Abstract
Cu,Zn, superoxide dismutase (SOD1) is a ubiquitous enzyme localized in multiple cellular compartments, including mitochondria, where it concentrates in the intermembrane space (IMS). Similar to other small IMS proteins, the import and retention of SOD1 in the IMS is linked to its folding and maturation, involving the formation of critical intra- and intermolecular disulfide bonds. Therefore, the cysteine residues of SOD1 play a fundamental role in its IMS localization. IMS import of SOD1 involves its copper chaperone, CCS, whose mitochondrial distribution is regulated by the Mia40/Erv1 disulfide relay system in a redox-dependent manner: CCS promotes SOD1 maturation and retention in the IMS. The function of SOD1 in the IMS is still unknown, but it is plausible that it serves to remove superoxide released from the mitochondrial respiratory chain. Mutations in SOD1 cause familial amyotrophic lateral sclerosis (ALS), whose pathologic features include mitochondrial bioenergetic dysfunction. Mutant SOD1 localization in the IMS is not dictated by oxygen concentration and the Mia40/Erv1 system, but is primarily dependent on aberrant protein folding and aggregation. Mutant SOD1 localization and aggregation in the IMS might cause the mitochondrial abnormalities observed in familial ALS and could play a significant role in disease pathogenesis. Antioxid. Redox Signal. 13, 1375–1384.
Introduction
The free radical scavenger Cu,Zn superoxide dismutase (SOD1) is one of the first lines of defense against oxidative damage. It is an abundant cytosolic protein but is also present in the mitochondrial intermembrane space (IMS). For its activity, SOD1 requires three posttranslational modifications: copper and zinc acquisition, intramolecular disulfide bond formation, and dimerization. The copper chaperone for SOD1, CCS, is responsible for copper insertion and disulfide bond formation. CCS is also critical for modulating the localization of SOD1 in cytosol or mitochondria.
In this article, we review critically the current literature on import mechanisms of SOD1 and CCS into the IMS and the putative functions of these proteins in this mitochondrial compartment. In particular, we focus on the role of the disulfide relay system and the significance of oxidative mechanisms in dictating the partitioning of SOD1 and CCS between mitochondria and cytosol. Furthermore, we discuss the function of CCS as an oxygen sensor that determines its own cellular distribution, as well as SOD1 localization for efficient removal of superoxide in the cytosol or IMS. We also discuss the putative mechanisms underlying copper loading into CCS-SOD1 in the IMS. Last, we address the role of mitochondrial SOD1 in the context of familial amyotrophic lateral sclerosis (ALS), in which SOD1 mutations cause degeneration of motor neurons, leading to fatal paralysis.
SOD1 Structure and Function
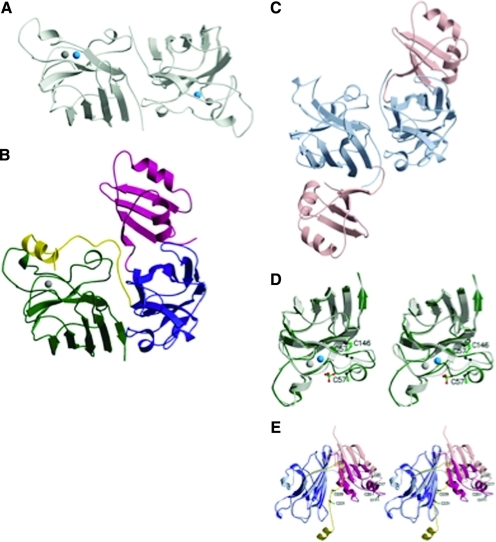
SOD1 is a ubiquitously expressed free radical scavenger that catalyzes the dismutation of superoxide to hydrogen peroxide and molecular oxygen (40). SOD1 is a relatively small protein of 154 amino acids that folds into an eight-stranded “Greek-key” β-barrel (33, 61) and binds one atom of copper and one of zinc (Fig. 1A). The copper ion, bound by histidine residues H46, H48, H63, and H120, is at the center of the catalysis reaction. Zinc, ligated by H63, H71, H80, and D83, is not necessary for enzymatic activity, but plays a structural role for the active site of the enzyme. A highly conserved intramolecular disulfide bridge is formed between C57 and C146 of SOD1 (Fig. 1D). This bond is necessary for SOD1 function and is very stable in the intracellular milieu, despite the highly reducing environment (3). The functional unit of SOD1 is a homodimer. Therefore, SOD1 maturation into the functional enzyme requires three posttranslational modifications: copper and zinc insertion, disulfide bond formation, and dimerization, all of which contribute significantly to SOD1 stability. The zinc-insertion mechanism is practically unknown, but it is likely that zinc transporters are needed because intracellular zinc concentration is tightly regulated (44). Copper insertion and oxygen-dependent disulfide bridge formation are facilitated by the copper chaperone for SOD1, CCS (Fig. 1C and E) (14, 21). Human CCS is a 274-amino acid protein that contains three domains; domain I has a classic CxxC motif for copper binding, which is not strictly necessary for protein function, whereas domain I as a whole is required for activity (35). Domain II has high sequence and structural similarities to SOD1 and is required for the docking of the two proteins (Fig. 1B) (33, 54). Domain III has a CxC copper-binding motif at the C terminal of CCS and contains the C229 residue involved in a transient disulfide bond with SOD1 (Fig. 1E) (33). Copper bound to solvent-exposed sulfur ligands of CCS is transferred to SOD1 (36). The transient intermolecular disulfide link between C229 of CCS and C57 of SOD1 is resolved by disulfide isomerization, resulting in the C57-C146 intramolecular disulfide bond in SOD1 (33). Whether copper insertion and disulfide oxidation happen as concurrent or sequential events is not known.
FIG. 1.
This figure and corresponding figure legend were reprinted by permission from the Macmillan Publishers Ltd; Nature Structural Biology (33), copyright (2001). (A) The yeast SOD1 homodimer (PDB accession code 1SDY) viewed looking down the dimer twofold axis. The copper ion is shown as a blue sphere, and the zinc ion, as a gray sphere. (B) The heterodimer shown with the SOD1 monomer in the same orientation as in (A). The SOD1 monomer is shown in green, and the three domains of the yCCS monomer are shown in magenta (I), blue (II), and yellow (III). (C) The yCCS homodimer (PDB accession code 1QUP) shown in the same orientation as (A and B). Only domains I and II are present in this structure. Domain I is shown in pink, and domain II, in light blue. (D) Superposition of SOD1 in the heterodimer (dark green) with SOD1 in the homodimer (light green). The zinc ion is shown as a gray sphere, and the copper ion (present only in the light green monomer), as a blue sphere. Cys residues involved in disulfide formation are shown as ball-and-stick representations. (E) Superposition of yCCS in the heterodimer (domain I in magenta and domain II in dark blue) with yCCS in the homodimer (domain I in pink and domain II in light blue). The figure was generated by superposing the two domain IIs. The Cys residues from the domain I MHCXXC motif and the domain III CXC motif are shown as ball-and-stick representations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
CCS is highly efficient in promoting SOD1 activation, because its molar concentration is at least one order of magnitude less than that of SOD1 (53). However, unlike yeast SOD1, metazoan SOD1 can be activated in a CCS- and oxygen-independent manner (7, 34). For example, genetic ablation of CCS in mice results in only a partial reduction of SOD1 activity (69), because a portion of the enzyme can receive copper through alternative pathways. These alternative mechanisms of SOD1 activation are thought to depend on reduced glutathione, as demonstrated both in yeast expressing human SOD1 and in mouse cells lacking CCS (7, 69). Interestingly, the inability to acquire copper in a CCS-independent manner in Saccharomyces cerevisiae SOD1 is associated with the presence of a proline residue (corresponding to position 144 in the human sequence), which controls the redox state of the protein (34). The evolutionary reasons behind the coexistence of CCS-dependent and -independent activation of SOD1 could be explained by the variable oxygen concentration to which different cells in multicellular organisms are exposed (35).
SOD1 Import into Mitochondria
SOD1 is localized predominantly in the cytosol, but a portion of it also is found in mitochondria and in other cellular compartments, including the nucleus (8) and the endoplasmic reticulum (32, 65). In mitochondria, SOD1 is concentrated in the IMS (26, 28, 39, 43), but it also is found in the matrix (68) and on the outer membrane (OM) (37, 46, 67).
Only recently, the mechanisms of SOD1 mitochondrial import have begun to unravel. Proteins destined for the mitochondrial matrix, IMS, inner membrane (IM), or OM, are imported by diverse mechanisms that are dictated by their targeting signals (MTS), which are typically N-terminal or internal amino acid motifs (24, 42, 48). However, some mitochondrial proteins, such as apocytochrome c, lack a recognizable MTS. Similarly, SOD1 and CCS have no known MTS or internal amino acid motif. Studies in yeast demonstrated that the SOD1 apoprotein is able to cross the OM only in its nonmetallated and disulfide reduced state. On import, the maturation of SOD1, facilitated by mitochondrially localized CCS, traps the protein in the IMS (19). This process of protein import and retention is common to many IMS proteins, whose mitochondrial import depends on trapping by folding (24). SOD1 IMS import was found to be subject to regulation by the disulfide relay system, because downregulation of Erv1 in yeast resulted in decreased SOD1 import (41). In the case of SOD1, it was proposed that CCS operates similar to Mia40 by promoting oxidative folding and cofactor acquisition (24, 41).
We addressed the mechanisms of SOD1 mitochondrial import in mammalian cells and showed that, also in this system, SOD1 mitochondrial localization depends on CCS-mediated oxidative folding (31). We proposed that, similar to other IMS proteins, such as Tim10 and COX19 (5), CCS import is dependent on the Mia40/Erv1 system, which is linked to the function of the mitochondrial respiratory chain (31). CCS does not contain a typical twin Cx3C or Cx9C motif required for import by the Mia40/Erv1 system. However, like Erv1, which contains two Cx2C repeats and is also imported by the disulfide relay system (63), the CxxC or CxC motifs in domains I and III of CCS, respectively, could be involved in the import by this system (35).
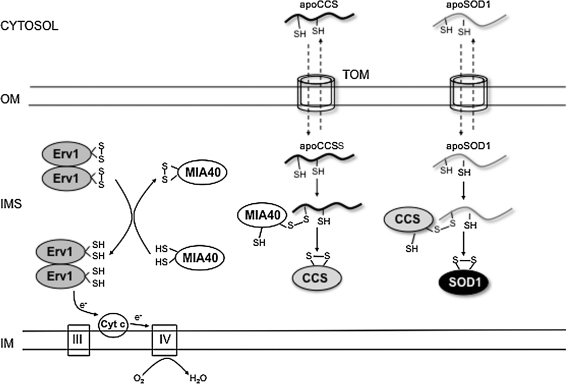
In yeast, mixed disulfide bonds between Ccs1 and Mia40 have been demonstrated (50). Based on this evidence, it was proposed that SOD1 import indirectly depends on Mia40/Erv1 and on respiratory chain function, through the interaction with mitochondrial CCS (31, 50). According to this proposed mechanism, apoCCS enters the IMS, where it forms a disulfide intermediate with Mia40. Mia40 interaction with CCS results in the formation of intramolecular disulfide bonds in CCS, which trap it in the IMS. In turn, mature CCS interacts with incoming apoSOD1 and promotes its folding and metallation, trapping SOD1 in the IMS. Erv1 function is to oxidize Mia40 and make it available for interactions with its substrates. Because Erv1 is oxidized through interaction with oxidized cytochrome c, the retention of CCS and SOD1 in the IMS is indirectly dependent on mitochondrial respiratory chain function (Fig. 2). In mammalian cells, this dependence was tested by using respiratory chain inhibitors: antimycin A (complex III inhibitor) increases the amount of oxidized cytochrome c, causing a sequential buildup of oxidized Erv1 and Mia40 and enhancing the efficiency of CCS and SOD1 import (29). Oxidative folding of SOD1 is critical for its retention in the IMS, because loss of the cysteines involved in the intramolecular disulfide bond (C57 or C146) results in a lack of mitochondrial SOD1 (19, 31).
FIG. 2.
Import of SOD1 and CCS into mitochondrial IMS. SOD1 and CCS are imported through the translocator of the outer membrane (TOM) into the mitochondrial IMS in their unfolded apoforms. In the IMS, apoCCS forms intermolecular disulfide bonds with the import receptor, Mia40, which results in the formation of CCS intramolecular disulfide bond that traps the protein in the IMS. Reduced Mia40 is reoxidized by the sulfhydryl oxidase, Erv1, which then donates its electron to cytochrome c to return to the oxidized state. SOD1 interacts with oxidized CCS through a transient intermolecular disulfide bond, which promotes the formation of the intramolecular disulfide, trapping SOD1 in the IMS. The resulting reduced CCS can then be reoxidized by the Mia40/Erv1 system.
The two other cysteine residues present in human SOD1, C6 and C111, also play a role in mitochondrial SOD1 localization, because their absence results in reduced mitochondrial SOD1 in mammalian cells (17, 31). Although the mechanisms whereby these residues modulate SOD1 mitochondrial import or retention are not fully understood, they are likely to involve disulfide-mediated interactions either with SOD1 itself or with other proteins of the IMS. These mechanisms may even be species specific, because rodent SOD1, which contains a serine instead of a cysteine at position 111, is imported and retained in mitochondria both in vivo (43) and in cultured cells (31).
Copper Insertion into Mitochondrial SOD1
Mitochondrial SOD1 and cytochrome c oxidase (COX), the final electron acceptor of the electron-transport chain, require copper for their activities. Because copper has high redox reactivity and could be toxic to cells, intracellular copper trafficking requires a well-coordinated and controlled network of copper-binding transporters/permeases, cupric reductases, and chaperones. Both cytosolic and mitochondrial SOD1 receive copper through their interactions with CCS, but, because both proteins enter mitochondria in their apo-form devoid of metals, the copper source for mitochondrial SOD1 is likely to reside inside mitochondria. A pool of copper in the mitochondrial matrix is available for mitochondrial metalloenzymes, bound by low-molecular-weight nonproteinaceous ligands (10, 11).
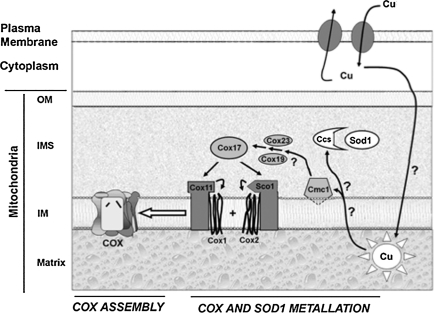
The mechanisms and players involved in copper trafficking across mitochondrial membranes, as well as those involved in copper transfer from the matrix pool to the IMS, are still open questions. Recently, a yeast protein embedded in the IM, Cmc1p, was shown to be critical for the distribution of copper to COX and SOD1 (27). It was shown that ablation of Cmc1p expression results in decreased COX assembly and activity and a concomitant increase in SOD1 activity in mitochondria. The increase in SOD1 enzymatic activity was not due to increased protein content, and therefore presumably linked to enhanced copper delivery to the enzyme. It was proposed that Cmc1p delivers copper to COX through its copper chaperones (cox17 and cox19, and Sco proteins), and that it may draw from the same copper pool in the IMS that CCS uses (27) (Fig. 3). Further work is needed to understand better the mitochondrial copper homeostasis, and it is likely that new molecular players soon will be identified. It is tempting to speculate that intramitochondrial copper partitioning among various cupro-enzymes could be regulated by redox-sensitive mechanisms. Such mechanisms may allow a coordinated regulation of protein import and copper distribution.
FIG. 3.
Proposed pathway of copper distribution to the mitochondrial cupro-enzymes, COX and SOD1 (27). This scheme was kindly provided by Drs. Horn and Barrientos (University of Miami School of Medicine). Copper is translocated across the plasma membrane by specific transporters. Intracellular chaperones are presumably involved in delivering copper to mitochondria; however, the mechanisms of copper translocation across two layers of membranes to the matrix are still unknown. From the matrix pool, copper is translocated to the IMS, where it is distributed between Cmc1p for delivery to the COX copper chaperone system (Cox23, Cox19, Cox17, Sco1 and Sco2), which then loads it into COX subunits 1 and 2, and CCS for delivery to SOD1. Several players and steps in this complex mitochondrial copper-partitioning system, indicated by question marks in the scheme, remain to be elucidated. Nevertheless, this proposed pathway suggests the possibility that CCS-SOD1 and COX17-COX may compete for the same pool of copper in mitochondria.
Moreover, a deeper knowledge of copper import, storage, and distribution mechanisms could help us to understand mitochondrial defects in the context of disease involving cupro-proteins, such as familial ALS.
SOD1 Function in the IMS
Mitochondria are primary producers of cellular free radicals, as an unavoidable byproduct of oxidative metabolism (59, 64). SOD2 (MnSOD) removes superoxide released into the mitochondrial matrix, where it resides. However, superoxide can also be released directly into the IMS (23). Because SOD1 localized in the IMS provides protection from superoxide in this mitochondrial compartment, the ability to distribute SOD1 in cytosol or mitochondria may be a critical function of cells coping with superoxide production to prevent free radical damage. Contrary to this view, one study in vitro suggests that presence of SOD1 in the IMS might promote the production of damaging free radicals (22). Conversely, in yeast, genetic ablation of SOD1 results in increased free radical damage in mitochondria (60). Despite the availability of mice devoid of SOD1, the specific impact of the lack of SOD1 on mitochondria has not yet been investigated in vivo. SOD1-knockout mice have a reduced life span and develop liver cancer (16), as well as a peripheral neuropathy (20).
To address the protective role of mitochondrial SOD1 against free radical damage in mammalian cells, it would be logical to investigate the effects of expressing transgenic SOD1 specifically targeted to the mitochondrial IMS, in cells and in mice lacking endogenous SOD1. The rationale of this experiment would be to test whether the deleterious effects caused by the lack of SOD1 could be prevented by the exclusive presence of SOD1 in mitochondria. These studies are ongoing and will help us to determine the functional impact of SOD1 localized in the mitochondrial IMS.
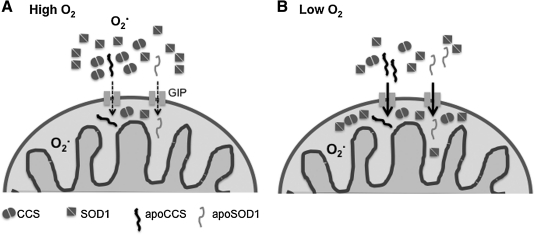
Because the activity of the Mia40/Erv1 system was shown to be dependent on oxygen concentration, it was proposed that the disulfide relay might work as an oxygen sensor for protein import in the IMS, whereby high oxygen concentration would promote SOD1 import in mitochondria (4). In our studies, we found that oxygen concentration does play a role in CCS and SOD1 intracellular distribution, but contrary to the proposal, CCS mitochondrial import is upregulated by low oxygen concentration and downregulated by high oxygen concentration (31). We speculated that high oxygen concentrations promote oxidative folding of CCS in the cytosol, which modulate cellular distribution of SOD1 (Fig. 4). A potential explanation of this phenomenon is that when oxygen tension is severely decreased, such as during severe hypoxia or anoxia, SOD1 accumulates in mitochondria in preparation for a burst of free radical production after reperfusion. This is a well-known event related to the accumulation of substrates unused by the mitochondrial respiratory chain in the absence of oxygen. When oxygen again becomes available, the accumulated substrates push ROS production from the respiratory chain to the point that the antioxidant defenses may not be able to protect the cell from oxidative damage (6).
FIG. 4.
Oxygen regulation of SOD1 and CCS import into the mitochondrial IMS. Both SOD1 and CCS cross the outer membrane through the general import pore (GIP) in their apo forms. In the IMS, mitochondrial CCS promotes folding and retention of SOD1. Under high-oxygen conditions (A), CCS folding is facilitated in the cytosol, where it enhances SOD1 folding and maturation. This allows cytosolic SOD1 to be effective in removal of superoxide produced outside of mitochondria. Under lower oxygen conditions (B), CCS and SOD1 maturation are delayed, and more apo-CCS and apo-SOD1 can enter mitochondria to remove superoxide released in this cell compartment.
Therefore, the proposed mechanism underlying oxygen-dependent mitochondrial IMS localization of SOD1 may contribute to protect the cells from the extremely damaging production of ROS generated by the mitochondrial respiratory chain on the outer side of the IM in the IMS. Conversely, in normoxia or hyperoxia, cytosolic CCS favors rapid SOD1 maturation in the cytosol, which could help to remove superoxide generated by extramitochondrial sources, such as NADPH-oxidases (9).
Finally, it cannot yet be excluded that SOD1 may serve alternative functions in mitochondria, in addition to superoxide dismutation. Several notable examples exist of IMS proteins with well-characterized dual roles, such as cytochrome c, which is involved in electron transfer and apoptosis, or the apoptosis-inducing factor (AIF), which is involved in respiratory chain complex I assembly and apoptosis (49, 66). At the moment, we do not have direct evidence of such dual function for SOD1, but it would be interesting to explore this possibility by expressing an inactive SOD1 protein targeted to the IMS of SOD1-deficient cells to test whether it can rescue some of the toxicity, unrelated to the dismutase activity.
From a technical point of view, the challenge will be to engineer a dismutase activity–deficient SOD1, without causing the protein to become misfolded and toxic.
Mutant SOD1 and Mitochondrial Dysfunction in Familial ALS
ALS is a fatal, paralytic disease resulting from the selective degeneration of upper and lower motor neurons in the spinal cord and brain. Although the vast majority of ALS cases are sporadic with no known etiology, mutations in SOD1 account for 20% of familial forms of ALS (51). To date, more than 140 different mutations throughout the whole protein have been linked to familial ALS (for a complete list, see http://alsod.iop.kcl.ac.uk). It is clear that SOD1-linked familial ALS is caused by a toxic gain of function of the mutant protein, rather than a loss of function, because enzymatic activity is not a disease determinant (mutant proteins with or without dismutase activity both cause ALS), and the disease is transmitted in an autosomal dominant manner. It is likely that multiple mechanisms and targets of mutant SOD1, which are still under investigation, lead to the selective death of motor neurons (47, 52). Most known SOD1 mutations cause protein misfolding and aggregation, which is thought to play a major role in familial ALS pathogenesis (30).
A large body of evidence of mitochondrial abnormalities, morphologic and bioenergetic, are observed in human familial and sporadic ALS, as well as in many of the cell-culture and rodent models of SOD1-linked ALS (25, 55). Like the wild-type protein, familial ALS-linked mutant SOD1 also accumulates in mitochondria, where it is thought to contribute directly to disease pathogenesis.
In our studies, we observed that an enzymatically inactive mutant SOD1 (G85R) was unresponsive to the physiologic modulations of import by oxygen and the Mia40/Erv1 system, and its localization in mitochondria was dictated mainly by formation of aggregates. Another mutant SOD1 protein (G93A), which is enzymatically active, only partially followed the physiologic modulation of import, and also formed aggregates in mitochondria (Fig. 5) (31). Expression of mutant forms of SOD1 in motor neuron–like NSC34 cells and transgenic mice results in disulfide-linked oligomeric forms of SOD1 that are trapped in mitochondria (15, 17). It was proposed that SOD1 aggregation involves disulfide cross-linking at C111, and substitution of C111 to serine reduces the formation of large-molecular-weight aggregates (12).
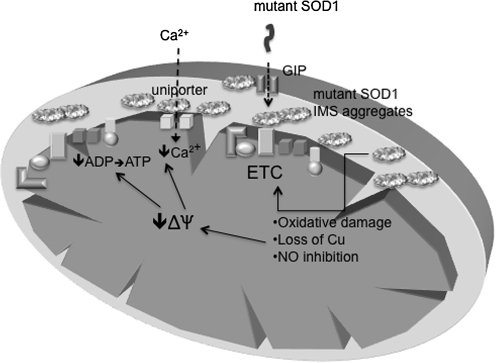
FIG. 5.
Aggregation of mutant SOD1 in mitochondria and its potential pathogenic consequences. Mutant SOD1 enters the IMS through the general import pore (GIP) and forms disulfide-linked oligomeric aggregates. Such aggregates have been proposed to cause mitochondrial damage in different ways, including free radical production, NO generation, and copper depletion. These events lead to mitochondrial electron-transfer chain (ETC) dysfunction and loss of membrane potential (ΔΨ), which results in decreased ATP synthesis and impaired calcium uptake through the calcium uniporter.
However, we found that the decrease in detergent-insoluble oligomeric forms of mutant SOD1, determined by the C111S substitution, did not affect mitochondrial localization of mutant SOD1 (31). We also found that a non–ALS-linked mutant SOD1 protein that exists solely in detergent-insoluble oligomers is degraded in the cytoplasmic milieu, but it is accumulated in the form of aggregates in mitochondria, where it is protected from proteosomal degradation (31).
Based on these observations, aggregation of mutant SOD1 in mitochondria overrides the physiologic import regulation and might cause the mitochondrial dysfunction that contributes to cell toxicity.
The exact mechanisms whereby mutant SOD1 causes mitochondrial damage and whether mitochondrial toxicity is a primary or secondary event in disease pathogenesis are just beginning to be unraveled (Fig. 5). In NSC34 cells, familial ALS-linked mutant SOD1 in mitochondria formed disulfide-linked oligomers, caused a shift in the mitochondrial redox state, and impaired respiratory chain activities (18). In transgenic mice, overexpression of CCS increased the accumulation of mutant G93A SOD1 in mitochondria, which caused extensive mitochondrial swelling and dramatic worsening of the disease phenotype (58). Similarly, CCS overexpression worsened the disease in another wild-type–like mutant SOD1 (G37R) mouse model, but not in mice expressing G85R or L126Z SOD1 mutants, which are enzymatically defective (56). In the G93A/CCS double-transgenic mice, the mutant SOD1 toxicity was attributed to COX deficiency, caused by functional and structural damage (57, 58).
How mutant G93A SOD1 causes COX deficiency is not clear, but it could be speculated that SOD1 and COX, both cupro-enzymes, compete for the same pool of copper in mitochondria. Supporting this hypothesis, as mentioned earlier, ablation of the IMS copper-transferring protein, Cmc1p, increases levels of active SOD1 but decreases COX activity (27). A recent study in NSC34 cells expressing inducible G93A mutant SOD1 suggested that COX deficiency is due to increased nitric oxide levels, rather than copper depletion, because copper supplementation did not rescue COX activity, but nitric oxide scavengers did (2). Nitric oxide can decrease COX activity by competitively displacing oxygen or, more significantly, can damage COX directly and irreversibly through peroxynitrite (29, 45). However, this does not exclude that in vivo, where copper is tightly regulated in the intracellular milieu, COX may become defective, if accumulating SOD1 subtracts the metal from the COX copper chaperones or if it interferes with the complex mechanisms of copper regulation in the IMS.
To test directly the pathogenic role of mutant SOD1 in mitochondria, the protein has been targeted specifically to different mitochondrial compartments in various neuronal cell-culture systems. Matrix localization of mutant SOD1 through a cleavable MTS caused apoptotic cell death in cultured neuronal cells (62). In our laboratory, we targeted mutant SOD1 to the IMS of NSC34 cells by using the cleavable yeast cytochrome b2 MTS. This resulted in mitochondrial fragmentation and alterations of mitochondrial dynamics in the neurites, as well as increased sensitivity to metabolic and oxidative stress conditions (38).
Similarly, mitochondrial localization of mutant SOD1 targeted by cytochrome c1 MTS in NSC34 cells caused mitochondrial morphologic abnormalities and reduced cell viability (13).
Taken together, this evidence suggests that mutant SOD1 localized in mitochondria plays a direct cytotoxic role in familial ALS. However, it will be important in the future to validate these findings in vivo, in mouse models expressing the IMS-targeted mutant SOD1.
Concluding Remarks
In recent years, our knowledge of the mechanisms underlying the localization of SOD1 in mitochondria, both in health and in disease, has grown significantly. However, many areas still must be further explored. The driving force that leads SOD1 across the outer mitochondrial membrane and whether the protein establishes any specific interaction with the mitochondrial-import machinery are still unknown. It cannot be excluded that, like other nuclear-encoded mitochondrial proteins, SOD1 could be co-translationally translocated across the outer membrane (1), when translated by perimitochondrial ribosomes. Alternatively, still unidentified amino acid motifs could determine the docking of the apo-protein on the mitochondrial import pore. Because of the strong tendency of SOD1 to fold in the cytosol and its extraordinary stability once it is folded, an active participation of cytosolic chaperones must keep it unfolded and allow its mitochondrial import. However, the nature of these chaperones is still unknown.
Another area of debate is the function of SOD1 in mitochondria, because its antioxidant requirement, especially in the IMS, has not been formally demonstrated. It is expected that ongoing experiments of targeting SOD1 exclusively to the IMS in mice lacking endogenous SOD1 will help in clarifying this issue. Nevertheless, the possibility exists that SOD1 in the IMS might have additional functions, besides of dismutation of superoxide. This is a provocative speculation, which could be tested by providing the IMS of SOD1-knockout mice with a different dismutation system, which would take care of superoxide without possessing all the potential properties of SOD1.
Finally, more work must be done to demonstrate conclusively that the presence of mutant SOD1 in mitochondria causes mitochondrial dysfunction, and that this dysfunction contributes to the pathogenesis of familial ALS. The ongoing development of animal models expressing mutant SOD1 exclusively in mitochondria will help to unravel this issue. Even more important will be to understand the molecular and biochemical basis of mitochondrial defects caused by mitochondrial mutant SOD1 and to devise approaches to treating them. A beneficial effect deriving from mitochondrial therapies would strongly support the role of mitochondrial dysfunction in the pathogenesis of the disease.
Abbreviations Used
- AIF
apoptosis-inducing factor
- ALS
amyotrophic lateral sclerosis
- CCS
copper chaperone for SOD1
- COX
cytochrome c oxidase
- ΔΨ
membrane potential
- ETC
electron-transfer chain
- GIP
general import pore
- IM
inner membrane
- IMS
intermembrane space
- MTS
mitochondrial targeting sequence
- OM
outer membrane
- SOD1
Cu,Zn superoxide dismutase
- SOD2
Mn superoxide dismutase
- TOM
translocator of the outer membrane
Acknowledgments
This work is supported by grants R01-NS051419, R01-NS062055, Muscular Dystrophy Association, and the Robert Packard ALS Research Center.
References
- 1.Ahmed AU. Fisher PR. Import of nuclear-encoded mitochondrial proteins: a cotranslational perspective. Int Rev Cell Mol Biol. 2009;273:49–68. doi: 10.1016/S1937-6448(08)01802-9. [DOI] [PubMed] [Google Scholar]
- 2.Arciello M. Capo CR. Cozzolino M. Ferri A. Nencini M. Carri MT. Rossi L. Inactivation of cytochrome c oxidase by mutant SOD1s in mouse motoneuronal NSC-34 cells is independent from copper availability but is because of nitric oxide. J Neurochem. 2010;112:183–192. doi: 10.1111/j.1471-4159.2009.06441.x. [DOI] [PubMed] [Google Scholar]
- 3.Arnesano F. Banci L. Bertini I. Martinelli M. Furukawa Y. O'Halloran TV. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 4.Bihlmaier K. Mesecke N. Kloeppel C. Herrmann JM. The disulfide relay of the intermembrane space of mitochondria: an oxygen-sensing system? Ann N Y Acad Sci. 2008;1147:293–302. doi: 10.1196/annals.1427.005. [DOI] [PubMed] [Google Scholar]
- 5.Bihlmaier K. Mesecke N. Terziyska N. Bien M. Hell K. Herrmann JM. The disulfide relay system of mitochondria is connected to the respiratory chain. J Cell Biol. 2007;179:389–395. doi: 10.1083/jcb.200707123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burwell LS. Nadtochiy SM. Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll MC. Girouard JB. Ulloa JL. Subramaniam JR. Wong PC. Valentine JS. Culotta VC. Mechanisms for activating Cu- and Zn-containing superoxide dismutase in the absence of the CCS Cu chaperone. Proc Natl Acad Sci USA. 2004;101:5964–5969. doi: 10.1073/pnas.0308298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang LY. Slot JW. Geuze HJ. Crapo JD. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol. 1988;107:2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H. Song YS. Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobine PA. Ojeda LD. Rigby KM. Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J Biol Chem. 2004;279:14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 11.Cobine PA. Pierrel F. Bestwick ML. Winge DR. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J Biol Chem. 2006;281:36552–36559. doi: 10.1074/jbc.M606839200. [DOI] [PubMed] [Google Scholar]
- 12.Cozzolino M. Amori I. Pesaresi MG. Ferri A. Nencini M. Carri MT. Cysteine 111 affects aggregation and cytotoxicity of mutant Cu,Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:866–874. doi: 10.1074/jbc.M705657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cozzolino M. Pesaresi MG. Amori I. Crosio C. Ferri A. Nencini M. Carri MT. Oligomerization of mutant SOD1 in mitochondria of motoneuronal cells drives mitochondrial damage and cell toxicity. Antioxid Redox Signal. 2009;11:1547–1558. doi: 10.1089/ars.2009.2545. [DOI] [PubMed] [Google Scholar]
- 14.Culotta VC. Klomp LW. Strain J. Casareno RL. Krems B. Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 15.Deng HX. Shi Y. Furukawa Y. Zhai H. Fu R. Liu E. Gorrie GH. Khan MS. Hung WY. Bigio EH. Lukas T. Dal Canto MC. O'Halloran TV. Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elchuri S. Oberley TD. Qi W. Eisenstein RS. Jackson Roberts L. Van Remmen H. Epstein CJ. Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- 17.Ferri A. Cozzolino M. Crosio C. Nencini M. Casciati A. Gralla EB. Rotilio G. Valentine JS. Carri MT. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc Natl Acad Sci U S A. 2006;103:13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferri A. Gabbianelli R. Casciati A. Celsi F. Rotilio G. Carri MT. Oxidative inactivation of calcineurin by Cu,Zn superoxide dismutase G93A, a mutant typical of familial amyotrophic lateral sclerosis. J Neurochem. 2001;79:531–538. doi: 10.1046/j.1471-4159.2001.00558.x. [DOI] [PubMed] [Google Scholar]
- 19.Field LS. Furukawa Y. O'Halloran TV. Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J Biol Chem. 2003;278:28052–28059. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 20.Flood DG. Reaume AG. Gruner JA. Hoffman EK. Hirsch JD. Lin YG. Dorfman KS. Scott RW. Hindlimb motor neurons require Cu/Zn superoxide dismutase for maintenance of neuromuscular junctions. Am J Pathol. 1999;155:663–672. doi: 10.1016/S0002-9440(10)65162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa Y. Torres AS. O'Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO J. 2004;23:2872–2881. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldsteins G. Keksa-Goldsteine V. Ahtoniemi T. Jaronen M. Arens E. Akerman K. Chan PH. Koistinaho J. Deleterious role of superoxide dismutase in the mitochondrial intermembrane space. J Biol Chem. 2008;283:8446–8452. doi: 10.1074/jbc.M706111200. [DOI] [PubMed] [Google Scholar]
- 23.Han D. Williams E. Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann JM. Hell K. Chopped, trapped or tacked: protein translocation into the IMS of mitochondria. Trends Biochem Sci. 2005;30:205–211. doi: 10.1016/j.tibs.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Hervias I. Beal MF. Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve. 2006;33:598–608. doi: 10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- 26.Higgins CM. Jung C. Ding H. Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci. 2002;22:1–6. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn D. Al-Ali H. Barrientos A. Cmc1p is a conserved mitochondrial twin CX9C protein involved in cytochrome c oxidase biogenesis. Mol Cell Biol. 2008;28:4354–4364. doi: 10.1128/MCB.01920-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaarsma D. Rognoni F. van Duijn W. Verspaget HW. Haasdijk ED. Holstege JC. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol (Berl) 2001;102:293–305. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson J. Duchen MR. Hothersall J. Clark JB. Heales SJ. Induction of mitochondrial oxidative stress in astrocytes by nitric oxide precedes disruption of energy metabolism. J Neurochem. 2005;95:388–395. doi: 10.1111/j.1471-4159.2005.03374.x. [DOI] [PubMed] [Google Scholar]
- 30.Karch CM. Prudencio M. Winkler DD. Hart PJ. Borchelt DR. Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS. Proc Natl Acad Sci USA. 2009;106:7774–7779. doi: 10.1073/pnas.0902505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamata H. Manfredi G. Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum Mol Genet. 2008;17:3303–3317. doi: 10.1093/hmg/ddn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi H. Almer G. Yamashita S. Guegan C. Nagai M. Xu Z. Sosunov AA. McKhann GM., 2nd Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc Natl Acad Sci USA. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamb AL. Torres AS. O'Halloran TV. Rosenzweig AC. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat Struct Biol. 2001;8:751–755. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 34.Leitch JM. Jensen LT. Bouldin SD. Outten CE. Hart PJ. Culotta VC. Activation of Cu,Zn-superoxide dismutase in the absence of oxygen and the copper chaperone CCS. J Biol Chem. 2009;284:21863–21871. doi: 10.1074/jbc.M109.000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leitch JM. Yick PJ. Culotta VC. The right to choose: multiple pathways for activating copper,zinc superoxide dismutase. J Biol Chem. 2009;284:24679–24683. doi: 10.1074/jbc.R109.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H. Zhu H. Eggers DK. Nersissian AM. Faull KF. Goto JJ. Ai J. Sanders-Loehr J. Gralla EB. Valentine JS. Copper(2+) binding to the surface residue cysteine 111 of His46Arg human copper-zinc superoxide dismutase, a familial amyotrophic lateral sclerosis mutant. Biochemistry. 2000;39:8125–8132. doi: 10.1021/bi000846f. [DOI] [PubMed] [Google Scholar]
- 37.Liu J. Lillo C. Jonsson PA. Velde CV. Ward CM. Miller TM. Subramaniam JR. Rothstein JD. Marklund S. Andersen PM. Brannstrom T. Gredal O. Wong PC. Williams DS. Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Magrane J. Hervias I. Henning MS. Damiano M. Kawamata H. Manfredi G. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattiazzi M. D'Aurelio M. Gajewski CD. Martushova K. Kiaei M. Beal MF. Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 40.McCord JM. Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 41.Mesecke N. Terziyska N. Kozany C. Baumann F. Neupert W. Hell K. Herrmann JM. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell. 2005;121:1059–1069. doi: 10.1016/j.cell.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 43.Okado-Matsumoto A. Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 44.Outten CE. O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 45.Parihar A. Vaccaro P. Ghafourifar P. Nitric oxide irreversibly inhibits cytochrome oxidase at low oxygen concentrations: evidence for inverse oxygen concentration-dependent peroxynitrite formation. IUBMB Life. 2008;60:64–67. doi: 10.1002/iub.12. [DOI] [PubMed] [Google Scholar]
- 46.Pasinelli P. Belford ME. Lennon N. Bacskai BJ. Hyman BT. Trotti D. Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Pasinelli P. Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 48.Pfanner N. Wiedemann N. Meisinger C. Lithgow T. Assembling the mitochondrial outer membrane. Nat Struct Mol Biol. 2004;11:1044–1048. doi: 10.1038/nsmb852. [DOI] [PubMed] [Google Scholar]
- 49.Pospisilik JA. Knauf C. Joza N. Benit P. Orthofer M. Cani PD. Ebersberger I. Nakashima T. Sarao R. Neely G. Esterbauer H. Kozlov A. Kahn CR. Kroemer G. Rustin P. Burcelin R. Penninger JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Reddehase S. Grumbt B. Neupert W. Hell K. The disulfide relay system of mitochondria is required for the biogenesis of mitochondrial Ccs1 and Sod1. J Mol Biol. 2009;385:331–338. doi: 10.1016/j.jmb.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 51.Rosen DR. Siddique T. Patterson D. Figlewicz DA. Sapp P. Hentati A. Donaldson D. Goto J. O'Regan JP. Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 52.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 53.Rothstein JD. Dykes-Hoberg M. Corson LB. Becker M. Cleveland DW. Price DL. Culotta VC. Wong PC. The copper chaperone CCS is abundant in neurons and astrocytes in human and rodent brain. J Neurochem. 1999;72:422–429. doi: 10.1046/j.1471-4159.1999.0720422.x. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt PJ. Ramos-Gomez M. Culotta VC. A gain of superoxide dismutase (SOD) activity obtained with CCS, the copper metallochaperone for SOD1. J Biol Chem. 1999;274:36952–36956. doi: 10.1074/jbc.274.52.36952. [DOI] [PubMed] [Google Scholar]
- 55.Shi P. Gal J. Kwinter DM. Liu X. Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2010;1802:45–51. doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Son M. Fu Q. Puttaparthi K. Matthews CM. Elliott JL. Redox susceptibility of SOD1 mutants is associated with the differential response to CCS over-expression in vivo. Neurobiol Dis. 2009;34:155–162. doi: 10.1016/j.nbd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Son M. Leary SC. Romain N. Pierrel F. Winge DR. Haller RG. Elliott JL. Isolated cytochrome c oxidase deficiency in G93A SOD1 mice over-expressing CCS protein. J Biol Chem. 2008;283:12267–12275. doi: 10.1074/jbc.M708523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Son M. Puttaparthi K. Kawamata H. Rajendran B. Boyer PJ. Manfredi G. Elliott JL. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci USA. 2007;104:6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sturtz LA. Diekert K. Jensen LT. Lill R. Culotta VC. A fraction of yeast cu,zn-superoxide dismutase and its metallochaperone, ccs, localize to the intermembrane space of mitochondria. a physiological role for sod1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 61.Tainer JA. Getzoff ED. Beem KM. Richardson JS. Richardson DC. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982;160:181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi H. Kobayashi Y. Ishigaki S. Doyu M. Sobue G. Mitochondrial localization of mutant superoxide dismutase 1 triggers caspase-dependent cell DEATH in a cellular model of familial amyotrophic lateral sclerosis. J Biol Chem. 2002;277:50966–50972. doi: 10.1074/jbc.M209356200. [DOI] [PubMed] [Google Scholar]
- 63.Terziyska N. Grumbt B. Bien M. Neupert W. Herrmann JM. Hell K. The sulfhydryl oxidase Erv1 is a substrate of the Mia40-dependent protein translocation pathway. FEBS Lett. 2007;581:1098–1102. doi: 10.1016/j.febslet.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Urushitani M. Sik A. Sakurai T. Nukina N. Takahashi R. Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 66.Vahsen N. Cande C. Briere JJ. Benit P. Joza N. Larochette N. Mastroberardino PG. Pequignot MO. Casares N. Lazar V. Feraud O. Debili N. Wissing S. Engelhardt S. Madeo F. Piacentini M. Penninger JM. Schagger H. Rustin P. Kroemer G. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23:4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vande Velde C. Miller TM. Cashman NR. Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc Natl Acad Sci USA. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vijayvergiya C. Beal MF. Buck J. Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong PC. Waggoner D. Subramaniam JR. Tessarollo L. Bartnikas TB. Culotta VC. Price DL. Rothstein J. Gitlin JD. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA. 2000;97:2886–2891. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]