Abstract
Background
Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX) syndrome is characterized by severe systemic autoimmunity caused by mutations in the FOXP3 (Forkhead Box P3) gene. Hematopoietic cell transplantation is currently the only viable option for long-term survival, but patients are frequently very ill and may not tolerate traditional myeloablative conditioning regimens.
Objective
Here, we present the outcome of hematopoietic cell transplantation using a low intensity, nonmyeloablative conditioning regimen in two patients with IPEX syndrome and significant pre-transplant risk factors.
Methods
Two high-risk patients with IPEX syndrome received HLA-matched related bone marrow or unrelated peripheral blood stem cell grafts following conditioning with 90 mg/m2 fludarabine and 4 Gy total body irradiation. Postgrafting immunosuppression consisted of mycophenolate mofetil and cyclosporine. Immune reconstitution and immune function was evaluated by measurement of donor chimerism, regulatory T-cell numbers, absolute lymphocyte subsets and T-cell proliferation assays.
Results
Both patients experienced minimal conditioning toxicity and successfully engrafted after hematopoietic cell transplantation. With a follow-up of 1 and 4 years, respectively, patients 1 and 2 have full immune function and normal FOXP3 protein expression.
Conclusion
A low intensity, nonmyeloablative conditioning regimen can establish stable engraftment and correct the life-threatening immune deficiency and enteropathy of IPEX syndrome despite the presence of comorbidities that preclude conventional hematopoietic cell transplantation.
Keywords: Hematopoietic cell transplantation, Nonmyeloablative conditioning regimen, Immunodeficiency, Immune dysregulation, Autoimmunity, Regulatory T-cells, Immune reconstitution
INTRODUCTION
IPEX syndrome is a rare and lethal genetic disease characterized by immune dysregulation, polyendocrinopathy, enteropathy and X-linked inheritance. It is caused by mutations in the gene encoding the DNA binding protein, FOXP3, which is located on the X chromosome at Xp11.23.(1) Patients with IPEX syndrome often present in early infancy with autoimmune disorders, most commonly severe enteropathy, type 1 diabetes mellitus, thyroiditis, eczematous or psoriatic dermatitis, and autoimmune cytopenias. Additional clinical features may include pneumonitis, nephritis, hepatitis, arthritis, myositis, alopecia, lymphadenopathy and splenomegaly.(2)
Without therapeutic intervention, affected male patients usually die within the first or second year of life. Supportive therapy including total parenteral nutrition, insulin, antibiotics, and blood transfusions is required in virtually all cases. Immunosuppressive therapy with steroids, cyclosporine (CSP), tacrolimus (FK506), and sirolimus (rapamycin) has been used with variable improvement in symptoms. Even with aggressive treatment, patients often do not survive beyond the second or third decade of life.(3;4) Correction of the dysregulated immune system can be achieved by hematopoietic cell transplantation (HCT) using an suitable donor;(5;6) however, there appears to be a high mortality rate associated with conventional conditioning regimens, presumably because most patients with IPEX syndrome have developed serious medical complications by the time of the transplant procedure. Thus, it seems reasonable, particularly in this group of high-risk patients, to evaluate a novel conditioning regimen designed to reduce toxicity.
PATIENTS and METHODS
Both patients developed classic complications of IPEX as listed in Table I. Patient 1 presented at 2 months of age with diabetes, profuse diarrhea with eosinophilic enteropathy, failure to thrive, hemolytic anemia, and bacterial infections. Immunologic studies showed a marked eosinophilia (1000–2000 cells/mm3) and an elevated IgE level (183 IU/mL). Sequencing of the FOXP3 gene identified a canonical splice mutation downstream of exon 1 in the FOXP3 gene (c.210_210+1GG>AC). This mutation leads to abnormal messenger RNA splicing, and lack of FOXP3 protein expression. Despite aggressive medical therapy, including tacrolimus and solumedrol, the autoimmune and infectious complications persisted. In the few months prior to HCT he received treatment for a bacterial brain abscess, candida albicans septicemia, and cytomegalovirus (CMV) reactivation (initial CMV polymerase chain reaction [PCR] 3200 copies/mL, reduced to 220 copies/mL at HCT).
Table I.
Pre-transplant patient characteristics
| Characteristic | Patient 1 | Patient 2 |
|---|---|---|
| Age at | ||
| Diagnosis | 2 months | 11 years |
| HCT | 9 months | 16 years |
| IPEX features | ||
| Immune dysregulation | Eosinophilia (1000–2000/mm3) | Eosinophilia (>2000/mm3) |
| Hyper-IgE (183 IU/mL) | Hyper-IgE (842 IU/mL) | |
| Anticardiolipin antibodies (DVT) | Hypogammaglobulinemia (204 mg/dL) | |
| Hemolytic anemia | Anemia | |
| Infections | Infections | |
| Membranous nephropathy | ||
| Polyendocrinopathy | Diabetes | Diabetes |
| Hypothyroidism | ||
| Growth Hormone Deficiency | ||
| Enteropathy | Diarrhea | Diarrhea |
| Failure to thrive | Failure to thrive | |
| Other | Steroid dependent interstitial lung disease | |
| Osteopenia | ||
| Seizure disorder | ||
| FOXP3 gene | ||
| Mutation* | c.210_210+1GG>AC | c.816+7G>C |
| Mutation significance | Abnormal mRNA splicing | Abnormal mRNA splicing |
| Protein expression | Absent | Reduced |
| Infections | ||
| Bacterial | Skin abscesses | Recurrent pneumonias |
| Staphylococcus aureus | ||
| MRSA | ||
| Brain abscess | ||
| Sepsis | ||
| Group A streptococcus | ||
| Enterococcus Faecalis | ||
| Coagulase negative | ||
| Staphylococcus | ||
| Fungal | Fungemia | Skin abscesses |
| Candida albicans | Alternaria | |
| Viral | Viremia | Viremia |
| Cytomegalovirus | Cytomegalovirus | |
| EBV | ||
| EBV-lymphoproliferative disorders of lungs and intestinal tract |
Mutation nomenclature per den Dunnan and Antonarakis.(25)
EBV, Epstein-Barr virus; HCT, hematopoietic cell transplantation; MRSA, methicillin-resistant Staphylococcus aureus
Patient 2 was clinically diagnosed with IPEX around 11 years of age. Sequencing of genomic DNA identified a potential donor splice-site mutation downstream of exon 7 in the FOXP3 gene (c.816+7G>C). cDNA sequencing confirmed that this mutation leads to abnormal FOXP3 mRNA splicing with a majority of transcripts lacking exon 7 which encodes the C-terminal portion of the critical leucine-zipper domain. The patient had a complicated medical history (Table 1) including bloody diarrhea with failure to thrive, diabetes mellitus, steroid dependent interstitial lung disease, and significant infections including recurrent invasive Alternaria fungal abscesses in his leg that required nine separate debridements followed by a skin graft, CMV infection and reactivation, and Epstein Barr virus (EBV) lymphoproliferative disorder of the lungs and gastrointestinal tract. He developed marked eosinophilia (>2000 cells/mm3), elevated IgE (842 IU/mL), and hypogammaglobulinemia (IgG 204 mg/dL) requiring intravenous immunoglobulin supplementation. Recurrent EBV viremia (1500 copies/ml) was detected 4 weeks before HCT and treated with rituximab, resulting in complete resolution of EBV at the time of HCT.
Transplant procedure
The conditioning regimen consisted of 30 mg/m2/day fludarabine for 3 consecutive days followed by total body irradiation (TBI), 4 Gy (2Gy BID), as previously described, except that a slightly higher dose of TBI (4 Gy versus 2 Gy) was given.(7) Postgrafting immunosuppression consisted of mycophenolate mofetil (MMF; 15 mg/kg three times a day from day 0 to day 40, followed by a taper to day 96 if there was no evidence of GVHD) and CSP (day −3 to day 100, adjusted to achieve serum trough levels between 400 and 500 ng/mL, followed by taper to day 180 if no GVHD).(7) Diagnosis and clinical grading of acute and chronic GVHD were performed according to established criteria.(8–11) Patients were given granulocyte colony stimulating factor (G-CSF) mobilized peripheral blood stem cells (patient 1) or bone marrow (patient 2) matched by high resolution typing at HLA-A, B, C, DRB1, and DQB1 (Table II). Following HCT, patients received supportive care that included antibiotics to treat or prevent opportunistic infections, prophylactic fluconazole, trimethoprim-sulfamethoxazole, intravenous immunoglobulin, and foscarnet/ganciclovir due to CMV reactivation pre HCT (patient 1).(12) Adverse events were graded by the Common Toxicity Criteria version 3.(13)
Table II.
Transplant characteristics and Post-transplant patient outcomes
| Patient 1 | Patient 2 | |
|---|---|---|
| Transplant Characteristics | ||
| Donor | HLA-matched unrelated | HLA- matched related |
| Stem Cell Source | Peripheral Blood | Bone Marrow |
| Cell Dose/kg | ||
| TNC | 15 × 108 | 5.1 × 108 |
| CD34 | 10 × 106 | 10 × 106 |
| CD3 | 5.4 × 108 | ND |
| Post – Transplant Patient Outcomes | ||
| Time to Engraftment (days) | ||
| Neutrophils (ANC>0.5 × 10^9/L) | 17 | 16 |
| Platelets (>50 × 10^9/L) | 11 | 17 |
| Transfusions Post-transplant | ||
| Red cells, Units | 17 | 5 |
| Platelets, Units | 4 | 2 |
| GVHD | ||
| Acute, Grade | II | II |
| Chronic (NIH global severity) | Severe | None |
| Lansky performance score- last follow up | 90% | 100% |
| Follow-up, month | 48 | 12 |
ANC, absolute neutrophil count; Kg, kilogram; NIH, National Institutes of Health, ND, not done
Immunologic evaluation
Recoveries of T and B cell numbers were evaluated by standard flow cytometry methods using specific monoclonal antibodies to CD3, CD4, CD8, and CD19. Lymphocyte function was assessed by measuring lymphocyte proliferation to mitogen [phytohemagglutinin (PHA)] responses. Proliferation was measured by [H3] thymidine incorporation according to standard methods.(14)
Analysis of FOXP3 protein expression in T cells
Pre-transplant FOXP3 protein expression in patient 1 was evaluated in fixed and permeabilized peripheral blood mononuclear cells (PBMC’s) by flow cytometry using a polyclonal anti-FOXP3 antibody as previously described.(15) All other samples for both patients were evaluated by flow cytometry using the Alexa Fluor® 488 anti-human FOXP3 flow kit with monoclonal antibody 259D according to the manufacturer’s recommendations (Biolegend, San Diego, CA). Samples were simultaneously stained for CD4 and CD25 and all samples were analyzed on a LSRII flow cytometer (BD, Franklin Lakes, NJ).
Analysis of FOXP3 gene sequence
Sequences of all exons, exon/intron boundaries, and the polyadenylation site of the FOXP3 gene were evaluated in genomic DNA isolated from peripheral blood using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Gene segments were amplified by polymerase chain reaction (PCR) using specific intronic oligonucleotide primers as previously described.(16) Purified PCR products were directly sequenced using the BigDye Terminator Cycle Sequencing Kit (PE Applied Biosystems, Boston, MA) and the sequences analyzed using the BioEdit software package.
Approval was obtained from the institutional review board and informed consent was obtained in accordance with the Declaration of Helsinki.
RESULTS
Engraftment
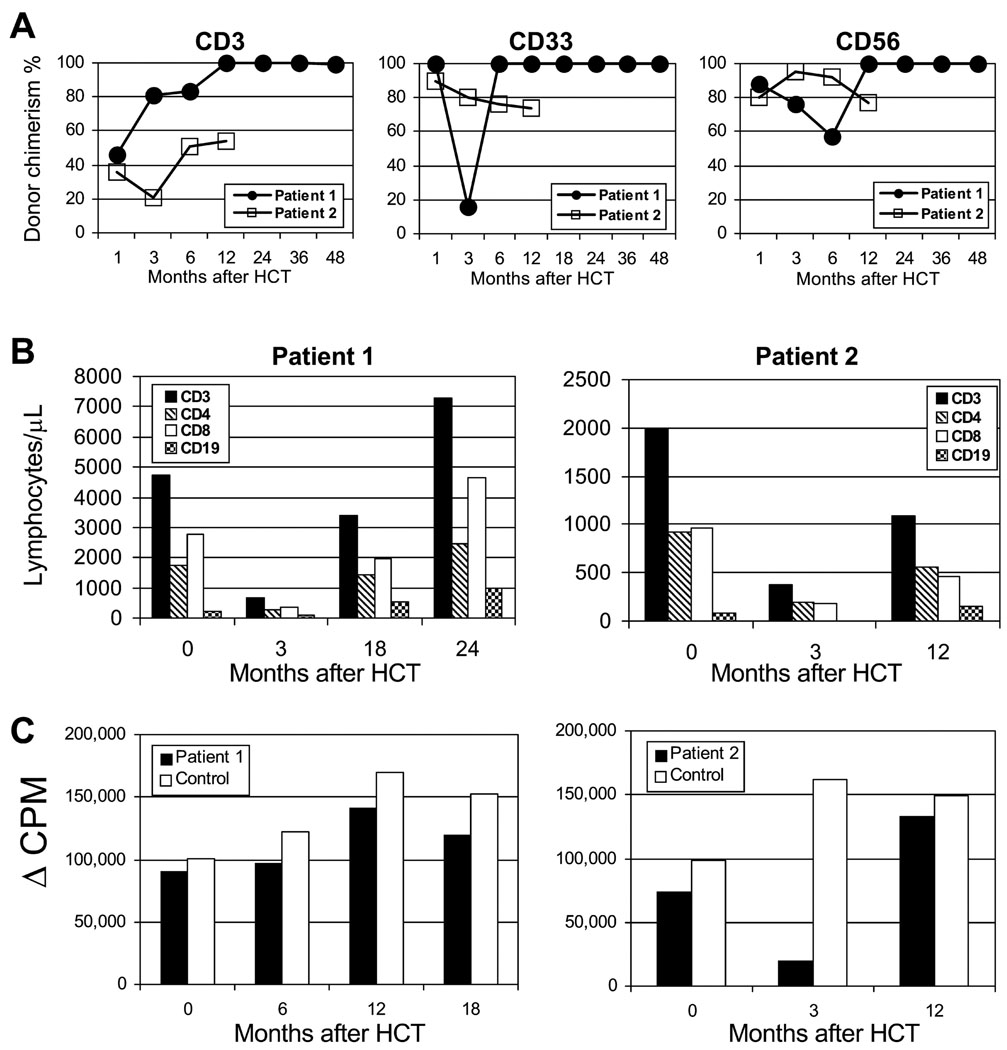
Recovery of peripheral blood absolute neutrophil counts (ANC; > 0.5 × 109/L) occurred on day +17 and day +16, for patients 1 and 2, respectively. Recovery of platelet counts (>50 × 109/L) occurred at days +11 and +17, respectively. Patient 1 developed episodic neutropenia as a side effect of ganciclovir, which resolved with G-CSF. The total number of packed red blood cell transfusions was 17 and 5, and the total number of platelet transfusions was 4 and 2, for patients 1 and 2, respectively. Both patients developed stable multi-lineage donor engraftment after HCT (Fig 1A).
FIG 1. Engraftment kinetics and immune recovery after HCT with nonmyeloablative conditioning in IPEX syndrome.
A) Percent CD3 T cell, CD33 myeloid, and CD56 NK cell donor chimerism levels in sorted peripheral blood cell subsets following HCT. B) Recovery of CD3+, CD4+, CD8+, and CD19 absolute lymphocyte counts following HCT. C) Recovery of T-cell function following HCT assessed by T cell proliferative responses to PHA.
Transplant-related toxicity and infection
Severe adverse events included one grade 4, pulmonary toxicity (patient 2) that resolved with diuretics. New infections were limited to bacteremias (three for patient 1 and one for patient 2) that resolved with appropriate antibiotics. Patient 1 was given ganciclovir, caspofungin, and antibiotics after HCT which prevented recurrence of his pre-existing infectious complications (CMV, candidemia, and brain abscess).
Graft-vs.-host disease
Both patients developed acute GVHD grade II, limited to the skin (patient 1) or gut (patient 2), which resolved with corticosteroid therapy. Extensive chronic GVHD developed in patient 1, who was treated with prednisone and sirolimus. At 4 years following HCT, patient 1 had no evidence of active chronic GVHD and was tapering off immunosuppression. Patient 2 has tapered off all immunosuppression and had no signs of chronic GVHD at 1 year after HCT.
Immunologic recovery
Neither patient has developed serious opportunistic infections after HCT. With respect to clinical symptoms associated with IPEX, the enteropathy and hemolytic anemia completely resolved in patient 1; however, there has been no improvement in control of diabetes. Patient 2 has had resolution of the interstitial lung disease, enteropathy and anemia; however there has been no improvement in control of diabetes and he remains on Synthroid for hypothyroidism.
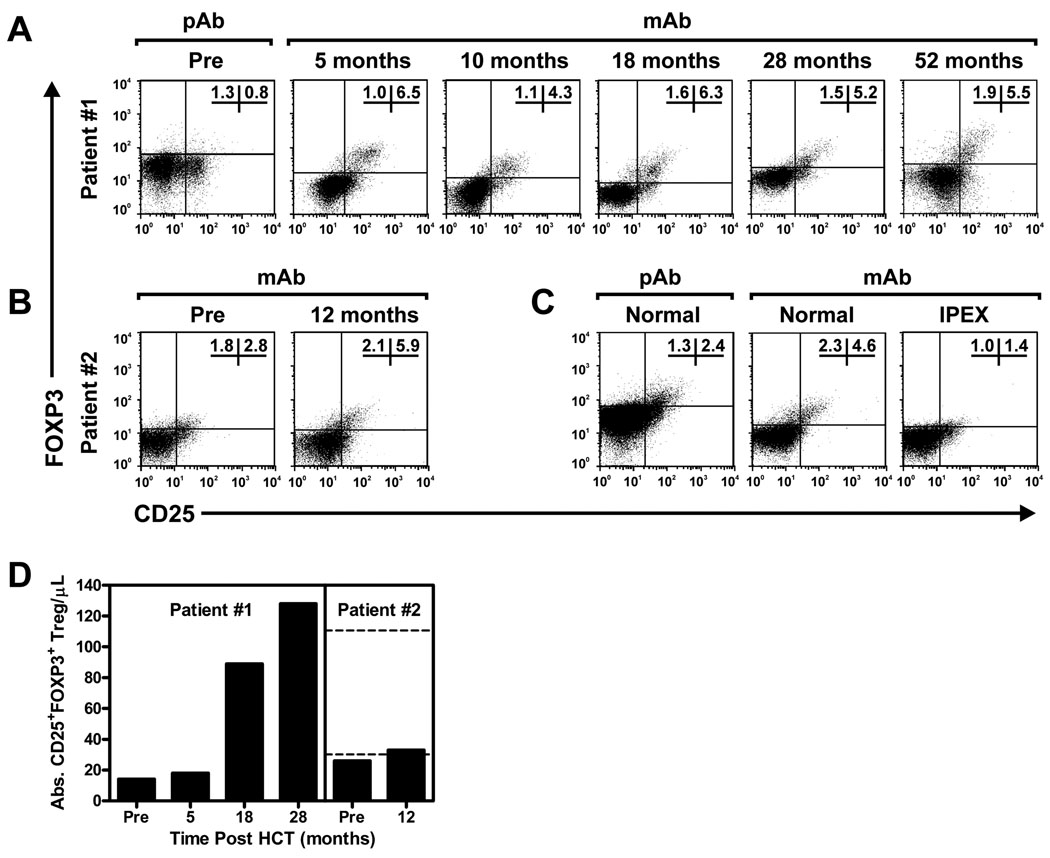
Recoveries of lymphocyte subsets and function following HCT are shown in Fig 1, B,C. Both patients had normal or above normal T and B lymphocyte counts for age at the time of last follow up. At 4 years after HCT, patient 1 has normal immunoglobulin production (IgG 817, IgM 166, IgA 99, and IgE 13) and high level protective immunization responses to hepatitis B surface antigen, haemophilus influenza Type B (HiB), pneumococcus, and tetanus toxoid off IVIG. Patient 2 tapered off all immunosuppression around 1 year after HCT, however, remains on IVIG until B cell function is evaluated. Flow cytometric evaluation demonstrated normal percentages of FOXP3 expressing regulatory T cells in the CD4+ T-cell population for both patients (Fig 2).
FIG 2. HCT restores a long-term, stable population of CD4+CD25+FOXP3+ regulatory T cells in IPEX syndrome.
A, B, C) Flow cytometry plots showing CD25 and FOXP3 expression in CD4+ T cells prior to, and at various time points after, successful HCT in patient 1 (Panel A) and in patient 2 (Panel B). Pre-transplant samples in patient 1 were stained with a rabbit polyclonal antibody (pAb) to FOXP3 as previously described.(15) For comparison, PBMC’s from a normal control individual stained with this polyclonal antibody are shown to the left in Panel C. All other samples were stained with a mouse monoclonal (clone 259D) antibody (mAb) to FOXP3 as indicated in Patients and Methods. For comparison, mAb staining of PBMC’s from a normal control individual and an IPEX patient with a FOXP3 mutation known to abrogate FOXP3 protein expression are shown in (Panel C). D) Absolute numbers of CD4+CD25+FOXP3+ Tregs are shown pre- and post-transplant in both patients. Dashed lines indicate the normal range for adults (mean ± 2 SD). Normal age-specific ranges for younger children are not yet well established.
DISCUSSION
The first case reports of the treatment of IPEX syndrome with HCT provided proof of principle that engraftment of normal marrow could restore immune homeostasis and ameliorate the disease symptoms caused by dysregulated autoimmune lymphocytes.(5;6) Unfortunately, these patients died of transplant related causes attributed in part to toxicity from intensive conditioning. Patients with IPEX understandably have a higher risk for poor outcome with conventional HCT regimens, since organ dysfunction and infections invariably are present at diagnosis and may not be controllable prior to HCT. The advent of new regimens designed to reduce organ toxicity may hold promise for the treatment of patients with IPEX disease; however, the absence of T regulatory cells theoretically creates a milieu of unregulated alloreactivity, and consequently a higher risk for graft rejection. To address this concern, we modified the nonmyeloablative regimen, previously reported by the Seattle group, to include a dose of 4 Gy TBI compared to the “standard” 2 Gy.(7;17–19)
Our experience suggests that mortality can be reduced using a non-toxic conditioning regimen, akin to the 2007 report by Rao and colleagues that described results with a different nonmyeloablative regimen. Bone marrow was transplanted into four patients with IPEX syndrome following conditioning with alemtuzumab (Campath 1-H), fludarabine, and melphalan. Mixed (n=2) or full (n=2) donor engraftment was established in all patients, who survived 6–25 months after HCT.(20) Others have also reported encouraging results with disease response in patients with both mixed and full donor chimerism using reduced intensity approaches that incorporate T-cell depleting agents.(21–23) Although effective in establishing engraftment, the long acting T-cell depleting agent alemtuzumab has been associated with increased risk of infection and may not be suitable in IPEX syndrome, when disseminated opportunistic infections are established before HCT. Our regimen of fludarabine and low-dose TBI shows promise as an acceptable, non-T-cell depleted alternative. Taken together, these results support the use of nonmyeloablative HCT for treatment of this rare life-threatening disorder.
In IPEX syndrome, the autoimmune manifestations are caused by absence of CD4+CD25+ FOXP3+ regulatory T cells (Tregs) which are important for sustaining self-tolerance. The mechanisms by which Tregs control autoimmune reactions without dampening protective immune responses to pathogens are not completely understood, yet it is clear that absence of Tregs results in severe autoimmune-mediated organ dysfunction. Tregs arise from pluripotent hematopoietic stem cells that differentiate into lymphoid progenitors, which then undergo selection in the thymus. Since FOXP3 expression is limited to lymphoid tissue, it is reasonable to assume that transplantation of normal bone marrow will ultimately generate Tregs. Indeed normal numbers of FOXP3 expressing CD4+CD25+ cells were observed in both patients, with full and mixed chimerism, respectively.
In our study, one patient had stable full donor multi-lineage engraftment following G-CSF mobilized PBSC infusion, and the other had stable mixed chimerism following bone marrow infusion. The long term engraftment potential of this approach in patients with IPEX syndrome is not entirely known. To date, our experience in patients with other primary immunodeficiency disorders using this approach has demonstrated that the majority of patients who reject the graft do so within the first month after HCT. Whether there will be late graft rejection or a decrease in donor engraftment to the point of inadequate disease response is not known in these patients and therefore longer follow up is needed.
The degree of donor chimerism required for disease response in patients with IPEX is also not entirely clear. Several case reports have demonstrated resolution of many of the manifestations of IPEX disease in patients with either mixed or full donor chimerism.(5;20–24) Some have reported that even low-level donor chimerism or donor chimerism only in the Treg compartment may be sufficient for achieving disease response.(5;22) Durability of this response in the setting of limited multi-lineage or single-lineage chimerism remains to be seen.
Both patients in our study had resolution of many of their underlying disease manifestations including enteropathy, anemia, failure to thrive, and propensity to infections. In contrast, the diabetes was irreversible even in the setting of full donor chimerism, likely the consequence of autoimmune-mediated islet cell destruction before HCT. Interestingly, the second patient reported by Rao et al. (2007) had improvement in diabetes, suggesting that some patients may have reversible or incomplete damage to islet cells.(20) There is not enough experience yet to judge the effect of HCT on other types of organ dysfunction in IPEX syndrome, although it is likely that the thyroid gland and other organs may also have irrevocable damage prior to HCT. Nonetheless, the ability of reduced intensity conditioning and HCT to ameliorate the most life-threatening complications of IPEX syndrome including enteropathy, failure to thrive, disseminated autoimmunity, and opportunistic infections supports the use of this therapy; particularly for this high-risk group of patients. Based on the fact that we have observed stable donor engraftment and excellent disease response in both of the reported patients, we believe that this low-toxicity approach is preferable for medically complex IPEX patients. While these pilot results demonstrate proof of principle, larger numbers of patients are needed to draw firmer conclusions.
Acknowledgements
We thank Gresford Thomas for data management; Michelle Bouvier, research nurse; the nursing and clinical staff for their dedicated care of patients; and Bonnie Larson, Helen Crawford, and Sue Carbonneau for help with manuscript preparation.
Supported in part by grants HL36444, K23 HL085288, CA15704, K08-AI063267 and N01-AI30070 from the NIH, Bethesda, MD, U.S.A., and grants from the Immunodeficiency Foundation and Jeffrey Modell Foundation.
Abbreviations
- ANC
Absolute neutrophil count
- BM
Bone marrow
- CMV
Cytomegalovirus
- CSP
Cyclosporine
- EBV
Epstein Barr virus
- FK506
Tacrolimus
- FOXP3
Forkhead Box P3
- G-CSF
Granulocyte colony stimulating factor
- GVHD
Graft versus Host Disease
- HCT
Hematopoietic Cell Transplantation
- IPEX
Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked
- MMF
Mycophenolate mofetil
- PBMC
Peripheral blood mononuclear cells
- PCR
Polymerase chain reaction
- PHA
Phytohemagglutinin
- TBI
Total body irradiation
- TREG
Regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Implications:
A low-intensity conditioning regimen associated with minimal toxicity is sufficient to establish stable, long-term engraftment in patients with IPEX syndrome following hematopoietic cell transplantation from either HLA-matched related or unrelated donors.
Financial Disclosure: The authors have no financial relationship with a biotechnology and/or pharmaceutical manufacturer that has an interest in the subject matter or materials discussed in this work.
REFERENCES
- 1.Bennett CL, Yoshioka R, Kiyosawa H, Barker DF, Fain PR, Shigeoka AO, et al. X-Linked syndrome of polyendocrinopathy, immune dysfunction, and diarrhea maps to Xp11.23–Xq13.3. Am J Hum Genet. 2000;66(2):461–468. doi: 10.1086/302761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wildin RS, Freitas A. IPEX and FOXP3: clinical and research perspectives. J Autoimmun. 2005;25 Suppl.:56–62. doi: 10.1016/j.jaut.2005.04.008. (Review). [DOI] [PubMed] [Google Scholar]
- 3.Taddio A, Faleschini E, Valencic E, Granzotto M, Tommasini A, Lepore L, et al. Medium-term survival without haematopoietic stem cell transplantation in a case of IPEX: insights into nutritional and immunosuppressive therapy. Eur J Pediatr. 2007;166(11):1195–1197. doi: 10.1007/s00431-006-0395-6. [DOI] [PubMed] [Google Scholar]
- 4.Powell BR, Buist NR, Stenzel P. An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr. 1982;100(5):731–737. doi: 10.1016/s0022-3476(82)80573-8. [DOI] [PubMed] [Google Scholar]
- 5.Baud O, Goulet O, Canioni D, Le Deist F, Radford I, Rieu D, et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344(23):1758–1762. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 6.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39(8):537–545. doi: 10.1136/jmg.39.8.537. (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burroughs LM, Storb R, Leisenring WM, Pulsipher MA, Loken MR, Torgerson TR, et al. Intensive postgrafting immune suppression combined with nonmyeloablative conditioning for transplantation of HLA-identical hematopoietic cell grafts: results of a pilot study for treatment of primary immunodeficiency disorders. Bone Marrow Transplant. 2007;40:633–642. doi: 10.1038/sj.bmt.1705778. [DOI] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, Klingemann H-G, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 9.Sullivan KM, Agura E, Anasetti C, Appelbaum FR, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 10.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. Diagnosis and Staging Working Group report. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Mielcarek M, Martin PJ, Leisenring W, Flowers MED, Maloney DG, Sandmaier BM, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102(2):756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 12.Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 13.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063–4071. [PubMed] [Google Scholar]
- 14.Fletcher MA, Urban RG, Asthana D, Walling J, Friedlander A, Page JB. Lymphocyte proliferation. In: Rose NR, De Macario EC, Folds JD, Lane HC, Nakamura RM, editors. Clinical Diagnosis and Management by Laboratory Methods. Washington,DC: ASM Press; 1997. pp. 313–319. [Google Scholar]
- 15.Gavin MA, Torgerson TR, Houston E, deRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. PNAS. 2006;103(17):6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi I, Shiari R, Yamada M, Kawamura N, Okano M, Yara A, et al. Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX) J Med Genet. 2001;38(12):874–876. doi: 10.1136/jmg.38.12.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 18.Sorror ML, Maris MB, Sandmaier BM, Storer BE, Stuart MJ, Hegenbart U, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23(16):3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 19.Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin lymphoma. J Clin Oncol. 2008;28(2):211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 20.Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J, et al. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood. 2007;109(1):383–385. doi: 10.1182/blood-2006-05-025072. [DOI] [PubMed] [Google Scholar]
- 21.Zhan H, Sinclari J, Adams S, Cale CM, Murciano D, Perroni L, et al. Immune reconstitutioin and recovery of FOXP3 (forkhead box P3)-expressing T cells after transplantation for IPEX (immune dysregulation polyendocrinopathy, enteropathy, x-linked) syndrome. Pediatrics. 2008;121(4):e998–e1002. doi: 10.1542/peds.2007-1863. [DOI] [PubMed] [Google Scholar]
- 22.Seidel MG, Fritsch G, Lion T, Jurgens B, Heitger A, Bacchetta R, et al. Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood. 2009;113(22):5689–5691. doi: 10.1182/blood-2009-02-206359. [DOI] [PubMed] [Google Scholar]
- 23.Dorsey MJ, Petrovic A, Morrow MR, Dishaw LJ, Sleasman JW. FOXP3 expression following bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Immunol Res. 2009;44(1–3):179–184. doi: 10.1007/s12026-009-8112-y. [DOI] [PubMed] [Google Scholar]
- 24.Mazzolari E, Forino C, Fontana M, D'Ippolito C, Lanfranchi A, Gambineri E, et al. A new case of IPEX receiving bone marrow transplantation. Bone Marrow Transplant. 2005;35(10):1033–1034. doi: 10.1038/sj.bmt.1704954. [DOI] [PubMed] [Google Scholar]
- 25.Den Dunnen JT, Antonarakis SE. Nomenclature for the description of human sequence variations. Hum Genet. 2001;109(1):121–124. doi: 10.1007/s004390100505. [DOI] [PubMed] [Google Scholar]