Abstract
Based upon previous reports of alterations in white matter integrity and gray matter density in smokers, we examined these markers in a large, well-matched sample of smokers and non-smokers. We further investigated the effect of heavy cigarette exposure by using pack-years and the effects of two relatively stable, highly heritable traits in smokers (Fagerström Test of Nicotine Dependence (FTND), a measure of severity of nicotine dependence so and Toronto Alexithymia Scale (TAS-20), measuring a stable personality trait related to smoking. Forty-eight nicotine-dependent subjects and 48 matched controls were included in the analyses, with smokers also subdivided into high/low dependence and high/low pack-years smokers. White matter integrity (fractional anisotropy (FA)) and gray matter density (voxel-based morphometry (VBM)) were measured and compared across groups. Gray matter density was lower in left prefrontal cortex (PFC) in high pack-years smokers and was inversely related to pack-years. In contrast, left insular cortex gray matter density was higher in smokers and associated with TAS-20 total score and with difficulty-identifying-feelings factor. Further, the most highly dependent smokers showed lower prefrontal FA, which was negatively correlated with FTND. There was no correlation between pack-years and FTND in our smoker population. These data suggest chronic tobacco use is correlated with prefrontal gray matter damage , while differences in insula gray matter and PFC white matter appear to reflect stable and heritable differences between smokers and non-smokers.
Introduction
Cigarette smoke is a major source of toxic chemical exposure to humans. Previous studies (Fagerstrom, 2002; reviewed by Swan and Lessov-Schlaggar, 2007) report that tobacco smoking affects multiple organ systems with risk varying with total exposure. Numerous heritable factors (e.g. DRD4 (McClernon et al., 2007), DRD2 (Radwan et al., 2007), COMT (Beuten et al., 2006; Guo et al., 2007), etc) also clearly influence nicotine dependence.
Structural differences in both brain white and gray matter have been reported between smokers and non-smokers. Nicotine dependence is a risk factor for white-matter degeneration (Swan and Lessov-Schlaggar, 2007), and smoking history is related to severity of periventricular white matter hyper-intensities (Fukuda and Kitani, 1996; Longstreth, Jr. et al., 2000) and white matter integrity in the corpus callosum (Paul et al., 2008). Further, there are reports of reduced gray matter volume and/or lower gray matter density in frontal cortical regions in chronic cigarette smokers as a function of total cigarette load (i.e. pack years) (Brody et al., 2004; Gallinat et al., 2006). Not all of the structural differences noted above between smokers and non-smokers demonstrate a dose-response relationship with cigarette exposure levels, suggesting that some of these morphological differences may reflect other neurodevelopmental and/or genetic factors independent of or interacting with tobacco smoking.
The severity of nicotine addiction is a relatively stable trait in established smokers who are not trying to quit (Janson, 1999; McCarthy, 2001). Further, severity of addiction, as measured by the Fagerström Test of Nicotine Dependence (FTND) (Dijkstra and Tromp, 2002), has been shown to be highly heritable (Feng et al., 2004; Kendler et al., 1999; Li, 2008; Vink et al., 2005). Alexithymia is also a relatively stable (Salminen et al., 2006) and possibly heritable (Jorgensen et al., 2007) personality characteristic related to one’s ability to identify and process emotions. The Toronto Alexithymia Scale (TAS-20) (Bagby et al., 1994) is a psychometrically valid measurement of alexithymia and includes three subscales: difficulty identifying feelings (DIF), difficulty describing ones’ feelings (DDF), and the presence of externally oriented thinking (EOT). A significantly higher DIF score has been found in smokers compared to controls (Carton et al., 2008; Grabowska et al., 2005). Alexithymia has also been associated with other substance use disorders (Cecero and Holmstrom, 1997; Loas et al., 1997). Further, several studies (Kano et al., 2007; Karlsson et al., 2008) show alexithymia to be associated with increased activation to emotional stimuli in the insula, a region associated with smoking and other drug use behavior (Naqvi et al., 2007) and which is known to respond to cigarette cues (Franklin et al., 2007). FTND and TAS-20 are smoking-related stable and heritable traits and potentially useful endophenotypes to aid in characterizing anatomical brain differences between smokers and non-smokers.
Voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) are two unbiased neuroimaging techniques that have been applied to examine structural brain alterations in various neuropsychological disorders (Ashburner and Friston, 2000; Lim and Helpern, 2002; Versace et al., 2008). VBM is especially useful in detecting gray matter differences (Ashburner and Friston, 2000; Good et al., 2001); it is less robust as a measure of white matter (Filippi et al., 2001). However, by measuring the freedom of movement of water molecules, DTI reliably assesses white matter integrity (Basser and Pierpaoli, 1996). Fractional anisotropy (FA) assesses orientational coherence and is typically higher in fibers with a homogeneous or linear structure than in tissue with an inhomogeneous structure (Lim and Helpern, 2002; Neumann-Haefelin et al., 2000). While conventional DTI is susceptible to image distortion in some brain regions (LeBihan D., 2003), new techniques for whole-brain alignment can improve results significantly (Geng et al., 2009).
In the present study, we simultaneously assessed both gray matter density and white matter integrity in a relatively large cohort of smokers and matched non-smokers using VBM and DTI with a recently published, improved registration method (Geng et al., 2009; Smith et al., 2004; Smith et al., 2006). Based on previous behavioral, functional and anatomical data, we hypothesized that cigarette smokers possess specific gray and white matter alterations. Further, as less exposed/dependent smokers may have intermediate brain phenotypes and combining smokers into one group could obscure more subtle differences in users, we divided our large smoking group into those that were most heavily exposed smokers, as defined by pack-years, and again into those most heavily dependent, as defined by FTND. Finally we correlated VBM and FA from regions identified in the above analyses with pack-years, FTND and TAS-20 total and its subscores to identify regional differences relating to intensity of cigarette exposure and stable traits of smokers.
Methods and Materials
Participants
Data were collected from 48 cigarette smokers and 48 healthy non-smoking controls matched by age, gender and education years (see demographics in Table 1). None of the smokers were currently trying to quit or seeking smoking cessation treatment and were required to abstain from smoking for two hours before the MRI session (See supplement I for assessment of impact of nicotine withdrawal). No controls had smoked more than 25 cigarettes in their lifetime and none in the past ten years. All participants were recruited through newspaper advertisements, flyers, and referrals. After complete description of the study to the subjects, written informed consent approved by the NIDA-IRP Institutional Review Board was obtained. Screening procedures included a history and physical exam and a comprehensive laboratory panel (CBC, blood chemistries, liver function tests, thyroid function screening, erythrocyte sedimentation rate, HIV antibody test, syphilis screening test, urinalysis, pregnancy test (females) and a comprehensive urine drug screen). FTND (smokers), a computerized self-report version of the Structured Clinical Interview for DSM-IV Axis-I Disorders with follow-up clinical interview, TAS-20, and a drug use survey were collected on each participant. Interview based assessments were conducted by a master’s level clinician and/or psychiatrist. TAS-20 scores are inadvertently omitted on two smokers and two controls. Vocabulary subtest of the WASI was collected on 35 controls and 41 smokers; no group difference was found.
Table 1.
Participant demographic data
| Smokers | Controls | HP | HF | LP | LF | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | C | S | C | S | C | S | C | |||||||||||||
| Number | 48 | 48 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | ||||||||||
| Gender | 24 F | 24 F | 12 F | 12 F | 12 F | 12 F | 12 F | 12 F | 12 F | 12 F | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 31.4 | 8.1 | 31.1 | 8.8 | 36.8 | 8.0 | 36.6 | 6.5 | 30.3 | 8.1 | 30.3 | 8.5 | 25.5 | 5.2 | 26.2 | 5.8 | 32.0 | 9.5 | 32.6 | 8.5 |
| Education level |
13.1 | 2.2 | 13.5 | 1.8 | 13.4 | 1.8 | 13.3 | 1.8 | 13.0 | 2.6 | 13.5 | 2.0 | 12.7 | 2.4 | 13.7 | 1.8 | 13.1 | 1.6 | 13.5 | 1.7 |
| Smoking Years |
12.8 | 7.4 | - | - | 18.4 | 5.8 | - | - | 11.5 | 6.4 | - | - | 7.2 | 3.5 | - | - | 14.1 | 8.2 | - | - |
| CPD | 20.9 | 6.6 | - | - | 21.4 | 8.2 | - | - | 22.9 | 8.0 | - | - | 20.2 | 4.5 | - | - | 18.8 | 4.0 | - | - |
| FTND | 5.4 | 1.9 | - | - | 5.6 | 1.9 | - | - | 7.0 | 1.2 | - | - | 5.3 | 1.8 | - | - | 3.9 | 0.9 | - | - |
| Initial smoking |
15.6 | 3.4 | - | - | 15.4 | 3.1 | - | - | 16.0 | 4.1 | - | - | 15.8 | 3.8 | - | - | 15.2 | 2.6 | - | - |
| age Pack- years |
12.9 | 7.9 | - | - | 18.6 | 7.1 | - | - | 13.2 | 8.7 | - | - | 7.2 | 3.2 | - | - | 12.6 | 7.2 | - | - |
CPD: cigarettes smoked per day
FTND: Fagerström Test for Nicotine Dependence
Pack-years: Smoking years X CPD / 20
F/M: female/male
HP/HF/LP/LF: High Pack-years/High FTND score/Low Pack-years /Low FTND score
S/C: smoker/control
Participants were excluded if they had any major medical illnesses (e.g. obstructive lung disease, which could independently effect our dependent measures), major psychiatric disorders, neurological illnesses, were left-handed or if their T1 weighted images revealed gross structural abnormalities. No participant had a history of dependence (current or past) on any drug other than nicotine. There was no significant difference between number of smokers and controls who were social drinkers, past social drinkers or casual drug users (Supplement II).
Data acquisition
Experiments were performed on a Siemens 3T Allegra MRI (Erlangen, Germany) at the NIDA-IRP in Baltimore, MD. A standard birdcage RF head coil was used to acquire whole-brain T1-weighted structural images (MPRAGE) for VBM analyses (1-mm3 isotropic voxels, TR=2500ms, TE=4.38ms, flip angle=8°). Whole-brain DTI was acquired using a single-shot, spin-echo echo-planar imaging technique (TR=5000ms, TE=87ms, BW=1700Hz/Pixel, FOV=220×220mm, matrix size=128×128, 35 slices, thickness=4mm, b value= 0 or 1000s/mm2). Thirteen unique volumes were collected to compute the tensor: a b=0s/mm2 image and 12 images with diffusion gradients applied in 12 noncollinear directions (Gx, Gy, Gz: [1.0, 0.0, 0.5], [0.0, 0.5, 1.0], [0.5, 1.0, 0.0], [1.0, 0.5, 0.0], [0.0, 1.0, 0.5], [0.5, 0.0, 1.0], [1.0, 0.0, −0.5], [0.0, −0.5, 1.0], [−0.5, 1.0, 0.0], [1.0, −0.5, 0.0], [0.0, 1.0, −0.5], [−0.5, 0.0, 1.0]). Subject head movement was minimized by the use of soft foam padding, a vacuum bag, and/or hardened polyurethane foam.
Data analysis
Participant characteristics
The relationship between pack-years and FTND score was assessed with a Pearson’s correlation test. In order to explore the effects of high levels of cigarette exposure and nicotine dependence, we performed two median splits of the smoker group, one based on pack-years and one on FTND score (details in Supplement III). The control subjects were also subdivided to match the four smoker subgroups (high pack-years, high FTND, low pack-years and low FTND) based on age, education, and gender (Table 1). TAS-20 total and subscale scores were compared using t-test.
DTI
A DTI analysis method based on tract-based spatial statistics (TBSS) (Smith et al., 2006) was employed with a new improved alignment method (Geng et al., 2009). TBSS is an observer-independent method of FA image analysis to allow voxel-wise comparisons. The raw diffusion weighted images were corrected for eddy current distortion using FSL (Jenkinson and Smith, 2001). FA images calculated according to Pierpaoli et al., (1996) were created by fitting the eddy current corrected diffusion data to a tensor model using AFNI (Cox, 1996). After affine aligning to the MNI space using FSL, all FA datasets were simultaneously registered onto an implicit reference corresponding to the group average using implicit reference-based group (IRG) registration (Geng et al., 2009). Compared with conventional methods, which register each subject’s image to a selected reference, the IRG registration eliminates the bias associated with reference selection and produces smaller registration errors. The mean FA image was built to create a mean FA skeleton via FSL 3.0 (Smith et al., 2004). This skeleton represents the centers of all tracts common to the group. Each participant's aligned FA data were then projected onto this skeleton.
VBM
T1-weighted structural data were analyzed with FSL-VBM (Smith et al., 2004), a voxel-based morphometric analysis package (Ashburner and Friston, 2000). Briefly, structural images were skull stripped (Smith, 2002) and then tissue segmented into gray matter, white matter, and CSF (Zhang et al., 2001). The resulting gray matter partial volume images were aligned to MNI152 standard space using an affine followed by a nonlinear registration with the Image Registration Toolkit (Rueckert et al., 1999). The resulting images were averaged to create a study-specific template, to which the native gray matter images were then non-linearly re-registered. The registered partial volume segmented images were modulated (to correct for local expansion or contraction) by the Jacobian of the warp field and smoothed with an isotropic Gaussian kernel with FWHM=8mm.
Due to EPI distortion, DTI data were not aligned to the structural data. The registration of FA and T1-weighted data were performed separately to obtain the best alignment of each measure.
Statistical Analyses
Whole brain analysis
According to the recommendations in the FSL software package (Smith et al., 2004), permutation-based testing (Nichols and Holmes, 2002) was performed to build statistical maps (p<0.05, FWE correction) between smokers (whole group and subgroups) and controls for DTI and VBM data in this whole brain analysis. Significant DTI and VBM clusters from these statistical maps were considered as ROIs and subjected to the following secondary ROI analyses.
ROI analysis
A correlation analysis (Pearson test) was applied to the DTI and VBM ROI data and smoking variables (pack-years and FTND score). Because our ROIs were defined based on the high FTND and high pack-years subgroups, this correlation analysis was only performed in these smoker subgroups in order to avoid biasing the correlation (see Supplementary IV for the whole smoker group results). To examine the influence between the two smoking variables, FTND (or pack-years) was added as a co-variable in pack-years (or FTND) correlation analyses for each ROI. Additionally, correlation analyses were performed between TAS-20 scores (total and sub-scores) and the neuroimaging data from these ROIs in both smokers and controls. Age, gender, and education years were employed as co-variables for all analyses. All statistical tests were two-tailed.
Results
Participants’ characteristics
FTND scores and pack-years were not correlated with each other in the smoker cohort (r[48]=0.190, p=0.195; Figure S1). The high pack-year smoker subgroup included 12 male (pack-years: 11.25–42.5) and 12 female (12.0–26.6) smokers with the highest pack-years for each gender. The other 12 male (1.5–11) and 12 female (1.8–12) smokers comprised the low pack-year smoker subgroup. Similarly, the high FTND smoker subgroup included 12 male (FTND scores: 6–9) and 12 female (FTND scores: 5–9) smokers with highest FTND scores for each gender. The other 12 male (FTND scores: 2–5) and 12 female (FTND scores: 3–5) smokers were in the low FTND smoker subgroup. By design, the high FTND smoker subgroup had higher FTND scores (t[46]=10.288, p<0.001) than did the low FTND subgroup; while pack-years (t[46]=0.287, p=0.775) and age of initial smoking (t[46]=0.821, p=0.416) did not differ between these subgroups. Similarly, the high pack-years smoker subgroup smoked more total cigarettes in their lifetime (t[46]=7.115, p<0.001) than did the low pack-years smoker group while their FTND score (t[46]=0.615, p=0.542) and age of initial smoking (t[46]=0.482, p=0.632) did not differ. The DIF subscale in smokers was significantly higher than that in controls (t[90]=2.32, p=0.024); no significant group differences were found in DDF, EOT or total TAS-20 scores (see Table 2).
Table 2.
Participant TAS-20 data
| Controls | Smokers | Group t-test | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| DIF | 7.8 | 1.4 | 9.0 | 3.5 | 2.321 | 0.024 |
| DDF | 8.4 | 3.2 | 8.1 | 3.6 | 0.425 | 0.672 |
| EOT | 17.3 | 4.0 | 16.6 | 3.6 | 0.846 | 0.400 |
| Total | 33.4 | 5.6 | 33.7 | 7.3 | 0.225 | 0.823 |
DIF: difficulty identifying feelings
DDF: difficulty describing ones’ feelings
EOT: externally oriented thinking
Total: Total=DIF+DDF+EOT.
Whole brain analyses
DTI
No significant differences were found between the smoker and control groups. However, there was a significant decrease in FA in the left PFC in the high FTND smokers compared to their matched controls (Figure 1A and Table 3; pFWE_corrected<0.001). No significant differences were found in any other subgroup comparison.
Figure 1.
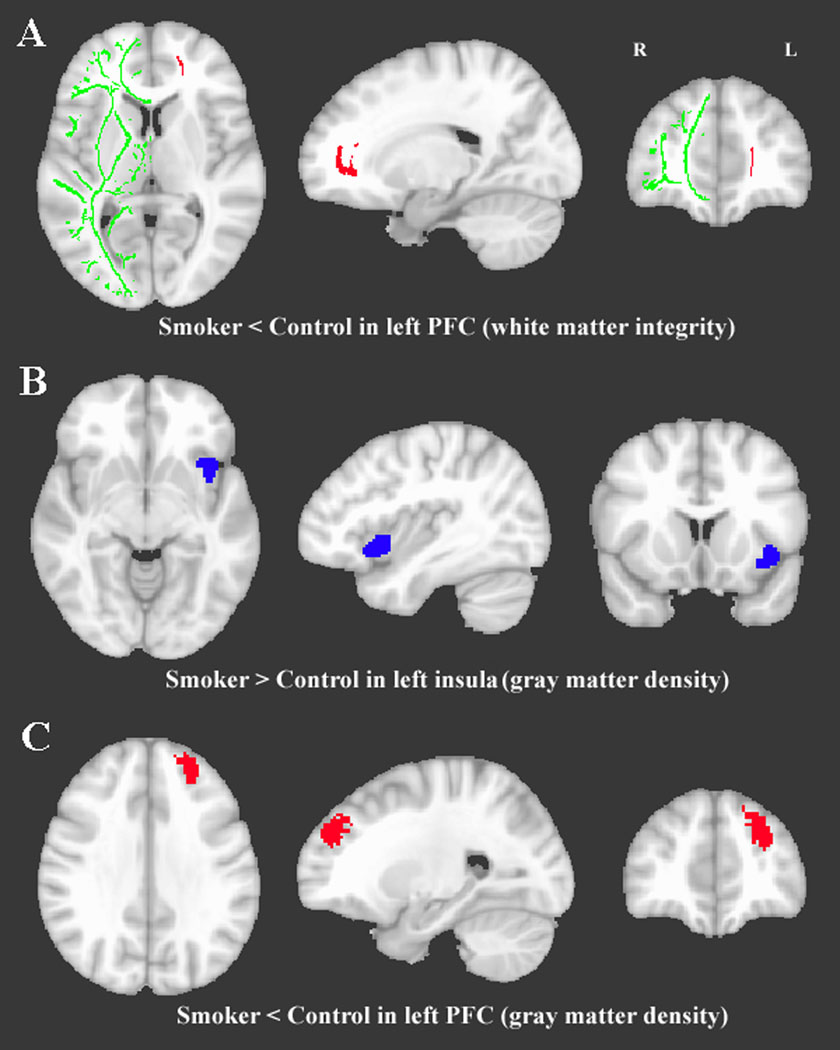
Clusters that showed a significant difference between smokers and controls. A. Lower white matter integrity (i.e., FA) in the left prefrontal area in high FTND smoker group, compared to high FTND control group. The FA DTI analysis is projected onto a white matter skeleton (shown in green) of the right hemisphere MNI brain. B. Higher gray matter density in the left insula in all smokers compared with all controls. C. Lower gray matter density in the left prefrontal cortex in high pack-years smoker group vs. high pack-years control group.
Table 3.
ROI coordinates of significant gray matter density and FA ROIs
| ROI | MNI coordinate (mm) | Smokers | Controls | |||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Mean | SD | Mean | SD | ||
| Gray matter density | Left Insula | 38.2 | −14.1 | −6.7 | 0.720 | 0.097 | 0.637 | 0.088 |
| Left Prefrontal | 24.3 | −46.1 | 32.4 | 0.560 | 0.145 | 0.620 | 0.128 | |
| FA | Left Prefrontal | 18.7 | −45.3 | 5.7 | 0.529 | 0.052 | 0.562 | 0.058 |
VBM
There was an increase in left insula gray matter density in the smoker group compared with their matched controls (Figure1B and Table 3, pFWE_corrected=0.036). Further, there was a decrease in gray matter density in the left PFC in the high pack-years smokers when compared with their matched control subgroup (Figure1C and Table 3, pFWE_corrected=0.015). No other significant subgroup differences in gray matter density were found.
ROI analyses
DTI
With normality of the data supported by the K–S test, parametric statistics were applied to the ROI analyses. The FA in the left PFC from all participants are shown in Figure S2. Confirming the whole brain analysis, FA in this PFC ROI of smokers was significantly lower than that in controls (t[94]=2.978, p=0.004). While the prefrontal FA in high FTND smokers did not correlate with pack-years (r[19]=−0.172, p=0.456), it did negatively correlate with FTND (r[19]=−0.518, p=0.016; Figure 2A), even when using pack-years as an additional covariant (r[18]=−0.511, p=0.021).
Figure 2.

A. Significant correlation between white matter integrity (FA) in the left prefrontal area and FTND score in high FTND smokers. B. Significant correlation between gray matter density in the left prefrontal cortex and pack-years in high pack-years smokers. All data were corrected for age, gender and years of education.
VBM
VBM ROI data were also normally distributed, as supported by the K–S test, allowing for parametric statistical analyses. Confirming the whole brain analysis, the insula gray matter density in smokers was significantly higher than in controls (t[94]=4.415, p<0.001), while the PFC gray matter density in smokers was significantly lower than in controls (t[94]=2.120, p=0.037). However, insular gray matter density did not correlate with any smoking marker (FTND: r[43]=−0.141, p=0.355; pack-years: r[43]=0.148, p=0.332). The gray matter density in the left PFC from all participants is showed in Figure S3. Prefrontal gray matter density negatively correlated with pack-years (r[19]=−0.469, p=0.032; Figure2B), but not with FTND (r[19]=−0.247, p=0.281) in the high pack-year smoker subgroup, the former remaining significant even when using FTND as an additional covariant (r[18]=−0.455, p=0.044).
TAS-20
Total TAS-20 score (r[41]=0.353, p=0.020; Figure3A) and the DIF subscale (r[41]=0.414, p=0.006; Figure 3B) were positively correlated with insula gray matter density in smokers but not in controls (Total: r[41]=−0.084, p=0.593; DIF: r[41]=−0.091, p=0.560). The lack of correlation in the control group may reflect the small range of scores in this particular control population (Table 2). No other significant associations were found between TAS-20 scores and the imaging data.
Figure 3.
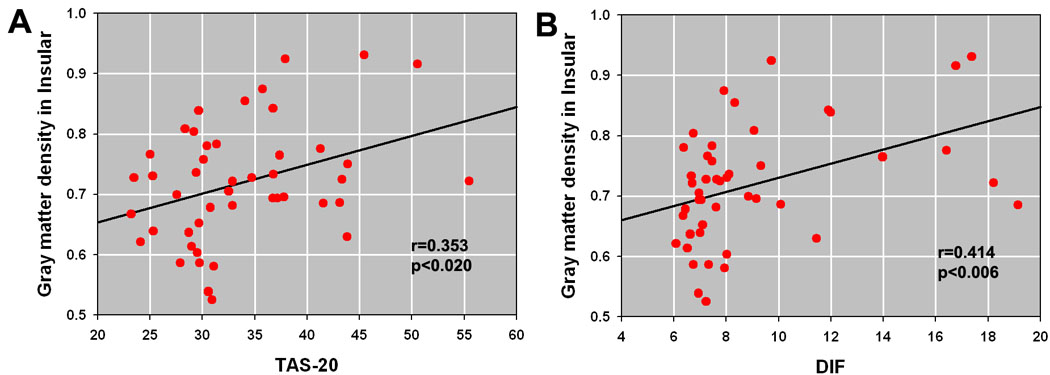
A. Significant correlation between gray matter density in the left insula and TAS-20 (total score). B. Significant correlation between gray matter density in the left insula and TAS-20 DIF subscale. Data were corrected for age, gender and years of education.
Discussion
Using VBM and DTI, the present study demonstrates the following brain structural differences between smokers and non-smokers: a) greater insular gray matter density in smokers correlated with alexithymia scores b) lower PFC white matter integrity negatively correlated with nicotine dependence severity in highly dependent smokers and c) lower PFC gray matter density negatively correlated to total lifetime cigarette exposure in heavily exposed smokers.
Only the left anterior insula differed between the entire smoker cohort and matched controls. Smokers had higher gray matter density, which also correlated with TAS-20 total and Difficulty-Identifying-Feelings subscale scores. The insula is a key structure in the perception of bodily needs and helps to regulate motivated behaviors (Contreras et al., 2007; Craig, 2002). Multiple imaging studies have shown that cigarette cues induce subjective drug craving that correlates with activity in the insula (Bonson et al., 2002; Brody et al., 2002; Franklin et al., 2007). Further, alexithymia has also been show to be associated with greater insula activation to emotional cues (Kano et al., 2007; Karlsson et al., 2008). Consistent with previous studies (Carton et al., 2008; Grabowska et al., 2005), we observed significantly higher DIF scores in smokers compared to controls. Thus, since alexithymia relates insular activation to emotional cues and cigarette cues acquire emotional salience as nicotine dependency develops, our insula gray matter difference relating to alexithymia may underlie insular activation to cigarette cues previously reported (Franklin et al., 2007). It is possible that increased gray matter density in the anterior insula may underlie a greater reliance on bodily sensations for the experience of emotion, to the detriment of an ability to verbalize emotions, i.e., alexithymia. This may facilitate nicotine dependence as nicotine administration and withdrawal both elicit considerable bodily sensations that may drive emotional responses towards cigarettes and in avoidance of withdrawal, thus sustaining nicotine dependence.
Loas et al., (1997) found the DIF significantly predicted nicotine abstinence. Combined with the observations by Naqvi et al., (2007) that cerebrovascular strokes in smokers limited to the insula lead to a profound reduction in post recovery smoking behavior lends support to our anatomical observations and adds to the growing literature suggesting a central role for the insula in neuronal processing related to affective components of nicotine dependence. Thus, the insula may be a key region to understand the relationship between nicotine dependence and other psychiatric illnesses. While Naqvi reported disruption of smoking behavior following strokes localized on either side of the insula, results were more striking for right-sided insular strokes (5/6 after right-sided vs. 7/13 after left-sided strokes), Franklin et al., (2007) demonstrated greater left but not the right insula activity in smokers during exposure to smoking cues compared to nonsmoking cues, consistent with the laterality observation in the present study. The role of laterality of insula function, especially as it relates to smoking, warrants further exploration.
Further brain anatomy differences between smokers and nonsmokers were only evident by parsing subjects into the most highly dependent or exposed subgroups. When so divided, a region in left PFC showed significantly lower FA in the most dependent smoker cohort. Further, this PFC FA correlated negatively with FTND. Reduced FA is generally thought to indicate less structural integrity and compromised fiber tracts and is often associated with brain pathology (Lim and Helpern, 2002). While FA differences were previously reported in smokers using an ROI strategy (Paul et al., 2008), this is the first demonstration based on a whole brain analysis. In fact, most previous DTI studies in drug dependent populations have used such an ROI approach, generally focusing on the genu of the corpus callosum to infer prefrontal white matter differences (Lim et al., 2002; Lim et al., 2008; Liu et al., 2008; Moeller et al., 2005; Moeller et al., 2007; Pfefferbaum et al., 2000; Pfefferbaum et al., 2008). In contrast, our whole brain approach directly identified prefrontal white matter impairment related to severity of dependence. As FTND is a stable (Janson, 1999; McCarthy, 2001) and highly heritable trait in those smokers with an established habit and not currently trying to quit (Feng et al., 2004; Kendler et al., 1999; Li, 2008; Vink et al., 2005), this result suggests a stable and heritable difference between smokers and non-smokers, which may, at least in part, be premorbid to smoking behavior.
We also observed lower left PFC gray matter density in those smokers with heavy cigarette exposure, which negatively correlated with lifetime cigarette usage quantified as pack-years. Several previous studies have reported similar findings. For example, Brody et al., (2004) and Galinat et al., (2006) demonstrated decreased PFC gray matter density that correlated negatively with pack-years. A variety of cognitive constructs generally associated with PFC functioning decline in elderly smokers at rates that increase as a function of pack-years of smoking (Galanis et al., 1997; Ott et al., 2004). There is also evidence that smoking cessation may mitigate these cognitive declines (reviewed in Swan and Lessov-Schlaggar, 2007). Further, the dose-effect relationship demonstrated in numerous studies using different methodologies suggests a toxic effect of cigarette smoking on the prefrontal cortex. Thus, our result supports a growing literature suggesting that cigarette smoking selectively impairs the prefrontal lobe, either from nicotine or one of the more than four thousand chemicals present in tobacco smoke, about 400 of which, including nicotine and CO, are known toxins (Swan and Lessov-Schlaggar, 2007),. The strength of the correlation in our study is, however, modest (r=−0.469), which is in keeping with the published literature. This is likely due to two factors: imprecision in the assessment of pack-years likely due to accuracy of recall of events, and such other factors as differences in subject IQ and socioeconomic status, which are known to affect gray matter density (Andreasen et al., 1993; Gianaros et al., 2007).
It has been argued that correlations with pack-years may not imply toxicity of cigarette smoke because smokers with high pack-years are likely to be heavier smokers and, thus, likely to be more dependent, which could explain differences related to pack-years. Since we have a measure of severity of dependence (FTND) as well as a measure of cumulative exposure (pack-years) which do not correlate with each other we are able to test this hypothesis directly. Our significant correlation with pack-years, even when controlling for severity of dependence suggests, while not providing mechanistic proof, that cumulative exposure to smoke is responsible for the observed relationship.
Another potential interpretation of these data is that people with prefrontal gray matter atrophy are self-medicating with nicotine to ameliorate consequences of their tissue alteration. However, if true, then the gray matter density should be related to cigarettes per day, not to pack-years because a self-medication effect is likely related to the number of cigarettes smoked in a unit time rather than to the duration itself. Further, as prefrontal gray matter density decreases with age (Raz et al., 1997) and pack-years is biased by age, if prefrontal gray matter density in smokers is associated with age-related traits, such an alternative hypothesis may also be possible. However, we employed age as a covariant in the correlation analysis between gray matter density and pack-years, thus mitigating any contribution of age in the correlation.
One limitation of the current result merits consideration. The correlation coefficients between PFC gray matter density and FTND (r=−0.172) and PFC FA and pack-years (r=−0.247) might well have reached significance with a larger sample size. Of note, our current sample size is roughly double that of previous studies yet failed to reproduce previous findings in smokers versus controls and only concurs with previous findings after parsing our smoking cohort into those most heavily exposed or dependent. Therefore, further investigation is certainly warranted. Nevertheless, when using FTND as a covariate in the pack-year correlation analysis (or vice-versa), the correlation results remained significant, lending further support to our interpretation,
In summary, by simultaneously measuring both VBM and DTI in a large, well-matched cohort of cigarette smokers and demographically matched non-smokers, we confirm previous results associating PFC impairment with exposure to cigarette smoke (e.g., pack-years). We also identified impaired white matter in the left PFC related to FTND, a stable and heritable measure of nicotine dependence severity. Finally, we identified reduced insular gray matter density in smokers that relates to the ability to process and describe one’s feelings, also a relatively stable trait of the adult personality. Although not providing mechanistic evidence, these results are consistent with 1) cigarette exposure induced toxicity to PFC gray matter and 2) insular gray matter and PFC white matter alterations that reflect stable and possibly heritable differences between smokers and controls. While alterations in smoker neuroanatomy likely result from a complex interaction of genes and environment, determining which differences relate more strongly to amount of exposure and which to stable and heritable factors is important to the development of more efficacious strategies to prevent and treat nicotine dependence.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH. We thank Kimberly Modo and Loretta Spurgeon for their assistance in the conduct of the study and Mary Pfeiffer for editing assistance. The authors reported no financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Reference List
- Andreasen NC, Flaum M, Swayze V, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. Intelligence and brain structure in normal individuals. Am.J.Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J.Psychosom.Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson.B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beuten J, Payne TJ, Ma JZ, Li MD. Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology. 2006;31:675–684. doi: 10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol.Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch.Gen.Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Carton S, Bayard S, Jouanne C, Lagrue G. Emotional Awareness and Alexithymia in Smokers Seeking Help for Cessation: A Clinical Analysis. The Journal of Smoking Cessation. 2008;3:2. [Google Scholar]
- Cecero JJ, Holmstrom RW. Alexithymia and affect pathology among adult male alcoholics. Journal of Clinical Psychology. 1997;53:201–208. doi: 10.1002/(sici)1097-4679(199704)53:3<201::aid-jclp2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput.Biomed.Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat.Rev.Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dijkstra A, Tromp D. Is the FTND a measure of physical as well as psychological tobacco dependence? J Subst.Abuse Treat. 2002;23:367–374. doi: 10.1016/s0740-5472(02)00300-8. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K. The epidemiology of smoking - Health consequences and benefits of cessation. Drugs. 2002;62:1–9. doi: 10.2165/00003495-200262002-00001. [DOI] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N, Xu X. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. Am.J.Hum.Genet. 2004;75:112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304–311. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Kitani M. Cigarette smoking is correlated with the periventricular hyperintensity grade of brain magnetic resonance imaging. Stroke. 1996;27:645–649. doi: 10.1161/01.str.27.4.645. [DOI] [PubMed] [Google Scholar]
- Galanis DJ, Petrovitch H, Launer LJ, Harris TB, Foley DJ, White LR. Smoking history in middle age and subsequent cognitive performance in elderly Japanese-American men. The Honolulu-Asia Aging Study. Am.J.Epidemiol. 1997;145:507–515. doi: 10.1093/oxfordjournals.aje.a009138. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur.J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Geng X, Christensen GE, Gu H, Ross TJ, Yang Y. Implicit reference-based group-wise image registration and its application to structural and functional MRI. Neuroimage. 2009;47:1341–1351. doi: 10.1016/j.neuroimage.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Critchley HD, Manuck SB, Hariri AR. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc.Cogn Affect.Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grabowska P, Targowski T, Rozynska R, Mierzejewska J, From S. Alexithymia and depression: relationship to cigarette smoking, nicotine dependence and motivation to quit smoking. Przegl.Lek. 2005;62:1004–1006. [PubMed] [Google Scholar]
- Guo S, Chen dF, Zhou DF, Sun HQ, Wu GY, Haile CN, Kosten TA, Kosten TR, Zhang XY. Association of functional catechol O-methyl transferase (COMT) Val108Met polymorphism with smoking severity and age of smoking initiation in Chinese male smokers. Psychopharmacology (Berl) 2007;190:449–456. doi: 10.1007/s00213-006-0628-4. [DOI] [PubMed] [Google Scholar]
- Janson H. Longitudinal patterns of tobacco smoking from childhood to middle age. Addict.Behav. 1999;24:239–249. doi: 10.1016/s0306-4603(98)00061-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med.Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jorgensen MM, Zachariae R, Skytthe A, Kyvik K. Genetic and environmental factors in alexithymia: a population-based study of 8,785 Danish twin pairs. Psychother.Psychosom. 2007;76:369–375. doi: 10.1159/000107565. [DOI] [PubMed] [Google Scholar]
- Kano M, Hamaguchi T, Itoh M, Yanai K, Fukudo S. Correlation between alexithymia and hypersensitivity to visceral stimulation in human. Pain. 2007;132:252–263. doi: 10.1016/j.pain.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Naatanen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. Br.J.Psychiatry. 2008;192:32–38. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol.Med. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- LeBihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat.Rev.Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Li MD. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Hum.Genet. 2008;123:119–131. doi: 10.1007/s00439-008-0473-0. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol.Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Helpern JA. Neuropsychiatric applications of DTI: a review. NMR Biomed. 2002;15:587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Li L, Hao Y, Cao D, Xu L, Rohrbaugh R, Xue Z, Hao W, Shan B, Liu Z. Disrupted white matter integrity in heroin dependence: a controlled study utilizing diffusion tensor imaging. Am.J.Drug Alcohol Abuse. 2008;34:562–575. doi: 10.1080/00952990802295238. [DOI] [PubMed] [Google Scholar]
- Loas G, Fremaux D, Otmani O, Lecercle C, Delahousse J. Is alexithymia a negative factor for maintaining abstinence? A follow-up study. Compr.Psychiatry. 1997;38:296–299. doi: 10.1016/s0010-440x(97)90063-8. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Arnold AM, Manolio TA, Burke GL, Bryan N, Jungreis CA, O'Leary D, Enright PL, Fried L. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people. The Cardiovascular Health Study. Collaborative Research Group. Neuroepidemiology. 2000;19:30–42. doi: 10.1159/000026235. [DOI] [PubMed] [Google Scholar]
- McCarthy W. Individual change amid stable smoking patterns in polydrug users over 3 years. Addictive Behaviors. 2001;26:143–149. doi: 10.1016/s0306-4603(00)00083-6. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology (Berl) 2007;194:433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Lane SD, Buzby M, Swann AC, Hasan KM, Kramer LA, Narayana PA. Diffusion tensor imaging in MDMA users and controls: association with decision making. Am.J.Drug Alcohol Abuse. 2007;33:777–789. doi: 10.1080/00952990701651564. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Wittsack HJ, Fink GR, Wenserski F, Li TQ, Seitz RJ, Siebler M, Modder U, Freund HJ. Diffusion- and perfusion-weighted MRI: influence of severe carotid artery stenosis on the DWI/PWI mismatch in acute stroke. Stroke. 2000;31:1311–1317. doi: 10.1161/01.str.31.6.1311. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum.Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Andersen K, Dewey ME, Letenneur L, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A, Launer LJ. Effect of smoking on global cognitive function in nondemented elderly. Neurology. 2004;62:920–924. doi: 10.1212/01.wnl.0000115110.35610.80. [DOI] [PubMed] [Google Scholar]
- Paul RH, Grieve SM, Niaura R, David SP, Laidlaw DH, Cohen R, Sweet L, Taylor G, Clark RC, Pogun S, Gordon E. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine.Tob.Res. 2008;10:137–147. doi: 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of Association and Projection White Matter Systems in Alcoholism Detected with Quantitative Fiber Tracking. Biol.Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin.Exp.Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di CG. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Radwan GN, El-Setouhy M, Mohamed MK, Hamid MA, Azem SA, Kamel O, Israel E, Loffredo CA. DRD2/ANKK1 TaqI polymorphism and smoking behavior of Egyptian male cigarette smokers. Nicotine.Tob.Res. 2007;9:1325–1329. doi: 10.1080/14622200701704889. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb.Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans.Med.Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Salminen JK, Saarijarvi S, Toikka T, Kauhanen J, Aarela E. Alexithymia behaves as a personality trait over a 5-year period in Finnish general population. J.Psychosom.Res. 2006;61:275–278. doi: 10.1016/j.jpsychores.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum.Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De LM, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De SN, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The Effects of Tobacco Smoke and Nicotine on Cognition and the Brain. Neuropsychol.Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JR, Hassel S, Walsh ND, Novelli M, Klein CR, Kupfer DJ, Phillips ML. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch.Gen.Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav.Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans.Med.Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


