Abstract
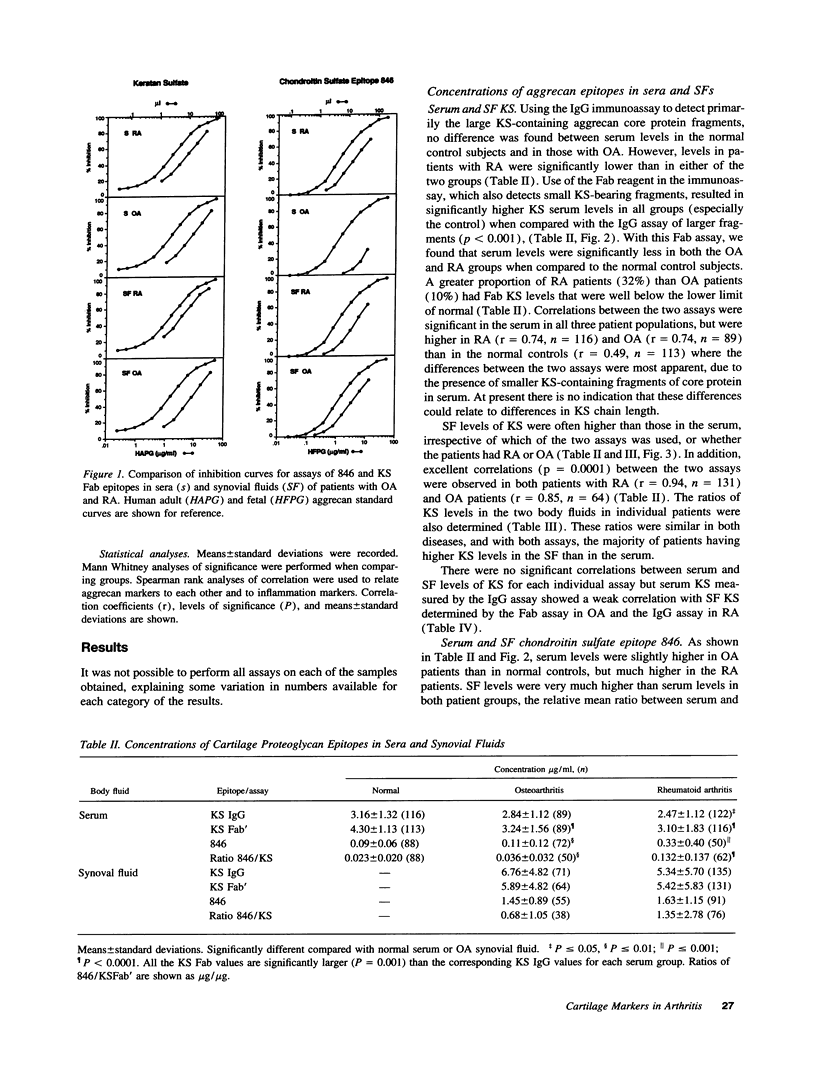
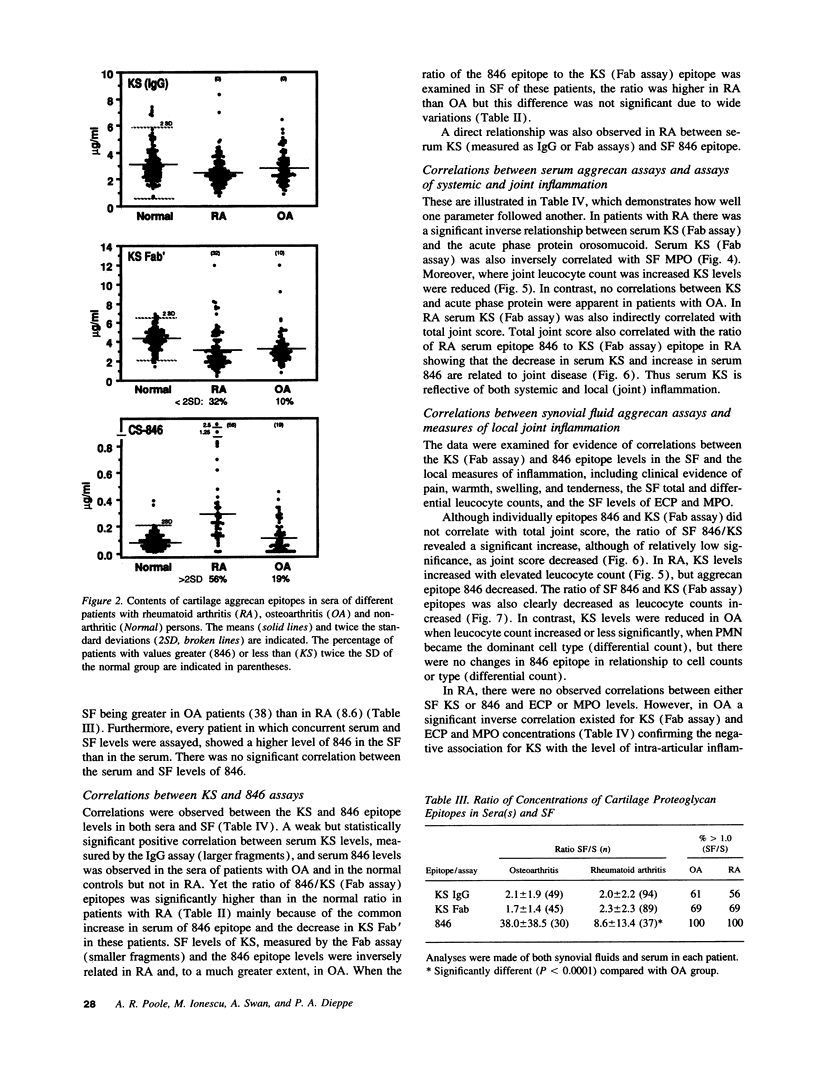
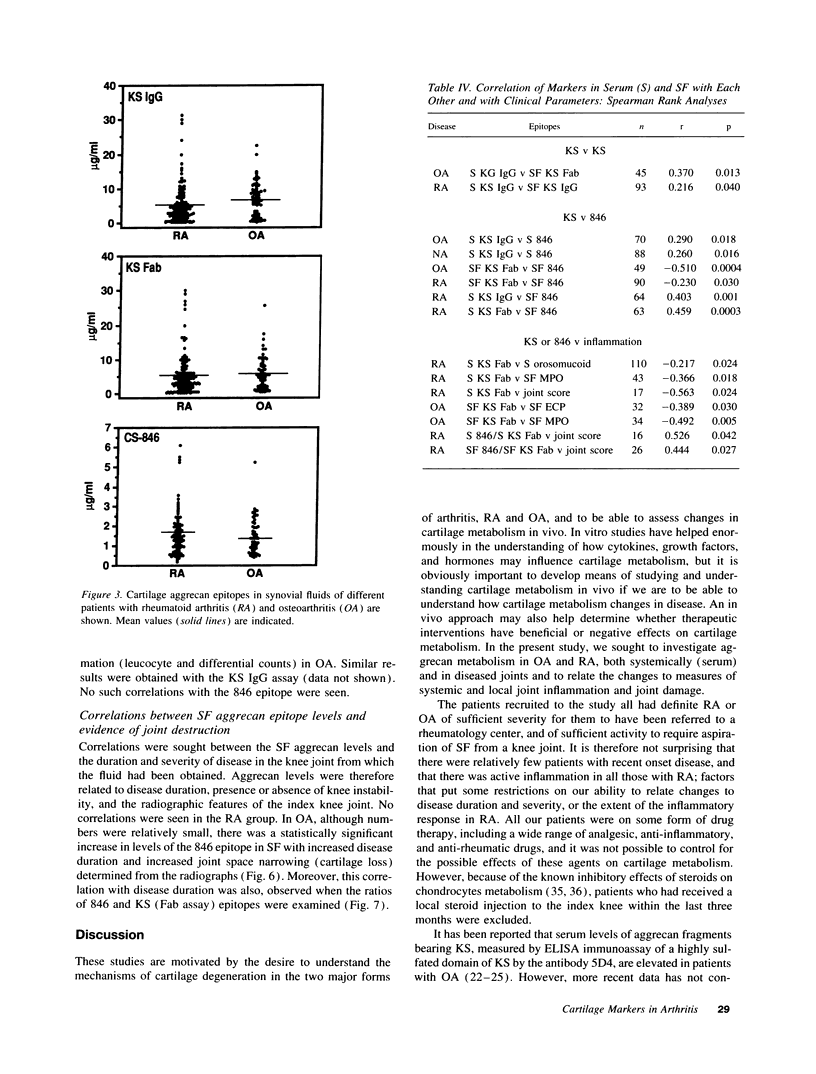
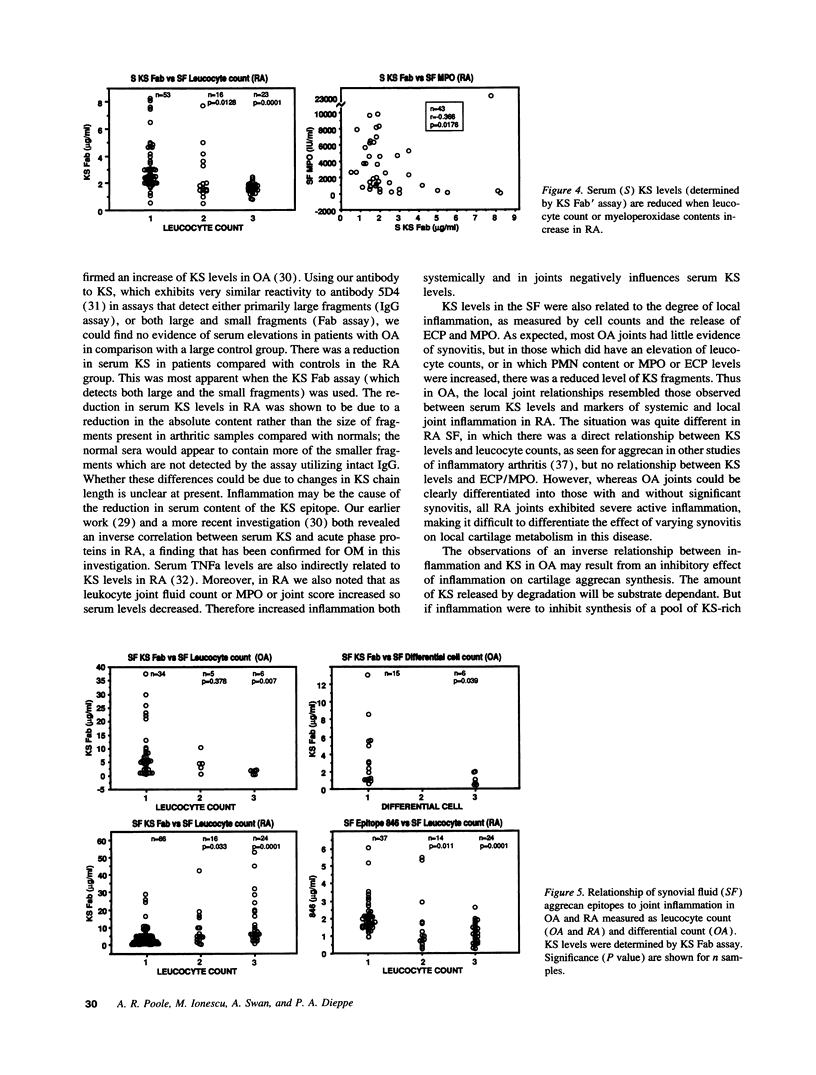
The metabolism of the cartilage proteoglycan aggrecan was studied in patients with osteoarthritis (OA, n = 83), rheumatoid arthritis (RA, n = 127), and in controls (n = 117) using monoclonal antibody-based radioimmunoassays for glycosaminoglycans in the serum and synovial fluid (SF) to detect epitope 846 on chondroitin sulfate (probably only on recently synthesized molecules) and a keratan sulfate (KS) epitope AN9PI, present on intact and degraded molecules. Epitope 846 levels were always elevated in SF over serum (mean 38-fold in OA and 8.6-fold in RA) being highest in OA patients with the longest disease duration and greatest loss of cartilage, and lowest in RA joints with high leucocyte counts. Serum levels were more often elevated in RA (56%) than in OA (19%) and probably reflect increased aggrecan synthesis in diseased joints. KS levels were higher in SF than in serum in 69% of patients (up to 2.3-fold); levels were inversely (OA) and directly (RA) related to SF leucocyte counts. Serum KS was reduced in both diseases and in RA was inversely related to both systemic and joint inflammation markers. SF 846 levels were inversely related to SF KS in both diseases. These epitopes may provide a measure of the balance between cartilage synthesis and degradation in these diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner E. C., Pratta M. A. Independent effects of interleukin-1 on proteoglycan breakdown, proteoglycan synthesis, and prostaglandin E2 release from cartilage in organ culture. Arthritis Rheum. 1989 Mar;32(3):288–297. doi: 10.1002/anr.1780320310. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- CHANDLER G. N., WRIGHT V. Deleterious effect of intra-articular hydrocortisone. Lancet. 1958 Sep 27;2(7048):661–663. doi: 10.1016/s0140-6736(58)92262-1. [DOI] [PubMed] [Google Scholar]
- Campion G. V., McCrae F., Schnitzer T. J., Lenz M. E., Dieppe P. A., Thonar E. J. Levels of keratan sulfate in the serum and synovial fluid of patients with osteoarthritis of the knee. Arthritis Rheum. 1991 Oct;34(10):1254–1259. doi: 10.1002/art.1780341008. [DOI] [PubMed] [Google Scholar]
- Caterson B., Mahmoodian F., Sorrell J. M., Hardingham T. E., Bayliss M. T., Carney S. L., Ratcliffe A., Muir H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990 Nov;97(Pt 3):411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Dahlberg L., Ryd L., Heinegård D., Lohmander L. S. Proteoglycan fragments in joint fluid. Influence of arthrosis and inflammation. Acta Orthop Scand. 1992 Aug;63(4):417–423. doi: 10.3109/17453679209154758. [DOI] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Roughley P. J., Buzás E., Poole A. R. Age-related changes in protein-related epitopes of human articular-cartilage proteoglycans. Biochem J. 1986 May 15;236(1):71–75. doi: 10.1042/bj2360071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M., Jayson M. I. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clin Exp Immunol. 1988 Jun;72(3):422–427. [PMC free article] [PubMed] [Google Scholar]
- Manicourt D. H., Triki R., Fukuda K., Devogelaer J. P., Nagant de Deuxchaisnes C., Thonar E. J. Levels of circulating tumor necrosis factor alpha and interleukin-6 in patients with rheumatoid arthritis. Relationship to serum levels of hyaluronan and antigenic keratan sulfate. Arthritis Rheum. 1993 Apr;36(4):490–499. doi: 10.1002/art.1780360409. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Conger K. A. The acute effects of intra-articular hydrocortisone on articular cartilage in rabbits. J Bone Joint Surg Am. 1966 Oct;48(7):1383–1388. [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The glycosaminoglycans of normal and arthritic cartilage. J Clin Invest. 1971 Aug;50(8):1712–1719. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehraban F., Finegan C. K., Moskowitz R. W. Serum keratan sulfate. Quantitative and qualitative comparisons in inflammatory versus noninflammatory arthritides. Arthritis Rheum. 1991 Apr;34(4):383–392. doi: 10.1002/art.1780340403. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Webber C., Reiner A., Roughley P. J. Studies of a monoclonal antibody to skeletal keratan sulphate. Importance of antibody valency. Biochem J. 1989 Jun 15;260(3):849–856. doi: 10.1042/bj2600849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Witter J., Roberts N., Piccolo F., Brandt R., Paquin J., Baron M. Inflammation and cartilage metabolism in rheumatoid arthritis. Studies of the blood markers hyaluronic acid, orosomucoid, and keratan sulfate. Arthritis Rheum. 1990 Jun;33(6):790–799. doi: 10.1002/art.1780330605. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Doherty M., Maini R. N., Hardingham T. E. Increased concentrations of proteoglycan components in the synovial fluids of patients with acute but not chronic joint disease. Ann Rheum Dis. 1988 Oct;47(10):826–832. doi: 10.1136/ard.47.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkalla G., Reiner A., Bogoch E., Poole A. R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992 Dec;90(6):2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., Mort J. S. Ageing and the aggregating proteoglycans of human articular cartilage. Clin Sci (Lond) 1986 Oct;71(4):337–344. doi: 10.1042/cs0710337. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Saxne T., Di Giovine F. S., Heinegård D., Duff G. W., Wollheim F. A. Synovial fluid concentrations of interleukin-1 beta and proteoglycans are inversely related. J Autoimmun. 1988 Aug;1(4):373–380. doi: 10.1016/0896-8411(88)90007-8. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A. Cartilage proteoglycans in synovial fluid and serum in patients with inflammatory joint disease. Relation to systemic treatment. Arthritis Rheum. 1987 Sep;30(9):972–979. doi: 10.1002/art.1780300903. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A. Therapeutic effects on cartilage metabolism in arthritis as measured by release of proteoglycan structures into the synovial fluid. Ann Rheum Dis. 1986 Jun;45(6):491–497. doi: 10.1136/ard.45.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. R., Leveille C. R., Stevens J. W., Oh W. H. Proteoglycan structure and metabolism in normal and osteoarthritic cartilage of guinea pigs. Arthritis Rheum. 1981 Dec;24(12):1528–1539. doi: 10.1002/art.1780241212. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Woodward L., Hall G. M., Hammond A., Williams A., Butler M. G., James I. T., Hart D. J., Thompson P. W., Scott D. L. Keratan sulphate in rheumatoid arthritis, osteoarthritis, and inflammatory diseases. Ann Rheum Dis. 1992 Oct;51(10):1134–1137. doi: 10.1136/ard.51.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet M. B., Coelho A., Schnitzler C. M., Schnitzer T. J., Lenz M. E., Jakim I., Kuettner K. E., Thonar E. J. Serum keratan sulfate levels in osteoarthritis patients. Arthritis Rheum. 1988 May;31(5):648–652. doi: 10.1002/art.1780310510. [DOI] [PubMed] [Google Scholar]
- Thompson P. W., Silman A. J., Kirwan J. R., Currey H. L. Articular indices of joint inflammation in rheumatoid arthritis. Correlation with the acute-phase response. Arthritis Rheum. 1987 Jun;30(6):618–623. doi: 10.1002/art.1780300603. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Jr, Oegema T. R., Jr Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am. 1979 Apr;61(3):407–416. [PubMed] [Google Scholar]
- Thonar E. J., Lenz M. E., Klintworth G. K., Caterson B., Pachman L. M., Glickman P., Katz R., Huff J., Kuettner K. E. Quantification of keratan sulfate in blood as a marker of cartilage catabolism. Arthritis Rheum. 1985 Dec;28(12):1367–1376. doi: 10.1002/art.1780281209. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Articular cartilage cultured with catabolin (pig interleukin 1) synthesizes a decreased number of normal proteoglycan molecules. Biochem J. 1985 May 1;227(3):869–878. doi: 10.1042/bj2270869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. A., Benton H. P. Synthesis of type II collagen is decreased in cartilage cultured with interleukin 1 while the rate of intracellular degradation remains unchanged. Coll Relat Res. 1988 Sep;8(5):393–405. doi: 10.1016/s0174-173x(88)80013-x. [DOI] [PubMed] [Google Scholar]
- Webber C., Glant T. T., Roughley P. J., Poole A. R. The identification and characterization of two populations of aggregating proteoglycans of high buoyant density isolated from post-natal human articular cartilages of different ages. Biochem J. 1987 Dec 15;248(3):735–740. doi: 10.1042/bj2480735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westacott C. I., Whicher J. T., Barnes I. C., Thompson D., Swan A. J., Dieppe P. A. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990 Sep;49(9):676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter J., Roughley P. J., Webber C., Roberts N., Keystone E., Poole A. R. The immunologic detection and characterization of cartilage proteoglycan degradation products in synovial fluids of patients with arthritis. Arthritis Rheum. 1987 May;30(5):519–529. doi: 10.1002/art.1780300506. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kobayashi K., Yamagata N., Iwabuchi H., Katsura T., Sugihara S., Negishi M., Ide H., Mori Y., Takahashi T. Inflammatory cytokines and enzymes in synovial fluid of patients with rheumatoid arthritis and other arthritides. Int Arch Allergy Immunol. 1992;98(4):286–292. doi: 10.1159/000236200. [DOI] [PubMed] [Google Scholar]
- van de Loo A. A., van den Berg W. B. Effects of murine recombinant interleukin 1 on synovial joints in mice: measurement of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis. 1990 Apr;49(4):238–245. doi: 10.1136/ard.49.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]



