Abstract
The HBx gene of hepatitis B virus has been shown to induce hepatic tumors in transgenic mice and is implicated in hepatocarcinogenesis in human hepatitis B virus infection. To further characterize the role of HBx gene in carcinogenesis, we established mouse fibroblast cell lines in which the expression of HBx gene could be controlled by glucocorticoid hormone and examined the effect of HBx gene expression on cell growth in vitro. Along with the expression of HBx gene, most cells in the G0/G1 phase moved into the S phase in 24 h, and the cell cycle progressed further toward 48 h. Induction of DNA synthesis was also demonstrated by bromo-deoxyuridine labeling analysis. These results indicate that HBx gene has a function to trigger the synthesis of cellular DNA and suggest that HBx gene may play a role in hepatocarcinogenesis in human infection by driving deregulated cell cycle progression.
Full text
PDF
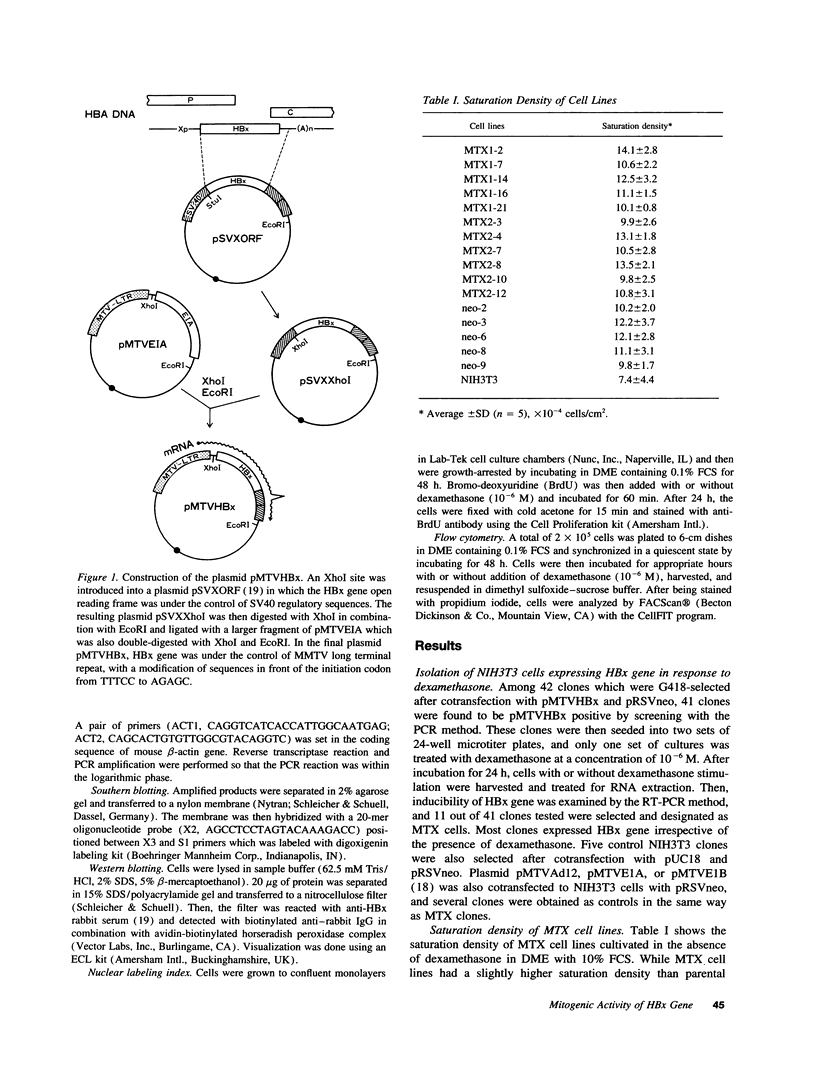


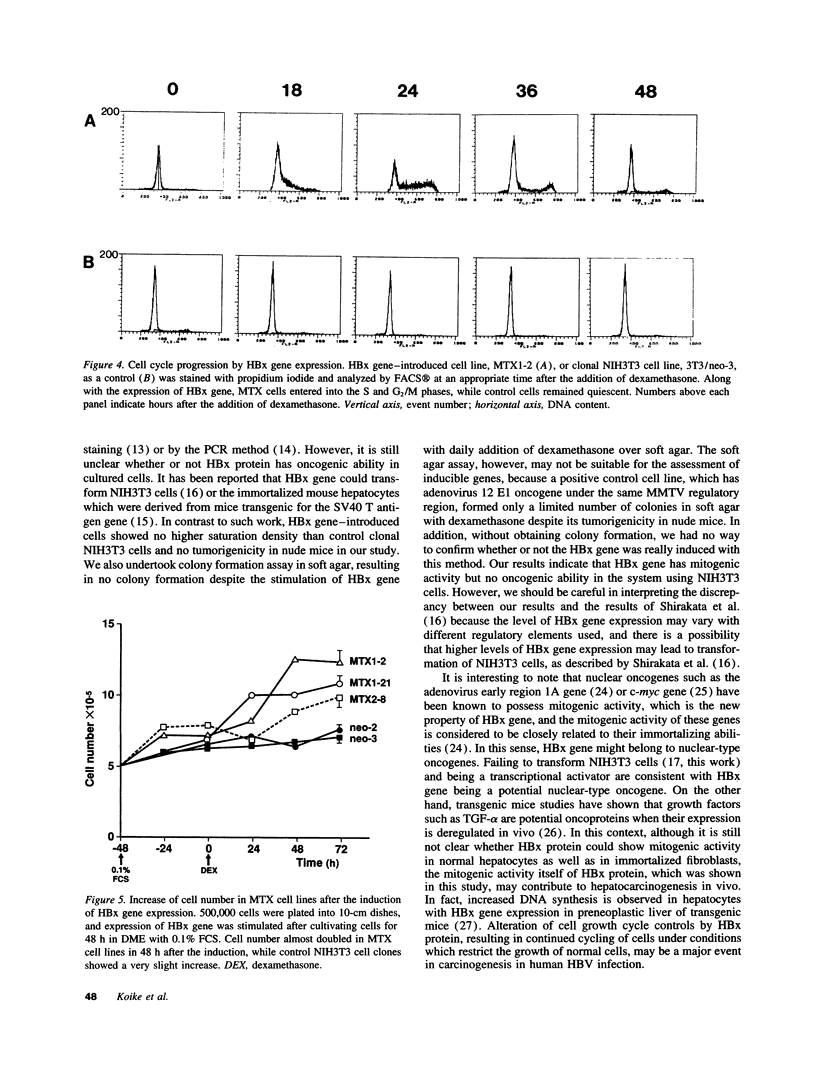

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Balsano C., Avantaggiati M. L., Natoli G., De Marzio E., Will H., Perricaudet M., Levrero M. Full-length and truncated versions of the hepatitis B virus (HBV) X protein (pX) transactivate the cmyc protooncogene at the transcriptional level. Biochem Biophys Res Commun. 1991 May 15;176(3):985–992. doi: 10.1016/0006-291x(91)90379-l. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Cheetham B. F., Li P., Parish C. R., Waldron-Stevens L. K., Bellett A. J. Adenovirus-induced alterations of the cell growth cycle: a requirement for expression of E1A but not of E1B. J Virol. 1983 Jan;45(1):192–199. doi: 10.1128/jvi.45.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colgrove R., Simon G., Ganem D. Transcriptional activation of homologous and heterologous genes by the hepatitis B virus X gene product in cells permissive for viral replication. J Virol. 1989 Sep;63(9):4019–4026. doi: 10.1128/jvi.63.9.4019-4026.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantis I. D., McGandy C. E., Chen T. J., Liaw Y. F., Gudat F., Bianchi L. Hepatitis B X-gene expression in hepatocellular carcinoma. J Hepatol. 1992 Jul;15(3):400–403. doi: 10.1016/0168-8278(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Finney R. E., Bishop J. M. Predisposition to neoplastic transformation caused by gene replacement of H-ras1. Science. 1993 Jun 4;260(5113):1524–1527. doi: 10.1126/science.8502998. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekulé A. S., Lauer U., Weiss L., Luber B., Hofschneider P. H. Hepatitis B virus transactivator HBx uses a tumour promoter signalling pathway. Nature. 1993 Feb 25;361(6414):742–745. doi: 10.1038/361742a0. [DOI] [PubMed] [Google Scholar]
- Kim C. M., Koike K., Saito I., Miyamura T., Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991 May 23;351(6324):317–320. doi: 10.1038/351317a0. [DOI] [PubMed] [Google Scholar]
- Koike K., Akatsuka T., Miyamura T. Characterization of hepatitis B virus X gene: in vitro translation of mRNA from COS-1 cells transfected with the X gene. Virology. 1988 Mar;163(1):233–235. doi: 10.1016/0042-6822(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Koike K., Hinrichs S. H., Isselbacher K. J., Jay G. Transgenic mouse model for human gastric carcinoma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5615–5619. doi: 10.1073/pnas.86.14.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Moriya K., Iino S., Yotsuyanagi H., Endo Y., Miyamura T., Kurokawa K. High-level expression of hepatitis B virus HBx gene and hepatocarcinogenesis in transgenic mice. Hepatology. 1994 Apr;19(4):810–819. [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Lee G. H., Merlino G., Fausto N. Development of liver tumors in transforming growth factor alpha transgenic mice. Cancer Res. 1992 Oct 1;52(19):5162–5170. [PubMed] [Google Scholar]
- Rogler C. E., Sherman M., Su C. Y., Shafritz D. A., Summers J., Shows T. B., Henderson A., Kew M. Deletion in chromosome 11p associated with a hepatitis B integration site in hepatocellular carcinoma. Science. 1985 Oct 18;230(4723):319–322. doi: 10.1126/science.2996131. [DOI] [PubMed] [Google Scholar]
- Seifer M., Höhne M., Schaefer S., Gerlich W. H. In vitro tumorigenicity of hepatitis B virus DNA and HBx protein. J Hepatol. 1991;13 (Suppl 4):S61–S65. doi: 10.1016/0168-8278(91)90026-8. [DOI] [PubMed] [Google Scholar]
- Seto E., Mitchell P. J., Yen T. S. Transactivation by the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature. 1990 Mar 1;344(6261):72–74. doi: 10.1038/344072a0. [DOI] [PubMed] [Google Scholar]
- Seto E., Yen T. S., Peterlin B. M., Ou J. H. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakata Y., Kawada M., Fujiki Y., Sano H., Oda M., Yaginuma K., Kobayashi M., Koike K. The X gene of hepatitis B virus induced growth stimulation and tumorigenic transformation of mouse NIH3T3 cells. Jpn J Cancer Res. 1989 Jul;80(7):617–621. doi: 10.1111/j.1349-7006.1989.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tokino T., Fukushige S., Nakamura T., Nagaya T., Murotsu T., Shiga K., Aoki N., Matsubara K. Chromosomal translocation and inverted duplication associated with integrated hepatitis B virus in hepatocellular carcinomas. J Virol. 1987 Dec;61(12):3848–3854. doi: 10.1128/jvi.61.12.3848-3854.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu J. S., Lai M. Y., Chen D. S., Robinson W. S. Activation of protooncogene c-jun by the X protein of hepatitis B virus. Virology. 1993 Jan;192(1):346–350. doi: 10.1006/viro.1993.1041. [DOI] [PubMed] [Google Scholar]
- Twu J. S., Schloemer R. H. Transcriptional trans-activating function of hepatitis B virus. J Virol. 1987 Nov;61(11):3448–3453. doi: 10.1128/jvi.61.11.3448-3453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. L., London W. T., Feitelson M. A. Hepatitis B x antigen in hepatitis B virus carrier patients with liver cancer. Cancer Res. 1991 Sep 15;51(18):4971–4977. [PubMed] [Google Scholar]
- Wollersheim M., Debelka U., Hofschneider P. H. A transactivating function encoded in the hepatitis B virus X gene is conserved in the integrated state. Oncogene. 1988 Nov;3(5):545–552. [PubMed] [Google Scholar]
- Yotsuyanagi H., Iino S., Koike K., Yasuda K., Hino K., Kurokawa K. Duration of viremia in human hepatitis A viral infection as determined by polymerase chain reaction. J Med Virol. 1993 May;40(1):35–38. doi: 10.1002/jmv.1890400108. [DOI] [PubMed] [Google Scholar]
- Zhou D. X., Taraboulos A., Ou J. H., Yen T. S. Activation of class I major histocompatibility complex gene expression by hepatitis B virus. J Virol. 1990 Aug;64(8):4025–4028. doi: 10.1128/jvi.64.8.4025-4028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]





