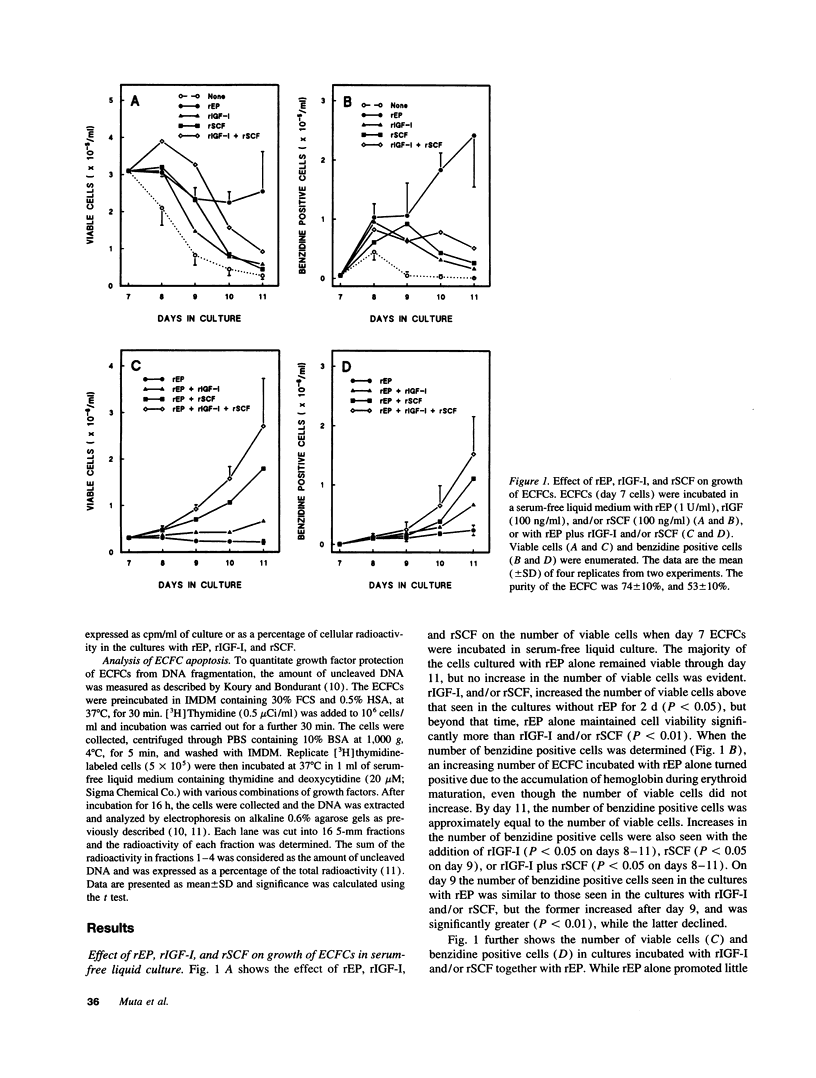
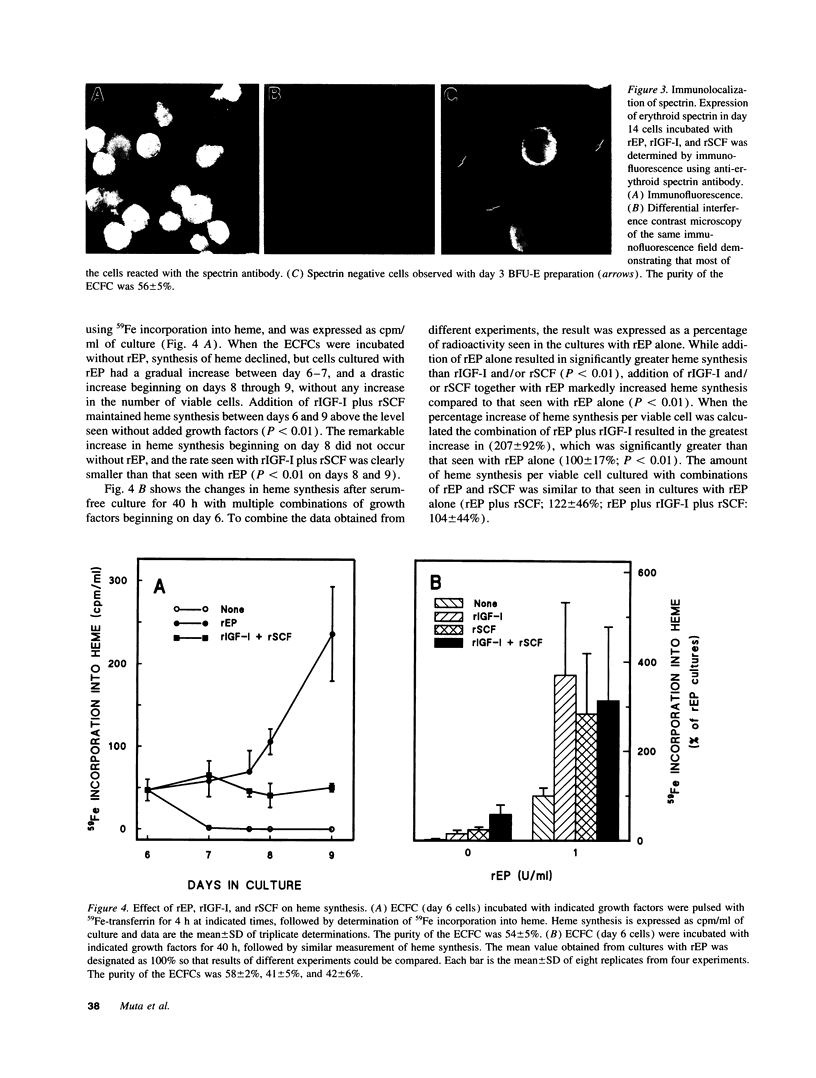
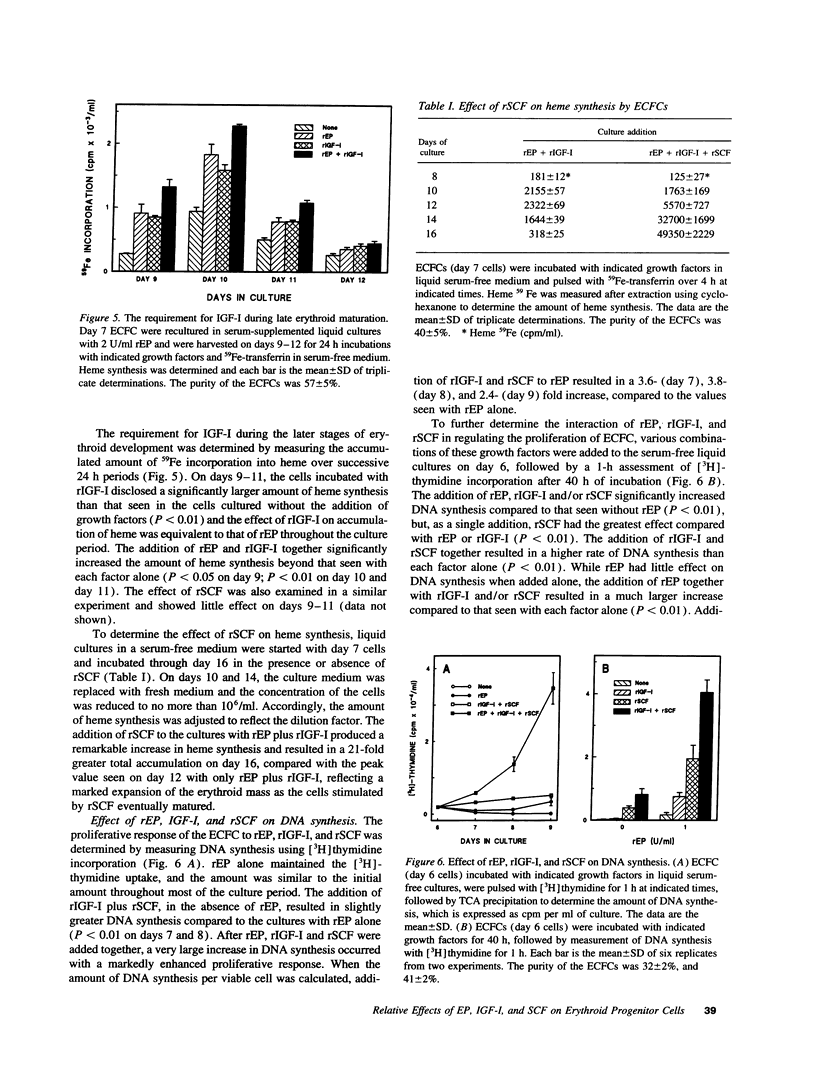
Abstract
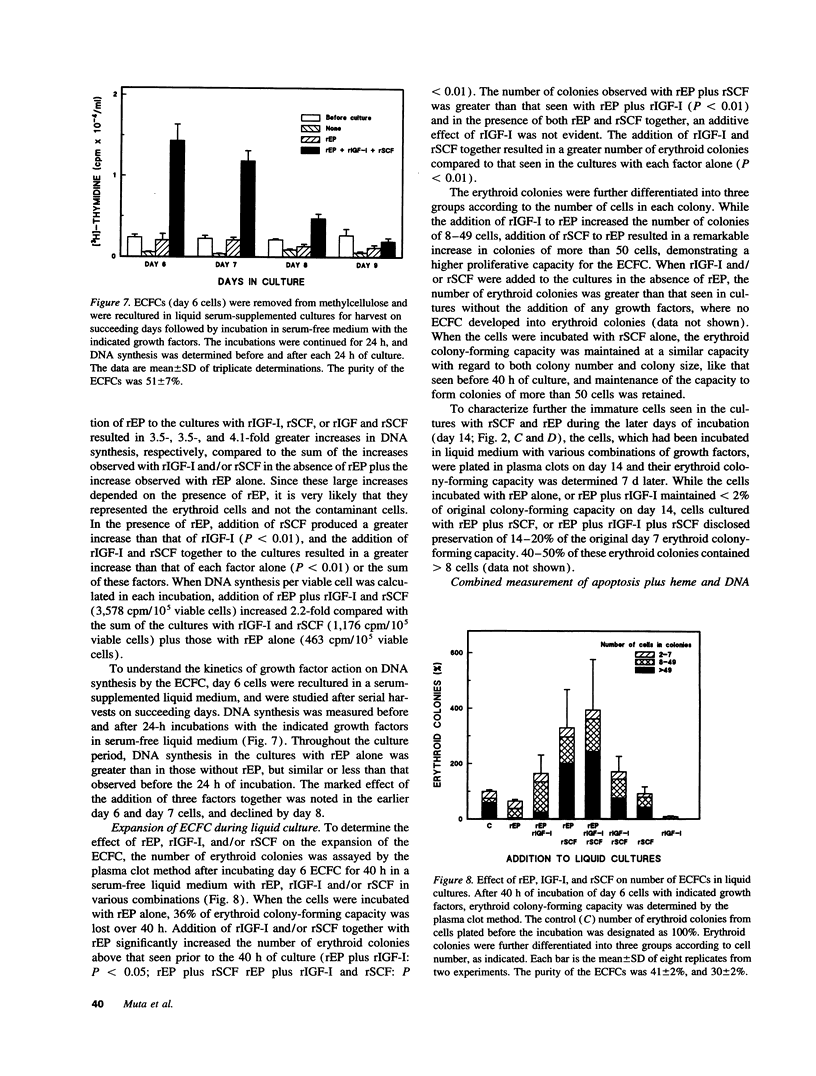
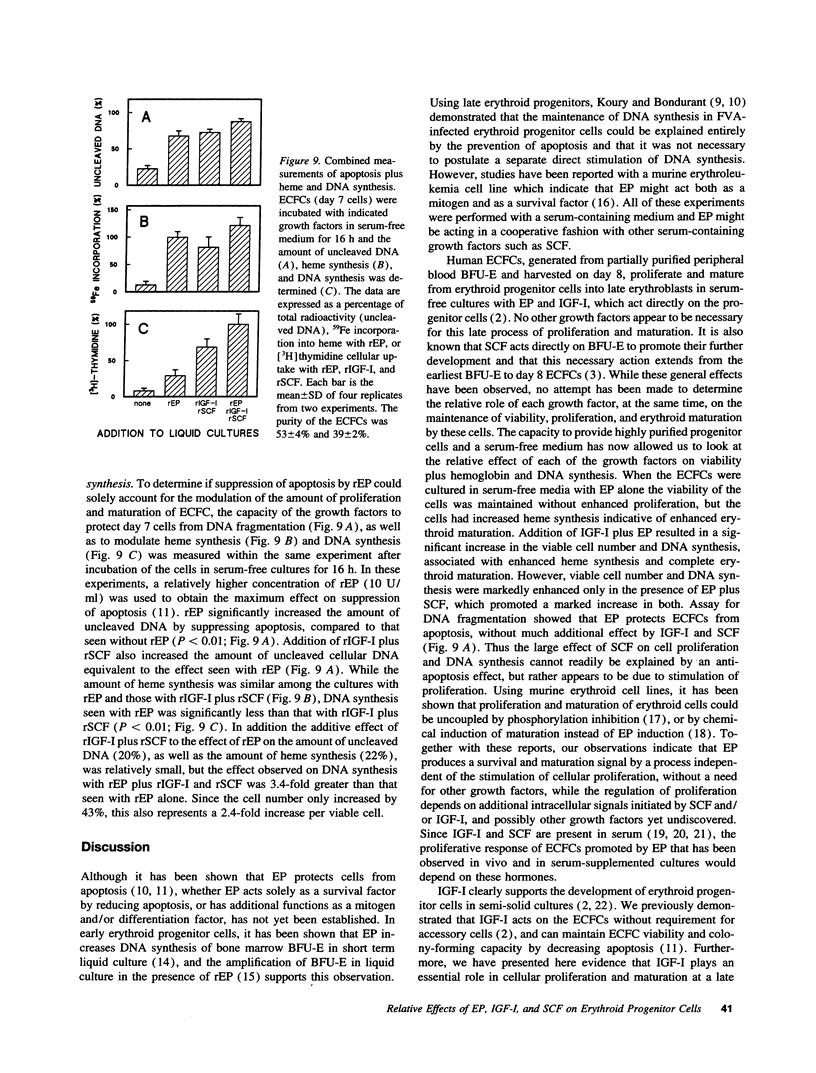
Erythropoietin (EP), insulin-like growth factor I (IGF-I) and stem cell factor (SCF) each reduce apoptosis of human erythroid progenitor cells. To determine if these growth factors have additional roles in stimulating erythropoiesis, the proliferation, maturation, and survival of highly purified human erythroid colony-forming cells (ECFCs) were studied during the application of different combinations of these growth factors in a serum-free liquid culture. EP maintained cell viability and supported heme synthesis during erythroid maturation, with little increase in viable cell number or stimulation of DNA synthesis. The addition of SCF with EP resulted in a substantial increase in DNA synthesis, which was greater than that seen with the addition of EP and was associated with a large expansion in the number of ECFCs. Thus EP, by itself, produces little increase in cell proliferation, and expansion of the number of erythroid cells depends upon the presence of SCF with EP. The addition of IGF-I with EP led to enhanced heme synthesis and moderate cellular proliferation, but also greatly enhanced nuclear condensation and enucleation in the late erythroblasts. Thus EP, by itself, is not sufficient for complete end-terminal nuclear condensation/enucleation and the presence of IGF-I is necessary for this complete process. While EP greatly reduced apoptosis during 16 h of incubation at 37 degrees C, the addition of SCF and IGF-I with EP had little additional effect, but these additions enhanced DNA synthesis > 3.4-fold. Thus SCF may have an additional role in directly stimulating proliferation through a process that is distinct from apoptosis. Our observations indicate that EP prevents apoptosis and maintains erythroid cell viability and development. IGF-I enhances erythroid maturation and proliferation, but the proliferation of erythroid progenitors is mainly controlled by the addition of SCF with EP, independent of an effect on apoptosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer S. H., Bishop T. R., Rogers O. C., Noyes A. N., Frelin L. P., Hobbs S. Roles of erythropoietin, insulin-like growth factor 1, and unidentified serum factors in promoting maturation of purified murine erythroid colony-forming units. Blood. 1992 Nov 15;80(10):2503–2512. [PubMed] [Google Scholar]
- Busfield S. J., Klinken S. P. Erythropoietin-induced stimulation of differentiation and proliferation in J2E cells is not mimicked by chemical induction. Blood. 1992 Jul 15;80(2):412–419. [PubMed] [Google Scholar]
- Correa P. N., Axelrad A. A. Production of erythropoietic bursts by progenitor cells from adult human peripheral blood in an improved serum-free medium: role of insulinlike growth factor 1. Blood. 1991 Dec 1;78(11):2823–2833. [PubMed] [Google Scholar]
- Dai C. H., Krantz S. B., Zsebo K. M. Human burst-forming units-erythroid need direct interaction with stem cell factor for further development. Blood. 1991 Nov 15;78(10):2493–2497. [PubMed] [Google Scholar]
- Dessypris E. N., Krantz S. B. Effect of pure erythropoietin on DNA-synthesis by human marrow day 15 erythroid burst forming units in short-term liquid culture. Br J Haematol. 1984 Feb;56(2):295–306. doi: 10.1111/j.1365-2141.1984.tb03957.x. [DOI] [PubMed] [Google Scholar]
- Her E., Frazer J., Austen K. F., Owen W. F., Jr Eosinophil hematopoietins antagonize the programmed cell death of eosinophils. Cytokine and glucocorticoid effects on eosinophils maintained by endothelial cell-conditioned medium. J Clin Invest. 1991 Dec;88(6):1982–1987. doi: 10.1172/JCI115524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani A. M., Nilsson G., Miettinen U., Craig S. S., Ashman L. K., Ishizaka T., Zsebo K. M., Schwartz L. B. Recombinant human stem cell factor stimulates differentiation of mast cells from dispersed human fetal liver cells. Blood. 1992 Dec 15;80(12):3009–3021. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Maintenance by erythropoietin of viability and maturation of murine erythroid precursor cells. J Cell Physiol. 1988 Oct;137(1):65–74. doi: 10.1002/jcp.1041370108. [DOI] [PubMed] [Google Scholar]
- Krantz S. B. Erythropoietin. Blood. 1991 Feb 1;77(3):419–434. [PubMed] [Google Scholar]
- Kurtz A., Härtl W., Jelkmann W., Zapf J., Bauer C. Activity in fetal bovine serum that stimulates erythroid colony formation in fetal mouse livers is insulinlike growth factor I. J Clin Invest. 1985 Oct;76(4):1643–1648. doi: 10.1172/JCI112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K. E., Bennett L. G., Wypych J., Yancik S. A., Liu X. D., Westcott K. R., Chang D. G., Smith K. A., Zsebo K. M. Soluble stem cell factor in human serum. Blood. 1993 Feb 1;81(3):656–660. [PubMed] [Google Scholar]
- McNiece I. K., Langley K. E., Zsebo K. M. Recombinant human stem cell factor synergises with GM-CSF, G-CSF, IL-3 and epo to stimulate human progenitor cells of the myeloid and erythroid lineages. Exp Hematol. 1991 Mar;19(3):226–231. [PubMed] [Google Scholar]
- Migliaccio G., Migliaccio A. R., Druzin M. L., Giardina P. J., Zsebo K. M., Adamson J. W. Effects of recombinant human stem cell factor (SCF) on the growth of human progenitor cells in vitro. J Cell Physiol. 1991 Sep;148(3):503–509. doi: 10.1002/jcp.1041480324. [DOI] [PubMed] [Google Scholar]
- Miura N., Okada S., Zsebo K. M., Miura Y., Suda T. Rat stem cell factor and IL-6 preferentially support the proliferation of c-kit-positive murine hemopoietic cells rather than their differentiation. Exp Hematol. 1993 Jan;21(1):143–149. [PubMed] [Google Scholar]
- Murphy M., Reid K., Williams D. E., Lyman S. D., Bartlett P. F. Steel factor is required for maintenance, but not differentiation, of melanocyte precursors in the neural crest. Dev Biol. 1992 Oct;153(2):396–401. doi: 10.1016/0012-1606(92)90124-y. [DOI] [PubMed] [Google Scholar]
- Muta K., Krantz S. B. Apoptosis of human erythroid colony-forming cells is decreased by stem cell factor and insulin-like growth factor I as well as erythropoietin. J Cell Physiol. 1993 Aug;156(2):264–271. doi: 10.1002/jcp.1041560207. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Fukumoto H., Mishina Y., Obinata M. Differentiation of erythroid progenitor (CFU-E) cells from mouse fetal liver cells and murine erythroleukemia (TSA8) cells without proliferation. Mol Cell Biol. 1988 Jun;8(6):2604–2609. doi: 10.1128/mcb.8.6.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Broudy V. C., Zsebo K. M. Isolation of c-kit receptor-expressing cells from bone marrow, peripheral blood, and fetal liver: functional properties and composite antigenic profile. Blood. 1991 Sep 15;78(6):1403–1412. [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dessypris E. N., Koury S. T., Sawyer S. T. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest. 1989 May;83(5):1701–1709. doi: 10.1172/JCI114070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Kans J. S., Dessypris E. N., Sawyer S., Glick A. D., Civin C. I. Purification of human erythroid colony-forming units and demonstration of specific binding of erythropoietin. J Clin Invest. 1987 Aug;80(2):357–366. doi: 10.1172/JCI113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak J. L., Pham T., Isaacs M., Hankins W. D. Erythropoietin is both a mitogen and a survival factor. Blood. 1991 Mar 15;77(6):1228–1233. [PubMed] [Google Scholar]
- Tanaka R., Koike K., Imai T., Shiohara M., Kubo T., Amano Y., Komiyama A., Nakahata T. Stem cell factor enhances proliferation, but not maturation, of murine megakaryocytic progenitors in serum-free culture. Blood. 1992 Oct 1;80(7):1743–1749. [PubMed] [Google Scholar]
- Umemura T., Papayannopoulou T., Stamatoyannopoulos G. The mechanism of expansion of late erythroid progenitors during erythroid regeneration: target cells and effects of erythropoietin and interleukin-3. Blood. 1989 May 15;73(7):1993–1998. [PubMed] [Google Scholar]
- Wickrema A., Krantz S. B., Winkelmann J. C., Bondurant M. C. Differentiation and erythropoietin receptor gene expression in human erythroid progenitor cells. Blood. 1992 Oct 15;80(8):1940–1949. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yu H., Bauer B., Lipke G. K., Phillips R. L., Van Zant G. Apoptosis and hematopoiesis in murine fetal liver. Blood. 1993 Jan 15;81(2):373–384. [PubMed] [Google Scholar]
- Zsebo K. M., Williams D. A., Geissler E. N., Broudy V. C., Martin F. H., Atkins H. L., Hsu R. Y., Birkett N. C., Okino K. H., Murdock D. C. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990 Oct 5;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]