Abstract
BACKGROUND AND PURPOSE
Bone morphogenetic proteins (BMPs) were first identified through their role in inducing bone and cartilage formation, but many other important functions have since been ascribed to BMPs, including dorsoventral patterning, angiogenesis and tissue homeostasis. Using dorsomorphin and LDN193189, selective small molecule inhibitors of BMP signalling, we investigated the role of BMP signalling in early vascular patterning in zebrafish.
EXPERIMENTAL APPROACH
The effects of dorsomorphin and LDN193189 on vascular endothelial growth factor-a (VEGF) and BMP signalling in developing zebrafish and in human pulmonary artery endothelial cells were determined using confocal microscopy, Western blotting and quantitative PCR.
KEY RESULTS
We showed that dorsomorphin, similar to the VEGF inhibitor SU5416, strongly inhibits intersegmental vessel formation in zebrafish and that this is due to inhibition of VEGF activation of VEGF receptor 2 (VEGFR2), leading to reduced VEGF-induced phospho-ERK (extracellular regulated kinase) 1/2 and VEGF target gene transcription. These effects occurred at concentrations of dorsomorphin that block BMP signalling. We also showed that LDN193189, an analogue of dorsomorphin, more potently blocks BMP signalling but has no effect on VEGF signalling in zebrafish and does not disrupt early vascular patterning.
CONCLUSIONS AND IMPLICATIONS
Dorsomorphin inhibits both BMP and VEGF signalling, whereas LDN193189 is a more selective BMP antagonist. Results obtained in cardiovascular studies using dorsomorphin need to be interpreted with caution, and use of LDN193189 would be preferable due to its selectivity. Our data also suggest that BMP signalling is dispensable for early patterning of intersegmental vessels in zebrafish.
Keywords: BMP, VEGF, VEGFR2, dorsomorphin, LDN193189, SU5416, zebrafish, dorsoventral patterning, angiogenesis
Introduction
The establishment of the cardiovascular system is a key component of embryonic development. The vasculature forms in two distinct phases. The first, vasculogenesis, involves the de novo formation of vessels. The subsequent remodelling, sprouting and growth of new vessels from the basic major vessels and plexuses formed by vasculogenesis is termed angiogenesis (Moser and Patterson, 2005). A variety of model systems both in vitro and in vivo have shown the importance of transforming growth factor-β (TGF-β) superfamily members in these processes. For example, bone morphogenetic protein (BMP)4 induces human embryonic stem cells to form a capillary like network of cells when grown on matrigel, and BMP2 increases tumour vascularization in mice (Langenfeld and Langenfeld, 2004; Boyd et al., 2007). Mice deficient in components of the BMP signal transduction pathway die in utero due to gastrulation defects and to a failure of vascular differentiation (Moser and Patterson, 2005). Conditional knockout of the mouse BMP type 1a receptor (BMPR1a, also known as ALK3) after initial patterning of the embryo has occurred confirms an important role for BMP signalling in vessel development (Park et al., 2006). In these mice there is poor vessel formation and/ or impaired recruitment of smooth muscle cells to the vasculature leading to severe abdominal haemorrhage. Finally, knockout of the key BMP target genes Id1 and Id3 in mice impairs tumour angiogenesis and metastasis (Lyden et al., 1999).
Bone morphogenetic proteins are members of the TGF-β superfamily. They signal through a heteromeric complex of two type I and two type II BMP receptors. After ligand binding, the type II receptor phosphorylates and activates the type I receptor. The activated type I receptor phosphorylates the signalling molecules Smads 1/5/8 that then form a complex with the co-Smad, Smad 4. This complex then translocates to the nucleus to alter gene transcription. Although originally named because of their ability to induce bone and cartilage, BMPs have other important wide-ranging functions including dorsoventral patterning, haematopoiesis, vascular development and homeostasis (Miyazono et al., 2010).
Vascular endothelial growth factor-a (VEGF) signalling through VEGF receptor 2 (VEGFR2) (also known as FLK1/KDR) is vital for both vasculogenesis and angiogenesis (Olsson et al., 2006). Loss of either VEGF or VEGFR2 in mice causes embryonic death due to failure of blood vessel formation (Shalaby et al., 1995; Carmeliet et al., 1996). In zebrafish loss of VEGF signalling leads to failure of intersegmental vessel (ISV) and dorsal aorta formation with expansion of the posterior cardinal vein (Covassin et al., 2006; Herbert et al., 2009). BMP signalling has been shown to interact with VEGF signalling: BMP4 increases VEGFR2 expression and activation in human dermal microvascular endothelial cells (Suzuki et al., 2008) and a study in zebrafish has shown that Smad binding elements control VEGF transcription (He and Chen, 2005).
Zebrafish have become a widely used model organism for studies of developmental biology and drug discovery. In zebrafish and other vertebrates, BMP signalling plays an essential role during gastrulation when it regulates dorsoventral patterning: high BMP signalling promotes ventral fates including blood, vasculature and pronephros. Loss of BMP signalling in the zebrafish causes dorsalization with loss of ventral fates and expansion of dorsal tissues such as neural ectoderm and trunk somites (Little and Mullins, 2006). Using this dorsalization phenotype a recent screen in zebrafish of over 7500 compounds found compound C (renamed dorsomorphin) to be a selective inhibitor of the type I BMP receptors ALK2, ALK3 and ALK6 without inhibiting the type I TGF-β receptors ALK4, ALK5 or ALK7 (Yu et al., 2008b). Previously, dorsomorphin was used solely as an AMP-activated kinase inhibitor, although it was subsequently found to inhibit platelet-derived growth factor receptor-β (PDGFR-β) (Yu et al., 2008a). Dorsomorphin is structurally related to a series of compounds that inhibit VEGFR2 (Fraley et al., 2002a,b;) but is reported not to inhibit the VEGF receptor VEGFR2 (Yu et al., 2008b). A structure–activity relationship study by the same authors found LDN193189, an analogue of dorsomorphin, to be a more potent and selective inhibitor of ALK2 and ALK3 (Cuny et al., 2008).
Here we show that dorsomorphin does not selectively inhibit BMP signalling as previously reported but also inhibits VEGFR2. LDN193189, an analogue of dorsomorphin, does not block VEGF signalling at concentrations that block BMP signalling in zebrafish.
Methods
Zebrafish husbandry
Wild-type AB and transgenic Tg(fli1a:egfp)y1 zebrafish were maintained under standard conditions (Nusslein-Volhard and Dahm, 2002), and embryos were staged according to Kimmel et al. (1995). Embryos were treated with dorsomorphin (1–10 µM, Merck Biosciences, Nottingham, UK), LDN193189 (250 nM–1 µM, a kind gift from Dr Paul Yu, Massachusetts General Hospital, Boston, MA, USA), SU5416 (1 µM, Sigma, Poole, Dorset, UK) or dimethylsulphoxide (DMSO, Sigma) as vehicle control at 4 h post fertilization (hpf) to determine the effect on dorsoventral axis patterning or at 12 hpf to study the effect on ISV formation. Embryos were scored for dorsalization class (Mullins et al., 1996) at 11 and 24 hpf and at 30 hpf for effect on vascular formation. All procedures undertaken conformed to Home Office requirements.
Cell culture
Human pulmonary artery endothelial cells (HPAECs) were purchased from Lonza (Wokingham, Berkshire, UK) and propagated according to the instructions supplied.
Western blotting
HPAECs were seeded in 6 cm dishes (4.4 × 105 cells per dish) and grown to confluence in EGM-2 (Lonza) before being placed in serum-restricted medium [M199/0.1% fetal bovine serum (FBS)] for 16 h. After 30 min pretreatment with either 0.1% FBS, DMSO vehicle control (10 µM), dorsomorphin (2.5 or 10 µM) or LDN193189 (1 µM), the cells were treated with either VEGF (25 ng·mL−1) for 10 min, BMP6 (50 ng·mL−1) for 1 h (R&D Systems, Abingdon, UK) or 0.1% FBS control for the same times. Cells were snap-frozen and lysed in 150 µL ice-cold lysis buffer [125 mM Tris-HCl, pH 7.4, 10% (v/v) glycerol, 2% (w/v) SDS containing an EDTA-free protease inhibitor cocktail (Roche Diagnostics Ltd., Lewes, East Sussex, UK)]. Lysates were sonicated and the protein assayed before Western blot analysis. Cell lysates (15–40 µg) were separated on SDS-PAGE gels and proteins transferred to polyvinylidene difluoride membranes (GE Amersham Biosciences, Little Chalfort, Bucks, UK) by semi-dry blotting. Blots were then blocked and probed with the relevant antibodies [rabbit monoclonals to phospho-Smad 1/5, Smad 1, phospho-ERK (extracellular regulated kinase) 1/2, ERK 1/2, Phospho-(Y1175) VEGFR2 (Cell Signalling Technology, Beverly, MA, USA) or goat polyclonal to VEGFR2 (R&D systems)]. All blots were re-probed with mouse monoclonal α-tubulin antibody (Sigma). Blots were incubated with horseradish peroxidase-linked antibodies specific for either rabbit, goat or mouse immunoglobulin and bound complexes visualized by chemiluminescence (ECL plus GE Amersham Biosciences).
RNA extraction and quantitative RT-PCR
HPAECs were seeded in 6-well plates (2 × 105 cells per well) and grown in EGM-2 for 2 days. The cells were placed in serum-restricted M199/0.1% FBS for 16 h and then pre-incubated with either 0.1% FBS, DMSO (10 µM), dorsomorphin (2.5 or 10 µM) or LDN193189 (1 µM) for 30 min. The cells were treated with VEGF (25 ng·mL−1) for 90 min, BMP6 (50 ng·mL−1) for 4 h or 0.1% FBS control for the same times. Total RNA was extracted using an RNeasy Kit (Qiagen, Crawley, West Sussex, UK) and after DNase treatment single-stranded cDNA was synthesized from 0.5 µg total RNA using M-MuLV RT (Roche) according to the manufacturer's instructions and diluted to 50 µL for RT-PCR. Quantitative RT-PCR was performed in duplicate in 10 µL reactions using 2.5 µL of cDNA, 1× Lightcycler Mastermix (Roche) and 0.5 mM forward and reverse specific primers on a Lightcycler LC480 (Roche) according to the manufacturer's instructions. The Nur77, Nor1 and GAPDH primer sequences were those used by Liu et al. (2003) Quantitect primers (Qiagen) were used for amplifying Id1. Expression levels were compared with a standard curve and values normalized to GAPDH and expressed as the fold change relative to the 0.1% serum control.
Statistical analysis
This was performed using a one-way anova with Tukey's multiple comparison post hoc test. A P-value <0.05 was deemed significant.
Imaging
A Leica APO dissecting microscope mounted with a Coolpix 4500 camera (Nikon) was used to image and photograph the dorsalized embryos and an Olympus FV1000 Inverted Confocal Microscope used to image Tg(fli1a:egfp)y1 embryos prior to image processing with Adobe software.
Receptor nomenclature
These comply with the British Journal of Pharmacology's guide to receptors and ion channels (Alexander et al., 2008).
Results
Dorsomorphin dorsalized zebrafish embryos
Dorsomorphin, a small molecule inhibitor, has recently been found to selectively block BMP signalling by inhibiting the type I BMP receptors ALK2, ALK3 and ALK6 (Yu et al., 2008b). This compound was used to investigate the role of BMP signalling in zebrafish blood vessel development. Before blood vessels form, BMP signalling is critical for dorsoventral patterning of the embryo. Loss or impaired BMP signalling during gastrulation causes loss of ventral structures and the embryos are defined as ‘dorsalized’ (Mullins et al., 1996). There are five classes of dorsalization (Figure 1A), with 1 being the least severe and 5 being the most. Using this dorsalization phenotype, 10 µM DM applied at 4 hpf was found to cause severe dorsalization (classes 4 and 5) in all embryos (Figure 1B), and this concentration was used in subsequent experiments.
Figure 1.
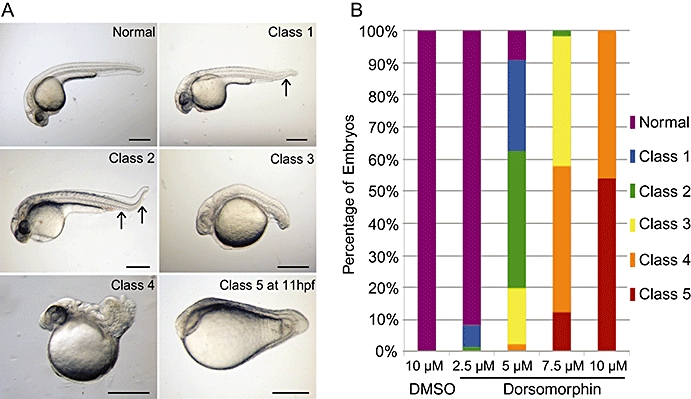
Dorsomorphin dorsalized zebrafish embryos. (A) Normal and classes of dorsalized embryos at 24 hpf (hours post fertilization) (unless stated). Lateral views with anterior to left. Class 1 only has loss of distal ventral fin, and class 2 has complete loss of ventral fin. Black arrows highlight loss of ventral fin tissue. Scale bars indicate 500 µm. (B) Summary of dorsalization class when embryos were exposed to different concentrations of dorsomorphin (DM) or 10 µM dimethylsulphoxide (DMSO) vehicle control from 4 hpf. Graph illustrates the combined results of class scoring from both 11 and 24 hpf; n= 121–143 embryos per group.
Dorsomorphin blocked ISV formation
The role of BMP signalling in early blood vessel patterning was investigated using Tg(fli1a:egfp)y1 zebrafish, in which the fli1a promoter drives green fluorescent protein expression in endothelial cells (Lawson and Weinstein, 2002). In control embryos by 30 hpf the dorsal aorta, posterior cardinal vein and ISVs have already formed (Figure 2B) and blood cells can be seen circulating (data not shown). Dorsomorphin was applied at 12 hpf because by this time point dorsoventral patterning is complete and therefore none of the embryos become dorsalized (data not shown). Dorsomorphin completely abolished ISV formation and also caused loss of the dorsal aorta with expansion of the posterior cardinal vein in all embryos (Figure 2C). The same phenotype was seen in all embryos when dorsomorphin was used at the lower concentration of 2.5 µM (data not shown), a concentration that induced only mild dorsalization (Figure 1B).
Figure 2.
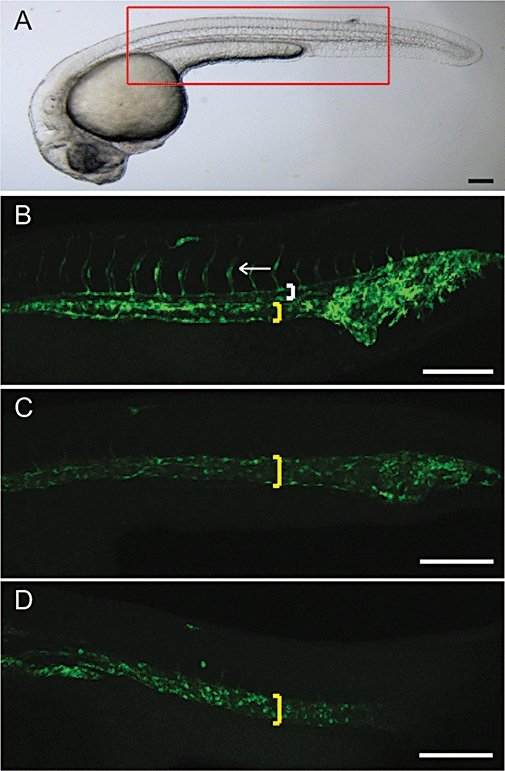
Dorsomorphin blocked intersegmental vessel (ISV) formation. (A) Normal embryo at 30 hpf (hours post fertilization) with red box illustrating the location where the confocal images were taken. Representative confocal z-stack images of Tg(fli1a:egfp)y1 zebrafish. (B) Normal with dorsal aorta (white bracket), posterior cardinal vein (yellow bracket) and ISV (white arrow) highlighted. Zebrafish exposed to 10 µM dorsomorphin (C) or 1 µM SU5416 (a pan vascular endothelial growth factor-a receptor inhibitor) (D) from 12 hpf have loss of the dorsal aorta and ISVs and expansion of the posterior cardinal vein. All are lateral views with anterior to left. Scale bars indicate 200 µm; n= 60–80 each group.
We note that this phenotype was also evident in all embryos treated with SU5416, an inhibitor of VEGFR (Figure 2D) and has previously been observed when VEGF signalling is inhibited (Covassin et al., 2006; Herbert et al., 2009). This suggests that dorsomorphin might block VEGFR2 directly, or that loss of BMP signalling inhibits VEGF signalling. There are data to support such an interaction of BMP and VEGF signalling: Smad binding elements on the VEGF promoter can control VEGF transcription (He and Chen, 2005) and BMP signalling can alter VEGFR2 expression and activation (Suzuki et al., 2008).
Dorsomorphin inhibited ERK and blocked transcription of VEGF target genes in HPAECs
ERK activation is important for the angiogenic effects of VEGF signalling (Olsson et al., 2006). We therefore investigated the effect of dorsomorphin on VEGF-induced ERK phosphorylation. VEGF caused up-regulation of phospho-ERK 1/2 on Western blots with no change in total ERK 1/2 (Figure 3A). This response was substantially inhibited by dorsomorphin, although not back to baseline levels (Figure 3A). We also examined if the reduction in phospho-ERK 1/2 induction was accompanied by altered VEGF-induced gene transcription. VEGF has previously been shown to induce the nuclear receptor 4A members Nor1 and Nur77 (Liu et al., 2003), although the function of their activation in endothelial cells is not known. VEGF induced both genes strongly compared with 0.1% FBS control (Nor1: 174.6 ± 74.9-fold, P < 0.001; Nur77: 17.3 ± 7.9-fold, P < 0.001). Dorsomorphin completely blocked these inductions at both concentrations (Figure 3B). To confirm that the concentrations of dorsomorphin used in the VEGF experiments also inhibit BMP signalling we treated HPAECs with BMP6. Dorsomorphin inhibited both the activation of phospho-Smad1/5 (Figure 3C) and induction of the BMP target gene, Id1 (Yang et al., 2008) (Figure 3D) in a concentration-dependent manner.
Figure 3.

Dorsomorphin inhibited signalling pathways induced by vascular endothelial growth factor-a (VEGF) and bone morphogenetic protein (BMP)6. Human pulmonary artery endothelial cells were pretreated with 0.1% fetal bovine serum (FBS), 10 µM dimethylsulphoxide (DMSO) (vehicle control), 2.5 or 10 µM dorsomorphin (DM) and then with 0.1% FBS or 25 ng·mL−1 VEGF (A, B) or 0.1% FBS or 50 ng·mL−1 BMP6 (C, D). VEGF induced phosphorylation of ERK 1/2 (A), VEGF target genes Nor1 and Nur77 (B) when cells were pretreated with 0.1% FBS and 10 µM DMSO, but this was completely inhibited by both concentrations of dorsomorphin. BMP6 induced phosphorylation of Smad 1/5 (C) and Id1 (D) when cells were pretreated with 0.1% FBS and 10 µM DMSO but again this was completely inhibited when cells were treated with dorsomorphin. VEGF induced phosphorylation of VEGF receptor 2 (VEGFR2) at tyrosine 1175 but this was blocked by 10 µM dorsomorphin (E). A, C and E are representative blots and B and D show mean ± SD, all n= 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with 0.1%/0.1% serum control.
These results show that dorsomorphin blocks both VEGF and BMP6 signal transduction. However, it is not clear whether the effect of dorsomorphin on VEGF signalling is at the level of the receptor or ERK activation because BMPs have been shown to activate ERK (Yang et al., 2008). To address this question we examined the autophosphorylation of VEGFR2 in response to VEGF directly. The key tyrosine phosphorylation site on VEGFR2 upstream of ERK 1/2 activation is Y1175 and, using a specific antibody for this phosphorylated tyrosine on VEGFR2, we confirmed that dorsomorphin blocks VEGF receptor auotphosphorylation (Figure 3E). It is therefore not possible to determine whether BMP signalling has any effect on ISV formation using dorsomorphin.
LDN193189 dorsalized zebrafish embryos but had no effect on ISV formation
LDN193189 is a more potent and selective inhibitor of BMP signalling in vitro than dorsomorphin (Cuny et al., 2008). Its effect on zebrafish development is currently unknown. Embryos were treated with different concentrations of LDN193189 at 4 hpf to determine its effects on dorsoventral patterning and they were scored as before. LDN193189 at 1 µM caused embryos to develop with the most severe dorsalized phenotypes (Figure 4A). Embryos treated from 12 or 24 hpf with this concentration appeared to be morphologically normal at 30 hpf, apart from no blood cells can be seen circulating. At 48 hpf they look morphologically normal apart from the development of significant pericardial oedema due to impaired heart formation and no circulating blood cells (data not shown). These phenotypes are predictable because BMP signalling is known to be important for cardiac development in other animal models (van Wijk et al., 2007) and for blood formation in zebrafish (Wilkinson et al., 2009).
Figure 4.

LDN193189 dorsalized zebrafish embryos but had no effect on vascular development. (A) Summary of combined dorsalization class at 11 and 24 hpf (hours post fertilization) when embryos were exposed to different concentrations of LDN193189 1 µM or dimethylsulphoxide (DMSO) vehicle control from 4 hpf; n= 130–153 embryos per group. (B) Representative confocal z-stack image (location as per red box in Figure 2A) of a Tg(fli1a:egfp)y1 zebrafish embryo taken as per Figure 2A exposed to 1 µM LDN193189 from 12 hpf with normal blood vessel development at 30 hpf. Lateral view with anterior to left. Scale bar indicates 200 µm; n= 60.
To assess for effects on ISV formation, Tg(fli1a:egfp)y1 embryos were exposed to 1 µM LDN193189 at 12 hpf. In contrast to specimens treated with dorsomorphin or SU5416, ISV and dorsal aorta formation in such embryos were normal (Figure 4B). To confirm that LDN193189 has no effect on VEGF signalling HPAECs were pretreated with LDN193189 prior to VEGF stimulation. LDN193189 (1 µM) had no effect on VEGF-induced phospho-ERK or nor1 and nur77 gene induction (Figure 5A,B) but potently inhibited BMP6-stimulated phospho-Smad 1/5 and Id1 gene induction (Figure 5C,D). These results confirm that BMP signalling is not essential for ISV formation and that LDN193189 does not inhibit VEGFR2 at a concentration that potently inhibits BMP signalling in vivo or in vitro and is therefore a more selective BMP inhibitor than dorsomorphin.
Figure 5.
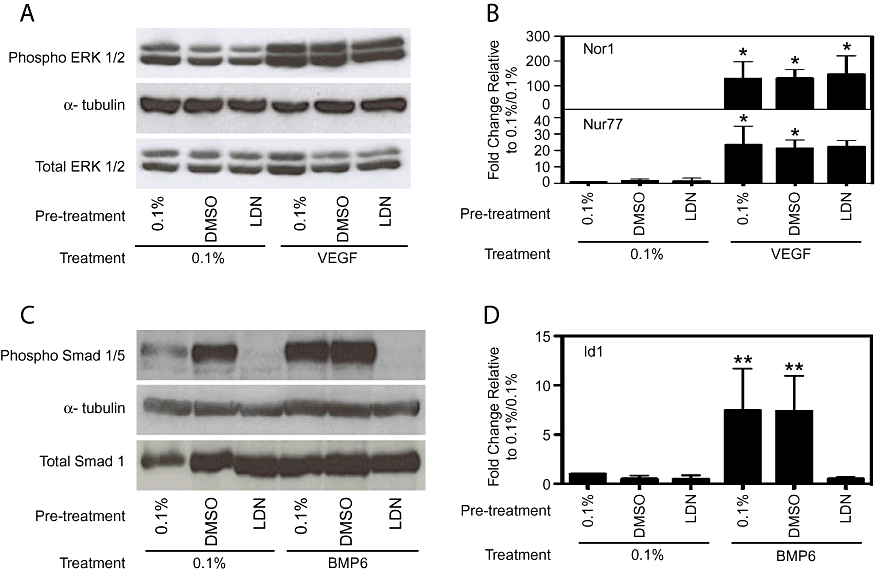
LDN193189 inhibited signalling pathways induced by bone morphogenetic protein (BMP)6 but not those induced by vascular endothelial growth factor-a (VEGF). Human pulmonary artery endothelial cells were pretreated with 0.1% fetal bovine serum (FBS), 1 µM dimethylsulphoxide (DMSO) (vehicle control) or 1 µM LDN193189 (LDN) and then with 0.1% FBS or 25 ng·mL−1 VEGF (A, B) or 0.1% FBS or 50 ng·mL−1 BMP6 (C, D). VEGF induced phosphorylation of extracellular regulated kinase (ERK) 1/2 (A), VEGF target genes Nor1 and Nur77 (B) when cells were pretreated with 0.1% FBS, 1 µM DMSO and 1 µM LDN193189. BMP6 induced phosphorylation of Smad 1/5 (C) and Id1 (D) when cells were pretreated with 0.1% FBS and 1 µM DMSO, but this was completely inhibited when cells were treated with LDN193189. A and C are representative blots and B and D show mean ± SD, all n= 3. *P < 0.05, **P < 0.01, compared with 0.1%/0.1% serum control.
Discussion
Dorsomorphin, originally known as an AMPK inhibitor, was identified as the first selective BMP inhibitor using a screen in zebrafish, but has also been shown to inhibit PDGFR-β (Yu et al., 2008a,b;). Our data highlight one of the potential difficulties in performing small molecule screens in that significant off-target effects may not be initially apparent even when using in vivo models. We present data using zebrafish and cultured HPAECs demonstrating that dorsomorphin also blocks VEGFR2 at concentrations that inhibit BMP signalling. We also report that LDN193189, an analogue of dorsomorphin known to block BMP signalling, has no effect on VEGF signalling at a concentration that blocks BMP signalling in the zebrafish in vivo model system. Both dorsomorphin and LDN193189 are structurally related to a series of compounds that inhibit VEGFR2 (Fraley et al., 2002a,b;). A recent study by Hao et al., who generated and analysed other analogues of dorsomorphin, found some to be selective inhibitors for either VEGFR2 or BMP type I receptors, but others that inhibited both including dorsomorphin (Hao et al., 2010). They also reported that LDN193189 inhibits VEGF signalling at 20 µM, which is 20× the concentration used in our studies. This highlights the importance of using the lowest effective concentration to minimize off-target effects.
VEGF-a signalling through VEGFR2 is critical for endothelial cell differentiation and mice lacking either display severely reduced endothelial cell numbers and fail to form blood vessels (Shalaby et al., 1995; Carmeliet et al., 1996). Similarly, in zebrafish, loss of VEGF signalling leads to failure of ISV and dorsal aorta formation and expansion of the posterior cardinal vein (Herbert et al., 2009). Like VEGFs, BMPs have been implicated in blood vessel formation. Although only limited data are available from zebrafish, mice deficient in components of the BMP signal transduction pathway die in utero due to gastrulation defects and to a failure of vascular differentiation (Moser and Patterson, 2005). BMPs can also regulate VEGF signalling via induction of VEGF ligand transcription (He and Chen, 2005) or through VEGFR2 expression and activation (Suzuki et al., 2008). With these results in mind, we were surprised that we were unable to see any vascular phenotype by 30 hpf when LDN193189 was applied at 12 hpf in the zebrafish. These data indicate that BMP signalling is dispensable for vessel formation in zebrafish, but it remains possible that it is needed for vessel maturation by recruitment of mural cells. This possibility derives from data in the mouse, where loss of either ALK3 or BMPRII leads to poor vessel coverage by mural cells leading to abdominal haemorrhage (Park et al., 2006; Liu et al., 2007). In the zebrafish, primitive mural cells can only be found surrounding some blood vessels from 4 days post fertilization (Santoro et al., 2009).
In conclusion, we demonstrate, both in vitro and in vivo, that dorsomorphin inhibits BMP and VEGF receptors whereas LDN193189 only inhibits BMP type I receptors. There are now several reports where dorsomorphin has been used to inhibit BMP signalling (Hao et al., 2008; van Dinther et al., 2009; Bai et al., 2010; Kamiya et al., 2010), and in view of its potent ability also to inhibit VEGFR2, this compound should be used with great caution in studies of cardiovascular development and regulation, and in other investigations. LDN193189 is a more selective reagent to dissect the role of BMP signalling without interfering with VEGF or TGF-β signalling. Our data further suggest that BMP signalling is dispensable for early vascular patterning in the zebrafish.
Acknowledgments
The authors thank Dr Paul Yu (Massachusetts General Hospital, Boston, MA, USA) for supply of LDN193189. This work was supported by a Wellcome Trust Programme Grant (JCS), a British Heart Foundation Programme Grant (NWM) and a NIHR Cambridge Biomedical Research Centre Clinical Training Fellowship and Sackler Fellowship (JEC).
Glossary
Abbreviations
- BMP
bone morphogenetic protein
- ERK
extracellular regulated kinase
- HPAECs
human pulmonary artery endothelial cells
- hpf
hours post fertilization
- ISV
intersegmental vessel
- PDGFR-β
platelet-derived growth factor receptor-β
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor-a
- VEGFR2
VEGF receptor 2
Conflicts of interest
The authors declare they have no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Gao Y, Arzigian M, Wojchowski DM, Wu W-S, Wang ZZ. BMP4 regulates vascular progenitor development in human embryonic stem cells through a smad-dependent pathway. J Cell Biochem. 2010;109:363–374. doi: 10.1002/jcb.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd NL, Dhara SK, Rekaya R, Godbey EA, Hasneen K, Rao RR, et al. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 2007;232:833–843. [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci USA. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, et al. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans M-J, de Boer J, et al. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2009 doi: 10.1359/jbmr.091110. Epub ahead of print: DOI 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- Fraley ME, Hoffman WF, Rubino RS, Hungate RW, Tebben AJ, Rutledge RZ, et al. Synthesis and initial SAR studies of 3,6-disubstituted pyrazolo[1,5-a]pyrimidines: a new class of KDR kinase inhibitors. Bioorg Med Chem Lett. 2002a;12:2767–2770. doi: 10.1016/s0960-894x(02)00525-5. [DOI] [PubMed] [Google Scholar]
- Fraley ME, Rubino RS, Hoffman WE, Hambaugh SR, Arrington KL, Hungate RW, et al. Optimization of a pyrazolo[1,5-a]pyrimidine class of KDR kinase inhibitors: improvements in physical properties enhance cellular activity and pharmacokinetics. Bioorg Med Chem Lett. 2002b;12:3537–3541. doi: 10.1016/s0960-894x(02)00827-2. [DOI] [PubMed] [Google Scholar]
- Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. Plos ONE. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Chen X. Transcription regulation of the vegf gene by the BMP/Smad pathway in the angioblast of zebrafish embryos. Biochem Biophys Res Commun. 2005;329:324–330. doi: 10.1016/j.bbrc.2005.01.133. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, et al. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, et al. Wnt inhibitors Dkk1 and sost are downstream targets of BMP signaling through the Type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res. 2010;25:200–210. doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res C Embryo Today. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- Liu D, Jia H, Holmes DIR, Stannard A, Zachary I. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arterioscler Thromb Vasc Biol. 2003;23:2002–2007. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- Liu D, Wang J, Kinzel B, Mueller M, Mao X, Valdez R, et al. Dosage-dependent requirement of BMP type II receptor for maintenance of vascular integrity. Blood. 2007;110:1502–1510. doi: 10.1182/blood-2006-11-058594. [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Moser M, Patterson C. Bone morphogenetic proteins and vascular differentiation: BMPing up vasculogenesis. Thromb Haemost. 2005;94:713–718. doi: 10.1160/TH05-05-0312. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden EJ, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Dahm R, editors. Zebrafish: A Practical Approach. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Park C, Lavine K, Mishina Y, Deng C-X, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Santoro MM, Pesce G, Stainier DY. Characterization of vascular mural cells during zebrafish development. Mech Dev. 2009;126:638–649. doi: 10.1016/j.mod.2009.06.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Montagne K, Nishihara A, Watabe T, Miyazono K. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem. 2008;143:199–206. doi: 10.1093/jb/mvm215. [DOI] [PubMed] [Google Scholar]
- van Wijk B, Moorman AFM, van den Hoff MJB. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Wilkinson RN, Pouget C, Gering M, Russell AJ, Davies SG, Kimelman D, et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Davies RJ, Southwood M, Long L, Yang X, Sobolewski A, et al. Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res. 2008;102:1212–1221. doi: 10.1161/CIRCRESAHA.108.173567. [DOI] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008a;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008b;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]


