Abstract
The SNARE-binding protein complexin (Cpx) has been demonstrated to regulate synaptic vesicle fusion. Previous studies are consistent with Cpx functioning either as a synaptic vesicle fusion clamp to prevent premature exocytosis, or as a facilitator to directly stimulate release. Here we examined conserved roles of invertebrate and mammalian Cpx isoforms in the regulation of neurotransmitter release using the Drosophila neuromuscular junction as a model synapse. We find that SNARE binding by Cpx is required for its role as a fusion clamp. All four mammalian Cpx proteins (mCpx), which have been demonstrated to facilitate release, also function as fusion clamps when expressed in Drosophilacpx null mutants, though their clamping abilities varies between isoforms. Moreover, expression of mCpx I, II or III isoforms dramatically enhance evoked release compared to mCpx IV or Drosophila Cpx. Differences in the clamping and facilitating properties of complexin isoforms can be partially attributed to differences in the C-terminal membrane tethering domain. Our findings indicate that the function of complexins as fusion clamps and facilitators of fusion are conserved across evolution, and that these roles are genetically separable within an isoform and across different isoforms.
Keywords: Exocytosis, Synapse, Neurotransmitter Release, Synaptic Vesicle, SNARE complex
INTRODUCTION
Neurotransmitter release is a tightly regulated process accompanied by cycles of assembly and disassembly of SNARE complexes during rounds of synaptic vesicle fusion (Littleton et al., 1998; Sollner et al., 1993). The requirement for the core SNARE machinery to form a parallel four-helix bundle to bring fusing membranes together is shared by most intracellular fusion processes. At synapses, SNARE-mediated fusion is uniquely regulated to allow rapid and calcium-triggered synaptic vesicle fusion. These adaptations to the core fusion machinery require synapse specific SNARE-binding proteins, including Synaptotagmin 1 (Syt 1) and complexin (Cpx). Syt 1 is a synaptic vesicle protein that binds to SNARE complexes and membrane phospholipids in a calcium-dependent manner, functioning as the calcium sensor for fast synchronous neurotransmitter release (Geppert et al., 1994; Xu et al., 2007; Yoshihara and Littleton, 2002). In contrast with the established function for Syt 1, Cpx's role in synaptic vesicle fusion is less clear. Cpx is a small cytosolic α-helical protein that binds assembled SNARE complexes (Bracher et al., 2002; Chen et al., 2002; McMahon et al., 1995; Pabst et al., 2000). Association with SNAREs has been suggested to allow Cpx to function as a facilitator for synaptic vesicle fusion (Reim et al., 2001; Xue et al., 2007; Xue et al., 2008) or as a fusion clamp to prevent premature exocytosis in the absence of calcium (Huntwork and Littleton, 2007; Maximov et al., 2009).
Evidence supporting a role for Cpx as a synaptic vesicle fusion clamp has been suggested by both biochemical studies (Giraudo et al., 2006; Schaub et al., 2006) and genetic knockouts in Drosophila (Huntwork and Littleton, 2007). Drosophila has a single Cpx homolog (DmCpx) that is enriched in presynaptic nerve terminals. Deletion of Drosophilacpx results in a dramatic increase in spontaneous synaptic vesicle fusion, with a corresponding reduction in evoked release (Huntwork and Littleton, 2007), indicating that Cpx functions as a vesicle clamp to prevent calcium-independent fusion. The fusion clamp model is also supported by biochemical studies demonstrating that Cpx can inhibit SNARE-mediated fusion in cell-cell or liposome fusion assays (Giraudo et al., 2006; Giraudo et al., 2009; Schaub et al., 2006). In contrast to the single Cpx isoform in Drosophila, mammals have four Cpx genes encoded in their genome (Reim et al., 2005). Removal of Cpxs at mammalian synapses has been shown to increase spontaneous release in cortical neurons (Maximov et al., 2009) or decrease spontaneous release at autapses or GABAergic synapses (Reim et al., 2001; Strenzke et al., 2009; Xue et al., 2007; Xue et al., 2008). Similar to Drosophila, mammalian synapses lacking Cpxs also exhibit reduced evoked release. The distinct effects of Cpx on spontaneous release at different synapses within and across species suggest the protein may play multiple roles in synaptic vesicle trafficking depending upon the synaptic environment. Alternatively, Cpx may have a conserved role in fusion that manifests differently depending upon the presence or absence of other synaptic components that modify the fusion properties of synaptic vesicles. A key test of these two models is determining whether the distinct roles of Cpx in clamping fusion and promoting release can be genetically separated, which would argue for multiple roles of Cpx during the synaptic vesicle cycle. Recent studies suggest Cpx contains both facilitating and inhibitory functions that are differentially manifest between invertebrate and mammalian isoforms (Xue et al., 2009).
To further examine these alternative models for Cpx function, we extended our previous analysis of Drosophila Cpx and tested whether mammalian Cpx (mCpx) isoforms can functionally substitute for the loss of Cpx at Drosophila neuromuscular junction (NMJ) synapses. We demonstrate that SNARE binding by Cpx is required for its roles as both a fusion clamp and a facilitator of vesicle fusion. All mCpx isoforms can partially rescue the cpx mini frequency phenotype, suggesting the ability of Cpx to act as a fusion clamp is evolutionarily conserved. Strikingly, mCpx IV, like the Drosophila homolog, can potently clamp spontaneous release compared to the other mCpx isoforms. To examine properties that might confer the ability of mCpx IV and DmCpx to function as potent fusion clamps, we investigated the C-terminal CAAX-box prenylation motif conserved in mCpx III, mCpx IV, and DmCpx. We find that the CAAX-box motif is necessary but not sufficient for Cpx to act as a fusion clamp. Finally, we show that mCpxs I, II, and III dramatically promote enhanced evoked release, whereas mCpx IV or DmCpx do not. Unexpectedly, expression of DmCpx lacking the CAAX-box motif in the cpx nulls enhances evoked release compared to WT DmCpx, suggesting that prenylation may partially mask facilitatory properties of Cpx. These experiments suggest that the dual function of Cpxs as fusion clamps and facilitators of release is evolutionarily conserved, requires SNARE binding, and can be genetically separated.
RESULTS
DmCpx function as a fusion clamp requires SNARE binding
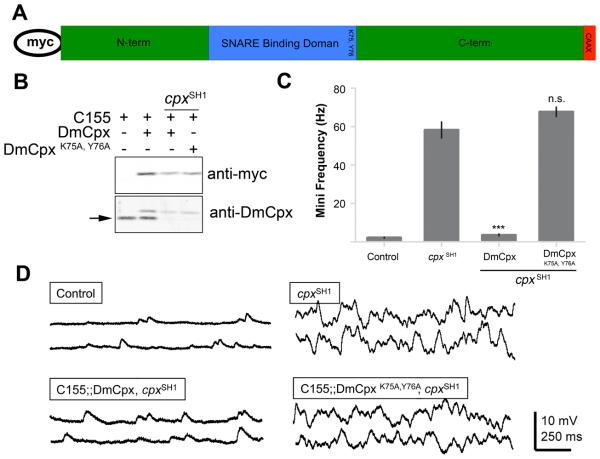
Genetic approaches have suggested two potential roles for Cpxs in synaptic exocytosis – as a fusion clamp and/or a facilitator of vesicle fusion. To further define the role of Cpx in neurotransmitter release, we examined similarities and differences in the function of Drosophila and mammalian Cpx isoforms at Drosophila NMJ synapses. Cpx is a small cytoplasmic protein with a central α-helix that binds the SNARE complex (Bracher et al., 2002; Chen et al., 2002) (Figure 1A). The SNARE binding domain of Cpx is highly conserved across evolution (Brose, 2008) and is essential for Cpx's role in facilitating synaptic vesicle fusion at mammalian synapses. Lysine 75 and tyrosine 76 flank the SNARE binding domain and are critical for Cpx-SNARE binding (Bracher et al., 2002; Chen et al., 2002; Xue et al., 2009; Xue et al., 2007). To determine if DmCpx must also engage SNARE complexes for its role in clamping spontaneous synaptic vesicle fusion, we generated transgenic lines expressing DmCpx with lysine 75 and tyrosine 76 mutated to alanine (DmCpxK75A, Y76A) in the cpx null background under control of the GAL4-UAS system. We used the phiC31-attP recombination system to insert all cpx transgenes into the same integration site on the 3rd chromosome, allowing identical transcriptional levels for each transgene. Cpx was also tagged with an N-terminal myc epitope to allow unambiguous identification of the transgenic protein in western and immunocytochemical studies. Western analysis demonstrates that DmCpxK75A,Y76A is expressed at similar levels to wildtype (WT) DmCpx in transgenic lines (Figure 1B), and that expression of Syt 1 or the t-SNARE syntaxin are unaltered (Supplementary Figure 1A). In addition, DmCpxK75A,Y76A localizes normally to synapses at Drosophila NMJs (Supplementary Figure 2), indicating SNARE binding is not required for Cpx targeting or retention at synapses. To test the functional effects of DmCpxK75A,Y76A on synaptic transmission, we performed electrophysiological recordings at 3rd instar larval abdominal muscle 6 synapses in cpx null mutants, and compared synaptic properties between the mutant and WT cpx transgenes expressed pan-neuronally with elav-GAL4. In cpx null mutants, miniature excitatory junctional potential (mini) frequency is greatly enhanced compared to WT controls (58.2 ± 4.2 Hz compared to 2.2 ± 0.3 Hz at control synapses, Figures 1C, D). Transgenic expression of WT DmCpx in the cpx null background significantly reduces mini frequency to near control levels (3.5 ± 0.5 Hz, Figures 1C, D). In contrast, expression of DmCpxK75A,Y76A does not rescue the dramatic increase in spontaneous fusion (67.7 ± 2.5 Hz, Figures 1C, D). These data demonstrate that SNARE binding is required for the function of Cpx as a fusion clamp at Drosophila synapses, similar to the requirement for SNARE binding for the function of mCpx as a facilitator and DmCpx as a clamp at mammalian synapses (Xue et al., 2009; Xue et al., 2007).
Figure 1.
DmCpx function as a fusion clamp requires the SNARE binding residues lysine 75 and tyrosine 76. (A) Schematic of Cpx protein domains. All transgenic lines generated in this study encoded a Cpx with an N-terminal myc tag. The SNARE binding domain is indicated in blue, and the localization of K75 and Y76 is shown. The C-terminal CAAX-box motif contained in some Cpx isoforms is shown in red. (B) The DmCpxK75A,Y76A transgenic protein is expressed at levels similar to the WT DmCpx transgenic protein and endogenous Cpx in lysates from adult transgenic heads. The top blot shows western probed with anti-myc antisera that recognizes the transgenic proteins driven by C155elav-Gal4 in WT or cpxSH1 background as indicated. The bottom blot is probed with anti-Drosophila Cpx antisera, which recognizes both endogenous and transgenic protein. Myc-tagged Cpx runs slower and can be separated from endogenous Cpx (indicated by the arrow). (C) Summary of mean mini frequency (Hz ± SEM). n = control (10), cpxSH1 (7), DmCpx (8), DmCpxK75A,Y76A (6). Statistical significance was determined by Student's t-test. (D) Sample traces of minis in control, cpxSH1, and rescue lines expressing WT DmCpx or DmCpxK75A,Y76A.
mCpxs rescue the cpx mini phenotype at Drosophila NMJ
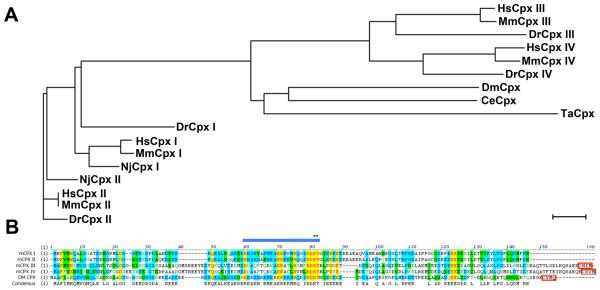
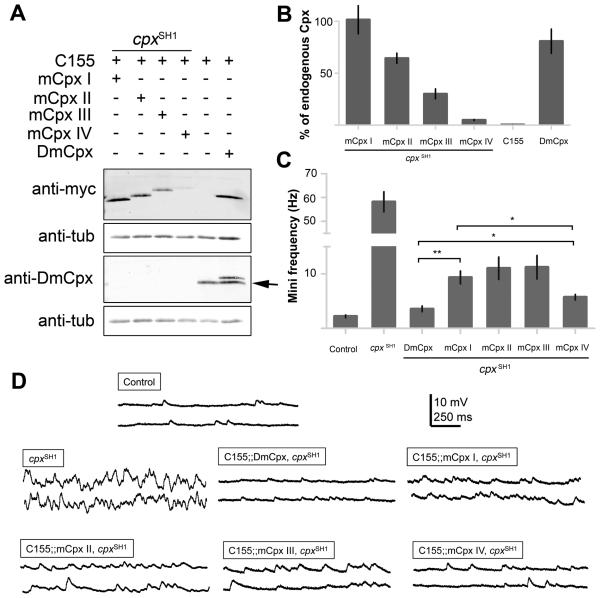
Given the conserved role of SNARE binding in Cpx function, we next examined whether mammalian Cpx isoforms could substitute for Drosophila Cpx as a fusion clamp. Phylogenetic analysis of Cpxs reveals two subgroups consisting of Cpx I and II, and Cpx III and IV (Figure 2A). DmCpx is phylogenetically more similar to Cpx III and IV. In addition, DmCpx and mCpxs III and IV share a C-terminal CAAX-box motif (Figure 2B) required for post-translational prenylation by addition of either a farnesyl (15-carbon) or a geranylgeranyl (20-carbon) moiety at the cysteine residue (Zhang and Casey, 1996). From an evolutionary perspective, both Cpx and Syt are absent from the single cell stage of evolution and are first found in primitive metazoan branches before the emergence of neurons and synapses (Barber et al., 2009). The first Cpx isoform in eukaryotes appears in the genome of the simple placozoan, Trichoplax adhaerens (Srivastava et al., 2008). This Cpx also contains a CAAX-box motif, suggesting that the prenylated version of Cpx represents the ancestral form of the protein. To test whether mCpxs can functionally substitute for DmCpx, we generated transgenic Drosophila expressing myc-tagged versions of the four mCpx isoforms. Although all transgenes generated in this study were integrated into the same AttP locus of the 3rd chromosome to avoid expression variation due to positional effects, mCpxs are expressed at different levels when driven with elav-GAL4 (mCpx I = 101.5 ± 13.6 %, mCpx II = 64.4 ± 5.0 %, mCpx III = 30.1 ± 4.8 %, mCpx IV = 5.0 ± 0.5 %, all relative to endogenous DmCpx; see Figure 3A, B). Since positional effects at the genomic level can be excluded, it is likely that expression differences between the mCpx isoforms reflect differential stability of the proteins. Although the transgenic proteins are expressed at distinct levels, all four isoforms target to presynaptic boutons (Supplementary Figure 3). In addition, the expression levels of syntaxin and Syt 1 are unaltered in Cpx transgenic rescues lacking endogenous DmCpx (Supplementary Figure 1B).
Figure 2.
Comparison of Cpx proteins. (A) Phylogenetic analysis of Cpx from the indicated species. Hs = Homo sapiens, Mm = Mus musculus, Dr = Danio rerio, Nj = Narke japonica, Ce = Caenorhabditis elegans, Dm = Drosophila melanogaster, Ta = Trichoplax adhaerens. The scale bar shows the percentage (5%) of amino acid substitutions required to generate the corresponding tree distances. (B) Alignment analysis of mouse Cpx isoforms and DmCpx. The SNARE-binding domain is indicated by the blue bar. Residues within the SNARE binding region that were mutated (see Figure 1) are indicated by *. The C-terminal CAAX-box motifs in mCpx III and IV and DmCpx is indicated by red box. Color code: black resides are non-similar residues. Blue residues on cyan are consensus amino acids derived from a block of similar residues at a given position. Black residues on green are consensus amino acids derived from the occurrence of greater than 50% of a single residue at a given position. Red residues on yellow are consensus residues derived from a completely conserved amino acid at a given position. Green residues are similar to the consensus amino acid at a given position.
Figure 3.
mCpxs rescue the cpxSH1 mutant mini frequency phenotype. (A) Expression of mCpx isoforms in Drosophila. The top blot shows western from adult head lysates probed with anti-myc antisera detect transgenic protein driven by C155elav-Gal4 in cpxSH1 or WT background as indicated. The bottom blot is probed with anti-Drosophila Cpx antisera. The last lane shows myc-tagged DmCpx expressed in the WT background. Anti-DmCpx detects myc-tagged DmCpx as well as endogenous DmCpx (arrow), allowing comparable expression analysis of all myc-tagged Cpxs with endogenous Cpx levels. (B) Summary of relative levels of transgenic myc-tagged Cpxs compared to endogenous DmCpx (% of endogenous DmCpx ± SEM, n = 3). Anti-DmCpx antisera detects both myc-tagged DmCpx and endogenous DmCpx (see Figure 3A last lane), allowing for relative expression analysis. (C) Summary of mean mini frequency (Hz ± SEM) for each line. n = control (10), cpxSH1 (7), DmCpx (8), mCpx I (12), mCpx II (5), mCpx III (5), mCpx IV (9). Horizontal lines indicate statistical comparisons determined by Student's t-test. (D) Sample traces of minis in control, cpxSH1, and rescue lines expressing DmCpx and the indicated mCpxs.
Functional rescue of the mCpx isoforms was first assayed by analyzing the cpx mutant mini phenotype in transgenic animals expressing each mCpx. All four mCpx isoforms significantly reduce mini frequency compared to cpx null mutants (mCpx I = 9.4 ± 1.2 Hz, mCpx II = 11.0 ± 2.0 Hz, mCpx III = 11.2 ± 2.2 Hz, and mCpx IV = 5.7 ± 0.5 Hz; Figure 3C, D). However, mini frequencies are significantly elevated in the rescue lines compared to control and the WT DmCpx rescue (2.2 ± 0.3 Hz and 3.6 ± 0.5 Hz, respectively). Interestingly, mCpx III and mCpx IV, despite expressing at much lower levels, rescue the fusion clamp phenotype to a degree comparable (mCpx III) or stronger (mCpx IV) than mCpx I and II. The ability of mCpx IV to rescue the mini phenotype is particularly striking, as its expression is much lower (5.0% of endogenous DmCpx) than the other Cpx transgenes, but rescues most similarly to DmCpx (DmCpx = 3.6 ± 0.5 compared to mCpx IV = 5.7± 0.5 Hz; Figure 3C, D). These results indicate that mCpxs have conserved functions as fusion clamps, with mCpx IV being particularly effective as a synaptic vesicle fusion clamp.
DmCpx clamping function requires C-terminal prenylation motif
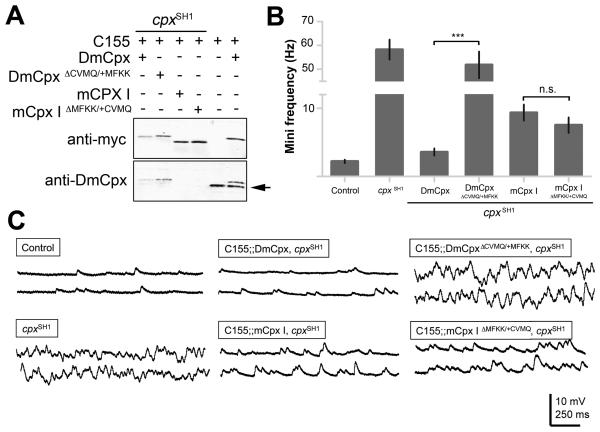
Given the ability of mCpx IV and mCpx III to rescue the mini phenotype of cpx null mutants despite their low expression levels, we assayed whether the prenylation CAAX-box motif plays a key role in clamping spontaneous synaptic vesicle fusion. Prenylation of proteins allows targeting of CAAX-box proteins to membranes (Zhang and Casey, 1996) and could potentially increase the local concentration of Cpx at release sites. To characterize the functional significance of Cpx prenylation, we generated chimeras of DmCpx and mCpx I that interchanged the last 4 residues, removing the CAAX box from DmCpx and adding it to mCpx I (DmCpx ΔCAAX/+MFKK and mCpx I ΔMFKK/+CAAX), allowing us to determine how prenylation impacts their function. All transgenes are expressed at similar levels compared with each other, and with endogenous DmCpx, as assayed by western analysis (Figure 4A). In addition, the transgenic proteins target and localize to presynaptic boutons of larval NMJs (Supplementary Figure 2). mCpx I localizes to synaptic boutons with or without the CAAX-box (Supplementary Figure 2), indicating mCpx I is targeted to presynaptic boutons independent of prenylation. To analyze the functional significance of Cpx prenylation, we assayed the ability of the transgenes to rescue the cpx null phenotype. Transgene expression of DmCpx in the cpx mutant significantly reduces the mini frequency to near control levels (3.5 ± 0.5 Hz, Figures 4B, C). In contrast, expression of DmCpx ΔCAAX/+MFKK does not rescue the mutant phenotype (51.9 ± 5.6 Hz, Figures 4B, C), demonstrating that the CAAX-box motif is required for DmCpx function as a vesicle clamp. mCpx I partially rescues the mini phenotype, but to a lesser degree than DmCpx (9.4 ± 1.2 Hz compared to 3.5 ± 0.5 Hz, Figures 4B, C). Addition of the CAAX-box motif to mCpx I does not significantly enhance its ability to rescue the cpx phenotype (7.5 ± 1.1 Hz). These data indicate that Drosophila Cpx requires prenylation for its in vivo function as a clamp, and that mCpx I and II have evolved distinct targeting mechanisms to enrich the protein at release sites, or alternative mechanisms for clamping fusion.
Figure 4.
DmCpx function as a fusion clamp requires the C-terminal CAAX-box motif. (A) Expression of DmCpx, mCpx I and chimeras that interchanged the last 4 residues of each protein (DmCpxΔCVMQ/+MFKK and mCpx IΔMFKK/+CVMQ). The top blot shows western probed with anti-myc antisera, which detects myc-tagged transgenic proteins driven by C155elav-Gal4 in cpxSH1 or the WT background as indicated. The bottom blot is probed with anti-Drosophila Cpx antisera. Endogenous Cpx is highlighted with the arrow. (B) Summary of mean mini frequency (Hz ± SEM) for each line. n = control (10), cpxSH1 (7), DmCpx (8), DmCpxΔCVMQ/+MFKK (6), mCpx I (12), mCpx IΔMFKK/+CVMQ (7). Horizontal lines indicate statistical comparisons determined by Student's t-test. (C) Sample traces of minis in control, cpxSH1 and rescue strains expressing the indicated Cpxs and chimeric proteins.
Differences in Cpx function as facilitators depends on C-terminal prenylation motif
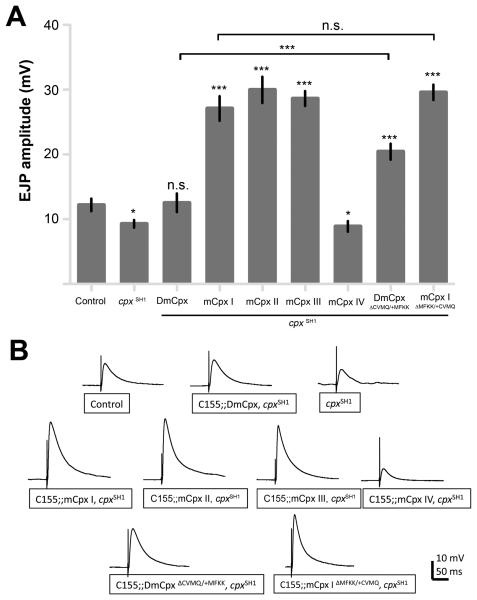
Our physiological analysis indicates that mammalian Cpxs can function as fusion clamps at Drosophila synapses, though with a lower efficiency than DmCpx. In addition to suppressing spontaneous release, Cpx also functions as a positive facilitator of transmitter release at both Drosophila and mammalian synapses (Huntwork and Littleton, 2007; Maximov et al., 2009; Reim et al., 2001; Xue et al., 2007). To determine whether mCpxs isoforms can rescue evoked defects at cpx null synapses, and if prenylation plays any role in its function similar to that observed with the regulation of spontaneous release, we examined evoked responses (EJPs) in the transgenic rescues. cpx mutants exhibit reduced EJP amplitudes compared to controls (9.3 ± 0.6 mV versus 12.2 ± 1.0 mV at control synapses in 0.2 mM extracellular calcium; Figures 5A, B). Transgene expression of DmCpx in cpx null mutants rescues the reduced evoked phenotype (12.5 ± 1.4 mV). Unexpectedly, we observed dramatic differences amongst the mCpxs in the rescue of evoked release in cpx null mutants (Figures 5A, B). mCpxs I, II, and III greatly enhance EJP amplitude (27.1 ± 1.9 mV, 30.0 ± 2.0 mV, and 28.6 ± 1.1 mV, respectively) in cpx mutants, far more than rescue with endogeneous Drosophila Cpx. In contrast, mCpx IV exhibits no rescue of the reduced evoked response (8.9 ± 0.8 mV). These data indicate mCpxs I, II, and III are strong activators of synaptic vesicle exocytosis at Drosophila synapses, while retaining some basic fusion clamp properties. In contrast, mCpx IV can effectively function as a fusion clamp, even at low expression levels, but does not display a robust rescue of the activating function of Cpx in synaptic vesicle fusion.
Figure 5.
mCpxs exhibit dramatic differences in facilitating evoked release in cpx null mutants. (A) Summary of mean EJP amplitude (mV ± SEM). n = control (12), cpxSH1 (7), DmCpx (11), mCpx I (12), mCpx II (15), mCpx III (10), mCpx IV (22), DmCpxΔCVMQ/+MFKK (15), and mCpx IΔMFKK/+CVMQ (19). Statistical comparisons were made with control, unless otherwise indicate by horizontal lines, using Student's t-test. (B) Averaged traces of EJPs in 0.2 mM calcium from control, cpxSH1 and rescued strains expressing the indicated transgenic protein.
Could C-terminal prenylation of Cpxs play a functional role in differential regulation of evoked responses? To test this hypothesis, we measured EJPs from cpx null mutants expressing DmCpx ΔCAAX/+MFKK and mCpx I ΔMFKK/+CAAX chimeras (Figures 5A, B). Remarkably, expression of DmCpx ΔCAAX/+MFKK, which does not rescue the mutant mini phenotype, enhances EJP amplitude (20.4 ± 1.2 mV) compared to rescue by WT DmCpx. In contrast, expression of mCpx I ΔMFKK/+CAAX exhibits a similar enhancement of EJP amplitude (29.6 ± 1.3 mV) compared to WT mCpx I. These data provide evidence that the two functions of Cpx in regulating neurotransmitter release – as a fusion clamp and facilitator of fusion – are separable in vivo, and that the distinct functionality of Cpx can be partially accounted for by C-terminal prenylation.
DISCUSSION
Synaptic transmission requires several SNARE-interacting proteins to modify the basic cellular fusion machinery to meet the unique requirements for rapid calcium-activated synaptic vesicle fusion. The best understood of these synapse-specific regulators is the calcium sensor synaptotagmin. In addition to Syt I, Cpx has been shown to bind 1:1 in a stoichiometric fashion to the neuronal SNARE complex and to regulate synaptic vesicle fusion. However, Cpx's specific role in vesicle fusion is unclear, as genetic studies in mice and Drosophila have suggested distinct functions. Likewise, in vitro fusion assays employing Cpx and SNAREs as the fusion machinery have suggested potentially divergent roles (Giraudo et al., 2006; Malsam et al., 2009; Schaub et al., 2006; Seiler et al., 2009). Using autaptic neuronal cultures, loss of Cpx I and II in mice was found to specifically reduce fast synchronous evoked fusion, without altering spontaneous release rates or synaptic vesicle pool sizes (Reim et al., 2001). These findings argued that Cpx plays a facilitating role in the calcium-triggering step of fusion. Supporting studies in acute brain slices also revealed a specific defect in calcium-triggered evoked fusion (Xue et al., 2008). The function of Cpx as a fusion clamp was supported by our analysis of null mutants in the sole Drosophila cpx homolog (Huntwork and Littleton, 2007), which revealed a dramatic increase in spontaneous fusion rates (>20-fold). Additional studies of mass cultured mouse cortical neurons lacking Cpxs also found a large increase in spontaneous fusion (Maximov et al., 2009). In addition to the increase in spontaneous fusion, Drosophila synapses lacking Cpx show reduced evoked fusion. Structure-functions studies of mammalian Cpxs demonstrated that the N-terminus of Cpx contains both stimulatory and inhibitory subdomains (Xue et al., 2010; Xue et al., 2007), arguing that both clamping and facilitatory roles of Cpx may co-exist and express differently depending upon the synapse being analyzed.
To address these issues in greater detail, we examined the conservation of Cpx function across species at the Drosophila NMJ. Our first goal was to determine if the role of Cpx as a fusion clamp required SNARE binding, as has been shown for its facilitatory function at mammalian synapses. Lysine 75 and tyrosine 76 flank the right side of SNARE-binding domain and are essential for the SNARE-Cpx interaction (Xue et al., 2009; Xue et al., 2007). Consistent with SNARE binding being required for Cpx function, we found that expressing Cpx with K75 and Y76 mutated in the cpx null background fails to rescue the dramatic increase in spontaneous fusion rates, indicating Cpx binding to SNARE complexes is a prerequisite for its ability to act as a fusion clamp. Our findings are consistent with findings that show DmCpx's ability to inhibit spontaneous release in cultured autaptic neurons from mice requires SNARE binding (Xue et al., 2009).
We next examined whether mammalian Cpx isoforms could function as fusion clamps in Drosophila. We found that mCpx IV rescues the increased mini frequency in Drosophila cpx mutants despite expression levels much lower than mCpxs I and II. mCpxs I, II and III exhibit partial rescue of the fusion clamp function, as spontaneous release rates are still elevated 3 to 4-fold compared to controls. Like Drosophila Cpx, mCpxs III and IV have a C-terminal CAAX-box prenylation motif (Reim et al., 2005). We find that disruption of the DmCpx C-terminal CAAX-box motif can completely phenocopy the SNARE-binding defective DmCpx mutant. The differential abilities of mCpxs to act as fusion clamps, together with the requirement of DmCpx's CAAX-box, suggest that prenylation may regulate Cpx's ability to function as a fusion clamp. In mammalian cultured neurons, the CAAX-box motif is necessary for proper targeting of mCpx III and IV to axonal terminals (Reim et al., 2005). The DmCpx CAAX-box mutant protein is still properly targeted to synaptic boutons (Supplementary Figure 2), indicating prenylation may be necessary for subcellular targeting beyond the limits of confocal microscopy, or for flexibility of the Cpx C-terminal domain that may affect its functionality. Alternative mechanisms to support Cpx fusion clamp properties must also exist given that mCpx I and II lack a CAAX-box motif, but retain some ability to function as fusion clamps in Drosophila. The C-terminal CAAX-box motif of Cpx is also required to inhibit spontaneous release in mouse autaptic neurons (Xue et al., 2009).
The mCpx I N-terminal α-helical accessory domain flanking the SNARE binding region has been proposed to inhibit fusion (Giraudo et al., 2009; Maximov et al., 2009; Xue et al., 2009; Xue et al., 2007). This region may clamp release by incorporating into a portion of the 4-helix bundle of the SNARE complex, mimicking the v-SNARE synaptobrevin and preventing full zippering of the SNARE complex (Giraudo et al., 2009; Maximov et al., 2009). This hypothesis was based on the observation that the mCpx I N-terminal accessory domain sequence is similar to the C-terminal membrane proximal synaptobrevin region that contributes to full SNARE complex assembly, including three shared hydrophobic residues. The orthologous region of Drosophila n-synaptobrevin is highly conserved compared with mammals. Moreover, all the residues in the hydrophobic layers of synaptobrevin that are proposed to compete with the mCpx I accessory helix for SNARE binding are conserved. Therefore, mCpx I may function as a fusion clamp in Drosophila by competing with Drosophila synaptobrevin to prevent full zippering of the SNARE complex. Do the other mCpxs and DmCpx clamp fusion similarly? This seems unlikely given the N-terminal accessory domains of mCpx III and IV and DmCpx are not similar, and nearly all of the residues that make up the hydrophobic layers proposed to interface with the t-SNARES and compete with synaptobrevin are charged in these isoforms. As such, there is likely to be alternative Cpx clamping mechanisms that function beyond the current competition model between Cpx and Synaptobrevin.
Our studies also reveal that mCpx I, II, and III, in addition to acting as a fusion clamp, strongly facilitate evoked fusion when expressed in Drosophila synapses compared to mCpx IV and DmCpx. This separation of the clamping and facilitatory roles is observed for all the mCpxs. mCpxs I, II, and III strongly facilitate evoked release and partially clamp spontaneous release at Drosophila synapses. In contrast, mCpx IV fully clamps spontaneous release, but does not facilitate evoked fusion. These data indicate that the facilitating role for Cpx can be genetically separated from its fusion clamp properties, arguing that the increase in spontaneous release and defective evoked responses observed in Drosophila cpx null mutants are likely to be only partially related. Xue et al. (2009) have recently made similar observations with regards to minis and evoked responses at Drosophila NMJs that express mCpx isoforms in cpx nulls (Xue et al., 2009). The mCpxs examined by Xue et al. (mCpx I and mCpx III) strongly facilitates evoked release and partially clamps spontaneous release at Drosophila NMJ, similar to our analysis. For the first time, we report that mCpx IV is a unique member of the mCpxs. At the Drosophila NMJ, mCpx IV acts as a strong vesicle clamp, but does not rescue evoked release in cpx null mutants. mCpxs IV is only expressed in the mammalian visual system, and enriched at rod synapses (Reim et al., 2005). The distinct properties of mCpx IV may contribute to the differential release kinetics exhibited at these synapses, where the fast component of exocytosis is 10-fold slower at rod synapses compared to cone synapses (Rabl et al., 2005).
Our in vivo experiments are consistent with recent in vitro studies that have demonstrated distinct abilities of mCpx isoforms and DmCpx to promote liposomal fusion. mCpx I and II strongly increase the kinetics of SNARE-mediated liposomal fusion, while the other mCpx isoforms and DmCpx have either no effect or even delay fusion (Malsam et al., 2009). An interaction of the C-terminal α-helical domain of mCpx I with lipids is required for its ability to facilitate fusion in these in vitro assays (Seiler et al., 2009). All Cpxs have a C-terminal α-helical domain that may interact with lipid bilayers of the vesicle and/or plasma membrane to modulate SNARE-mediated fusion. Prenylation of the C-terminus of DmCpx and mCpx IV may override or block the facilitatory nature of the α-helical domain by sterically hindering it from associating with membranes. Consistent with this idea, DmCpx lacking the prenylation CAAX-box motif expressed in cpx nulls exhibit enhanced evoked release compared with WT DmCpx. However, mCpx III, which also has a CAAX-box motif, exhibits evoked responses similar to the non-prenylated mCpx I and II. Interestingly, adding the prenylation motif to mCpx I has no effect on evoked release. These data suggest that mCpx III may share facilitatory properties that exist in mCpx I and II, and that these properties likely map outside of the C-terminus.
How can Cpx's general role in clamping fusion be correlated with the divergent properties of mCpxs in their ability to stimulate calcium-dependent vesicle fusion? Biochemical and electrophysiological experiments suggest that Cpx and Syt 1, the calcium sensor for exocytosis, compete for SNARE binding (Tang et al., 2006). Cpx is proposed to clamp synaptic vesicles at the active zone in a metastable state. Syt 1, in response to calcium, displaces Cpx at the SNARE complex and promotes vesicle fusion. The integration of specific Cpx N-terminal and C-terminal domains in the context of SNARE complex dynamics likely confer distinct functional properties. Specific Cpx isoforms may generate different metastable SNARE complex states that result in SNARE complex substrates differentially activated by Syt 1 in response to calcium. The strength of Cpx as a fusion clamp or release facilitator is also likely modified by the presence and function of other SNARE associated proteins, including Munc-18 (Deak et al., 2009). Moreover, post-translational modifications such as prenylation (Reim et al., 2005) and phosphorylation (Hill et al., 2006) may further modulate Cpx function in an activity-dependent manner. In summary, our results indicate that Cpx plays multiple roles in synaptic transmission that are genetically separable and may contribute to synapse diversity and plasticity.
EXPERIMENTAL METHODS
Drosophila genetics and molecular cloning
Drosophila melanogaster were cultured on standard medium at 25°C. mCpx I and II were cloned from a mouse cDNA library. mCpx III and IV were cloned from mouse cDNA clones obtained from Open Biosystems (Clone IDs 4500804 and 5357047). DmCpx was cloned from a Drosophila cDNA clone obtained from the Drosophila Genomics Research Center (Clone ID GH27718). QuickChange XL (Stratagene, La Jolla, CA) was used for site directed mutagenesis on the existing cloned WT DmCpx to generate DmCpx K75A,Y76A. The following primers were used to clone Cpx isoforms and mutants:
mCpx I:
5′CATATGGAGTTCGTGATGAAACAAGC,
3′TCTAGATTACTTCTTGAACATGTCCTGC;
mCpx IΔMFKK/+CVMQ:
5′CATATGGAGTTCGTGATGAAACAAGC,
3′TCTAGATCACTGCATGACACAGTCCTGCAGTGGCCCAGGCAGG;
mCpx II:
5′ TCTAGATTACTTCTTGAACATGTCCTGC
3′ CATATGGACTTCGTCATGAAGCAAGC
mCpx III:
5′CATATGGCGTTCATGGTGAAGTCC
3′TCTAGATCACATGATATGGCACTTCTCAGC
mCpx IV:
5′CATATGGCTTTCTTTGTGAAAAATAT
3′ TCTAGATCACATCACAGAACACTTCTGC
DmCpx:
5′CATATGGCGGCCTTCATAGCTAAGC
3′TCTAGATCAATGCATGACACATTTTCCCT
DmCpxΔCVMQ/+MFKK:
5′CATATGGCGGCCTTCATAGCTAAGC
3′TCTAGATTACTTCTTGAACATTTTTCCCTCTATTTGAGTTT
DmCpxK75A,Y76A:
5′GAGAGGATGAGGCAAGACATTCGCGATGCTGCAAACATCAAGAAGAAGGAGGAG ATCGTG
3′CACGATCTCCTCCTTCTTCTTGATGTTTGCAGCATCGCGAATGTCTTGCCTCATCTT CTC
PCR products were subcloned into a modified pValum construct with an N-terminal myc tag, allowing use of the Gal4/UAS expression system (Brand and Perrimon, 1993). These constructs (UASCpx) were injected into a yv; attP third chromosome docking strain as described (Ni et al., 2008) by Genetic Services Inc. (Cambridge, MA). Homozygous third chromosome UAS lines were then recombined into the cpxSH1 null mutant (Huntwork and Littleton, 2007). The C155 elav-GAL4 driver was used for neuronal expression of transgenes. The phylogeny analysis shown in Figure 2A was performed using ClustalW2 (Larkin et al., 2007). Alignment was performed using Invitrogen Vector NTI (Carlsbad, CA).
Immunostaining and Western Analysis
Immunostaining was performed on third instar larvae at wandering stage after rearing at 25°C as described previously (Huntwork and Littleton, 2007). Anti-myc (Clontech, Mountain View, CA), anti-DmCpx (1:5000, (Huntwork and Littleton, 2007)) and anti-HRP (1:10000, Jackson ImmunoResearch, West Grove, PA) were used for immunostaining. Immunoreactive proteins were visualized on a Zeiss Pascal Confocal with PASCAL software (Carl Zeiss MicroImaging, Inc.) using fluorescent secondary antibodies (Molecular Probes, Carlsbad, CA). Western blotting of whole adult head lysates was performed using standard laboratory procedures with anti-myc (1:1000, 1:500, GeneTex, San Antonio, TX) and anti-DmCpx (1:5000, (Huntwork and Littleton, 2007)) anti-syntaxin (1:1000, Developmental Studies Hybridoma Bank, Iowa City, IA), anti-Syt 1 (1:200, (Littleton et al., 1993) antisera. Western blots were visualized on a Li-Cor Odyssey infrared imaging system.
For quantitative Western blot analysis, adult heads were collected and lysed in 10 μl of RIPA buffer/head (50 mM Tris-HCl, 150 mM NaCl, 1.5 mM MgCl2, 0.5 % Sodium Deoxycholate, 1% NP-40, 0.1% ZSDS, pH 7.4). The equivalent of 1 head was loaded per lane. Equal loading was assayed using anti-tubulin clone B-5-1-2 at 1:60000 (T5168; Sigma-Aldrich). Western blots were imaged using an Odyssey infrared scanner (Li-Cor). The mean integrated density of each band (mean intensity/pixel multiplied by the area of the band) corresponding to myc-tagged transgene was quantified using ImageJ (Rasband, 1997-2009) and normalized to the mean integrated density of tubulin. All values were then normalized to myc-DmCpx. These values were then multiplied by the ratio of the tubulin normalized mean integrated density values of myc-DmCpx and endogenous DmCpx obtained from the C155;;UAS-myc-tagged DmCpx lysates when blotted using anti-DmCpx (see Figure 3A last lane). The resultant value is the relative level of myc-tagged transgenes compared to endogenous DmCpx expressed in the C155;;UAS-myc-tagged DmCpx line.
Electrophysiological analysis
Electrophysiological analysis of wandering stage 3rd instar larva was performed in Drosophila saline containing (in mM): 70 NaCl, 5 KCl, 4 MgCl2, 10 NaHCO3, 5 Trehalose, 115 sucrose, 5 HEPES-NaOH, 0.2 CaCl, pH 7.2, modified from HL3 as previously described (Rieckhof, 2003). Evoked EJPs were recorded intracellularly from muscle fiber 6 of segment A3 using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA). Data acquisition and analysis was performed using Clampfit 9.0 software (Axon Instruments, Foster City, CA) as previously described (Huntwork and Littleton, 2007). All error bars are standard error of the mean (SEM). n = number of recordings from muscle 6 from at least 3 animals. Statistical significance was determined by Student's t-test (n.s. = P>0.05, * = P<0.05, ** = P<0.01, *** = P<0.001).
Supplementary Material
Cpx transgenic rescues do not exhibit altered levels of syntaxin or Syt 1 levels. All western blots were performed on adult head lysates, and were probed with anti-myc, anti-DmCpx, anti-syntaxin (stx), or anti-Syt I. Equal loading was assayed by anti-tubulin (tub), with representative blots shown. (A) DmCpxK75A,Y76A expressed in the cpxSH1 background does not alter syntaxin or Syt 1 levels. (B) mCpxs expressed in the cpxSH1 background does not alter syntaxin or Syt 1 levels. (C) DmCpxΔCVMQ/+MFKK and mCpx IΔMFKK/+CVMQ in the cpxSH1 background does not alter syntaxin or Syt 1 levels.
WT DmCpx, mCpx and the indicated mutant transgenic proteins driven by C155elav-Gal4 are localized properly to muscle 6/7 NMJs. Third instar wandering larvae of the indicated genotypes were dissected, fixed and costained with anti-HRP, and rabbit anti-DmCpx or mouse anti-myc antisera as indicated. (A) Control larvae were stained with anti-DmCpx to detect endogenous Cpx co-stained with anti-HRP. (B) – (F) transgenic lines were stained anti-HRP, and anti-myc to detect the following neuronally expressed transgenes: (B) WT DmCpx, (C) DmCpx K75A, Y76A, (D) DmCpx ΔCVMQ/+MKFF, (E) mCpx I, and (F) mCpx I ΔMFKK/+CVMQ. Scale bar = 20 μm.
DmCpx and mCpxs transgenic proteins driven by the pan-neuronal driver C155elav-Gal4 are localized properly to muscle 6/7 NMJs. Third instar wandering larvae of the following transgenic lines were prepared as described in SI Figure 1, and costained with anti-HRP and anti-myc antisera: (A) WT DmCpx, (B) mCpx I, (C) mCpx II, (D) mCpx III, (E) mCpx IV. Scale bar = 20 μm.
ACKNOWLEDGMENTS
We thank Ramon A. Jorquera, Lauren Barr, and Sarah Huntwork for helpful discussions. This work was supported by NIH grant NS064750 to R.W.C., and NIH grant NS40296 to J.T.L.
Abbreviations
- NMJ
neuromuscular junction
- EJP
excitatory junctional potential
- Cpx
complexin
- m
mammalian
- SNARE
soluble N-ethylmaleimide-sensitive fusion attachment protein receptor
- Dm
Drosophila melanogaster
- SEM
standard error of the mean
- WT
wildtype
- HRP
horse radish peroxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barber CF, Jorquera RA, Melom JE, Littleton JT. Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains. J Cell Biol. 2009;187:295–310. doi: 10.1083/jcb.200903098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher A, Kadlec J, Betz H, Weissenhorn W. X-ray structure of a neuronal complexin-SNARE complex from squid. J Biol Chem. 2002;277:26517–26523. doi: 10.1074/jbc.M203460200. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brose N. For better or for worse: complexins regulate SNARE function and vesicle fusion. Traffic. 2008;9:1403–1413. doi: 10.1111/j.1600-0854.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- Deak F, Xu Y, Chang WP, Dulubova I, Khvotchev M, Liu X, Sudhof TC, Rizo J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Callaghan DA, Ding W, Kelly JF, Chakravarthy BR. Identification of okadaic acid-induced phosphorylation events by a mass spectrometry approach. Biochem Biophys Res Commun. 2006;342:791–799. doi: 10.1016/j.bbrc.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Chapman ER, Kreber R, Garment MB, Carlson SD, Ganetzky B. Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron. 1998;21:401–413. doi: 10.1016/s0896-6273(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Malsam J, Seiler F, Schollmeier Y, Rusu P, Krause JM, Sollner TH. The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc Natl Acad Sci U S A. 2009;106:2001–2006. doi: 10.1073/pnas.0812813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, Villalta C, Booker M, Perkins L, Perrimon N. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5:49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst S, Hazzard JW, Antonin W, Sudhof TC, Jahn R, Rizo J, Fasshauer D. Selective interaction of complexin with the neuronal SNARE complex. Determination of the binding regions. J Biol Chem. 2000;275:19808–19818. doi: 10.1074/jbc.M002571200. [DOI] [PubMed] [Google Scholar]
- Rabl K, Cadetti L, Thoreson WB. Kinetics of exocytosis is faster in cones than in rods. J Neurosci. 2005;25:4633–4640. doi: 10.1523/JNEUROSCI.4298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. Image J. U. S. National Institutes of Health. Bethesda, MD: 1997-2009. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Reim K, Wegmeyer H, Brandstatter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K, Brose N. Structurally and functionally unique complexins at retinal ribbon synapses. J Cell Biol. 2005;169:669–680. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat Struct Mol Biol. 2006;13:748–750. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- Seiler F, Malsam J, Krause JM, Sollner TH. A role of complexin-lipid interactions in membrane fusion. FEBS Lett. 2009;583:2343–2348. doi: 10.1016/j.febslet.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS. The Trichoplax genome and the nature of placozoans. Nature. 2008;454:955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- Strenzke N, Chanda S, Kopp-Scheinpflug C, Khimich D, Reim K, Bulankina AV, Neef A, Wolf F, Brose N, Xu-Friedman MA, Moser T. Complexin-I is required for high-fidelity transmission at the endbulb of held auditory synapse. J Neurosci. 2009;29:7991–8004. doi: 10.1523/JNEUROSCI.0632-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Sudhof TC. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Xue M, Craig TK, Xu J, Chao HT, Rizo J, Rosenmund C. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol. 2010;17:568–575. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Lin YQ, Pan H, Reim K, Deng H, Bellen HJ, Rosenmund C. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron. 2009;64:367–380. doi: 10.1016/j.neuron.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Reim K, Chen X, Chao HT, Deng H, Rizo J, Brose N, Rosenmund C. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat Struct Mol Biol. 2007;14:949–958. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc Natl Acad Sci U S A. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cpx transgenic rescues do not exhibit altered levels of syntaxin or Syt 1 levels. All western blots were performed on adult head lysates, and were probed with anti-myc, anti-DmCpx, anti-syntaxin (stx), or anti-Syt I. Equal loading was assayed by anti-tubulin (tub), with representative blots shown. (A) DmCpxK75A,Y76A expressed in the cpxSH1 background does not alter syntaxin or Syt 1 levels. (B) mCpxs expressed in the cpxSH1 background does not alter syntaxin or Syt 1 levels. (C) DmCpxΔCVMQ/+MFKK and mCpx IΔMFKK/+CVMQ in the cpxSH1 background does not alter syntaxin or Syt 1 levels.
WT DmCpx, mCpx and the indicated mutant transgenic proteins driven by C155elav-Gal4 are localized properly to muscle 6/7 NMJs. Third instar wandering larvae of the indicated genotypes were dissected, fixed and costained with anti-HRP, and rabbit anti-DmCpx or mouse anti-myc antisera as indicated. (A) Control larvae were stained with anti-DmCpx to detect endogenous Cpx co-stained with anti-HRP. (B) – (F) transgenic lines were stained anti-HRP, and anti-myc to detect the following neuronally expressed transgenes: (B) WT DmCpx, (C) DmCpx K75A, Y76A, (D) DmCpx ΔCVMQ/+MKFF, (E) mCpx I, and (F) mCpx I ΔMFKK/+CVMQ. Scale bar = 20 μm.
DmCpx and mCpxs transgenic proteins driven by the pan-neuronal driver C155elav-Gal4 are localized properly to muscle 6/7 NMJs. Third instar wandering larvae of the following transgenic lines were prepared as described in SI Figure 1, and costained with anti-HRP and anti-myc antisera: (A) WT DmCpx, (B) mCpx I, (C) mCpx II, (D) mCpx III, (E) mCpx IV. Scale bar = 20 μm.