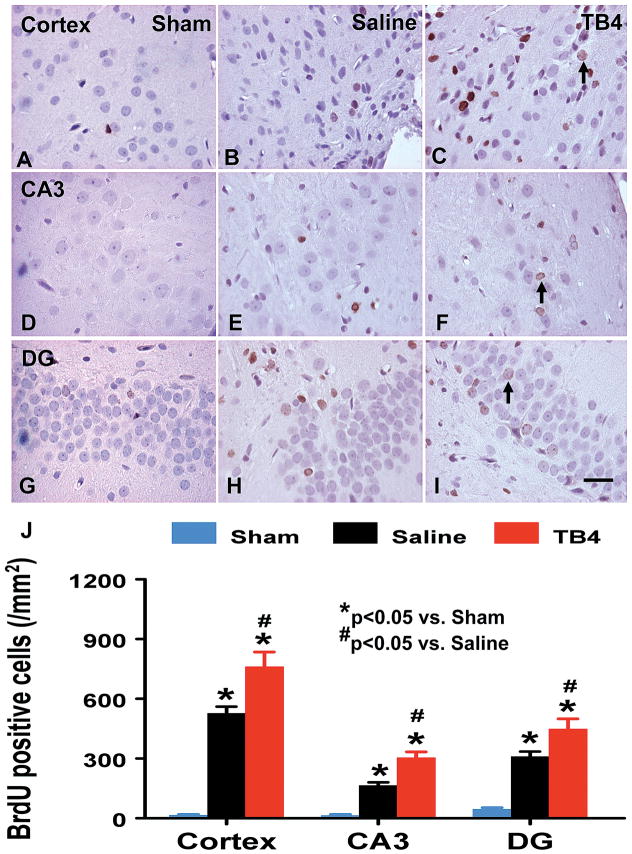
An analog of thymidine, BrdU can be incorporated into the newly synthesized DNA of replicating cells during the S phase of the cell cycle, substituting for thymidine during DNA replication. Staining with BrdU is commonly used to detect proliferating cells. The number of BrdU+ cells found in the ipsilateral cortex (p = 0.003), dentate gyrus ((p = 0.001), and CA3 (p = 0.005) areas was significantly increased at 35 days after TBI, compared with sham controls (Fig. 4). However, Tβ4 treatment further increased the number of BrdU+ cells in the cortex (p = 0.005), dentate gyrus (p = 0.011), and CA3 region (p = 0.023) after TBI compared with saline controls (Fig. 4).
Fig. 4.
The effect of TB4 on BrdU+ cells in the injured cortex, ipsilateral CA3, and dentate gyrus 35 days after TBI. TBI alone (B, E, and H) significantly increases the number of BrdU+ cells in the ipsilateral cortex, CA3 and dentate gyrus compared to sham controls (A, D, and G) (p < 0.05). TB4 treatment significantly increases the number of BrdU+ cells in these regions (C, F, and I; arrow as example showing BrdU+ cell) compared to the saline-treated groups (p < 0.05). The number of BrdU+ cells is shown in (J). Scale bar = 25μm (I, applicable to A–I). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Saline group. N (rats/group) = 6 (Sham); 9 (Saline); and 10 (TB4).
Treatment With Tβ4 Increases Mature Oligodendrocytes in the Brain of Rats That Sustained TBI
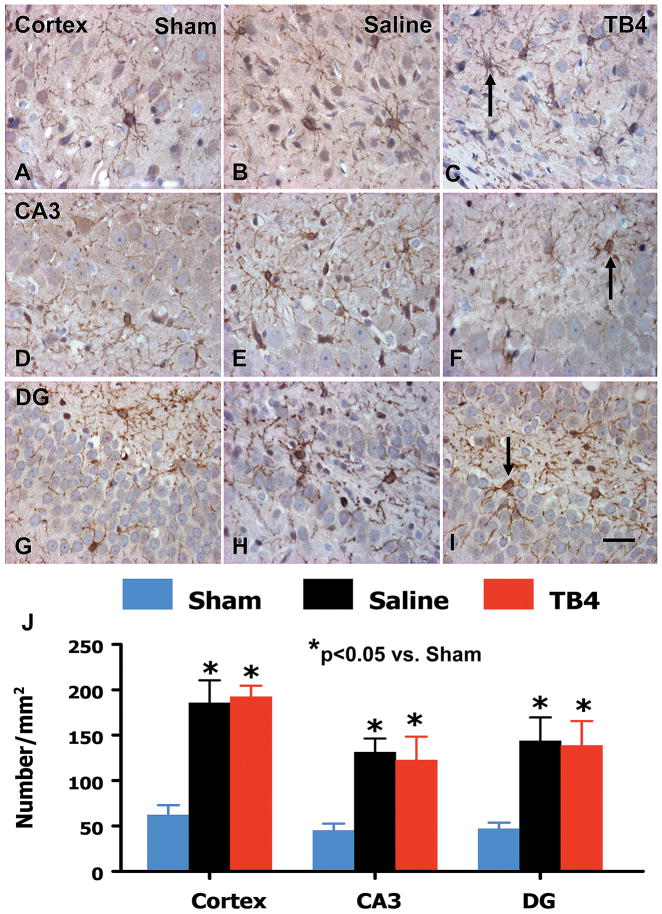
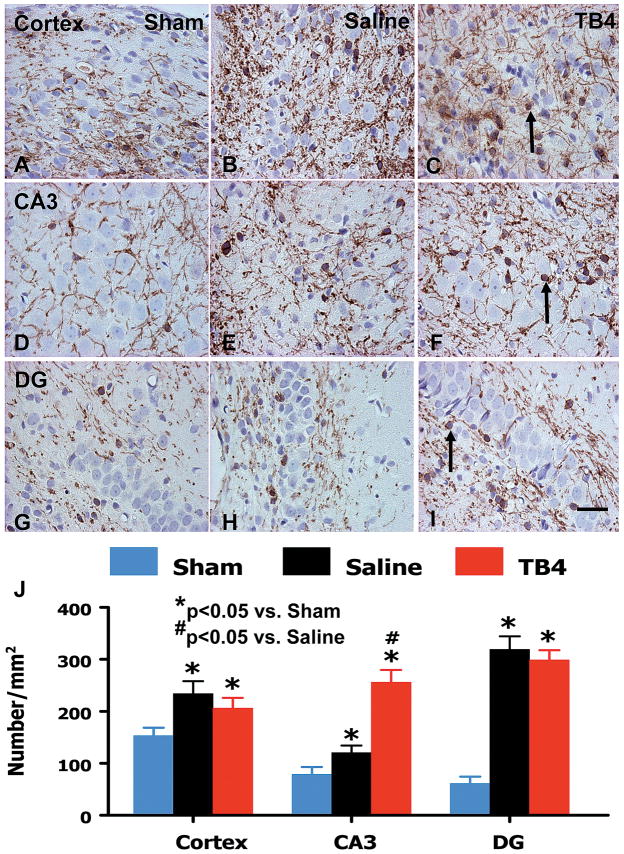
Using OPC marker NG2 staining, we found that TBI alone significantly increased NG2+ cells in the lesion boundary zone (p = 0.015), dentate gyrus (p = 0.006), and CA3 (p = 0.001) compared with the sham controls (Fig. 5). However, Tβ4 treatment did not have significant effects on OPC proliferation. The mature oligodendrocytes, the CNPase+ cells, were significantly increased in these regions (p < 0.05) (Fig. 6). Treatment with Tβ4 significantly increased the number of mature oligodendrocytes only in the CA3 region but not in other regions.
Fig. 5.
The effect of TB4 on oligodendrocyte progenitor cells (NG2+) in the injured cortex, ipsilateral CA3 and dentate gyrus 35 days after TBI. TBI alone (B, E, and H) significantly increases the number of NG2+ cells in the ipsilateral cortex, CA3 and dentate gyrus compared to sham controls (A, D, and G) (p < 0.05). TB4 treatment does not affect the number of NG2+ cells in these regions (C, F, and I; arrow as example showing NG2+ cell) compared to the saline-treated groups. The number of BrdU+ cells is shown in (J). Scale bar = 25μm (I, applicable to A–I). Data represent mean ± SD. *p < 0.05 vs Sham group. N (rats/group) = 6 (Sham); 9 (Saline); and 10 (TB4).
Fig. 6.
The effect of TB4 on mature oligodendrocytes (CNPase+) in the injured cortex, ipsilateral CA3, and dentate gyrus 35 days after TBI. TBI alone (B, E, and H) significantly increases the number of CNPase+ cells in the ipsilateral cortex, CA3, and dentate gyrus compared to sham controls (A, D, and G) (p < 0.05). TB4 treatment does not affect the number of CNPase+ cells in the cortex (C, arrow as example showing CNPase+ cell) and DG (I) but significantly increases the number of CNPase+ cells in the CA3 region (F) compared to saline groups. The number of BrdU+ cells is shown in (J). Scale bar = 25μm (I, applicable to A–I). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Saline group. N (rats/group) = 6 (Sham); 9 (Saline); and 10 (TB4).
Treatment With Tβ4 Promotes Oligodendrogenesis in the CA3 Region and Neurogenesis in the Injured Cortex and Dentate Gyrus
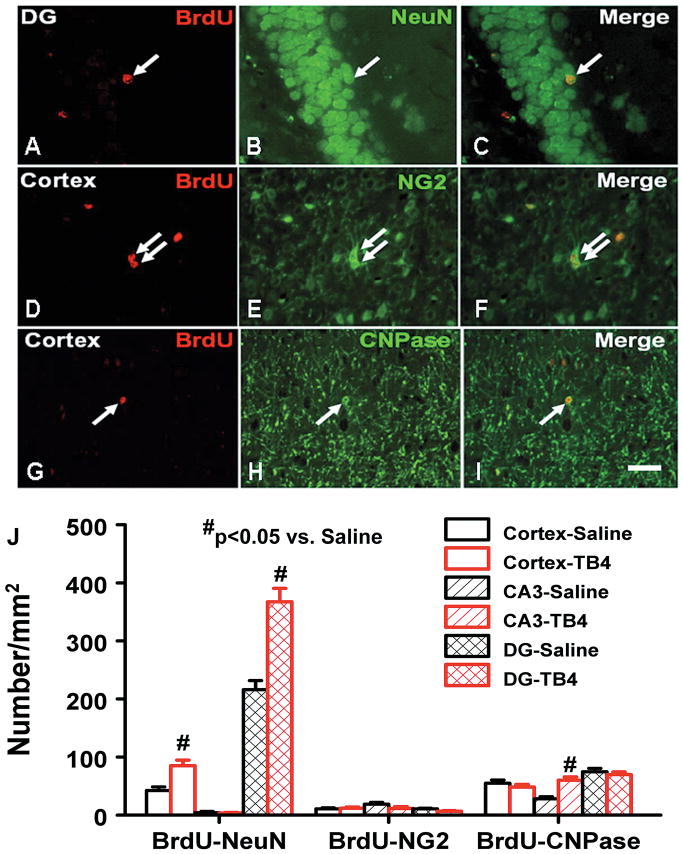
Double immunostaining using a cell proliferating marker (BrdU) and an OPC marker (NG2) revealed proliferating OPCs (NG2+/BrdU+cells) in the TBI rat brains (Fig. 7). Treatment with Tβ4 did not affect the OPC proliferation. Double immunostaining using BrdU and a mature oligodendrocyte marker (CNPase) revealed that CNPase+/BrdU+ cells were present in the brains of rats that sustained TBI. Treatment with Tβ4 significantly increased CNPase+/BrdU+ cells in the CA3 region but not in the injured cortex and dentate gyrus region.
Fig. 7.
Double immunofluorescent staining for BrdU (red) and NeuN (green) to identify newborn neurons (yellow after merge) in the brain (A–C), for BrdU (red) and NG2 (green) to identify newborn OPCs in the brain (D–F), and for BrdU (red) and CNPase (green) to identify newborn mature oligodendrocytes in the brain (G–I). The arrows indicate newborn neurons (A–C), OPCs (D–F) and mature oligodendrocytes (G–I). Bar = 25 um (for all panels). J: Bar graph showing the numbers of NeuN+, NG2+, and CNPase+ cells colabeled with BrdU.
Scale bar = 25μm (I, applicable to A–I). Data represent mean ± SD. #p < 0.05 vs Saline group. N (rats/group) = 6 (Sham); 9 (Saline); and 10 (TB4).
To identify newly generated neurons, double labeling for BrdU (proliferating marker) and NeuN (mature neuronal marker) was performed. The number of NeuN/BrdU-colabeled cells (newborn neurons) was significantly higher in the injured cortex (p = 0.023) and dentate gyrus (p = 0.006) after TBI compared to sham controls. Treatment with Tβ4 significantly increased the number of newborn neurons in the injured cortex (p = 0.023) and dentate gyrus (p = 0.009) compared with saline controls.
Discussion
We demonstrate for the first time that delayed (24 hours postinjury) Tβ4 treatment after TBI significantly improves long-term behavioral benefits. This finding was reflected in improvements in spatial learning and sensorimotor functional recovery, as evaluated by Morris water maze and foot fault tests as well as mNSSs. Delayed Tβ4 treatment does not reduce lesion volume. The improvements in spatial learning and sensorimotor function may derive from the effect of Tβ4 treatment on reducing hippocampal cell loss and increasing angiogenesis, neurogenesis, and CA3 oligodendrogenesis. These data suggest that Tβ4 treatment is a potential therapy for patients who have sustained TBI.
Many factors including serious side effects, significant risks, and poor brain penetration of the drugs studied may contribute to unsuccessful TBI clinical trials.34 Whether injected Tβ4 stays in the cerebral vasculature or crosses the blood-brain barrier into the brain parenchyma has not been investigated. However, studies with intraperitoneal administration of Tβ4 have demonstrated passage of Tβ4 into the brain.31 For example, the pharmacokinetic study of TB4 in mice demonstrates that the peak concentration is found in the brain homogenate at 40 minutes and 2 hours following intraperitoneal administration of Tβ4.31 In addition, the intravenous administration of Tβ4 over a dose range of 0.6–60mg/kg is safe and tolerated without evidence of dose-limiting toxicity in all study animals.13 Thymosin β4 protects human corneal epithelial cells against oxidative injury,24 induces hair growth via stem cell migration and differentiation,39 promotes epicardium-derived neovascularization in the adult heart,43,47 reduces rat hippocampal neuronal loss by kainic acid excitotoxicity,40 reduces apoptotic death of chick and rat neurons,11 and inhibits inflammation.49 These studies suggest that Tβ4 is a multifunctional peptide (that is, it has angiogenic, anti-apoptotic, anti-inflammatory, anti-oxidative, and tissue-protective effects). Detrimental factors including inflammation, oxidative injury, excitotoxicity, apoptosis, and hypoxia/ischemia have been suggested in secondary injury after TBI.58 Thymosin β4 seems to be capable of targeting these factors. In the present study, we first investigated the functional therapeutic effect of Tβ4 for treating TBI in rats and then evaluated its effects on cell loss, angiogenesis, neurogenesis, and oligodendrogenesis.
Our data demonstrate that delayed Tβ4 treatment significantly improves sensorimotor and spatial learning functional recovery after TBI; however, it does not reduce TBI-induced lesion volume. This is in agreement with our previous studies60 and others,21 which demonstrated that delayed pharmacological treatments do not reduce lesion volume after TBI. Our present study shows that Tβ4 treatment promotes not only angiogenesis but also neurogenesis in the injured cortex and hippocampus. TB4 is a strong angiogenic peptide which promotes angiogenesis after ischemic injury.46 Thymosin β4 also reduces cardiac infarction by promoting cell migration and myocyte survival.7 Our previous studies demonstrate that treatments promoting angiogenesis and neurogenesis significantly improve functional recovery after stroke12 and TBI.60 Angiogenesis and neurogenesis are well coupled in the neurovascular niche.69 The therapeutic benefits of Tβ4 may be derived from increased angiogenesis and neurogenesis. Neurogenesis normally occurs in 2 neurogenic areas – the subventricular zone (SVZ)-olfactory bulb pathway and the subgranular zone of the dentate gyrus.70 TBI alone enhances neurogenesis in the dentate gyrus and SVZ.29,51,57,60 The newly formed neurons in the dentate gyrus are able to project axons to the CA3 region to establish anatomical connections.52 Thymosin β4 further increases neurogenesis in the dentate gyrus. In addition, Tβ4 treatment reduces neuronal loss in the dentate gyrus and CA3 regions of the hippocampus. The hippocampus is involved in spatial learning and memory processing. This suggests that increased neurogenesis in the dentate gyrus and reduced neuronal loss in the dentate gyrus and CA3 regions may play a role in improving spatial learning after Tβ4 treatment. Our present and previous studies have shown that TBI and treatment also induce cortical neurogenesis.29,57,60 Cortical neurogenesis may be mediated by neuroblasts migrating from the SVZ into the lesion boundary zone after TBI36 and stroke.65,67,68 Increased cortical angiogenesis and neurogenesis may partially contribute to sensorimotor functional recovery after brain injury.
Our recent study demonstrates that Tβ4 treatment improves functional recovery and stimulates oligodendrogenesis in mice with experimental autoimmune encephalomyelitis (EAE), which causes an immune-mediated myelin destruction and axonal loss.63 In the present study, we examined the effect of Tβ4 therapy on OPCs (NG2+ cells) and mature oligodendrocytes (CNPase+ cells) at Day 35 after TBI. TBI alone significantly increased the number of OPCs in the injured cortex and hippocampus. Similarly, TBI alone significantly increased the number of mature oligodendrocytes in the injured cortex and hippocampus. The number of NG2+/BrdU+ cells that represent the newly generated OPCs is much lower than the number of NG2+ cells in the present study. The newly generated mature oligodendrocytes (CNPase+/BrdU+ cells) is also lower than the number of CNPase+ cells. These data suggest that many OPCs and mature oligodendrocytes come from OPC proliferation and differentiation 10 days later after TBI because BrdU injection was given during the first 10 days after TBI. Increased OPC proliferation and differentiation indicate that the myelinating process is stimulated after TBI. This process may partially underline spontaneous functional recovery seen after TBI. Treatment with Tβ4 did not increase the number of NG2+ cells in the injured cortex and hippocampus. The Tβ4 effect on OPCs in the present TBI study differs from that reported in our previous EAE study in which Tβ4 stimulated OPCs.63 The discrepancy may derive from the difference in animal models (TBI vs EAE), species (rats vs. mice), treatment timing (posttraumatic treatment for TBI vs preventive treatment for EAE), and brain regions studied (cortex/hippocampus vs SVZ/striatum). Thymosin β4 did not promote OPC differentiation in the injured cortex and dentate gyrus, but increased OPC differentiation in the CA3 region. This may facilitate myelination of axons projected from new neurons generated in the dentate gyrus. Our present data indicate that the Tβ4 effects are region-dependent after TBI. TB4 treatment promotes angiogenesis in the injured cortex, dentate gyrus and CA3 regions of the hippocampus and increases neurogenesis in the dentate gyrus and injured cortex while it promotes OPC differentiation in the CA3 region.
Thymosin β4 is a pleiotropic peptide and is mainly recognized as a regulator of actin polymerization by sequestering G-actin. It forms a 1:1 complex with actin dissociated from actin filaments, and thereby helps to maintain an actin pool for filament reorganization.53 The peptide has been implicated in lymphocyte maturation, carcinogenesis, apoptosis, angiogenesis, blood coagulation, and wound healing25,53 The peptide is also involved in lesion-induced neuroplasticity through microglia upregulation and it participates in the growth of neuronal processes.35 Increased expression of Tβ4 was found in the microglia in the entorhinally denervated zones of the hippocampus,17 suggesting that Tβ4 may participate in the process of activated microglia, the earliest event of lesion-induced plasticity. Intracerebroventricular administration of Tβ4 significantly reduced hippocampal neuronal loss induced by kainic acid.40 Upregulation of Tβ4 in the pyramidal neurons of the hippocampus may be related to restoration of neurite circuits after focal ischemic damage.56 The signal pathways underlying these benefits remain largely unknown. Thymosin β4 induces expression and release of plasminogen activator inhibitor type 1 (PAI-1) in endothelial cells via activation of the mitogen-activated protein kinase cascade, which leads to enhanced c-Fos/c-Jun binding to the AP-1-like element present in the PAI-1 promoter.6 A recent study shows that Tβ4 induces endothelial progenitor cell migration via the PI3K/Akt/eNOS signal transduction pathway, which may mediate angiogenesis.41 Internalization of exogenous Tβ4 is essential for the antiapoptotic effects of exogenous Tβ4 on human corneal epithelial cells.23 Internalization of the exogenous Tβ4 and a consequential activation of integrin-linked kinase and Akt by this peptide seem to be the major mechanisms that promote the survival of cardiomyocytes.5,19 Recently, the Ku80 subunit of ATP-dependent DNA helicase II was found to be associated with Tβ4; it functions as a novel receptor for Tβ4 and mediates its intracellular activity.4 These signal pathways may also mediate neuroprotection and neurorestoration of Tβ4. Further verification is required.
Conclusions
The therapeutic efficacy of delayed Tβ4 treatment for TBI in the present study, along with the fact that multifunctional Tβ4 without evidence of dose-limiting toxicity in all study animals can enter the brain,31 suggests that Tβ4 is a potential therapy for patients who have sustained TBI. These data warrant further investigation of the optimal dose and therapeutic window of Tβ4 treatment for TBI and the associated underlying mechanisms.
Acknowledgments
This work was supported by NIH Grant Nos. R01 NS62002 (to Dr. Xiong) and PO1 NS42345 (to Drs. Mahmood and Chopp). Drs. Z. G. Zhang, Morris, and Chopp have applied for a patent for the use of Tβ4 as a treatment of stroke, neurological disease, and injury.
Sources of financial support: NINDS grants RO1 NS62002 (Ye Xiong) and PO1 NS42345 (Asim Mahmood, Michael Chopp).
References
- 1.Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. doi: 10.1016/0166-4328(90)90122-u. [DOI] [PubMed] [Google Scholar]
- 2.Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 3.Beauchamp K, Mutlak H, Smith WR, Shohami E, Stahel PF. Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol Med. 2008;14:731–740. doi: 10.2119/2008-00050.Beauchamp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bednarek R, Boncela J, Smolarczyk K, Cierniewska-Cieslak A, Wyroba E, Cierniewski CS. Ku80 as a novel receptor for thymosin beta4 that mediates its intracellular activity different from G-actin sequestering. J Biol Chem. 2008;283:1534–1544. doi: 10.1074/jbc.M707539200. [DOI] [PubMed] [Google Scholar]
- 5.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 6.Boncela J, Smolarczyk K, Wyroba E, Cierniewski CS. Binding of PAI-1 to endothelial cells stimulated by thymosin beta4 and modulation of their fibrinolytic potential. J Biol Chem. 2006;281:1066–1072. doi: 10.1074/jbc.M506303200. [DOI] [PubMed] [Google Scholar]
- 7.Cavasin MA. Therapeutic potential of thymosin-beta4 and its derivative N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) in cardiac healing after infarction. Am J Cardiovasc Drugs. 2006;6:305–311. doi: 10.2165/00129784-200606050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Choi SY, Noh MR, Kim DK, Sun W, Kim H. Neuroprotective function of thymosin-beta and its derivative peptides on the programmed cell death of chick and rat neurons. Biochem Biophys Res Commun. 2007;362:587–593. doi: 10.1016/j.bbrc.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 13.Crockford D. Development of thymosin beta4 for treatment of patients with ischemic heart disease. Ann N Y Acad Sci. 2007;1112:385–395. doi: 10.1196/annals.1415.051. [DOI] [PubMed] [Google Scholar]
- 14.Davis AE. Mechanisms of traumatic brain injury: biomechanical, structural and cellular considerations. Crit Care Nurs Q. 2000;23:1–13. doi: 10.1097/00002727-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Day LB, Weisand M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- 16.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 17.Dong JH, Ying GX, Liu X, Wang WY, Wang Y, Ni ZM, et al. Expression of thymosin beta4 mRNA by activated microglia in the denervated hippocampus. Neuroreport. 2005;16:1629–1633. doi: 10.1097/01.wnr.0000183326.21241.48. [DOI] [PubMed] [Google Scholar]
- 18.Fine JD. Epidermolysis bullosa: a genetic disease of altered cell adhesion and wound healing, and the possible clinical utility of topically applied thymosin beta4. Ann N Y Acad Sci. 2007;1112:396–406. doi: 10.1196/annals.1415.017. [DOI] [PubMed] [Google Scholar]
- 19.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 20.Guarnera G, ADER, Camerini R. Thymosin beta-4 and venous ulcers: clinical remarks on a European prospective, randomized study on safety, tolerability, and enhancement on healing. Ann N Y Acad Sci. 2007;1112:407–412. doi: 10.1196/annals.1415.003. [DOI] [PubMed] [Google Scholar]
- 21.Hartley CE, Varma M, Fischer JP, Riccardi R, Strauss JA, Shah S, et al. Neuroprotective effects of erythropoietin on acute metabolic and pathological changes in experimentally induced neurotrauma. J Neurosurg. 2008;109:708–714. doi: 10.3171/JNS/2008/109/10/0708. [DOI] [PubMed] [Google Scholar]
- 22.Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Muller S, et al. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho JH, Chuang CH, Ho CY, Shih YR, Lee OK, Su Y. Internalization is essential for the antiapoptotic effects of exogenous thymosin beta-4 on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:27–33. doi: 10.1167/iovs.06-0826. [DOI] [PubMed] [Google Scholar]
- 24.Ho JH, Tseng KC, Ma WH, Chen KH, Lee OK, Su Y. Thymosin beta-4 upregulates anti-oxidative enzymes and protects human cornea epithelial cells against oxidative damage. Br J Ophthalmol. 2008;92:992–997. doi: 10.1136/bjo.2007.136747. [DOI] [PubMed] [Google Scholar]
- 25.Huff T, Muller CS, Otto AM, Netzker R, Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 26.Kupatt C, Bock-Marquette I, Boekstegers P. Embryonic endothelial progenitor cell-mediated cardioprotection requires Thymosin beta4. Trends Cardiovasc Med. 2008;18:205–210. doi: 10.1016/j.tcm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 28.Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61:596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. discussion 602–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu D, Mahmood A, Zhang R, Copp M. Upregulation of neurogenesis and reduction in functional deficits following administration of DEtA/NONOate, a nitric oxide donor, after traumatic brain injury in rats. J Neurosurg. 2003;99:351–361. doi: 10.3171/jns.2003.99.2.0351. [DOI] [PubMed] [Google Scholar]
- 30.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–1193. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 31.Mora CA, Baumann CA, Paino JE, Goldstein AL, Badamchian M. Biodistribution of synthetic thymosin beta 4 in the serum, urine, and major organs of mice. Int J Immunopharmacol. 1997;19:1–8. doi: 10.1016/s0192-0561(97)00005-2. [DOI] [PubMed] [Google Scholar]
- 32.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 33.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 34.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulussen M, Landuyt B, Schoofs L, Luyten W, Arckens L. Thymosin beta 4 mRNA and peptide expression in phagocytic cells of different mouse tissues. Peptides. 2009;30:1822–1832. doi: 10.1016/j.peptides.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Petraglia AL, Marky AH, Walker C, Thiyagarajan M, Zlokovic BV. Activated protein C is neuroprotective and mediates new blood vessel formation and neurogenesis after controlled cortical impact. Neurosurgery. 66:165–171. doi: 10.1227/01.NEU.0000363148.49779.68. discussion 171–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philp D, Goldstein AL, Kleinman HK. Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mech Ageing Dev. 2004;125:113–115. doi: 10.1016/j.mad.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Philp D, Nguyen M, Scheremeta B, St-Surin S, Villa AM, Orgel A, et al. Thymosin beta4 increases hair growth by activation of hair follicle stem cells. Faseb J. 2004;18:385–387. doi: 10.1096/fj.03-0244fje. [DOI] [PubMed] [Google Scholar]
- 39.Philp D, St-Surin S, Cha HJ, Moon HS, Kleinman HK, Elkin M. Thymosin beta 4 induces hair growth via stem cell migration and differentiation. Ann N Y Acad Sci. 2007;1112:95–103. doi: 10.1196/annals.1415.009. [DOI] [PubMed] [Google Scholar]
- 40.Popoli P, Pepponi R, Martire A, Armida M, Pezzola A, Galluzzo M, et al. Neuroprotective effects of thymosin beta4 in experimental models of excitotoxicity. Ann N Y Acad Sci. 2007;1112:219–224. doi: 10.1196/annals.1415.033. [DOI] [PubMed] [Google Scholar]
- 41.Qiu FY, Song XX, Zheng H, Zhao YB, Fu GS. Thymosin beta4 induces endothelial progenitor cell migration via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol. 2009;53:209–214. doi: 10.1097/FJC.0b013e318199f326. [DOI] [PubMed] [Google Scholar]
- 42.Reti R, Kwon E, Qiu P, Wheater M, Sosne G. Thymosin beta4 is cytoprotective in human gingival fibroblasts. Eur J Oral Sci. 2008;116:424–430. doi: 10.1111/j.1600-0722.2008.00569.x. [DOI] [PubMed] [Google Scholar]
- 43.Riley PR, Smart N. Thymosin beta4 induces epicardium-derived neovascularization in the adult heart. Biochem Soc Trans. 2009;37:1218–1220. doi: 10.1042/BST0371218. [DOI] [PubMed] [Google Scholar]
- 44.Royo NC, Schouten JW, Fulp CT, Shimizu S, Marklund N, Graham DI, et al. From cell death to neuronal regeneration: building a new brain after traumatic brain injury. J Neuropathol Exp Neurol. 2003;62:801–811. doi: 10.1093/jnen/62.8.801. [DOI] [PubMed] [Google Scholar]
- 45.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 47.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin beta-4 is essential for coronary vessel development and promotes neovascularization via adult epicardium. Ann N Y Acad Sci. 2007;1112:171–188. doi: 10.1196/annals.1415.000. [DOI] [PubMed] [Google Scholar]
- 48.Smart N, Rossdeutsch A, Riley PR. Thymosin beta4 and angiogenesis: modes of action and therapeutic potential. Angiogenesis. 2007;10:229–241. doi: 10.1007/s10456-007-9077-x. [DOI] [PubMed] [Google Scholar]
- 49.Sosne G, Qiu P, Kurpakus-Wheater M. Thymosin beta-4 and the eye: I can see clearly now the pain is gone. Ann N Y Acad Sci. 2007;1112:114–122. doi: 10.1196/annals.1415.004. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava D, Saxena A, Michael Dimaio J, Bock-Marquette I. Thymosin beta4 is cardioprotective after myocardial infarction. Ann N Y Acad Sci. 2007;1112:161–170. doi: 10.1196/annals.1415.048. [DOI] [PubMed] [Google Scholar]
- 51.Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, et al. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Sun W, Kim H. Neurotrophic roles of the beta-thymosins in the development and regeneration of the nervous system. Ann N Y Acad Sci. 2007;1112:210–218. doi: 10.1196/annals.1415.013. [DOI] [PubMed] [Google Scholar]
- 54.Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- 55.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 56.Vartiainen N, Pyykonen I, Hokfelt T, Koistinaho J. Induction of thymosin beta(4) mRNA following focal brain ischemia. Neuroreport. 1996;7:1613–1616. doi: 10.1097/00001756-199607080-00017. [DOI] [PubMed] [Google Scholar]
- 57.Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, et al. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, et al. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2009 doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang H, Cheng X, Yao Q, Li J, Ju G. The promotive effects of thymosin beta4 on neuronal survival and neurite outgrowth by upregulating L1 expression. Neurochem Res. 2008;33:2269–2280. doi: 10.1007/s11064-008-9712-y. [DOI] [PubMed] [Google Scholar]
- 62.Yarmola EG, Klimenko ES, Fujita G, Bubb MR. Thymosin beta4: actin regulation and more. Ann N Y Acad Sci. 2007;1112:76–85. doi: 10.1196/annals.1415.008. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Zhang ZG, Morris D, Li Y, Roberts C, Elias SB, et al. Neurological functional recovery after thymosin beta4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164:1887–1893. doi: 10.1016/j.neuroscience.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 65.Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, et al. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, et al. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55:345–352. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]