Abstract
In evolution, exploitative strategies often create a paradox in which the most successful individual strategy within the group is also the most detrimental strategy for the group, potentially resulting in extinction. With regards to sexual conflict, the overexploitation of females by harmful males can yield similar consequences. Despite these evolutionary implications, little research has addressed why sexual conflict does not ultimately drive populations to extinction. One possibility is that groups experiencing less sexual conflict are more productive than groups with greater conflict. However, most studies of sexual conflict are conducted in a single isolated group, disregarding the potential for selection among groups. We observed Aquarius remigis water striders in a naturalistic multigroup pool in which individuals could freely disperse amongst groups. The free movement of individuals generated variation in aggression and sex-ratio among groups, thereby increasing the importance of between-group selection compared to within-group selection. Females dispersed away from local aggression, creating more favorable mating environments for less-aggressive males. Furthermore, the use of contextual analysis revealed that individual male aggression positively predicted fitness while aggression at the group level negatively predicted fitness, empirically demonstrating the conflict between levels of selection acting on mating aggression.
Keywords: Multilevel selection, Group selection, Water strider, Sexual conflict, Altruism, contextual analysis
INTRODUCTION
Adaptations that increase relative fitness at the local scale can have detrimental consequences at a larger scale and even lead to extinction (Haldane 1932, Rankin et al. 2007, Wilson and Wilson 2007). The overexploitation of food resources illustrates this conflict, which Hardin (1968) termed the tragedy of the commons, and the framework has been widely applied to the evolution of social behaviors such as cooperation.
A conflict among levels of selection is also salient to competition for females among males (Rankin et al. 2007, Eldakar et al. 2009a, 2009b), which often results in the reduction of female fitness and therefore the reproductive output of the group as a whole. (Bauer et al. 2004, Le Galliard et al. 2005, Rankin and López-Sepulcre 2005, Sih and Watters 2005, Rankin and Kokko 2006, Eldakar et al. 2009a, 2009b). This observation was brought to the fore by Rice (1996), who demonstrated the negative fitness consequences to female Drosophila melanogaster of paternity competition among males. It has since been widely replicated across taxa (Chapman et al. 2003, Arnqvist and Rowe 2005, Rankin and López-Sepulcre 2005, Ronkainen et al. 2010). Despite these evolutionary implications, little research has addressed why sexual conflict does not drive more species to the precipice of extinction. A possible explanation is that laboratory studies do not accurately represent evolutionary counterforces to sexual conflict that exist in natural populations.
Most studies of sexual conflict are conducted in a single isolated group, precluding the potential role of a multigroup population structure in countering selection within groups. In multigroup populations, the local advantage of overexploitation by individuals can be balanced by the advantages of non-exploitation beyond the local scale (Wilson and Wilson 2007). In the case of sexual conflict, individuals within groups of males who do not harass females are more productive, compared to those within groups in which females are harassed more, despite the within-group advantage of harassment. This effect has been previously demonstrated in two species known to engage in sexual conflict. Wigby and Chapman (2005) showed in D. melanogaster, that sex-peptide deficient populations produce more offspring than populations comprised of high sex-peptide producing males, despite the selective advantage of sex-peptide production within populations. Research on the water strider Aquarius remigis has shown that aggressive males acquire more matings than less aggressive males within groups, but group productivity declines steeply with the proportion of aggressive males (Sih and Watters 2005, Eldakar et al. 2009b).
Both of these studies artificially created variation among groups and did not address the question of how the variation might arise in natural multigroup populations. One possibility is that variation arises through the contingent movement of individuals. Just as foragers leave patches when food levels are low or when they encounter a predator, individuals can be expected to leave patches in response to harsh social environments (Pepper 1997, Wilson and Dugatkin 1997, Pepper and Smutts 2002, Aktipis 2004).
In the case of sexual conflict, the distribution of females amongst groups can be greatly influenced by the distribution of highly aggressive males (Bauer et al. 2004, Sih and Watters 2005, Eldakar et al. 2009a, 2009b, Turlure and Van Dyck 2009). Recently, Eldakar et al. (2009a) demonstrated this in A. remigis by measuring the fitness consequences of mating aggression in an isolated group vs. a more naturalistic multigroup population in which individuals could freely disperse. Eldakar et al. (2009a) demonstrated that selection strongly favored aggressive mating within isolated groups; however, in the multigroup population, the free movement of individuals amongst groups favored reduced aggression. Female dispersal created distribution patterns in which females clustered around less aggressive males, providing a favorable local sex ratio, while leaving more aggressive males with male-biased sex ratios and reduced mating opportunities.
The natural habitat of the water strider A. remigis provides a model system to investigate sexual conflict in multigroup populations. A remigis are well known to engage in mating aggression and live in a multigroup population structure imposed by the alternating pools and riffles of moderately flowing streams. Aggressive males actively pursue females, leaping onto their backs and initiating an energetic battle in which the female attempts to escape (Arnqvist 1997). Males differ widely in their degree of aggression and individual differences are stable across time and most environmental conditions (Sih and Kruppa 1995, Kruppa and Sih 1998, Eldakar et al. 2009b). The presence of a spectrum of male behaviors in natural populations provides a hint that the local advantage of aggressive tactics within groups is not the only selective force at work.
Building on the findings of Eldakar et al. (2009a), we measured the effect that individual dispersal has on the subjectively experienced environment of males based on their aggressive phenotype (i.e. the unique environment experienced by each individual). Through measuring the spatial heterogeneity arising from dispersal we can better understand the costs and benefits of aggressive mating in a multigroup population. Here we used a similar experimental multigroup pool, with the addition of a top mounted camera, to track the movement of individuals in response to their social environment as well as the spatial distribution of individuals. We predicted that the free-movement of females would result in individual males experiencing different subjective sex ratios observable at multiple spatial scales and attenuating the mating success of aggressive males. We predicted (a) female dispersal away from groups with aggressive males would create more favorable sex ratios and greater mating opportunities in less aggressive groups. This will result in (b) higher aggression males experiencing more male-biased groups as well as (c) experience fewer females within groups than less aggressive males. In regards to females, we predicted (d) females would preferentially associate with less aggressive males, such that the average aggression score of males encountered by females would be less than the population average. These distribution patterns are expected to influence male mating success at multiple levels (Sih and Watters 2005, Eldakar et al. 2009a, 2009b) and produce the same unimodal relationship between male aggression and mating success as previously demonstrated by Eldakar et al. (2009a). Using contextual analysis to partition selection into the within and between group components, we expected individual male aggression score to positively predict mating success while his experience of aggression at the group level will negatively predict mating success.
METHODS AND MATERIALS
An experimental multigroup population was created using a large wading pool (3m×1.3m) with six sub-pools in the center and a current along the periphery. The current was created with adjustable water pumps and the sub-pools were created with dividers. A central divider bisected the pool longitudinally with two additional dividers perpendicular to the central divider creating a total of 6 equal-sized pools. Dividers remained open 10cm from the pool periphery allowing strider movement among the sub-pools via the peripheral current. Each sub-pool was supplied with a foam raft for resting. Striders were fed small crickets, Acheta domesticus (<1cm) every other day ad libitum.
72 (36 male, 36 female) adults were collected from White Clay Creek at the Stroud Water Research Center in Avondale PA and placed in the experimental pool. Striders were individually marked with enamel paint and observed for mating behavior and mating success for a period of 10 days. Each individual was observed in random order for five, five-minute focal sampling periods, totaling 25 minutes (Martin and Bateson 1993). Behavioral data taken for males are described in table 1. Lunge-ats, lunge-jump-ons and jump-ons were considered to represent increasing levels of aggression (as per Wilcox and Ruckdeschel 1982, Eldakar et al. 2009b). Behavioral data taken for females consisted of recording how many times a female was the recipient of the male behaviors described in table 1, in addition to pool switches and the number strides taken. Using the average occurrences of lung-ats, jump-ons and mating attempts, we calculated an aggression index for each male as by Eldakar et al. (2009b). Dispersal tendency was considered as the average number of pool switches performed by an individual during focal sampling. During periods of observation, all successful matings by other males in addition to the focal individual were also recorded.
Table 1.
Ethogram of observed behaviors during focal observations.
| Classification | Definition |
|---|---|
| lunge-at | Donor strider quickly propels itself forward towards recipient strider, possibly making contact, but not ending up on top of the recipient strider’s body. |
| lunge-jump-on | Donor strider quickly propels itself forward towards recipient strider, ending up partially on top of the recipients body. |
| jump-on | Donor strider jumps off the water surface to land on top of the recipients body without traversing the space between the two striders. |
| mating attempt | A male strider lunges or jumps on a female strider and attempts to hold himself on the female’s back while trying to insert his penis into the female’s spermatheca. The female eventually shakes the male off during her pre-copulatory struggle. |
| Mating | A mating in which a female ceases her pre-copulatory struggles. |
| Dispersal tendency | The number of times an individual changed sub-pools. |
| Strides | The number of times an individual propells forward by use of the mesothoracic legs. |
Following the 10 days of behavioral observations, a 12 megapixel digital camera was mounted above the pool facing the water surface in order to track the movement of individuals. Photos were taken in five-minute intervals (starting at time zero) for an hour randomly chosen between 12-3pm in which striders peaked in activity. Photos continued for 10 consecutive days, totaling 130 photos. Sub-pool level data obtained from the photos included calculating the sex-ratio and the average aggression score of the males within each of the 6 sub-pools for each time period. Data obtained for individual males included identifying his sub-pool location (of the 6), the sex-ratio and average male aggression of his local sub-pool and sex of the nearest strider. Sex of the nearest strider was not considered if the nearest strider was further than 10 cm from the focal individual. The mean sub-pool sex-ratio experienced by each male—referred to as his sub-pool subjective frequency of females—indicated his quality of sub-pool mating environment throughout photo sampling. The mean sex-ratio of the nearest strider—referred to as his immediate subjective frequency of females—indicated the quality of his more immediate mating environment. These values are closely associated as males experiencing female bias sub-pools were more likely to experience female-bias nearest neighbor environments. A correlation between the difference of these two values (sub-pool subjective frequency of females – immediate subjective frequency of females) and individual male aggression scores reveals the additional sorting of females occurring within sub-pools.
The average sub-pool aggression experience for each male was used to investigate whether a male’s group environment (group phenotype) influences his mating success along with his individual aggression score (individual phenotype). These individual and group level effects were quantified using multilevel statistics in order to determine whether selection at multiple levels of biological organization influence individual character. There are two general methods of identifying natural selection at multiple levels, the most common method using the Price equation (Price 1970, 1972) as well as a related method which we use here known as contextual analysis (Heisler and Damuth 1987, Goodnight 1992, Okasha 2006). In the Price equation, the evolutionary response of a character is attributed to selection at the individual and group level, demonstrated mathematically by using the covariance between character and fitness at these multiple levels. Therefore, according to this formulation, group selection is considered to occur if a covariance exists between group character (average trait value of group members) and fitness at the group level (average fitness of group members). Although useful, this methodology may falsely identify a correlation at the group-level as selection at the group-level, whereby the correlation may simply be a byproduct of direct selection at the individual level (Okasha 2006). For example, Foster (2010) describes a hypothetical example of non-social snails that do not affect one another’s fitness and reside in a patchy environment. If larger snails have greater reproduction and survival and vary in their distribution amongst patches, then patches with larger snails will have greater productivity, producing a positive result for group selection in the Price equation albeit solely a byproduct of selection at the individual level (see also Sober 1984).
To resolve these issues, we use contextual analysis which is in essence a hierarchical expansion of the Price equation. This formulation uses partial regressions to control for potential cross level byproducts and attribute group selection to only those effects on fitness that cannot be explained by effects at the individual level (see Heisler and Damuth 1987, Goodnight 1992, Okasha 2006, Foster 2010). In regards to our current study, although group membership in this mixing population is transient, we define groups based on the trait group concept considering groups as fitness affecting interactions amongst individuals (Wilson 1975). Therefore, an individual male’s subjective social environment experienced through the study defines his group. Group selection is considered to occur if this social context explains a portion of individual fitness above and beyond that explained by individual phenotype. In other words, group level selection is identified if a male’s mating success is dependent not only on his individual behavior but on the behavior of his group as well. If the regression coefficients (Beta values) for the individual and group traits are of different signs, then the two forces are acting in opposition (Weinig et al. 2007).
Data obtained for females included identifying her sub-pool, sex of the nearest strider, as well as aggression score if the closest strider was male. The mean aggression score of the nearest strider (if male) was referred to as her subjective experience of male aggression. In addition, dispersal tendency from the photos (average number of pool switches per hour) were used to corroborate dispersal tendency obtained during focal sampling. All aggression data was log transformed for normality.
RESULTS
Dispersal mediated sexual conflict
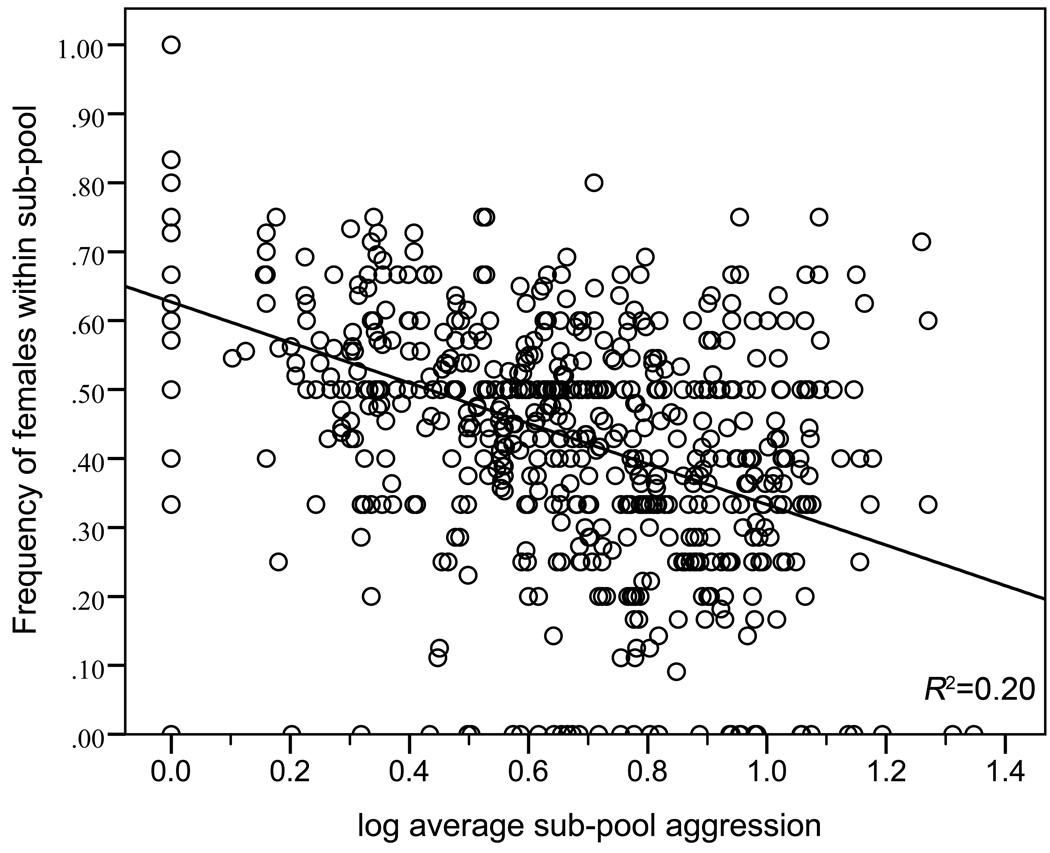
In agreement with our previous findings (2009a), the within group advantage of aggressive mating was countered by spatial heterogeneity imposed by the dispersal of individuals in a multigroup population. Females dispersed away from local aggression such that harassment in the form of mating attempts predicted female dispersal among the sub-pools in the experimental multigroup pool (Pearson r36= 0.737, p<0.001) as well as the number of strides taken by females during focal behavioural sampling (Pearson r36=0.400, p=0.016). Analysis of the time lapse photos revealed that the average aggression of males within sub-pools negatively correlated with the proportion of females within the sub-pools (Fig. 1) (Pearson r780= −0.451, p<0.001), indicating female dispersal away from aggression. Aggressive males countered female dispersal, although did not eliminate female-imposed variation amongst groups. Male aggression score positively correlated with male dispersal tendency from both focal (Pearson r36= 0.726, p<0.001) and photo sampling (Pearson r36=0.691, p<0.001).
Figure 1.
Individual dispersal in response to local aggression imposes sex-ratio heterogeneity amongst sub-pools. The frequency of females within sub-pools decreased as the average aggression score within sub-pools increased.
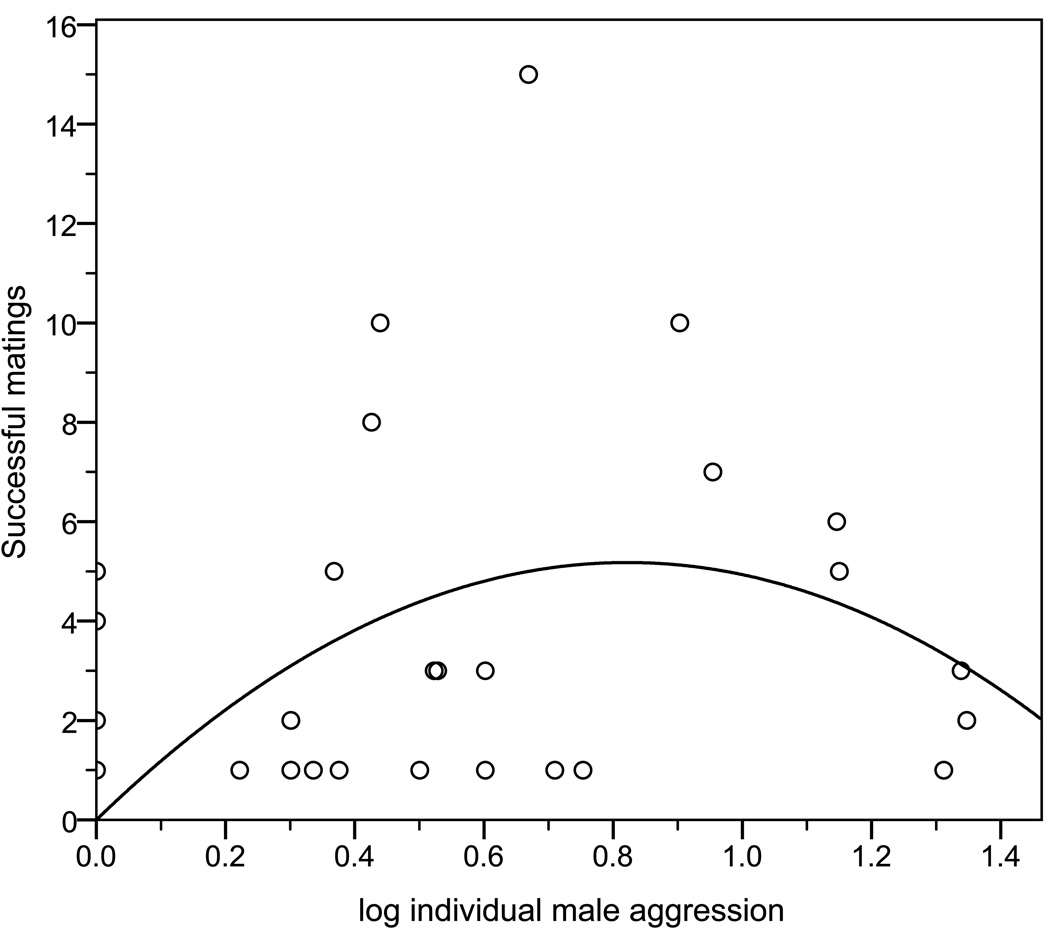
As expected, the net effect of dispersal leads to an overall quadratic unimodal relationship between individual male aggression and mating success in the total population (Fig 2. R2= 0.18, F=3.68, p=0.036), replicating the findings of Eldakar et al. (2009a). Using contextual analysis revealed that the overall quadradic relationship is based on directional within- and between-group selection acting in opposite directions. Individual male aggression positively predicted mating success (B= 0.752, p=0.019) while his average group environment (mean sub-pool aggression experienced by each male) negatively predicted mating success (B= −0.556, p=0.076). Although not statistically significant, group level selection was a clear factor and sufficient to balance individual level selection as observed in the non-linear relationship produced when not controlling for selection at the various levels.
Figure 2.
Male aggression had a quadratic unimodal relationship with the number of successful matings with selection favoring intermediate levels of male aggression.
Subjective experience of males
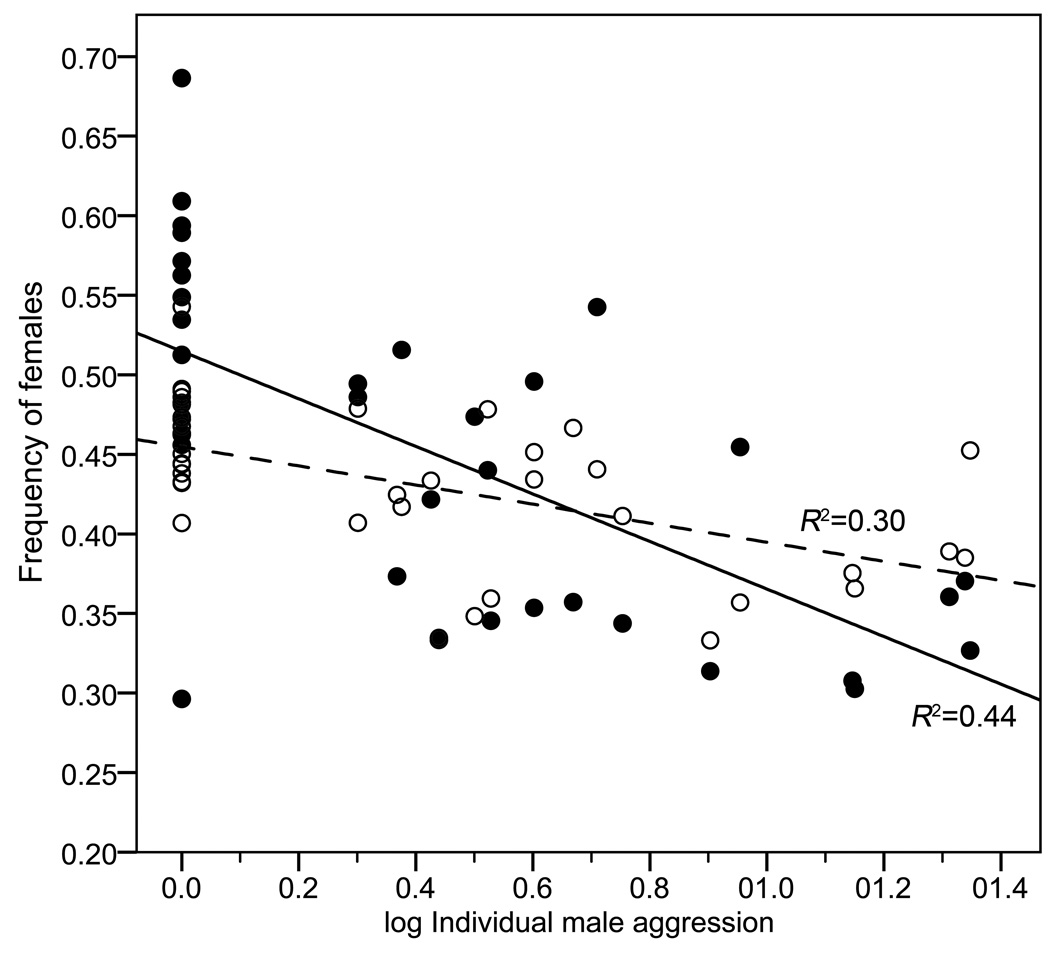
Another way to demonstrate the effects of population structure on the tradeoffs of male aggression is through the subjective environment experienced by individuals. Individual male aggression scores negatively correlated with the sub-pool subjective frequency of females (mean sub-pool sex-ratio experienced by each male) (Fig. 3) (Pearson r36= −0.505, p=0.002). At the finer scale, individual male aggression scores also negatively correlated with the immediate subjective frequency of females (mean sex-ratio of the nearest strider for each male) (Fig. 3) (Pearson r36= −0.627, p<0.001).
Figure 3.
Subjective frequency of females decreases as males increase in their aggression rank. More aggressive males experienced fewer females in their local sub-pool (open circles) as well as in nearest neighbor interactions (filled circles) than less aggressive males. Dashed and solid fit lines correspond to local sub-pool and nearest neighbor frequencies respectively.
As expected, amongst males, the sub-pool subjective frequency of females positively correlated with his immediate subjective frequency of females. However, male aggression score positively correlated with the difference between these values (sub-pool subjective frequency – immediate subjective frequency) (Pearson r36= −0.450, p=0.006), indicating that females impose spatial heterogeneity within sub-pools in addition to amongst sub-pools.
Subjective experience of females
Spatial heterogeneity is also readily observed through the subjective experience of females (aggression score of nearest strider if male). The average female experience of male aggression (x̄ = 2.64, 95% CI= 2.24—3.04) was significantly lower than if females were to interact with males at random (male population average, x̄= 4.09). Specifically, the subjective experience of male aggression by 30 of the 36 females was less than the male population average (binomial p<0.001). Overall, males of an aggression score of 0 comprised 58.2% of the nearest male neighbor interactions experienced by females despite only comprising 36.2% of the actual experimental population.
DISCUSSION
Countless studies have demonstrated sexual conflict at the scale of local social interactions, but few to date have addressed the counterforces mediating the conflict (Sih and Watters 2005, Wigby and Chapman 2005, Eldakar et al. 2009a, 2009b). Researchers often provide a single-group population structure in their experiments, making local social interactions the only interactions, and precluding the possibility of selection acting beyond the local scale (Eldakar et al. 2009a). Recently, Eldakar et al. (2009a) suggested that the contingent movement of individuals has a profound impact on spatial heterogeneity and evolutionary dynamics of structured populations (see also Pepper 1997, Pepper and Smutts 2002, Aktipis 2004). Eldakar et al. (2009a) elicited dramatic shifts in the observed patterns of mating success by altering the ability of individuals to disperse in a multigroup population. When dispersal was blocked, isolating sub-pools, aggression strongly predicted mating success. However, this relationship was muted when allowing the free movement of individuals between sub-pools, indicating that heterogeneity within sub-pools alone is not sufficient to prevent aggressive males from having the local advantage. These findings were also recently supported in observations of wild populations whereby differently structured natural populations selected for different phenotypic distributions of aggressiveness in males amongst streams (Eldakar et al. 2010).
The current study replicates the previous study with the addition of an overhead mounted camera to capture spatial heterogeneity and its consequences to male mating success imposed by individual dispersal in greater detail. Overall, male aggression score did not linearly predict mating success, demonstrating a non-linear unimodal relationship with a slight peak at intermediate levels of aggression indicating stabilizing selection on male aggression. When using contextual analysis to control for both individual and group level effects of aggression on mating success, we found that individual male aggression score positively predicted mating success while his average group environment of aggression predicted mating success in the opposite direction. Unlike scenarios explaining the stable equilibrium of alternative mating strategies based on local negative frequency dependent selection (Shuster and Wade 2003), stabilizing frequency dependence does not occur at the local scale in the A. remigis system. Thus, the stabilizing relationship reveals the net balance of tradeoffs at multiple levels on male aggression.
We attribute the tradeoffs of male aggression to the spatial heterogeneity imposed by the contingent movement of individuals. Females dispersed down aggression gradients imposing sex-ratio heterogeneity both among and within sub-pools, creating favorable mating environments for less aggressive males. The overall subjective experience of females to local male aggression was much less than the male average, indicating that females associated more with less aggressive males than what would be expected at random. Although the mechanism by which females recognize aggressive males is unknown, the simplest possibility is that the heterogeneity is caused entirely by avoidance of aggressive males without any recognition of less aggressive males. Females may just react to the frequency of their own physical encounters with males and disperse accordingly. Other more elaborate assessment mechanisms may exist such as the use of surface water waves (Wilcox 1979) used to detect increased ripple signaling of males in search of mates or perceiving waves resulting from nearby precopulatory struggles.
Although females dispersed away from local aggression, aggressive males also moved between groups, yet did not entirely counteract female movement in its effect on local sex-ratio. Individual males experienced considerably different subjective sex ratios, and thus mating success. Lower aggressive males experienced more female-bias sub-pools, more female-bias immediate social environments within sub-pools and potentially more mating opportunities than more aggressive males despite residing within the same multigroup population. Aside from the already unfavorable sex-ratios in aggressive pools, female behaviors may further reduce the quality of the mating environment. It has been previously demonstrated that high aggression pools exhibit decreased mating activity, resulting from an increase in mating reluctance and decrease in time spent on the water surface by females in response to harassment (Weigensberg and Fairbairn 1994, Sih and Watters 2005, Eldakar et al. 2009b). Furthermore, females have been shown to acquire fewer food items in high aggression pools (Eldakar et al. 2009b), potentially decreasing fecundity beyond the already negative consequences of multiple matings (demonstrated in Aquarius paludum, see Ronkainen et al. 2010) thus reducing the overall payoff of matings in high aggression regions.
Recently, it was suggested that sexual conflict can also be limited by density dependence (Kokko and Rankin 2006, Rankin 2007). Costly traits which confer a competitive advantage may prove superfluous as local competition is reduced. The recent model by Rankin (2007) demonstrated that conflict leads to reduced population density decreasing the advantage of costly competitive traits, potentially selecting against conflict in the population. This concept has been empirically supported regarding the costly traits of mandible size in the European earwig Forficula auricularia (Tompkins and Brown 2004) and sperm production in D. bifurca (Bjork et al. 2007). Although we did not modify overall population density, it may be interpreted that sex-ratio and therefore density of males relative to females varied considerably among the sub-pools in our study. According to Rankin’s model, male bias sub-pools should favor aggression by resulting in greater access to females through increased mating attempts. Conversely, female bias sex ratios should favor reduced aggression. Both of these scenarios exist concurrently as a result of dispersal in our multigroup population.
More research is required to determine the relative importance of these alternative factors, but our study suggests that dispersal induced heterogeneity in multigroup populations can be a major factor in mediating sexual conflict. It is interesting to relate our current study of dispersal and multilevel selection to theoretical models that led to the widespread rejection of group selection in the 1960's and 70's (see Wilson and Wilson 2007). In those models, dispersal was assumed to be random, thereby decreasing genetic and phenotypic variation among groups. This assumption led to the conclusion that group selection is likely to be significant only in isolated groups with little dispersal among groups. No one foresaw at the time that dispersal might increase genetic and phenotypic variation among groups when it is in response to local conditions, even though this makes good biological sense and is empirically supported by our study.
Statistical partitioning methods such as the Price equation and contextual analysis divide selection in the total population into within- and between-group components. Since selection is a causal process, any correlational method can misclassify certain cases, such as the example of nonsocial traits that are nevertheless segregated into different groups described earlier (see Okasha 2006 for a fuller discussion). In our case, we are clearly dealing with the kind of social behavior that has always been central to the group selection controversy, in which a behavior that is "for the good of the group" (non-aggression) is selectively disadvantageous within groups, requiring a multi-group population structure to evolve. Future studies of sexual conflict in all species should recognize that success in local interactions does not necessarily result in success in the population as a whole.
Acknowledgements
We thank K. Foster, W. Driscoll, J. Shepherd, A. Clark, A. Gallup, B. Eldakar, S. Wilcox, B. Dadayeva, D. Shender and the members of EvoS, Binghamton University’s Evolutionary Studies Program, for the helpful discussion. We also thank the Stroud Water Research Center for permitting the collection of striders. Funding was provided by the Center for Insect Science, University of Arizona, NIH grant 5 K12 GM000708.
REFERENCES
- 1.Aktipis CA. Know when to walk away: contingent movement and the evolution of cooperation. J. Theor. Biol. 2004;231:249–260. doi: 10.1016/j.jtbi.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Arnqvist G. Evolution of mating systems. In: Choe JC, Crespi BJ, editors. Social Competition and Cooperation in Insects and Arachnids: Vol. I. Cambridge: Cambridge University Press; 1997. pp. 146–163. [Google Scholar]
- 3.Arnqvist G, Rowe L. Sexual Conflict. NJ: Princeton University Press; 2005. [Google Scholar]
- 4.Bauer S, Samietz J, Berger U. Sexual harassment in heterogeneous landscapes can mediate population regulation in a grasshopper. Behav. Ecol. 2004;16:239–246. [Google Scholar]
- 5.Bjork A, Dallai R, Pitnick S. Adaptive modulation of sperm production rate in Drosophila bifurca, a species with giant sperm. Biol. Lett. 2007;3:517–519. doi: 10.1098/rsbl.2007.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. [Google Scholar]
- 7.Eldakar OT, Dlugos MJ, Pepper JW, Wilson DS. Population structure mediates sexual conflict in water striders. Science. 2009a;326:816. doi: 10.1126/science.1180183. [DOI] [PubMed] [Google Scholar]
- 8.Eldakar OT, Dlugos MJ, Wilcox RS, Wilson DS. Aggressive mating as a tragedy of the commons in the water strider Aquarius remigis. Behav. Ecol. Sociobiol. 2009b;64:25–33. [Google Scholar]
- 9.Eldakar OT, Dlugos MJ, Holt GP, Wilson DS, Pepper JW. Population structure influences sexual conflict in wild populations of water striders. Behaviour. 2010 doi: 10.1163/000579510X510520. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster KR. A defense of sociobiology. Cold Spring Harb Symp Quant Biol. 2010:1–16. doi: 10.1101/sqb.2009.74.041. [DOI] [PubMed] [Google Scholar]
- 11.Goodnight CJ, Schwartz JM, Stevens L. Contextual analysis of models of group selection, soft selection, hard selection, and the evolution of altruism. Am. Nat. 1992;140:743–761. [Google Scholar]
- 12.Haldane JBS. The Causes of Evolution. London: Longmans, Green & Co.; 1932. [Google Scholar]
- 13.Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- 14.Heisler IL, Damuth J. A method for analyzing selection in hierarchically structured populations. Am. Nat. 1987;130:582–602. [Google Scholar]
- 15.Kokko H, Rankin DJ. Lonely hearts or sex in the city? Density-dependent effects in mating systems? Phil Trans R Soc B. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krupa JJ, Sih A. Fishing spiders, green sunfish, and a stream-dwelling water strider: male-female conflict and prey responses to single versus multiple predator environments. Oecologia. 1998;117:258–265. doi: 10.1007/s004420050656. [DOI] [PubMed] [Google Scholar]
- 17.Le Galliard JF, Fitze PS, Ferriere R, Clobert J. Sex ratio bias, male aggression, and population collapse in lizards. Proc Natl Acad Sci USA. 2005;102:18231–18236. doi: 10.1073/pnas.0505172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. Cambridge: Cambridge Univ. Press; 1993. [Google Scholar]
- 19.Okasha S. Evolution and the levels of selection. New York: Oxford Univ. Press; 2006. [Google Scholar]
- 20.Pepper JW. Simple models of assortment through environmental feedback. Artificial Life. 1997;13:1–9. doi: 10.1162/artl.2007.13.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Pepper JW, Smuts BBB. A mechanism for the evolution of altruism among non-kin: positive assortment through environmental feedback. Am. Nat. 2002;160:205–213. doi: 10.1086/341018. [DOI] [PubMed] [Google Scholar]
- 22.Price GR. Selection and covariance. Nature. 1970;227:520–521. doi: 10.1038/227520a0. [DOI] [PubMed] [Google Scholar]
- 23.Price GR. Extension of covariance selection mathematics. Ann. Hum. Genet. 1972;35:455–458. doi: 10.1111/j.1469-1809.1957.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 24.Rankin DJ. Resolving the tragedy of the commons: the feedback between intraspecific conflict and population density. J. Evol. Biol. 2007;20:173–180. doi: 10.1111/j.1420-9101.2006.01211.x. [DOI] [PubMed] [Google Scholar]
- 25.Rankin DJ, López-Sepulcre A. Can adaptation lead to extinction? Oikos. 2005;111:616–619. [Google Scholar]
- 26.Rankin DJ, Kokko H. Sex, death and tragedy. Trends Ecol. Evol. 2006;21:225–226. doi: 10.1016/j.tree.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Rankin DJ, Bargum K, Kokko H. The tragedy of the commons in evolutionary biology. Trends Ecol. Evol. 2007;22:643–651. doi: 10.1016/j.tree.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Rice W. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 29.Ronkainen K, Kaitala A, Kivelä SM. Polyandry, multiple mating, and female fitness in a water strider Aquarius paludum. Behav. Ecol. Sociobiol. 2010;64:657–664. [Google Scholar]
- 30.Shuster S, Wade M. Mating Systems and Strategies. Princeton: Princeton Univ Press; 2003. [Google Scholar]
- 31.Sih A, Watters J. The mix matters: behavioral types and group dynamics in water striders. Behaviour. 2005;142 1423-143. [Google Scholar]
- 32.Sih A, Krupa JJ. Interacting effects of predation risk, sex ratio and density on male/female conflicts and mating dynamics of stream water striders. Behav. Ecol. 1995;6:316–325. [Google Scholar]
- 33.Sober E. The Nature of Selection: Evolutionary Theory in Philosophical Focus. Cambridge MA: MIT Press; 1984. [Google Scholar]
- 34.Turlure C, Van Dyck H. On the consequences of aggressive male mate-locating behaviour and micro-climate for female host plant use in the butterfly Lycaena hippothoe. Behav. Ecol. Sociobiol. 2009;64:1–11. [Google Scholar]
- 35.Wilcox RS. Sex discrimination in Gerris remigis: role of a surface wave signal. Science. 1979;206:1325–1327. doi: 10.1126/science.206.4424.1325. [DOI] [PubMed] [Google Scholar]
- 36.Tomkins JL, Brown GS. Population density drives the local evolution of a threshold dimorphism. Nature. 2004;431:1099–1103. doi: 10.1038/nature02918. [DOI] [PubMed] [Google Scholar]
- 37.Weigensberg I, Fairbairn DJ. Conflict of interest between the sexes: a study of mating interactions in a semiaquatic bug. Anim. Behav. 1994;48:893–901. [Google Scholar]
- 38.Weinig C, Johnston JA, Willis CG, Maloof JN. Antagonistic multilevel selection on size and architecture in variable density settings. Evolution. 2007;61:58–67. doi: 10.1111/j.1558-5646.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- 39.Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox RS, Ruckdeschel R. Food threshold territoriality in a water strider (Gerris remigis) Behav. Ecol. Sociobiol. 1982;11:85–90. [Google Scholar]
- 41.Wilson DS. A theory of group selection. Proc. Nat. Acad. Sci. 1975;72:143–146. doi: 10.1073/pnas.72.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson DS, Wilson EO. Rethinking the theoretical foundation of sociobiology. Q. Rev. Biol. 2007;82:327–348. doi: 10.1086/522809. [DOI] [PubMed] [Google Scholar]
- 43.Wilson DS, Dugatkin LA. Group selection and assortative interactions. Am. Nat. 1997;149:336–351. [Google Scholar]