Abstract
A distributed network of brain regions is linked to drug-related cue responding. However, the relationships between smoking cue-induced phasic activity and possible underlying differences in brain structure, tonic neuronal activity and connectivity between these brain areas are as yet unclear. Twenty-two smokers and 22 controls viewed smoking-related and neutral pictures during a functional arterial spin labeling scanning session. T1, resting functional, and diffusion tensor imaging data were also collected. Six brain areas, dorsal lateral prefrontal cortex (dlPFC), dorsal medial prefrontal cortex (dmPFC), dorsal anterior cingulate cortex/cingulate cortex, rostral anterior cingulate cortex (rACC), occipital cortex, and insula/operculum, showed significant smoking cue-elicited activity in smokers when compared with controls and were subjected to secondary analysis for resting state functional connectivity (rsFC), structural, and tonic neuronal activity. rsFC strength between rACC and dlPFC was positively correlated with the cue-elicited activity in dlPFC. Similarly, rsFC strength between dlPFC and dmPFC was positively correlated with the cue-elicited activity in dmPFC while rsFC strength between dmPFC and insula/operculum was negatively correlated with the cue-elicited activity in both dmPFC and insula/operculum, suggesting these brain circuits may facilitate the response to the salient smoking cues. Further, the gray matter density in dlPFC was decreased in smokers and correlated with cue-elicited activity in the same brain area, suggesting a neurobiological mechanism for the impaired cognitive control associated with drug use. Taken together, these results begin to address the underlying neurobiology of smoking cue salience, and may speak to novel treatment strategies and targets for therapeutic interventions.
Keywords: Smoking cue, anatomical, ASL, DTI, VBM, resting state functional connectivity
Introduction
Cigarette smoking is one of the most common preventable causes of death and disease in the United States (Fellows et al., 2002). Approximately 80% of smokers who try to quit smoking relapse within the first month and only about 3% remain abstinent for six months (Hughes et al., 1992). The model offered by Tiffany (1990; 2000) suggests that the main reason for the high relapse rate seen in smoking and other addictions may not be due to drug-stimuli induced conscious craving for the drug, but rather the induction of an automatized, at least in part, subconscious, drug-seeking drive state which, when coupled with an impaired ability to control/inhibit this impulse after addiction has developed (Garavan and Hester, 2007), may lead to continued drug-use. Therefore, a deeper understanding of addiction-related neural responses to and mechanisms underlying drug-related cues is important for the development of more efficacious addiction treatments.
A growing body of neuroimaging have examined the neural basis of smoking cue reactivity. While there is considerable variability in regions identified, likely due to differences in study population (active smokers, those attempting to quit, abstinent vs. smoking ad lib, gender, etc.) and differences in cue presentations (video, pictures, scripts/guided imagery, multimodal stimuli, etc.), most studies have identified core brain regions related to identification of salient stimuli, attention and motor preparation. Anterior cingulate cortex (ACC), prefrontal cortex (PFC), and such sub-cortical areas as amygdala are the most commonly reported loci of activation. Additional regions in occipital, parietal, and temporal lobes have also been reported studies (David et al., 2005; Due et al., 2002; Franklin et al., 2007; Lee et al., 2005; McClernon et al., 2008b; Smolka et al., 2006).
These brain areas are thought to play distinct roles in the cue response process. For example, Phillips et al., (2003) proposed that when exposed to salient affective/motivational cues (e.g., smoking-related stimuli to smokers), brain areas in a ventral system, including the rostral ACC and insula, activate to engage emotional/motivational identification processes, while brain areas in a dorsal system, including the dorsal PFC and dorsal ACC, play a role in emotional/motivational regulation. Of course, fMRI studies of cue responsivity can only identify those brain areas that are phasically responsive to salient stimuli; they cannot identify long-term, tonic drug-induced brain changes or pre-existing differences that may underlie the observed phasic activity to acute stimuli presentations.
Previous studies have found significantly reduced frontal cortex gray matter volume and/or density in chronic cigarette smokers when compared to nonsmokers (Brody et al., 2004; Gallinat et al., 2006). Further, resting regional cerebral blood flow (CBF) is lower in smokers compared to non-smokers (Kubota et al., 1983). However, whether these relatively static brain markers are related to or are reflective of specific smoking cue reactivity, general smoking-related neuronal alterations, or indeed have a causative relationship to smoking is still not clear. Therefore, the first objective of this study was to examine the relationship between smoking cue-induced activity and underlying tonic neurobiological features including brain gray matter density (as measured by voxel-based morphometry; VBM) and resting neuronal activity (as measured by resting CBF). It was hypothesized that smoking cue-induced phasic fMRI activity in smokers would correlate with tonic local alterations in resting neuronal activity and/or local gray matter density.
Nicotinic receptors are known to modulate anatomically and neurochemically widespread neuronal systems (Gotti et al., 2009). As such, potential chronic smoking-induced neuronal alterations might also be expected to have anatomically disparate loci, including brain areas involved in smoking cue processes (Mansvelder et al., 2006). Therefore, our second objective was to examine potential circuit level alterations in chronic smokers and their relationship to regional, phasic smoking cue activation. Synchronized spontaneous fluctuations in the resting-state fMRI signal are thought to reflect anatomically constrained, intrinsic, dynamic interactions between brain regions (Vincent et al., 2007). Resting state functional connectivity (rsFC) maps have been observed in specific brain circuits (Albert et al., 2009; Greicius, 2008; Waites et al., 2005), alterations of which have been demonstrated in such disorders as Alzheimer’s disease, schizophrenia, major depression and epilepsy (reviewed by Greicius, 2008). Although rsFC does not, by definition, measure brain activation during task performance, the strength of such resting connectivity has been demonstrated to predict performance on behavioral tasks requiring that circuit (Anticevic et al., 2010; Hampson et al., 2006; Kelly et al., 2008; Tambini et al., 2010). Therefore, rsFC was employed to determine possible circuit level characteristics of chronic smokers. It was hypothesized that brain areas activated by phasic smoking cues will exhibit altered rsFC in chronic smokers and that rsFC will be related to cue-induced activity.
While rsFC has been shown to be anatomically constrained (Greicius et al., 2009), it nevertheless does not provide direct anatomical information. We therefore employed diffusion tensor imaging (DTI), a neuroimaging technique that assesses white matter integrity by measuring the freedom of water movement. We previously found prefrontal white matter integrity alterations related to cigarette smoking (unpublished). Therefore, our third objective was to examine whether smoking-related impaired prefrontal white matter integrity is associated with prefrontal rsFC strength alterations and/or phasic regional smoking cue-elicited activity. It was hypothesized that there is a direct link between these anatomical and functional measures.
Materials and Methods
Participants
Data were collected from 22 smokers (11 females; mean (±SD) age: 31.0±9.4; range: 21 to 50) and 22 age and gender matched controls (10 females; mean age: 29.6±7.0; range: 21 to 42). Smokers used cigarettes for an average of 12.0±7.3 years (range: 2 to 28 years) and smoked a mean of 21.1±4.3 cigarettes/day (range: 15 to 30), with an average Fagerström Test for Nicotine Dependence (FTND) score of 5.4±1.9 (range: 3 to 10) and an average lifetime cigarette usage of 12.7±7.5 pack-years (range: 1.5 to 27). Smokers were required to not smoke for at least two hours before their MRI session and compliance was monitored by a member of the study team. A urine sample was collected and assessed for common drugs of abuse (TRIAGE®) before the scanning session. Participants were excluded if they had any major medical illnesses, current major psychiatric disorders, neurological illnesses, or if their T1 weighted images revealed gross structural abnormalities. No participants had a history of dependence (current or past) on any drug other than nicotine based on the computerized Structured Clinical Interview for DSM-IV, drug use survey and clinical interview.
All participants gave written informed consent to protocols approved by the NIDA-IRP Institutional Review Board and were compensated for their participation. Cue-elicited and resting Arterial Spin Label (ASL) cerebral blood flow (CBF) scans were obtained from all subjects along with DTI and anatomical T1 data. Resting state BOLD fMRI data were obtained on all but four smokers and four controls.
Experimental Paradigm
In a block design paradigm, participants were presented with two kinds of visual cues, i.e., smoking-related and neutral pictures. The neutral pictures were similar in content to the smoking-related ones, but absent any smoking-related properties (Figure 1). All pictures were selected from those of Mucha et al., (1999) and the International Smoking Image Series (Gilbert and Rabinovich, 1999). In each of two scanning runs within a single scanning session, thirteen 30s blocks, consisting of six cue and seven interspersed fixation/resting blocks, were presented (Figure 1). Each cue block consisted of 10 corresponding pictures, each presented for 1s with a 2s fixation gap, about 5°X5° visual angle. No subjective measurements (e.g., craving self-report) were collected during scanning because it has been proposed that to do so would engage top-down appraisal processes that would likely alter neural activity reflecting the automatic consequence of the smoking-related emotional stimuli (Ochsner and Gross, 2005; Taylor et al., 2003), which was the main focus of this study. The order of the neutral and the smoking-related blocks was counter-balanced across the participants.
Figure 1.

Experimental paradigm of an ASL scanning run
Data acquisition
Experiments were performed on a Siemens 3T Allegra MRI scanner equipped with a standard RF birdcage head coil to acquire the neuroimaging data. A pulsed ASL sequence (Luh et al., 1999; Wong et al., 1997) was used to quantify perfusion changes associated with brain activation in response to the visual stimuli. Eleven oblique 8mm thick axial slices encompassed most of the brain. Some parietal areas were not covered in some participants. The PASL parameters are as follows: the gap between the labeling slab and the proximal slice=10mm, TI1=700ms, TI1 stop-time=1300ms, TI2=1400ms, with crusher bipolar gradients switched between slice excitation and readout to reduce signal from large vessels. Further parameters were: TE/TR=15ms/3000ms, number of measurements=130/run, imaging matrix=64×64, FOV=220mm×220mm, partial (6/8) Fourier acquisition, BW=3004Hz/Px, echo spacing=0.4ms. Whole-brain T1-weighted structural images (MPRAGE) (1-mm3 isotropic voxels, TE/TR=4.38ms/2500ms, FA=8°) were acquired for anatomical reference and VBM analyses. A resting-state BOLD fMRI scanning run was acquired using a gradient-echo EPI sequence (150 volumes, TE/TR=27ms/2000ms, FA=80°, FOV=220mm×220mm, imaging matrix=64×64; 33 5mm-sagital slices in one smoker and two controls and 39 4mm-axial slices in the others). DTI data were acquired using a single-shot, spin-echo echo-planar imaging technique (TE/TR=87ms/5000ms, BW=1700Hz/Pixel, FOV=220mm×220mm, imaging matrix=128x128, 35 slices, thickness=4mm, b value= 0 and 1000s/mm2). Thirteen unique volumes were collected to compute the tensor: a b=0s/mm2 image and 12 images with diffusion gradients applied in 12 noncollinear directions (Gx, Gy, Gz: [1.0, 0.0, 0.5], [0.0, 0.5, 1.0], [0.5, 1.0, 0.0], [1.0, 0.5, 0.0], [0.0, 1.0, 0.5], [0.5, 0.0, 1.0], [1.0, 0.0, −0.5], [0.0, −0.5, 1.0], [−0.5, 1.0, 0.0], [1.0, −0.5, 0.0], [0.0, 1.0, −0.5], [−0.5, 0.0, 1.0]). Participant head movement was minimized by the use of soft foam padding, a vacuum bag, and/or hardened polyurethane foam.
Whole brain analysis for ASL fMRI data
Functional imaging data were analyzed with the Analysis of Functional Neuro-Images (AFNI) software package (Cox, 1996). After motion and acquisition time corrections, perfusion-weighted image series were generated by pair-wise subtraction (i.e., the effective TR is 6s.) of the label and control images (Liu and Wong, 2005), followed by conversion to absolute CBF based on Equation 1. The CBF data were subjected to a whole brain 2×2 repeated-measures analysis of variance (ANOVA) with group (smoker and control) as a between-subjects factor and cue (smoking and neutral) as a within-subject factor.
| (1)* |
*:ΔM is the difference signal (control–labeled); M0b is the fully relaxed magnetization of arterial blood†; TI1=0.7s, TI2=1.4s, and T1b=1.613s (the T1 decay time for labeled blood at 3 T (Lu et al., 2004)). †: M0b is calculated from the steady-state signal of the control images.
Secondary ROI analysis
Clusters showing significant interaction (FWE corrected p<0.05) between cue (smoking vs. neutral) and group (smokers vs. controls) in the whole-brain ASL results were considered as regions of interest (ROIs) and subjected to secondary analyses with an uncorrected threshold of p=0.05, which was chosen to reflect the exploratory nature of the post-hoc analyses. Age and gender were used as covariates for all correlation analyses in this secondary analysis.
Smoking cue-elicited changes in CBF [smoking-neutral] derived from the smoking and neutral cue conditions were averaged across voxels in each ROI from the corresponding blocks. Signal from the first effective TR in each block was removed to minimize the effects of hemodynamic delay. A paired t-test was applied to the CBF data between the smoking and the neutral cue conditions in smokers to determine if the whole brain analysis [cue X group] result reflected, at least in part, differential activation in smokers to smoking cues. Additional exploratory correlations were performed between cue-elicited CBF changes and lifetime cigarette usage and FTND.
Based on our first hypothesis, resting neuronal activity (in the form of resting CBF) and brain structure (gray matter density) were calculated for each ROI in each participant and compared between controls and smokers via group t-tests. The resting CBF was calculated from fixation blocks in the ASL scans. Similar to cue-elicited CBF data, the first effective TR in each block was also removed. The gray matter density was calculated as described below from T1-weighted structural data via FSL-VBM, a voxel-based morphometry analysis package (Ashburner and Friston, 2000). First, structural images were skull stripped and then tissue segmented into gray matter, white matter and CSF. The resulting gray matter partial volume images were aligned to standard space using an affine registration with the Image Registration Toolkit (Rueckert et al., 1999). The resulting images were averaged to create a study-specific template to which the native gray matter images were non-linearly re-registered. The registered partial volume images were then modulated (to correct for local expansion or contraction) by the Jacobian of the warp field and then smoothed with an isotropic Gaussian kernel with FWHM=8mm.
To test whether resting CBF and gray matter density were related to cue-elicited phasic CBF changes, Pearson’s correlation analyses were performed in each ROI between the cue-elicited CBF values (smoking-neutral condition) and both gray matter density and resting CBF. Additional exploratory correlations were performed between these measures and lifetime cigarette usage and FTND.
Based on our second hypothesis, rsFC strength was calculated between different cue-activated ROIs using resting-state BOLD fMRI data via AFNI and MATLAB (The MathWorks, Inc.). First, volumes were slice-timing aligned and motion corrected. After linear detrending of the time-course of each voxel, volumes were spatially normalized and resampled to Talairach space at 2×2×2mm3 and temporally low-pass filtered (f cutoff =0.1Hz) (Cordes et al., 2001). The time courses from all voxels in each ROI were averaged in each participant, followed by Pearson’s correlation analyses between the different ROIs. Fluctuations unlikely to be relevant to neuronal activity were regressed out as covariates including the six rigid head-motion parameter time-courses and the average time-courses in white matter and CSF (Fox et al., 2005; Lund et al., 2006). Correlation coefficients were transferred to z scores and then compared between groups and correlated to smoking cue-elicited CBF in corresponding ROIs and correlated with lifetime cigarette usage and FTND in exploratory within-smoker group analyses.
DTI analysis was based on tract-based spatial statistics (TBSS) (Smith et al., 2006), an automated, observer-independent method to allow group-wise comparisons, employed with an improved fractional anisotropy (FA) alignment method (Geng et al., 2009). According to the method of Pierpaoli et al., (1996), FA images were created by fitting the raw diffusion data to a tensor model. All FA datasets were simultaneously registered onto an implicit reference corresponding to the group average using implicit reference-based group (IRG) registration (Geng et al., 2009). Compared with conventional methods, which register each group image to a selected reference, the IRG registration eliminates the bias associated with reference selection and produces smaller registration error. All FA datasets were then transformed into standard space using an affine transformation. The mean FA image was built to create a mean FA skeleton via FSL (Smith et al., 2004). This skeleton represents the centers of all tracts common to the group. Each participant’s aligned FA data were then projected onto this skeleton.
Because our previous smoking-related FA results (unpublished) and the observed alterations in smoking cue-related rsFC strength (see Results) suggest only frontal cortex involvement, a frontal white matter skeleton mask was created and group comparisons with permutation-based testing (Nichols and Holmes, 2002) were performed within this mask to address our third hypothesis. The FA values from clusters showing significant group difference were averaged and correlated to the smoking cue-elicited CBF changes in frontal ROIs and the rsFC strengths between the corresponding ROIs.
Results
Whole brain CBF analysis of smoking cue provocation
Six clusters showed a significant (p<0.005 (F value>8.76) combined with a minimum cluster size of 1226 mm3 yields an FWE corrected p<0.05) interaction between group (smoker vs. control) and cue (smoking vs. neutral) from the whole brain ANOVA analysis. These clusters include bilateral dorsal medial prefrontal cortex (dmPFC), right dorsal lateral prefrontal cortex (dlPFC), bilateral dorsal anterior cingulate cortex/cingulate cortex (dACC/CC), right middle occipital gyrus (MOG), left insula/operculum (including operculum temporale into superior temporal gyrus and operculum frontoparietale into postcentral gyrus and inferior parietal)) (I/O) and bilateral rostral anterior cingulate cortex (rACC) (see Figure 2, Table 1). Data from each of these ROIs were used in subsequent secondary analyses.
Figure 2.
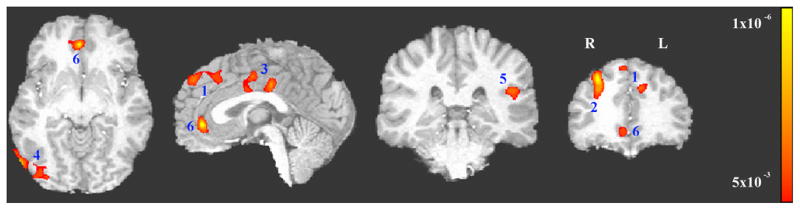
Significant interactions (FWE corrected p<0.05, i.e., uncorrected p<0.005 and minimal volume=1226mm3) between group (smokers vs. controls) and stimulus cue type (smoking vs. neutral). 1: bilateral dorsal medial prefrontal cortex (dmPFC), 2: right dorsal lateral prefrontal cortex (dlPFC); 3: bilateral dorsal anterior cingulate cortex/cingulate cortex (dACC/CC)), 4: right middle occipital gyrus (MOG), 5: left Insula/operculum, and 6: bilateral rostral anterior cingulate cortex (rACC).
Table 1.
Brain regions showing significant interaction between group (smokers vs. controls) and cue type (smoking vs. neutral)
| ROI | Brain region | Volume (mm3) | Talairach coordinate (mm) |
corrected P | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| dmPFC1 | Bilateral dorsal medial prefrontal cortex | 4166 | 3.9 | 41.2 | 39.7 | <0.001 |
| dlPFC1 | Right middle/superior prefrontal gyrus (dorsal lateral prefrontal cortex) | 3954 | 24.0 | 29.4 | 34.1 | <0.001 |
| dACC/CC | Bilateral dorsal anterior cingulate cortex | 4762 | 0.3 | −9.1 | 41.7 | <0.001 |
| MOG | Right middle occipital gyrus | 1957 | 47.5 | −76.7 | −0.3 | 0.003 |
| I/O | Insula;/left superior temporal gyrus/ | 1644 | −55.8 | −26.3 | 18.5 | 0.010 |
| rACC | Bilateral rostral anterior cingulate cortex | 1239 | 4.1 | 41.1 | −5.7 | 0.046 |
These clusters appeared connected at uncorrected p=0.005 and were separated at uncorrected p=.001 on the activation map based on structural independence (see supplementary materials).
Secondary ROI analyses
Smoking cue-elicited CBF phasic activity
The averaged CBF for each cue identified ROI during each of the cue conditions in both groups are listed in Table 2. There was a significant CBF increase in smokers to the smoking cue compared to the neutral cue condition in all six brain regions (see Table 2). Lifetime cigarette usage was positively correlated with the cue-elicited CBF change (smoking–neutral) in the rACC (r[18]=0.53, p=0.017; Figure 3A) and negatively related to that in the dlPFC (r[18]= −0.46, p=0.042; Figure 3B). There was no significant correlation between smoking cue-elicited CBF and FTND in any ROI.
Table 2.
Cue-elicited CBF (ml/100g/min) across groups and conditions
| ROI | Smoker | Control | Smoking vs neutral cue in smokers | Correlation with lifetime cigarette usage in smokers | Correlation with FTND in smokers | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Smoking cue | Neutral cue | Smoking cue | Neutral cue | |||||||
| mean±SD | mean±SD | mean±SD | mean±SD | t | p | R | p | r | p | |
| dmPFC | 44.7±14.9 | 38.7±13.6 | 39.9±17.3 | 45.2±15.5 | 6.15 | <0.001 | −0.08 | 0.725 | 0.16 | 0.494 |
| dlPFC | 29.1±9.0 | 25.1±7.9 | 26.1±10.8 | 33.6±10.6 | 6.29 | <0.001 | −0.46 | 0.042 | −0.17 | 0.477 |
| dACC/CC | 49.5±15.9 | 42.8±17.2 | 52.1±20.2 | 58.7±17.0 | 6.27 | <0.001 | 0.18 | 0.449 | −0.09 | 0.713 |
| MOG | 45.1±17.8 | 40.9±18.9 | 49.2±30.2 | 55.4±30.2 | 5.11 | <0.001 | −0.03 | 0.991 | −0.27 | 0.255 |
| I/O | 42.0±18.8 | 36.4±18.8 | 51.2±28.5 | 58.3±28.1 | 5.35 | <0.001 | 0.26 | 0.269 | −0.02 | 0.939 |
| rACC | 56.2±16.8 | 46.0±18.1 | 59.6±26.7 | 64.0±25.5 | 5.14 | <0.001 | 0.53 | 0.017 | 0.08 | 0.754 |
Figure 3.
A. The smoking cue-elicited CBF change in rACC was positively correlated with lifetime cigarette usage (pack-years). B. The smoking cue-elicited CBF change in dlPFC was negatively correlated with lifetime cigarette usage (pack-years). C. The smoking cue-elicited CBF change in dlPFC was positively correlated with gray matter density in the same area. All data were corrected for age and gender.
Resting CBF and gray matter density
To address our first hypothesis, gray matter density and baseline neuronal activity (i.e. resting CBF) and their relationship to smoking cue-elicited phasic CBF in each ROI were calculated (Table 3 and 4). Significantly lower resting CBF was found in the I/O (t[42]= −2.83, p=0.007), the rACC (t[42]= −2.79, p=0.008), and the dACC (t[42]= −2.15, p=0.037) in smokers compared to controls. There were no significant associations between resting CBF and the phasic smoking cue-elicited CBF changes, lifetime cigarette usage or FTND (see Table 3). Further, the gray matter density in dlPFC in smokers was significantly lower (t[42]= −2.62, p=0.012) than in controls and was positively correlated with smoking-related CBF cue changes (r[18]=0.54, p=0.013; Figure 3C), with a trend towards a negative correlation with lifetime cigarette usage (r[18]= −0.43, p=0.060). The gray matter in the I/O in smokers was also lower in controls (t[42]= −2.07, p=0.045). No other group differences or correlations were seen.
Table 3.
Resting CBF (ml/100g/min) and correlations with smoking cue-elicited CBF, lifetime cigarette usage and FTND
| ROI | Control | Smoker | Group t-test | Correlation to smoking cue-elicited CBF in smokers | Correlation with lifetime cigarette usage in smokers | Correlation to FTND in smokers | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean±SD | mean±SD | t | p | r | p | r | p | r | P | |
| dmPFC | 44.1±15.9 | 41.2±13.6 | −0.63 | 0.530 | 0.27 | 0.245 | 0.02 | 0.944 | 0.24 | 0.314 |
| dlPFC | 33.0±12.2 | 27.0±8.3 | −1.88 | 0.067 | 0.35 | 0.136 | 0.24 | 0.311 | 0.17 | 0.473 |
| dACC/CC | 57.8±18.1 | 46.7±16.2 | −2.15 | 0.037 | −0.25 | 0.297 | −0.09 | 0.710 | 0.19 | 0.421 |
| MOG | 50.1±28.7 | 40.6±18.6 | −1.31 | 0.200 | −0.19 | 0.415 | 0.30 | 0.197 | 0.39 | 0.091 |
| I/O | 57.3±26.8 | 38.4±16.2 | −2.83 | 0.007 | −0.24 | 0.320 | −0.16 | 0.505 | 0.14 | 0.554 |
| rACC | 68.7±22.5 | 52.7±14.8 | −2.79 | 0.008 | −0.25 | 0.279 | −0.26 | 0.270 | 0.04 | 0.884 |
Table 4.
Gray matter density and correlations with smoking cue-elicited CBF, lifetime cigarette usage and FTND
| ROI | Control | Smoker | Group t-test | Correlation to smoking cue-elicited CBF in smokers | Correlation with lifetime cigarette usage in smokers | Correlation to FTND in smokers | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean±SD | mean±SD | t | p | r | P | r | p | r | p | |
| dmPFC | 0.598±0.096 | 0.571±0.061 | −1.09 | 0.282 | 0.03 | 0.916 | −0.14 | 0.550 | 0.08 | 0.754 |
| dlPFC | 0.430±0.085 | 0.364±0.083 | −2.62 | 0.012 | 0.54 | 0.013 | −0.43 | 0.060 | −0.06 | 0.797 |
| dACC/CC | 0.631±0.072 | 0.673±0.104 | 1.57 | 0.125 | −0.06 | 0.796 | 0.35 | 0.127 | 0.44 | 0.050 |
| MOG | 0.579±0.130 | 0.575±0.096 | −0.11 | 0.912 | 0.12 | 0.626 | 0.11 | 0.655 | 0.12 | 0.604 |
| I/O | 0.597±0.113 | 0.533±0.090 | −2.07 | 0.045 | −0.39 | 0.090 | −0.25 | 0.289 | 0.22 | 0.346 |
| rACC | 0.652±0.132 | 0.585±0.103 | −1.87 | 0.069 | 0.23 | 0.323 | −0.03 | 0.903 | 0.23 | 0.320 |
RsFC
In reference to our second hypothesis, rsFC strength between the dlPFC and rACC was positively correlated to the cue-elicited CBF change in the dlPFC (r[14]=0.64, p=0.007; Figure 4A), while the rsFC strength between the dmPFC and dlPFC was positively related to the smoking cue-elicited CBF change in dmPFC (r[14]=0.62, p=0.010; Figure 4B). Further, rsFC strength between the dmPFC and the I/O was negatively correlated with the smoking cue-elicited CBF change in both dmPFC (r[14]= −0.55, p=0.027; Figure 4C) and I/O (r[14]= −0.55, p=0.026; Figure 4D). No group differences or additional significant correlations were found (Table 5).
Figure 4.
A. The rsFC strength between dlPFC and rACC was positively correlated with the smoking cue-elicited CBF change in dlPFC. B. The rsFC strength between dmPFC and dlPFC was positively correlated with smoking cue-elicited CBF change in dmPFC. C. The rsFC strength between dmPFC and Insula/operculum was negatively correlated with smoking cue-elicited CBF change in dmPFC. D. The rsFC strength between dmPFC and Insula/operculum was negatively correlated with smoking cue-elicited CBF change in Insula/operculum. All data were corrected for age and gender.
Table 5.
Averaged rsFC z scores between ROI pairs and correlations with smoking cue-elicited CBF, lifetime cigarette usage and FTND
| ROI to ROI | Smoker | Control | Group t-test | Correlation with smoking cue-elicited CBF changes in smokers |
Correlation with lifetime cigarette usage in smokers | Correlation to FTND in smokers | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First ROI | Second ROI | |||||||||||
| mean±SD | mean±SD | t | p | r | p | r | p | r | P | r | p | |
| dmPFC to dlPFC | 0.158±0.183 | 0.060±0.113 | 1.92 | 0.063 | 0.62 | 0.010 | 0.08 | 0.780 | 0.09 | 0.734 | 0.22 | 0.406 |
| dmPFC to dACC/CC | 0.098±0.161 | 0.110±0.096 | −0.28 | 0.784 | −0.15 | 0.574 | 0.41 | 0.117 | 0.02 | 0.947 | 0.07 | 0.810 |
| dmPFC to MOG | −0.005±0.085 | −0.007±0.090 | −0.40 | 0.694 | −0.36 | 0.169 | 0.05 | 0.860 | 0.13 | 0.645 | 0.22 | 0.412 |
| dmPFC to I/O | 0.048±0.123 | 0.047±0.094 | 0.03 | 0.977 | −0.55 | 0.027 | −0.55 | 0.026 | 0.11 | 0.696 | 0.11 | 0.690 |
| dmPFC to rACC/CC | 0.102±0.131 | 0.099±0.073 | 0.07 | 0.943 | 0.03 | 0.925 | 0.07 | 0.809 | −0.10 | 0.727 | −0.40 | 0.124 |
| dlPFC to dACC/CC | 0.032±0.162 | 0.030±0.095 | 0.05 | 0.960 | 0.10 | 0.726 | −0.10 | 0.714 | −0.25 | 0.353 | 0.26 | 0.327 |
| dlPFC to MOG | −0.021±0.100 | −0.002±0.066 | −0.67 | 0.512 | −0.24 | 0.371 | 0.36 | 0.170 | −0.30 | 0.268 | −0.13 | 0.645 |
| dlPFC to I/O | −0.010±0.105 | −0.001±0.074 | −0.29 | 0.774 | 0.11 | 0.696 | −0.13 | 0.643 | −0.20 | 0.461 | 0.23 | 0.387 |
| dlPFC to rACC | 0.025±0.126 | 0.008±0.117 | 0.42 | 0.680 | 0.64 | 0.007 | 0.04 | 0.888 | −0.27 | 0.319 | 0.23 | 0.384 |
| dACC/CC to MOG | 0.018±0.105 | 0.029±0.053 | −0.41 | 0.685 | 0.25 | 0.344 | −0.17 | 0.522 | 0.30 | 0.258 | 0.06 | 0.829 |
| dACC/CC to I/O | 0.165±0.115 | 0.218±0.114 | −1.40 | 0.171 | 0.41 | 0.115 | −0.10 | 0.721 | 0.29 | 0.283 | 0.04 | 0.872 |
| dACC to rACC | 0.075±0.110 | 0.067±0.091 | 0.26 | 0.800 | 0.39 | 0.989 | 0.06 | 0.834 | −0.28 | 0.292 | −0.11 | 0.696 |
| MOG to I/O | 0.022±0.090 | 0.039±0.102 | −0.53 | 0.599 | −0.14 | 0.602 | −0.01 | 0.984 | 0.20 | 0.461 | −0.13 | 0.644 |
| MOGto rACC | −0.041±0.078 | −0.003±0.049 | −1.75 | 0.090 | 0.33 | 0.209 | −0.31 | 0.247 | −0.26 | 0.323 | −0.05 | 0.852 |
| I/O to rACC | −0.005±0.103 | 0.030±0.086 | −1.11 | 0.274 | 0.11 | 0.679 | 0.49 | 0.056 | 0.29 | 0.270 | 0.29 | 0.269 |
White matter integrity (DTI)
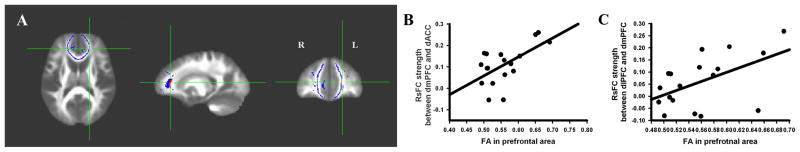
Consistent with our previous observations (unpublished), one cluster located in the left prefrontal area showed significantly lower FA in smokers than in controls (uncorrected p=0.005 and volume=45mm3 (yields an FWE corrected p=0.034), Talairach coordinate: x=19.5mm, y=−38.1mm, z=10.2mm; see Figure 5A). To address our third hypothesis, correlations between the FA data averaged from this cluster and the rsFC strength between frontal ROIs and phasic cue-elicited CBF activity in frontal ROIs were calculated. This regional FA was significantly correlated with the rsFC strength in controls between dACC and dmPFC (r[14]=0.59, p=0.015; Figure 5B) and between the dmPFC and the dlPFC (r[14]=0.53, p=0.036; Figure 5C). Finally, this FA reduction was not correlated with any other rsFC circuit between any of the frontal ROIs in either smokers or controls, nor did it correlate with phasic smoking cue-elicited activity in any frontal ROI in smokers (Table 6).
Figure 5.
A. The red cluster illustrates the significant FA difference between smokers and controls. The blue area is the prefrontal mask (based on the skeleton data). Because our previous results (unpublished) and all significant rsFC circuits were located in prefrontal cortex, only FA data within this prefrontal mask were included in the analysis. B. FA in this prefrontal cluster shown in A was positively correlated with the rsFC strength between dmPFC and dACC in non-smokers. C. FA in this prefrontal cluster shown in A was positively correlated with the rsFC strength between dmPFC and dlPFC in non-smokers. All data were corrected for age and gender.
Table 6.
Correlation between FA in the frontal cluster and rsFC strengths between frontal ROIs
| Control | Smoker | |||
|---|---|---|---|---|
| r | p | r | p | |
| dmPFC to rACC | 0.16 | 0.561 | 0.42 | 0.102 |
| dlPFC to rACC | −0.25 | 0.349 | −0.20 | 0.453 |
| dACC/CC to rACC | 0.45 | 0.078 | 0.13 | 0.643 |
| dmPFC to dlPFC | 0.53 | 0.036 | 0.39 | 0.134 |
| dmPFC to dACC/CC | 0.59 | 0.015 | −0.11 | 0.686 |
| dlPFC to dACC/CC | 0.01 | 0.962 | −0.31 | 0.237 |
Discussion
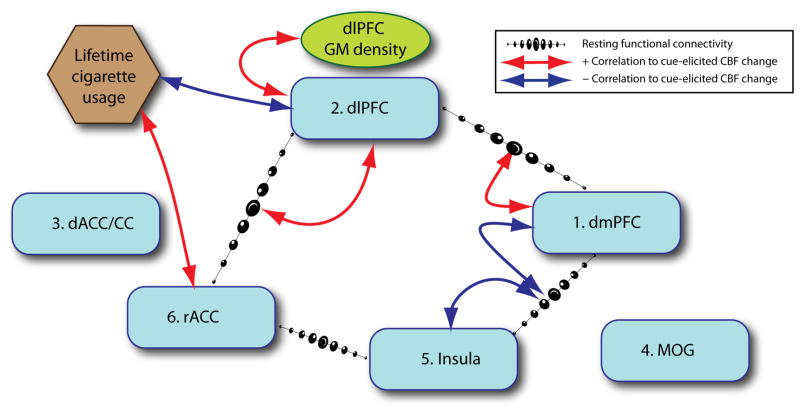
In this study, we measured changes in regional cerebral blood flow (CBF) while both smokers and non-smokers were exposed to smoking-related and matched neutral pictures. We found six brain areas that showed greater smoking cue-elicited activity in smokers, including dorsal medial prefrontal cortex (dmPFC), right dorsal lateral prefrontal cortex (dlPFC), dorsal anterior cingulate cortex/cingulate cortex (dACC/CC), right middle occipital gyrus (MOG), left insula/operculum and rostral anterior cingulate cortex (rACC). Using these 6 functionally identified ROIs as nodes in a rsFC analysis, we found that the connectivity strength between rACC and dlPFC was positively correlated with subsequent cue-elicited activity in dlPFC, while the rsFC strength between dlPFC and dmPFC was positively correlated with cue-elicited activity in dmPFC. Further, the rsFC strength between dmPFC and insula/operculum was negatively related to cue-elicited activity in both dmPFC and insula/operculum. In addition, gray matter density in the dlPFC was lower in smokers compared to controls and positively related to smoking cue-elicited activity in the same brain area. Further, lifetime exposure to cigarettes was correlated to smoking cue-elicited activity in dlPFC and rACC. Finally, prefrontal FA was reduced in smokers while resting CBF was reduced in three ROIs. Insofar as these secondary ROI analyses were not corrected for multiple comparisons, they should be considered exploratory. However the network and relationships they reveal are intriguing (see Figure 6). The implications of these results are discussed below.
Figure 6.
Summary of the correlations between functional connectivity, gray matter density, and lifetime cigarette usage to smoking cue-elicited CBF changes in smokers. The smoking cue-elicited CBF change in dlPFC was positively correlated to the rsFC between dlPFC and rACC, negatively with lifetime cigarette usage, and positively with gray matter density. The smoking cue-elicited CBF change in dmPFC was positively correlated to the rsFC between dlPFC and dmPFC and negatively with the rsFC between dmPFC and Insula/operculum. The smoking cue-elicited CBF change in Insula/operculum was negatively related to the rsFC between dmPFC and Insula/operculum. The smoking cue-elicited CBF change in rACC was positively associated with lifetime cigarette usage. Note, these hypothesized circuits and relationships should be considered exploratory.
Smoking cue-elicited CBF phasic changes
Six brain regions (dlPFC, dmPFC, dACC/CC, rACC, MOG, and insula/operculum, see Figure 2) demonstrated a significant increase in activation to cue presentation that was both group (smoker vs. control) specific and cue (smoking vs. neutral) selective. These functionally identified clusters were subjected to secondary ROI analysis (discussed below) in the present study partially because previous studies (David et al., 2005; Due et al., 2002; Franklin et al., 2007; Lee et al., 2005; McClernon et al., 2008b; Smolka et al., 2006) have not reported consistent results. For example, Franklin et al., (2007) and Lee et al., (2005) reported brain networks that had almost no overlapping areas. Lee et al., (2005) found activation almost entirely cortically located, including the prefrontal cortex, anterior cingulate gyrus, and supplementary motor area, while Franklin et al., (2007) reported predominantly sub-cortical areas, including ventral striatum, amygdala, hippocampus and medial thalamus. Further, McClernon et al. (2008b) and Smolka et al. (2006) both looked at correlations between smoking cue-evoked fMRI and FTND, and reported opposite results.
While there are many possible explanations for these discrepancies, different experimental paradigms (event-related design vs block design), neuroimaging tools (BOLD vs ASL), subject smoking status (ad lib smoking, time of abstinence), and/or data analysis method, are likely contributory. Indeed, Franklin et al., (2007) and Due et al., (2002) only analyzed data from a priori-selected brain areas, not from whole brain. In particular, Franklin et al., (2007) employed 10-minutes video/audio clip, which likely activated circuits distinct from those processing discrete pictures presented for only 5 sec by Lee et al., (2005). We also used a brief, discrete stimulus (1 sec in the present study), and interestingly, we too see predominantly cortical activation, with very similar regional distribution. Cue duration and the embedded context (complex in a video, discrete and isolated for a picture) likely activate and maintain (or not) different attentional networks that can have dramatic profound effects on cue processing.
The difference roles of these brain areas during smoking-related cue process may also help explain the disparate literature. Based on a prominent model of emotional processing (Phillips et al., 2003), these six brain areas may be divided into two groups, a ventral and a dorsal system. The ventral system, including rostral ACC and insula (and possibly opercular and occipital gyrus), has been implicated in emotional/motivational identification processes, and their activation in this study may thus reflect the motivational salience engaged in smokers by the smoking cues. The dorsal system, which includes the dorsal medial/lateral PFC and dorsal ACC, is thought to play a role in motivational regulation, suggesting that response regulatory resources, perhaps including response inhibition, are being engaged in response to smoking cues, especially inasmuch as subjects seeing these cues were required to remain in the scanner and in the lab for some time and could not freely act upon any cue-induced drive state.
Further, since McClernon et al., (2008a) and David et al., (2007) found that acute nicotine abstinence significantly alters BOLD activation to smoking cue in cigarette smokers, and since smokers in the current study were not permitted to smoke for at least 2 hours before scanning, our observed [smoker-nonsmoker] differences may reflect not only the learning-induced enhanced salience of the cues to smokers, but also the acute effects of very early nicotine withdrawal. Notably, the CBF difference between the smoking and neutral cue conditions in the rACC in smokers was positively correlated to total lifetime nicotine exposure, but not to FTND, a measure of addiction severity (Heatherton et al., 1991), suggesting a potential smoking-related compensation and/or learning effect.
Relationship between phasic cue activity and functional network/anatomical alterations
In an attempt to identify underlying structural and tonic functional brain alterations that might help explain the acute cue responses reported above, we performed exploratory correlation analyses between the phasic cue responses, rsFC circuits and gray matter density alterations. Each is discussed below.
Relationship between rsFC and smoking cue-induced phasic activation
It has recently been suggested that the strength of rsFC circuits reflect the ability to engage those circuits during active task processing and may thus serve as surrogates for behavioral performance and/or learning-induced plasticity. For example, motor learning modulates fronto-parietal resting state networks (Albert et al., 2009) and the performance on a language task affects resting state networks in regions associated with language (Waites et al., 2005). Further, Anticevic et al., (2010), Tambini et al., (2010), and Hampson et al., (2006) also reported that rsFC between posterior cingulate cortex, hippocampus, and frontal cortical regions can predict performance on working memory tasks.
In the present study, the rsFC strength between dlPFC and rACC was positively correlated with the increased phasic smoking cue activity magnitude in the dlPFC itself, suggesting that this circuit is engaged when a smoker is processing smoking cues. The rostral ACC belongs to the affective division of the cingulate cortex and is engaged by emotionally laden tasks in both normal healthy volunteers and in those with psychiatric disorders such as depression and drug dependence (Bush et al., 2000). Previous studies (Bush et al., 2000; Posner et al., 2007) have shown that the ventral/rostral ACC receives motivational/emotional information from sub-cortical regions (e.g., amygdala) and then transfers this information to and receives modulations from such higher-level cortical regions as the dlPFC. Further, convergent evidence supports a role for the dlPFC in cognitive control processes, including regulation of affective information induced by affective/emotional cues (reviewed by Badre, 2008; Phillips et al., 2003). Therefore, the positive correlation between rsFC strength of a dlPFC-rACC circuit and the increased dlPFC response to smoking cues suggests that this brain circuit is involved in processing the enhanced salience of smoking cues in smokers, most likely by regulating the salience-identifying response of the rACC. This raises the interesting possibility that connectivity in this circuit might be a potential target for therapeutic interventions.
Similarly, smoking cue-elicited activity in dmPFC was correlated to rsFC strength between dmPFC and dlPFC in smokers, further supporting a role for a dorsal PFC network in processing salient cues. It has been suggested that the dlPFC and dmPFC/ACC play different roles in cognitive control processes (Ridderinkhof et al., 2004), with the dmPFC/ACC involved in performance monitoring (e.g., cue-elicited arousal or motivation monitoring during cue provocation), while the dlPFC plays a role in subsequent performance control adjustments (e.g., arousal control/inhibition or action/motivation induced by cues). The positive connectivity strength between monitoring and control adjustment brain systems and their correlation with activity following cue presentation in the monitoring system component suggests a role for this circuit in salient cue processing.
Further, the rsFC strength between dmPFC and insula/operculum was negatively correlated with the phasic smoking cue activity magnitude in both dmPFC and insula/operculum. As discussed above, dmPFC plays a role in cue-elicited arousal or motivation monitoring. The insula is a key neural structure for representing the interoceptive effects of drug use, which are associated with conscious cue-induced urges (reviewed by Naqvi and Bechara, 2009). Naqvi et al., (2007) observed that cerebrovascular strokes in smokers limited to the insula lead to a profound reduction in post recovery smoking behavior and craving for cigarette. Further, previous studies also reported that the operculum (in particular opercular/SII) plays an important role in somatosensory and pain responding (Craig et al., 1996; Mazzola et al., 2006) and is related to anticipation of palatable food intake and consumption (Stice et al., 2009). The latter result appears consistent with our findings in that craving induced by cues is an anticipation of future consumption of a desirable commodity (cigarettes). Although the relationship of the dmPFC to the insula/operculum is not yet well understood, greater connectivity in the current study was associated with less cue reactivity in both regions, implying a sort of mutual inhibition of cue reactivity. Intriguingly, there was a trend towards a positive correlation between rsFC in the insula/operculum-rACC circuit and cue reactivity in the rACC (p=0.056), pointing to a possible mechanism for this region to influence (or be influenced by) cue reactivity in those prefrontal regions related to salience detection that is regulated by connectivity between insula and dmPFC, thus completing a brain circuit (i.e., rACC to dlPFC to dmPFC to insula to rACC) involved in smoking-related cue process.
Intriguingly and contrary to our hypothesis, while the cue provocation led to increased phasic activation in smokers, the rsFC strength in the above circuits did not differ between smokers and controls. It is thus possible that the rsFC relationship with regional cue-related activation may not be unique to smoking-related information per se, but rather a response characteristic of appetitive/motivational stimuli in general, of which smoking-related information in smokers is only one of many such categorical stimuli. As there were no appetitive/motivational stimuli presented to our subjects (e.g. appetitive food cues), this hypothesis cannot be tested using the current dataset, but would be of interest for future studies.
dlPFC in nicotine dependence
The dlPFC gray matter density was significantly lower in smokers compared to controls, which is consistent with previous reports (Brody et al., 2004; Gallinat et al., 2006) that also found a negative correlation with lifetime cigarette consumption. We also observed a similar negative correlation, but at a trend level (p = 0.060). Brody et al (2004) suggest that such group differences in dlPFC gray matter might reflect the cumulative effects of thousands of cigarette cue presentations or the pharmacological or neurotoxic properties of cigarettes. Given convergent data suggesting that smoking damages PFC functions (Ernst et al., 2001; Jacobsen et al., 2005) and the positive correlation of cue-induced activation with gray matter density (i.e., more ‘normalized’ gray matter density is associated with more activation to cues), it is possible that dlPFC activation to cues may reflect engagement of control systems to deal with the appetitive cue response, which weaken with extended exposure to cigarettes.
In further support of this interpretation, the dlPFC has been implicated in, among other functions, cognitive control and/or inhibitory processes, including regulation of information induced by affective/emotional cues (Badre, 2008; Phillips et al., 2003). Recently, Artiges et al., (2009) reported that smoking cues trigger an adaptive top-down process, e.g., cue-induced craving may induce inhibitory processing in smokers involving the right dlPFC. Further, dlPFC gray matter has been found to correlate positively to the inhibitory control necessary to perform the Stroop task (Haldane et al., 2008). We observed cue-induced activity in rACC that increases with lifetime exposure to cigarettes, while dlPFC cue-induced activity decreased with lifetime exposure, and is related to the strength of rsFC between rACC and dlPFC. In addition, dlPFC gray matter density trended towards a negative correlation with lifetime exposure, a result already demonstrated by several other groups. Taken together, a picture starts to emerge of possible loss of dlPFC regulation of cue responsivity in rACC related to continuous smoking behavior. The rACC cue response thus grows due to loss of descending regulatory input which, based on the relationship between rsFC and cue-induced activation, likely involves a circuit from rACC to dlPFC to dmPFC to insula/ST to rACC. Taking into account the exploratory character of our statistical analysis, this conclusion needs further empirical testing, perhaps using effective connectivity analysis.
Tonic brain differences unrelated to smoking cues
Finally, we identified a white matter cluster in the prefrontal lobe that was significantly reduced in smokers (Figure 5A), using a recently developed voxel-wise DTI analysis method (Geng et al., 2009; Smith et al., 2006). Further, the impaired prefrontal white matter integrity in this region was significantly correlated with rsFC strength between the dACC/CC and the dmPFC and between the dmPFC and the dlPFC in nonsmokers, suggesting that the absence of such a relationship in smokers may reflect the absence of coherence between white matter and rsFC that is normally present. Since FA in this cluster did not correlate with phasic cue-elicited activity in dACC/CC, dmPFC or dlPFC, the reduced FA is not likely to be specific to smoking cue reactivity. However, because the rsFC between dmPFC and dlPFC was significantly correlated to smoking-related cue activity (in smokers) and this FA cluster (in nonsmokers), this prefrontal white matter impairment may indirectly influence smoking-related cue processes via general smoking-related alterations in prefrontal areas, e.g., inhibition or control functions.
Further, rACC, insula/operculum and dACC/CC showed significantly lower resting CBF in smokers. This result is consistent with previous reports (Kubota et al., 1983; Siennicki-Lantz et al., 2002) of lower regional CBF in frontal and temporal regions in smokers. Interestingly, Kosten et al., (1998) and Pezawas et al., (2002) found a similar CBF decrease in mixed cocaine-alcohol and opioid users, respectively. The coupling between neuronal activity, metabolism and CBF is the basis of most functional neuroimaging methods (Villringer and Dirnagl, 1995; Wong et al., 1997). Therefore, lower regional CBF likely reflects lower tonic neural activity in smokers and is consistent with a previous study demonstrating reduced frontal brain metabolism in cocaine addicts (Volkow et al., 1992). Further, Peoples et al., (2007) reported that not only did prolonged cocaine self-administration reduce tonic accumbal neural activity in rats, short-term drug exposure was still able to increase activity in the same area, providing a potential mechanism underlying a similar CBF pattern in the present study, wherein we observed increased phasic cue-induced activity in the face of tonic decreased resting neural activity in the same areas.
While it is most likely that the resting CBF findings reflect lower tonic neural activity in smokers, one cannot eliminate potential alternative effects of chronic cigarette smoke and/or nicotine on rCBF regulatory mechanisms, vessel alterations due to embolism and/or vascular injury, alterations in blood viscosity or oxygen levels, and/or neuronal morphological alterations (Mathew and Wilson, 1991). A number of factors would argue against such ‘non-specific contributors to the observed signal changes. For example, reduced gray matter density was seen only in insula/operculum and dlPFC, limiting any morphological contributions to the regional effects reported. Further, although all our participants presented to scanning sessions with normal hematocrit and oxygen saturation levels, chronic smoking, perhaps due to elevated CO levels or other constituents in tobacco smoke, may nevertheless have damaged local cerebral vasculature (Leone, 2007). However, Murphy et al (2006) report that neuronal activity-BOLD coupling remains unchanged in chronic smokers and thus does not support an interpretation of ‘non-specific’ effect of nicotine and/or cigarette smoke on blood flow or alterations in coupling. As such, we suggest that alterations in resting CBF, rsFC and phasic CBF alterations to salient smoking pictures reported herein, accurately reflect alterations in neuronal activity.
Limitations and Conclusion
Several potential limitations of the present study merit consideration. As pointed out earlier, no direct behavioral measure of cue induced craving or drug seeking or control over this drive state were collected. According to Tiffany’s (2000) ‘cognitive process’ model, the drug-seeking drive elicited by cues becomes automatized, suggesting that drug-related stimuli presented in the current study should have resulted in an enhanced drive state. We elected not to gather craving ratings, as our primary focus was on neural responses to cues, and the very act of rating can alter such responses (Ochsner and Gross, 2005; Taylor et al., 2003). While this approach allowed us to assess neural responding to the cues unencumbered by other psychological processes, we have no measure that participant’s paid attention to the cues, or even looked at them. However, based on the commonality of regions we obtained and those seen in other cue reactivity studies, it would appear that subjects were compliant with task instructions, but there is no definitive verification of this in our design. Another limitation is the absence of smoking cue-elicited activity in sub-cortical areas, including the amygdala, hippocampus and nucleus accumbens is curious, as these regions have been reported in one or more drug-related cue studies (David et al., 2005; Franklin et al., 2007; Grant et al., 1996; Kilts et al., 2001; McClernon et al., 2007). Partial volume-induced signal reductions due to the relatively thick 8mm slices used in the ASL acquisition may help explain this null result. It is also possible, however, that, due to the complex interactions cited above for controlling responding to salient cues, some cues responses were obscured. Future connectivity studies, both at rest and during cue exposure, might help clarify this issue. Finally, multiple comparison correction was not employed in the secondary ROI analyses, reflecting the exploratory nature of this approach. As such, our findings should be seen as preliminary, with a large sample replication with more focused hypotheses warranted.
In conclusion, the present study attempted to expand our understanding of the neural basis for the enhanced salience of cues in chronic smokers by applying multi-modal imaging techniques to extend traditional differential activation to smoking cues. We found numerous changes in PFC, especially dlPFC, in smokers along with decreases in resting CBF, gray matter density, and the relationship between rsFC strength and phasic cue response magnitude. While considered preliminary, many of our findings are consistent with the extant literature, suggesting candidate neural circuitry underlying smoking cue responses along with changes in the circuitry with repeated smoking and daily environmental cue exposure. Understanding the changing role of the dlPFC and overall alterations in PFC function may be fruitfully applied in developing clinical strategies to assist with smoking cessation.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH. We thank Kimberly Modo and Loretta Spurgeon for their assistance in the conduct of some of the studies reported herein and Mary Pfeiffer for editing assistance.
Footnotes
Disclosure/Conflict of Interest
None
The authors reported no financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiges E, Ricalens E, Berthoz S, Krebs MO, Penttila J, Trichard C, Martinot JL. Exposure to smoking cues during an emotion recognition task can modulate limbic fMRI activation in cigarette smokers. Addict Biol. 2009;14:469–477. doi: 10.1111/j.1369-1600.2009.00167.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Mackillop J, Sweet LH, Cohen RA, Niaura R, Rogers RD, Matthews PM, Walton RT. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Fellows J, Trosclair A, Adams E, Rivera C. Annual smoking-attributable mortality, years of potential life lost, and economic costs: United States, 1995–1999. MMWR Morb Mortal Wkly Rep. 2002;51:300–303. [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Geng X, Christensen GE, Gu H, Ross TJ, Yang Y. Implicit reference-based group-wise image registration and its application to structural and functional MRI. Neuroimage. 2009;47:1341–1351. doi: 10.1016/j.neuroimage.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International Smoking Image Series (With Neutral Counterparts), version 1.2 ed. Carbondale: Integrative Neuroscience Laboratory; 1999. [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane M, Cunningham G, Androutsos C, Frangou S. Structural brain correlates of response inhibition in Bipolar Disorder I. J Psychopharmacol. 2008;22:138–143. doi: 10.1177/0269881107082955. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, Shea P, Solomon LJ, Flynn BS. Smoking cessation among self-quitters. Health Psychol. 1992;11:331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Cheeves C, Palumbo J, Seibyl JP, Price LH, Woods SW. Regional cerebral blood flow during acute and chronic abstinence from combined cocaine-alcohol abuse. Drug Alcohol Depend. 1998;50:187–195. doi: 10.1016/s0376-8716(98)00038-6. [DOI] [PubMed] [Google Scholar]
- Kubota K, Yamaguchi T, Abe Y, Fujiwara T, Hatazawa J, Matsuzawa T. Effects of smoking on regional cerebral blood flow in neurologically normal subjects. Stroke. 1983;14:720–724. doi: 10.1161/01.str.14.5.720. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- Leone A. Smoking, haemostatic factors, and cardiovascular risk. Curr Pharm Des. 2007;13:1661–1667. doi: 10.2174/138161207780831347. [DOI] [PubMed] [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52:679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Lund TE, Madsen KH, Sidaros K, Luo WL, Nichols TE. Non-white noise in fMRI: does modelling have an impact? Neuroimage. 2006;29:54–66. doi: 10.1016/j.neuroimage.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Substance abuse and cerebral blood flow. Am J Psychiatry. 1991;148:292–305. doi: 10.1176/ajp.148.3.292. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Mauguiere F. Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex. 2006;16:960–968. doi: 10.1093/cercor/bhj038. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2008a doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008b;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Pauli P. Modulation of craving by cues having differential overlap with pharmacological effect: evidence for cue approach in smokers and social drinkers. Psychopharmacology (Berl) 1999;147:306–313. doi: 10.1007/s002130051172. [DOI] [PubMed] [Google Scholar]
- Murphy K, Dixon V, LaGrave K, Kaufman J, Risinger R, Bloom A, Garavan H. A validation of event-related FMRI comparisons between users of cocaine, nicotine, or cannabis and control subjects. Am J Psychiatry. 2006;163:1245–1251. doi: 10.1176/ajp.2006.163.7.1245. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Kravitz AV, Lynch KG, Cavanaugh DJ. Accumbal neurons that are activated during cocaine self-administration are spared from inhibitory effects of repeated cocaine self-administration. Neuropsychopharmacology. 2007;32:1141–1158. doi: 10.1038/sj.npp.1301203. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Fischer G, Podreka I, Schindler S, Brucke T, Jagsch R, Thurnher M, Kasper S. Opioid addiction changes cerebral blood flow symmetry. Neuropsychobiology. 2002;45:67–73. doi: 10.1159/000048679. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di CG. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7:391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Siennicki-Lantz A, Lilja B, Elmstahl S. Cerebral perfusion deficits in age-associated memory impairment. The role of tobacco smoking. Aging Clin Exp Res. 2002;14:108–116. doi: 10.1007/BF03324424. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De LM, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De SN, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Villringer A, Dirnagl U. Coupling of brain activity and cerebral blood flow: basis of functional neuroimaging. Cerebrovasc Brain Metab Rev. 1995;7:240–276. [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van E, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed. 1997;10:237–249. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<237::aid-nbm475>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.