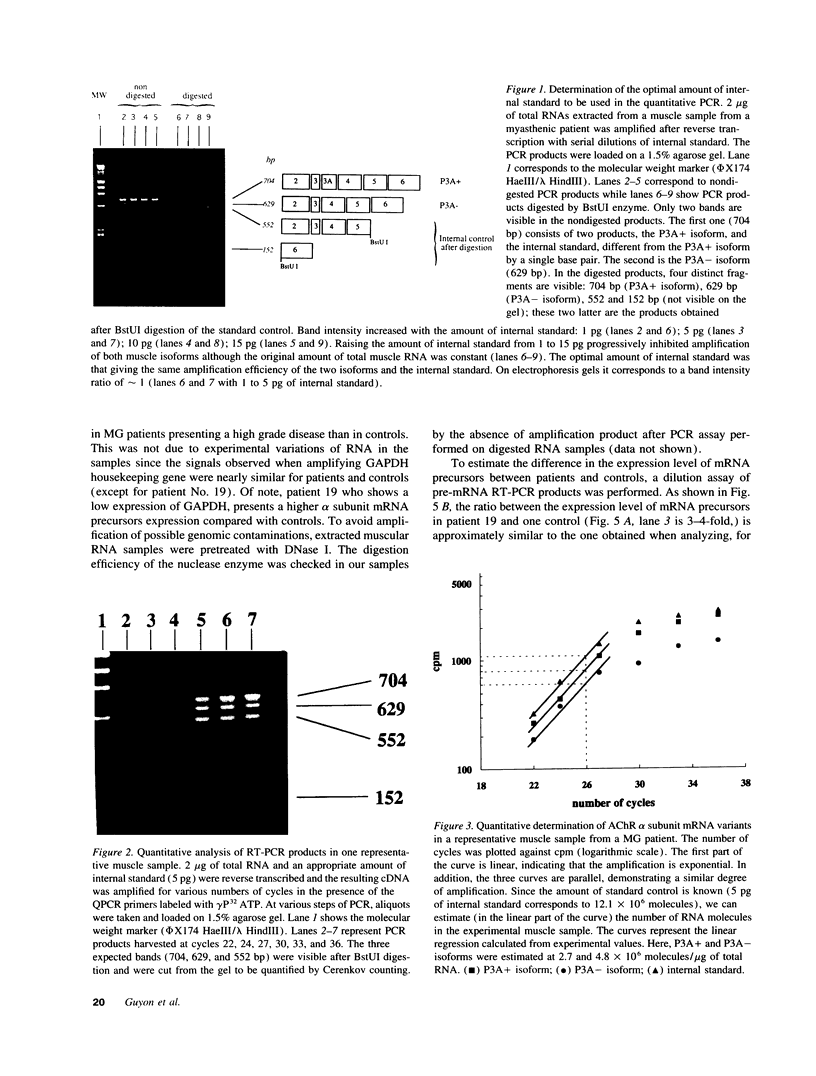
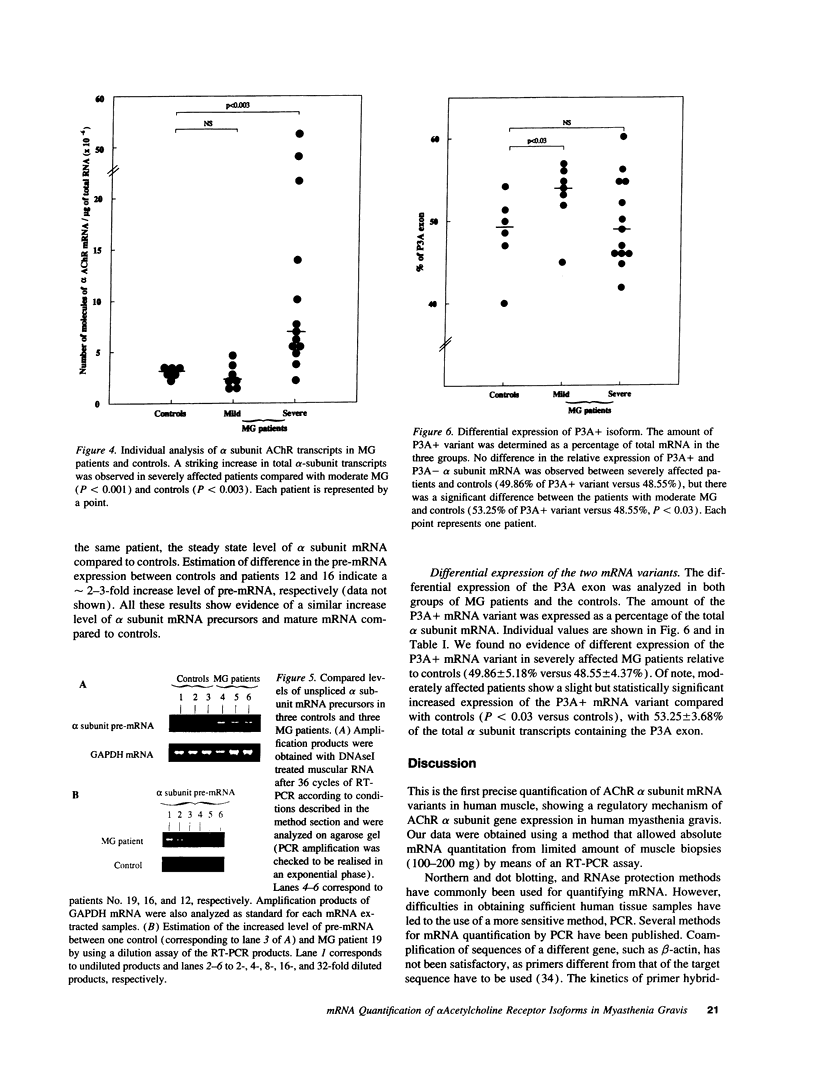
Abstract
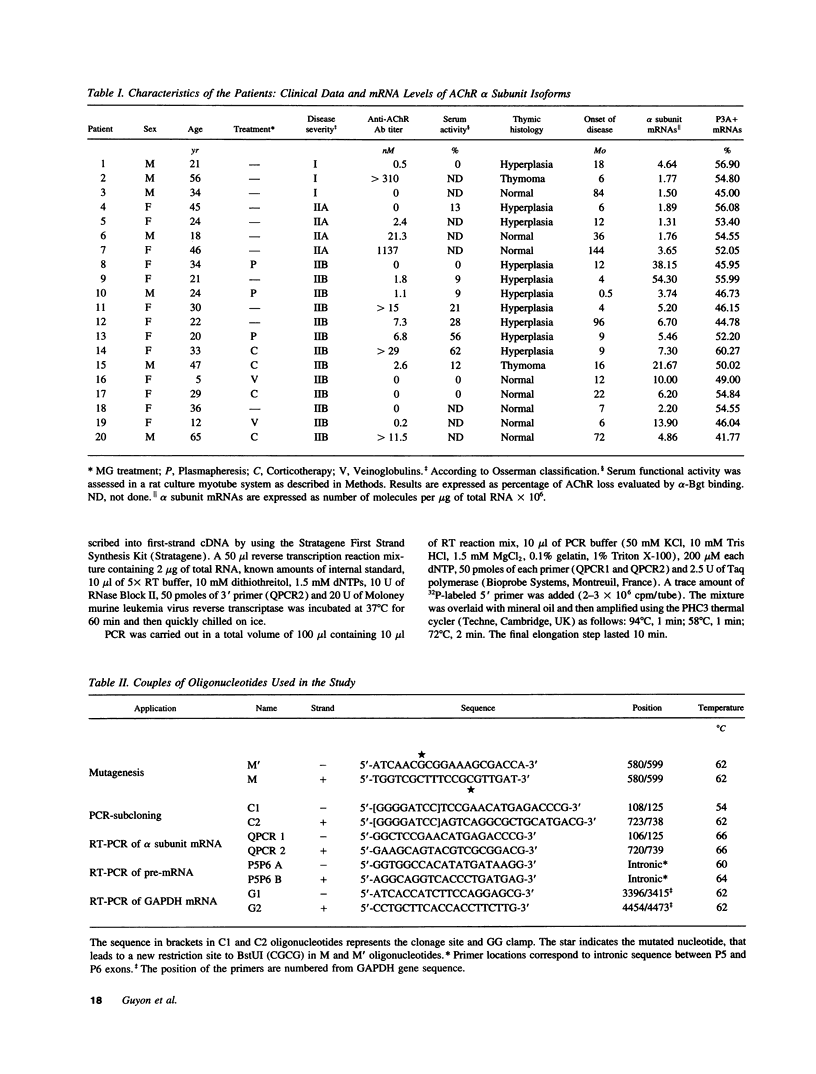
Myasthenia gravis (MG) is an autoimmune disease mediated by auto-antibodies that attack the nicotinic acetylcholine receptor (AChR). To elucidate the molecular mechanisms underlying the decrease in AChR levels at the neuromuscular junction, we investigated the regulation of AChR expression by analyzing mRNA of the two AChR alpha subunit isoforms (P3A+ and P3A-) in muscle samples from myasthenic patients relative to controls. We applied a quantitative method based on reverse transcription of total RNA followed by polymerase chain reaction (PCR), using an internal standard we constructed by site-directed mutagenesis. An increased expression of mRNA coding for the alpha subunit of the AChR isoforms was observed in severely affected patients (P < 0.003 versus controls) but not in moderately affected patients, independently of the anti-AChR antibody titer. Study of mRNA precursor levels indicates a higher expression in severely affected patients compared to controls, suggesting an enhanced rate of transcription of the message coding for the alpha subunit isoforms in these patients. We have also reported that mRNA encoding both isoforms are expressed at an approximate 1:1 ratio in controls and in patients. We have thus identified a new biological parameter correlated with disease severity, and provide evidence of a compensatory mechanism to balance the loss of AChR in human myasthenia gravis, which is probably triggered only above a certain degree of AChR loss.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Gallego M. E., Nadal-Ginard B. Generation of protein isoform diversity by alternative splicing: mechanistic and biological implications. Annu Rev Cell Biol. 1987;3:207–242. doi: 10.1146/annurev.cb.03.110187.001231. [DOI] [PubMed] [Google Scholar]
- Asher O., Kues W. A., Witzemann V., Tzartos S. J., Fuchs S., Souroujon M. C. Increased gene expression of acetylcholine receptor and myogenic factors in passively transferred experimental autoimmune myasthenia gravis. J Immunol. 1993 Dec 1;151(11):6442–6450. [PubMed] [Google Scholar]
- Asher O., Neumann D., Fuchs S. Increased levels of acetylcholine receptor alpha-subunit mRNA in experimental autoimmune myasthenia gravis. FEBS Lett. 1988 Jun 20;233(2):277–281. doi: 10.1016/0014-5793(88)80442-3. [DOI] [PubMed] [Google Scholar]
- Asher O., Neumann D., Witzemann V., Fuchs S. Acetylcholine receptor gene expression in experimental autoimmune myasthenia gravis. FEBS Lett. 1990 Jul 16;267(2):231–235. doi: 10.1016/0014-5793(90)80932-9. [DOI] [PubMed] [Google Scholar]
- Asher O., Provenzano C., Fuchs S. Regulation of acetylcholine receptor gene expression in rats treated with alpha-bungarotoxin. FEBS Lett. 1991 May 6;282(2):242–246. doi: 10.1016/0014-5793(91)80487-n. [DOI] [PubMed] [Google Scholar]
- Balvay L., Libri D., Gallego M., Fiszman M. Y. Intronic sequence with both negative and positive effects on the regulation of alternative transcripts of the chicken beta tropomyosin transcripts. Nucleic Acids Res. 1992 Aug 11;20(15):3987–3992. doi: 10.1093/nar/20.15.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkas T., Gabriel J. M., Mauron A., Hughes G. J., Roth B., Alliod C., Tzartos S. J., Ballivet M. Monoclonal antibodies to the main immunogenic region of the nicotinic acetylcholine receptor bind to residues 61-76 of the alpha subunit. J Biol Chem. 1988 Apr 25;263(12):5916–5920. [PubMed] [Google Scholar]
- Beeson D., Morris A., Vincent A., Newsom-Davis J. The human muscle nicotinic acetylcholine receptor alpha-subunit exist as two isoforms: a novel exon. EMBO J. 1990 Jul;9(7):2101–2106. doi: 10.1002/j.1460-2075.1990.tb07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrih-Aknin S., Morel E., Raimond F., Safar D., Gaud C., Binet J. P., Levasseur P., Bach J. F. The role of the thymus in myasthenia gravis: immunohistological and immunological studies in 115 cases. Ann N Y Acad Sci. 1987;505:50–70. doi: 10.1111/j.1749-6632.1987.tb51282.x. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Merlie J. P. Transcriptional regulation of nicotinic acetylcholine receptor genes during muscle development. J Biol Chem. 1986 Sep 5;261(25):11452–11455. [PubMed] [Google Scholar]
- Changeux J. P. Compartmentalized transcription of acetylcholine receptor genes during motor endplate epigenesis. New Biol. 1991 May;3(5):413–429. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Adams R. N., Josifek L. F., Self S. G. Functional activities of autoantibodies to acetylcholine receptors and the clinical severity of myasthenia gravis. N Engl J Med. 1982 Sep 23;307(13):769–775. doi: 10.1056/NEJM198209233071301. [DOI] [PubMed] [Google Scholar]
- Evans S., Goldman D., Heinemann S., Patrick J. Muscle acetylcholine receptor biosynthesis. Regulation by transcript availability. J Biol Chem. 1987 Apr 5;262(10):4911–4916. [PMC free article] [PubMed] [Google Scholar]
- Eymard B., de la Porte S., Pannier C., Berrih-Aknin S., Morel E., Fardeau M., Bach J. F., Koenig J. Effect of myasthenic patient sera on the number and distribution of acetylcholine receptors in muscle and nerve-muscle cultures from rat. Correlations with clinical state. J Neurol Sci. 1988 Aug;86(1):41–59. doi: 10.1016/0022-510x(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Drachman D. B., Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973 Oct 19;182(4109):293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Fontaine B., Changeux J. P. Localization of nicotinic acetylcholine receptor alpha-subunit transcripts during myogenesis and motor endplate development in the chick. J Cell Biol. 1989 Mar;108(3):1025–1037. doi: 10.1083/jcb.108.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Changeux J. P. Calcitonin gene-related peptide and muscle activity regulate acetylcholine receptor alpha-subunit mRNA levels by distinct intracellular pathways. J Cell Biol. 1987 Sep;105(3):1337–1342. doi: 10.1083/jcb.105.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Changeux J. P. Activity regulates the levels of acetylcholine receptor alpha-subunit mRNA in cultured chicken myotubes. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4558–4562. doi: 10.1073/pnas.82.13.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine G. D., Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol. 1978 Sep;9(5):495–515. doi: 10.1016/s0046-8177(78)80131-2. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Engel A. G., Seybold M. E., Lennon V. A., Lambert E. H. Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine recepotr antibodies. J Exp Med. 1976 Sep 1;144(3):739–753. doi: 10.1084/jem.144.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J. M., Lambert E. H. Content of acetylcholine receptor and antibodies bound to receptor in myasthenia gravis, experimental autoimmune myasthenia gravis, and Eaton-Lambert syndrome. Neurology. 1978 Feb;28(2):130–138. doi: 10.1212/wnl.28.2.130. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Lobos E. A., Rudnick C. H., Watson M. S., Isenberg K. E. Linkage disequilibrium study of RFLPs detected at the human muscle nicotinic acetylcholine receptor subunit genes. Am J Hum Genet. 1989 Apr;44(4):522–533. [PMC free article] [PubMed] [Google Scholar]
- Lu C. Z., Lu L., Hao Z. S., Xia D. G., Qain J., Arnason B. G. Antibody-secreting cells to acetylcholine receptor and to presynaptic membrane receptor in seronegative myasthenia gravis. J Neuroimmunol. 1993 Mar;43(1-2):145–149. doi: 10.1016/0165-5728(93)90085-d. [DOI] [PubMed] [Google Scholar]
- MacLennan C., Beeson D., Vincent A., Newsom-Davis J. Human nicotinic acetylcholine receptor alpha-subunit isoforms: origins and expression. Nucleic Acids Res. 1993 Nov 25;21(23):5463–5467. doi: 10.1093/nar/21.23.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Morel E., Raimond F., Goulon-Goëau C., Berrih S., Vernet-Der-Garabedian B., Harb J., Bach J. F. Le dosage des anticorps anti-récepteurs de l'acétylcholine dans la myasthénie. Etude de 329 sérums. Nouv Presse Med. 1982 May 22;11(24):1849–1854. [PubMed] [Google Scholar]
- Morris A., Beeson D., Jacobson L., Baggi F., Vincent A., Newsom-Davis J. Two isoforms of the muscle acetylcholine receptor alpha-subunit are translated in the human cell line TE671. FEBS Lett. 1991 Dec 16;295(1-3):116–118. doi: 10.1016/0014-5793(91)81399-s. [DOI] [PubMed] [Google Scholar]
- Moss S. J., Beeson D. M., Jackson J. F., Darlison M. G., Barnard E. A. Differential expression of nicotinic acetylcholine receptor genes in innervated and denervated chicken muscle. EMBO J. 1987 Dec 20;6(13):3917–3921. doi: 10.1002/j.1460-2075.1987.tb02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M., Wagner J., Dzau V. J. Gene expression of the renin-angiotensin system in human tissues. Quantitative analysis by the polymerase chain reaction. J Clin Invest. 1993 May;91(5):2058–2064. doi: 10.1172/JCI116428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlo V. P., Poskanzer D. C., Schwab R. S., Viets H. R., Osserman K. E., Genkins G. Myasthenia gravis: evaluation of treatment in 1,355 patients. Neurology. 1966 May;16(5):431–439. doi: 10.1212/wnl.16.5.431. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Self S. G. Measurement of junctional acetylcholine receptors in myasthenia gravis: clinical correlates. Muscle Nerve. 1985 Mar-Apr;8(3):245–251. doi: 10.1002/mus.880080311. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Changeux J. P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol Rev. 1984 Oct;64(4):1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Talib S., Okarma T. B., Lebkowski J. S. Differential expression of human nicotinic acetylcholine receptor alpha subunit variants in muscle and non-muscle tissues. Nucleic Acids Res. 1993 Jan 25;21(2):233–237. doi: 10.1093/nar/21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyka K. V., Brachman D. B., Pestronk A., Kao I. Myasthenia gravis: passive transfer from man to mouse. Science. 1975 Oct 24;190(4212):397–399. doi: 10.1126/science.1179220. [DOI] [PubMed] [Google Scholar]
- Tsay H. J., Neville C. M., Schmidt J. Protein synthesis is required for the denervation-triggered activation of acetylcholine receptor genes. FEBS Lett. 1990 Nov 12;274(1-2):69–72. doi: 10.1016/0014-5793(90)81331-h. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Changeux J. P. High affinity binding of alpha-bungarotoxin to the purified alpha-subunit and to its 27,000-dalton proteolytic peptide from Torpedo marmorata acetylcholine receptor. Requirement for sodium dodecyl sulfate. EMBO J. 1983;2(3):381–387. doi: 10.1002/j.1460-2075.1983.tb01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Kokla A., Walgrave S. L., Conti-Tronconi B. M. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67-76 of the alpha subunit. Proc Natl Acad Sci U S A. 1988 May;85(9):2899–2903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Sophianos D., Zimmerman K., Starzinski-Powitz A. Antigenic modulation of human myotube acetylcholine receptor by myasthenic sera. Serum titer determines receptor internalization rate. J Immunol. 1986 May 1;136(9):3231–3238. [PubMed] [Google Scholar]
- Vincent A. Immunology of acetylcholine receptors in relation to myasthenia gravis. Physiol Rev. 1980 Jul;60(3):756–824. doi: 10.1152/physrev.1980.60.3.756. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley L. M., Urso D., Tumas K., Maltzman J., Loh E., Levinson A. I. Molecular evidence for the expression of nicotinic acetylcholine receptor alpha-chain in mouse thymus. J Immunol. 1992 May 15;148(10):3105–3109. [PubMed] [Google Scholar]
- Yamamoto T., Sato T., Sugita H. Antifilamin, antivinculin, and antitropomyosin antibodies in myasthenia gravis. Neurology. 1987 Aug;37(8):1329–1333. doi: 10.1212/wnl.37.8.1329. [DOI] [PubMed] [Google Scholar]