Abstract
BACKGROUND AND PURPOSE
Anandamide and sphingosine-1-phosphate (S1P) both regulate vascular tone in a variety of vessels. This study aimed to examine the mechanisms involved in the regulation of coronary vascular tone by anandamide and S1P, and to determine whether any functional interaction occurs between these receptor systems.
EXPERIMENTAL APPROACH
Mechanisms used by anandamide and S1P to regulate rat coronary artery (CA) reactivity were investigated using wire myography. Interactions between S1P and the cannabinoid (CB)2 receptor were determined using human embryonic kidney 293 (HEK293) cells that stably over-express recombinant CB2 receptor.
KEY RESULTS
Anandamide and S1P induced relaxation of the rat CA. CB2 receptor antagonists attenuated anandamide-induced relaxation, while S1P-mediated relaxation was dependent on the vascular endothelium and S1P3. Anandamide treatment resulted in an increase in the phosphorylation of sphingosine kinase-1 within the CA. Conversely, anandamide-mediated relaxation was attenuated by inhibition of sphingosine kinase. Moreover, S1P3, specifically within the vascular endothelium, was required for anandamide-mediated vasorelaxation. In addition to this, S1P-mediated relaxation was also reduced by CB2 receptor antagonists and sphingosine kinase inhibition. Further evidence that S1P functionally interacts with the CB2 receptor was also observed in HEK293 cells over-expressing the CB2 receptor.
CONCLUSIONS AND IMPLICATIONS
In the vascular endothelium of rat CA, anandamide induces relaxation via a mechanism requiring sphingosine kinase-1 and S1P/S1P3. In addition, we report that S1P may exert some of its effects via a CB2 receptor- and sphingosine kinase-dependent mechanism, where subsequently formed S1P may have privileged access to S1P3 to induce vascular relaxation.
Keywords: cannabinoids, sphingosine-1-phosphate, sphingosine kinase
Introduction
The psychotropic effects of cannabinoids (CBs) are well established. There is also growing evidence for the role of endogenous CBs in the regulation of the cardiovascular system, with the release of endogenous CBs in response to stress such as during myocardial infarction reported as being cardioprotective, reducing tissue damage and arrhythmia (Hajrasouliha et al., 2008). CBs also appear to have a role in modulating vascular tone, and the endogenous CB N-arachidonylethanolamide (anandamide) has been reported to induce relaxation in a variety of vessels, both resistance and conduit, including aorta, mesenteric, hepatic and coronary arteries (CAs) (Zygmunt et al., 1999; White et al., 2001; Herradon et al., 2007; Ho and Randall, 2007). However, no clear consensus has been reached with regard to the mechanisms governing this relaxation.
CBs can exert their effects via activation of two specific G-protein-coupled receptors (GPCRs) designated CB1 and CB2, both of which are Gi/o-coupled and act to inhibit adenylyl cyclase (Howlett et al., 1986; Felder and Glass, 1998). Several studies have reported the importance of these CB receptors in mediating responses to anandamide, with vascular relaxation mainly attributed to activation of the CB1 receptor (O'Sullivan et al., 2004; Romano and Lograno, 2006). Conversely, a body of evidence also exists proposing that the effects of anandamide cannot be accredited to either CB receptor subtype (Grainger and Boachie-Ansah, 2001; White et al., 2001; Herradon et al., 2007). The existence of a novel endothelial receptor referred to as a ‘non-CB1/non-CB2’ CB receptor has been proposed, and this receptor has been reported to play a role in mediating relaxation to anandamide (Jarai et al., 1999; Offertaler et al., 2003; Herradon et al., 2007). In addition, anandamide has been shown to act as a ligand for transient receptor potential vanilloid type 1 channels (Smart et al., 2000), and in some arteries, such as rat mesenteric, the vasodilator effects of anandamide have been attributed to its interaction with this receptor, resulting in the release of the potent vasodilator peptide CGRP from sensory nerve endings (Zygmunt et al., 1999; Ho and Hiley, 2003). Furthermore, the role of the vascular endothelium in the vasodilator process is also unclear, as both endothelium-dependent and endothelium-independent mechanisms of anandamide-induced relaxation have been proposed (Pratt et al., 1998; Grainger and Boachie-Ansah, 2001; White et al., 2001; Ho and Hiley, 2003).
At an intracellular level, several mediators have been suggested to facilitate the effect of anandamide. For instance, in bovine ophthalmic arteries and rat aorta, generation of nitric oxide mediates relaxation to anandamide (Romano and Lograno, 2006; Herradon et al., 2007). Studies in a variety of other vessels have also implicated the enzyme COX in the vasoactive effects of anandamide; some proposing that COX metabolizes anandamide following its cellular uptake, resulting in the production of vasodilator eicosanoids (Grainger and Boachie-Ansah, 2001; Herradon et al., 2007; Ho and Randall, 2007). Moreover, release of endothelium-derived hyperpolarizing factor has also been suggested to play a role in anandamide-induced relaxation (O'Sullivan et al., 2004). In summary, the mechanisms mediating this vasodilator response to anandamide appear to vary significantly, depending on the vascular region and species under investigation.
The bioactive lipid mediator sphingosine-1-phosphate (S1P) is also cardioprotective and plays an important role in regulating vascular tone. Synthesized from sphingosine by the enzyme sphingosine kinase (SK1 and SK2), S1P can exert many of its biological effects via five specific GPCRs designated S1P1–5 (Chun et al., 2002; Pyne et al., 2009). In the vasculature S1P1, S1P2 and S1P3 are involved in modulating vascular tone, with the degree and type of vascular response varying between species and vascular beds (Alewijnse et al., 2004; Hemmings, 2006; Peters and Alewijnse, 2007). For example, mesenteric arteries from the rat have been reported to constrict in response to S1P, whereas rat aorta will dilate when exposed to similar concentrations of S1P (Hemmings et al., 2004; Roviezzo et al., 2006). The effect of S1P on vascular tone appears to depend on the receptor subtypes expressed within that vessel and the distribution of these receptors (Coussin et al., 2002). S1P1, which is highly expressed on vascular endothelium, can mediate vasorelaxation via endothelial NOS (eNOS) activation (Igarashi and Michel, 2000; Igarashi et al., 2001). Conversely, S1P is able to increase intracellular calcium levels and activate Rho kinase in smooth muscle cells via S1P2, leading to vasoconstriction (Hemmings, 2006; Peters and Alewijnse, 2007). Therefore, the receptor subtype expression within a vascular bed and the balance between vasodilator and vasoconstrictor signals stimulated by S1P will determine its overall effect on vascular tone.
S1P and CB receptors both belong to the lysolipid family of GPCRs and exhibit a sequence identity of approximately 20% (Sanchez and Hla, 2004). Furthermore, a link may exist between the CB and sphingolipid pathways as CBs have been shown to regulate sphingolipid metabolism, with activation of the CB1 receptor resulting in ceramide accumulation in a variety of cell types (Blazquez et al., 1999; Galve-Roperh et al., 2000). In addition to this, sphingosine and its analogue, the immunosuppressant FTY720, have also been reported to interact with the CB1 receptor (Paugh et al., 2006).
Therefore, given the role of both anandamide and S1P in mediating cardioprotection and regulating vascular tone, the present study aimed to examine the mechanisms involved in the regulation of rat coronary vascular tone mediated by anandamide and SIP, and to determine whether any functional interaction/overlapping of signalling pathways occurs between these systems.
Methods
Tissue preparation and myography
All experimental procedures complied with the United Kingdom Animals (Scientific Procedures) Act 1986 and the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication no. 85–23, revised 1996). Male Sprague Dawley rats (250–400 g body weight) were killed with a rising concentration of CO2, and the heart removed and transferred to cold modified Krebs–Henseleit solution (NaCl 118 mM, NaHCO3 25 mM, KCl 4.7 mM, KH2PO4 1.2 mM, MgSO4 1 mM, CaCl2 2.5 mM and glucose 11 mM). The left anterior descending and right CAs were then carefully dissected free from the heart tissue; 2 mm segments were mounted in a wire myograph and maintained at 37°C in Krebs–Henseleit solution with a constant supply of 95% O2/5% CO2. Vessels were then normalized to a tension equivalent to that generated at 90% of the diameter of the vessel at 100 mmHg (Mulvany and Halpern, 1977). Tissue was allowed to equilibrate for 30 min prior to challenge with 80 mM KCl to assess viability. In order to determine vascular endothelial function, vessels were pre-contracted with the thromboxane A2 mimetic U46619 (0.3 µM) and the response to 1 µM ACh observed. Relaxation to ACh > 50% denoted endothelium intact vessels. Where indicated, vessels were denuded of endothelium by gentle rubbing of the intimal surface with a human hair. Successful denudation was confirmed by a vasodilator response to ACh of <10%.
Myography experimental protocol
After 30 min equilibration, vessels were submaximally pre-contracted with U46619 (0.3 µM). When a stable level of contraction was achieved, concentration–response curves were generated by cumulative addition of agonist. Anandamide and S1P (1 nM–30 µM) were added in half-log molar concentrations in a cumulative manner with 5 min between each addition. In the case of anandamide, phenylmethanesulphonylfluoride (PMSF) (200 µM), an anandamide breakdown inhibitor, was added 10 min prior to the first addition. In experiments where the effects of inhibitors on agonist-induced relaxation were to be investigated, the inhibitor of interest was added 10 min before pre-contraction with U46619, and was present during construction of the concentration–response curve.
Cell culture
Human embryonic kidney 293 (HEK293) cells were maintained at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with essential amino acids, penicillin (50 U·mL−1), streptomycin (50 µg·mL−1) and 10% (v/v) fetal calf serum (FCS), whereas stable over-expressing HA-tagged CB2 receptor HEK293 (CB2HEK293) cells (gift from Prof K Mackie, Indiana University, IN, USA) were maintained in DMEM supplemented with 10% (v/v) FCS and 800 µg·mL−1 G418, also known as Geneticin). All HEK293 cells were quiesced in serum-free medium for 24 h before experimentation. MCF-7 cells were grown in DMEM supplemented with penicillin (50 U·mL−1), streptomycin (50 µg·mL−1), 10% (v/v) FCS, 0.4% G418 and 15 µg·mL−1 insulin, and maintained at 37°C with 5% CO2. MCF-7 cells were quiesced for 48 h in serum-free media before experimentation.
[35S]-GTPγS (guanosine 5′- [γ-[35S]thio]triphosphate) binding assay
HEK293 or CB2HEK293 cells were homogenized in buffer containing 20 mM HEPES (pH 7.5), 50 mM NaCl and 2 mM EDTA. Nuclei and cell debris were removed by centrifugation at 2000×g for 5 min at 4°C, and the resulting supernatant centrifuged at 100 000×g for 1 h at 4°C. The pellet containing the membrane fraction was then rinsed with and resuspended in buffer containing 50 mM HEPES (pH 7.5), 100 mM NaCl, 1 mM MgCl2 and 2 mM EDTA. Samples containing 20 µg of membrane protein were then incubated for 15 min at 30°C in buffer containing 50 mM HEPES (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 10 µM GDP, 2 mM DTT, 0.1 nM [35S]-GTPγS and the indicated concentrations of agonist and/or antagonist. Non-specific binding was determined in the presence of unlabelled GTPγS (20 µM). Reactions were terminated by rapid filtration through GF/C glass fibre filters using a 24-well Brandel Cell Harvester (Gaithersburg, MD, USA). Filters were then rinsed with wash buffer containing 20 mM Tris/HCl (pH 7.4), 120 mM NaCl and 25 mM MgCl2, and membrane-bound radioactivity was quantified by liquid scintillation counting. Samples were run in duplicate.
Immunoblotting
In experiments where rat CA tissue was used, vessel rings were mounted in a wire myograph and the tension on the vessels normalized, as already described, prior to stimulation with anandamide for 30 min. Vessel rings were then carefully removed from the myograph. Four CA rings were pooled together and homogenized in RIPA buffer [50 mM sodium HEPES (pH 7.5), 150 mM sodium chloride, 5 mM EDTA, 10 mM sodium fluoride, 10 mM sodium phosphate, 1% (v/v) Triton X-100, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 0.1 mM phenylmethylsulphonyl fluoride, 10 µg·mL−1 soy bean trypsin inhibitor, 10 µg·mL−1 benzamidine] to make one sample. Analysis of proteins by SDS–PAGE and immunoblotting was performed as previously described (Long et al., 2006) using anti-ERK2, anti-phosphorylated ERK1/2, anti-haemagglutinin (anti-HA) and anti-phospho-SK1 antibodies. Densitometry was performed on each resulting film exposure to determine normalized levels of the protein of interest using Molecular Analyst software version 2.1 (Bio-Rad Laboratories, Hemel Hempstead, UK).
Materials
Anandamide (in Tocrisolve 100), AM630, JTE907 and AM251 were purchased from Tocris Bioscience (Bristol, UK). S1P, VPC23019 and W146 were from Avanti Polar Lipid (Alabaster, AL, USA). 4-(4-(4-Chlorophenyl)thiazol-2-ylamino)phenol (SKi), U46619 and G418 were from Merck Biosciences Ltd (Nottingham, UK), and CAY10444 was from Axxora (Nottingham, UK). The following chemicals were purchased from Sigma-Aldrich (Poole, UK): indomethacin, acetylcholine, insulin and PMSF. Cell culture supplies were obtained from Invitrogen (Paisley, UK). Anti-ERK-2 and anti-phosphorylated ERK-1/2 antibodies were from BD Biosciences (Oxford, UK); anti-phospho-specific sphingosine kinase 1 (Ser-225) from ECM Biosciences (Versailles, KY); and anti-HA antibody from Sigma-Aldrich. GTPγS and [35S]-GTPγS (1250 Ci·mmol−1) were purchased from Bioquote Ltd (York, UK) and Perkin Elmer LAS (Beaconsfield, UK) respectively.
Statistical analysis
Relaxation responses are expressed as a percentage loss of the tone induced by U46619. EC50 values were obtained from individual concentration–response curves by fitting the data to a logistics equation (GraphPad PRISM version 4, GraphPad Software, La Jolla, CA, USA). Data are given as mean ± SEM of n, where n= the number of arteries from different animals. Statistical comparisons of concentration–response curves were made by two-way anova of the whole data set. Where appropriate, one-way anova with Newman–Keuls multiple comparison post-tests was employed. P values less than 0.05 were considered statistically significant.
None of the antagonists or enzyme inhibitors had a significant effect on U46619-induced contractions in rat CA (Table 1).
Table 1.
Effect of antagonists and enzyme inhibitors on U46619-induced contraction in rat CA
| Mean U46619-induced contraction (g) | |
|---|---|
| Vehicle | 0.35 ± 0.05 |
| AM251 | 0.37 ± 0.12 |
| AM630 | 0.35 ± 0.04 |
| JTE907 | 0.44 ± 0.09 |
| W146 | 0.37 ± 0.06 |
| CAY1044 | 0.36 ± 0.06 |
| SKi | 0.38 ± 0.07 |
| VPC20139 | 0.32 ± 0.05 |
| AM630 + CAY10444 | 0.41 ± 0.14 |
Data are expressed as mean ± SEM for n≥ 7 arteries from different animals. Statistical analysis using one-way anova with Newman–Keuls post-test revealed no significant differences between groups.
All drug/molecular target nomenclature conform to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2009).
Results
Anandamide induced vascular relaxation via the CB2 receptor subtype
Addition of anandamide (1 nM–30 µM) to rat U46619-pre-contracted intact CA resulted in a concentration-dependent, slowly developing relaxation, which reached a maximum of 42.1 ± 5.9%, EC50 2.36 × 10−8 M, n= 13. The relaxant effect of anandamide was not reduced by denudation of the endothelium (maximal relaxation 44.6 ± 4.1%, EC50 2.23 × 10−8, n= 7, Figure 1A).
Figure 1.
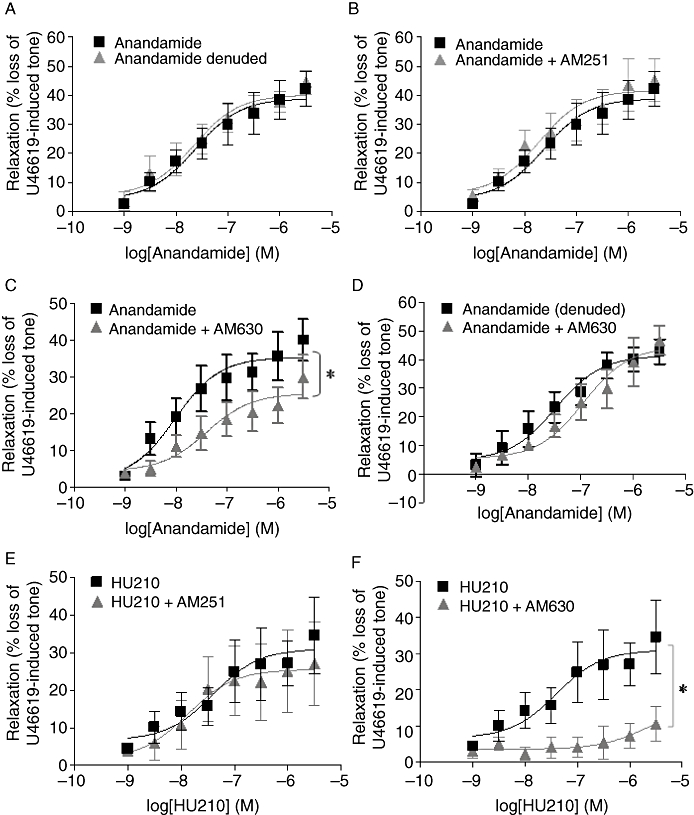
Anandamide induced vascular relaxation via the CB2 receptor. Concentration–response curves for the vasorelaxant effect of anandamide (1 nM–30 µM) in (A) endothelium-intact (n= 13) and endothelium-denuded (n= 7) rat CA and concentration–response curves for the relaxation of U46619-induced tone in endothelium-intact rat CA for anandamide (1 nM–30 µM) in the presence of (B) AM251, a selective CB1 antagonist (10 µM) (n= 6) (C) AM630, a selective CB2 antagonist (100 nM) (n= 5) and (D) AM630 (100 nM) (n= 4) in endothelium-denuded rat CA. (E) Concentration–response curves showing the vasorelaxant effect of HU210 alone (1 nM–30 µM) and in the presence of AM251 (10 µM) (n= 6) and (F) in the presence of AM630 (10 µM) (n= 4). Data are expressed as a percentage of U46619-induced tone and shown as mean ± SEM for n arteries from different animals. *P < 0.05 versus anandamide alone as determined by two-way anova.
The pretreatment of the vessels with the selective CB1 receptor antagonist AM251 (10 µM) did not significantly alter the magnitude of the vasorelaxation induced by anandamide (n= 6, Figure 1B, Table 2). However, the CB2-selective antagonist AM630 (100 nM) significantly attenuated the relaxant response to anandamide (n= 4, P < 0.05, Figure 1C). Initial experiments using AM630 at a higher concentration of 10 µM also attenuated anandamide-mediated relaxation, reducing the maximal relaxation to 24.99 ± 2.78%, n= 6, P < 0.05 (Table 2). Similarly, the CB2-selective inverse agonist JTE907 (10 µM) also markedly reduced relaxation in response to anandamide (maximal relaxation 9.7 ± 0.92%, n= 5, Table 2). However, in endothelium-denuded vessels, AM630 (100 nM) had no effect on the ability of anandamide to induce vascular relaxation (n= 4, Figure 1D; Table 2).
Table 2.
Effects of antagonist and enzyme inhibitors on anandamide and HU210-mediated relaxation in rat CA
| pEC50 | Rmax | n | |
|---|---|---|---|
| Anandamide | −7.63 ± 0.30 | 38.92 ± 4.93 | 6 |
| Anandamide + AM251 (10 µM) | −7.73 ± 0.33 | 41.71 ± 6.01 | 6 |
| Anandamide | −8.01 ± 0.33 | 35.41 ± 2.85 | 6 |
| Anandamide + AM630 (100 nM) | −7.39 ± 0.37* | 25.56 ± 2.88* | 5 |
| Anandamide | −7.72 ± 0.29 | 38.92 ± 3.77 | 6 |
| Anandamide + AM630 (10 µM) | −7.60 ± 0.46 | 24.99 ± 2.78* | 6 |
| Anandamide | −7.65 ± 0.29 | 41.09 ± 3.45 | 7 |
| Anandamide + JTE907 | −8.24 ± 0.42 | 9.724 ± 0.92* | 5 |
| Anandamide (denuded) | −7.48 ± 0.24 | 41.36 ± 3.17 | 6 |
| Anandamide + AM630 (10 µM) (denuded) | −6.95 ± 0.23 | 44.55 ± 4.24 | 4 |
| HU210 | −7.40 ± 0.5 | 31.03 ± 4.44 | 5 |
| HU210 + AM251 (10 µM) | −7.87 ± 0.69 | 25.51 ± 4.636 | 6 |
| HU210 | −7.40 ± 0.5 | 31.03 ± 4.44 | 5 |
| #HU210 + AM630 (10 µM) | −5.72 ± 1.19# | 14.71 ± 13.81# | 4 |
| Anandamide | −7.53 ± 0.35 | 47.50 ± 5.37 | 13 |
| Anandamide + indomethacin (10 µM) | −8.41 ± 0.43 | 22.50 ± 1.93* | 7 |
| Anandamide (denuded) | −7.51 ± 0.54 | 49.12 ± 7.32 | 7 |
| Anandamide + indomethacin (10 µM) (denuded) | −7.54 ± 0.37 | 46.50 ± 5.73 | 6 |
| Anandamide | −7.63 ± 0.30 | 38.92 ± 3.38 | 13 |
| Anandamide + SKi (10 µM) | NA | 2.3 ± 6.16* | 5 |
| Anandamide (denuded) | −7.59 ± 0.28 | 40.08 ± 3.28 | 6 |
| Anandamide + SKi (10 µM) (denuded) | −7.188 ± 0.36 | 37.57 ± 5.10 | 4 |
| Anandamide | −7.63 ± 0.28 | 38.92 ± 5.37 | 13 |
| Anandamide + VPC23019 (10 µM) | −7.57 ± 0.35 | 14.85 ± 1.36* | 8 |
| Anandamide (denuded) | −7.65 ± 0.25 | 40.19 ± 2.86 | 7 |
| Anandamide + VPC23019 (10 µM) (denuded) | −7.175 ± 0.21 | 36.74 ± 2.24 | 8 |
| Anandamide | −7.63 ± 0.3 | 38.92 ± 5.37 | 13 |
| Anandamide + W146 (10 µM) | −7.04 ± 0.28 | 34.76 ± 3.08 | 5 |
| Anandamide | −7.63 ± 0.3 | 38.92 ± 5.37 | 13 |
| Anandamide + CAY10444 (10 µM) | −7.99 ± 0.28 | 16.15 ± 2.90* | 5 |
| Anandamide (denuded) | −7.65 ± 0.25 | 40.19 ± 2.86 | 7 |
| Anandamide + CAY10444 (denuded) (10 µM) | −7.33 ± 0.26 | 47.33 ± 3.32 | 6 |
| Anandamide | −7.63 ± 0.29 | 38.92 ± 3.37 | 6 |
| Anandamide + AM630 (100 nM) + CAY10444 (10 µM) | −6.76 ± 0.32* | 25.51 ± 3.50* | 4 |
Data are expressed as mean ± SEM for n arteries from different animals.
P < 0.05 versus anandamide alone,
P < 0.05 versus HU210 alone as determined by two-way anova.
In addition to the vasodilator effects of anandamide, HU210, a non-selective CB1/2 agonist, also mediated relaxation of the rat CA. This response was not inhibited by the CB1 antagonist AM251 (n= 5, Figure 1E; Table 2). It was, however, significantly attenuated by the CB2 antagonist AM630 (10 µM) (n= 4, P < 0.05, Figure 1F; Table 2).
S1P stimulated vascular relaxation in an endothelium-dependent manner
To confirm that S1P is a vasorelaxant in rat CA, its effect on CA tone was directly assessed. In endothelium-intact U46619 pre-contracted arteries, S1P caused a concentration-dependent relaxation. In common with anandamide, this was a slow onset response resulting in a maximum relaxation of 46.1 ± 4.5%, n= 10 (Figure 2A; Table 3). In vessels where the endothelial layer was removed, relaxation in response to S1P was markedly reduced (maximal relaxation 23.7 ± 6.8%, n= 8, P < 0.05, Figure 2A; Table 3). Furthermore, responses to S1P were not significantly inhibited by the S1P1 antagonist W146 (Figure 2B; Table 3, n= 5) or JTE013, an S1P2 antagonist (Figure 2C; Table 3, n= 6). However, the S1P3 antagonist CAY10444 did significantly attenuate S1P-mediated relaxation (21.0 ± 2.7%, n= 5, P < 0.05, Figure 2D; Table 3).
Figure 2.
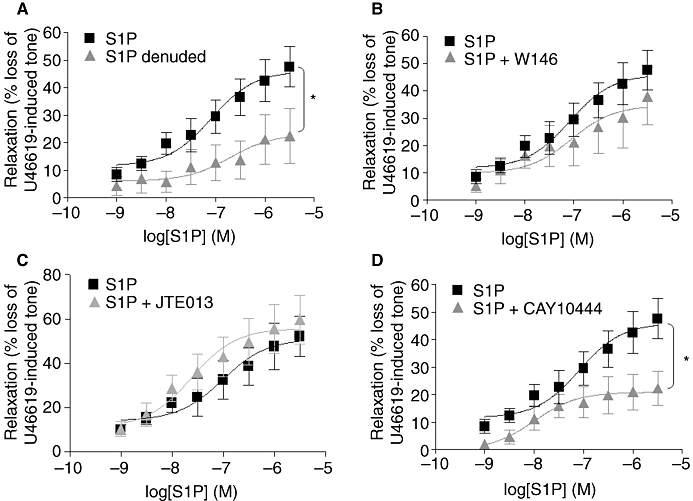
S1P stimulated vascular relaxation in an endothelium-dependent manner. (A) Concentration–response curves showing the vasorelaxant effect of S1P (1 nM–30 µM) on U46619-pre-contracted endothelium-intact (n= 10) and endothelium-denuded (n= 8) isolated CA and concentration–response curves showing the effect of S1P (1 nM–30 µM, n= 10) in the presence of (B) W146, a selective S1P1 antagonist (10 µM) (n= 5) (C) JTE013, a selective S1P2 antagonist (10 µM) (n= 6) and (D) CAY10444, a selective S1P3 antagonist (10 µM) (n= 5) in rat endothelium-intact U46619-pre-contracted CA. Data are expressed as a % of U46619-induced tone and shown as mean ± SEM for n arteries from different animals. *P < 0.05 versus S1P alone as determined by two-way anova.
Table 3.
Effects of antagonist and enzyme inhibitors on S1P-mediated relaxation in rat CA
| pEC50 | Rmax | n | |
|---|---|---|---|
| S1P | −7.09 ± 0.3 | 46.10 ± 4.48 | 10 |
| S1P (denuded) | −6.71 ± 0.77* | 23.15 ± 6.82* | 8 |
| S1P | −7.09 ± 0.3 | 46.10 ± 4.48 | 10 |
| S1P + W146 (10 µM) | −7.05 ± 0.58 | 34.74 ± 6.28 | 5 |
| S1P | −7.01 ± 0.37 | 51.02 ± 6.02 | 10 |
| S1P + JTE013 (10 µM) | −7.62 ± 0.35 | 55.79 ± 0.36 | 6 |
| S1P | −7.09 ± 0.3 | 46.10 ± 4.48 | 10 |
| S1P + CAY10444 (10 µM) | −7.99 ± 0.48 | 20.98 ± 2.65* | 5 |
| S1P | −7.09 ± 0.3 | 46.10 ± 4.48 | 10 |
| S1P + indomethacin (10 µM) | −7.29 ± 2.39 | 9.22 ± 7.77* | 4 |
| S1P | −7.01 ± 0.37 | 51.02 ± 6.02 | 10 |
| S1P + AM630 (10 µM) | −6.972 ± 0.42 | 23.48 ± 3.78* | 6 |
| S1P | −7.09 ± 0.3 | 46.10 ± 4.48 | 10 |
| *S1P + JTE907 (10 µM) | −7.19 ± 0.39 | 18.51 ± 2.44* | 5 |
| S1P | −7.09 ± 0.3 | 46.10 ± 4.48 | 10 |
| S1P + SKi (10 µM) | −7.82 ± 0.3 | 23.14 ± 4.52* | 5 |
Data are expressed as mean ± SEM for n arteries from different animals.
P < 0.05 versus S1P alone as determined by two-way anova.
Role for COX in the relaxation mediated by anandamide and S1P
The non-selective COX inhibitor indomethacin (10 µM) was utilized to determine whether COX plays a role downstream of anandamide and S1P in mediating vascular relaxation in the rat CA. Pre-incubation with indomethacin significantly reduced the vasodilator effect of anandamide in endothelium-intact vessels (maximal relaxation 22.5 ± 1.93%, n= 7, P < 0.05, Figure 3A; Table 2), but did not significantly alter responses to anandamide in denuded vessels (maximal relaxation 46.5 ± 5.73%, n= 4, Figure 3B; Table 2). Responses to S1P in intact vessels were also significantly attenuated in the presence of indomethacin (maximal relaxation 9.22 ± 7.77%, n= 4, P < 0.05, Figure 3C; Table 3), indicating the involvement of COX in the relaxation of rat CA to both anandamide and S1P.
Figure 3.
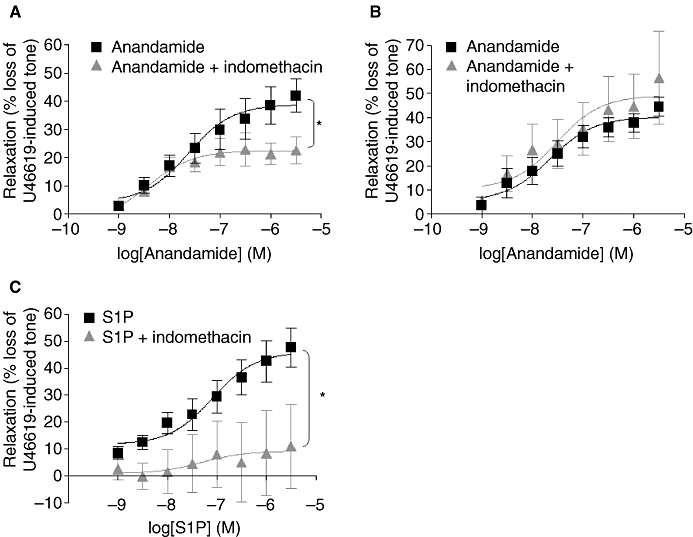
Role of COX in anandamide and S1P-mediated relaxation. Concentration–response curves for the vasorelaxant effect of anandamide alone (1 nM–30 µM, n= 13) and in the presence of indomethacin 10 µM in rat (A) endothelium-intact (n= 7) and in (B) endothelium-denuded (n= 6) U46619-pre-contracted CA. (C) Concentration–response curves showing the vasorelaxant effect of S1P alone (1 nM–30 µM, n= 10) and in the presence of indomethacin 10 µM (n= 4) in rat endothelium-intact U46619-pre-contracted CA. Data are expressed as a percentage of U46619-induced tone and shown as mean ± SEM for n arteries from different animals. *P < 0.05 versus anandamide or S1P alone as determined by two-way anova.
Sphingosine kinase was required for anandamide-induced relaxation
Stimulation of rat CA rings with anandamide resulted in a 50.9 ± 11.8% increase in the Ser225 phosphorylation of SK1 (n= 4, P < 0.05, Figure 4A). The phosphorylation of SK1 on Ser225 has been demonstrated before in response to agonist stimulation, and is catalysed by ERK-1/2 (Pitson et al., 2003). Indeed, anandamide stimulation of rat CA rings also increased the levels of phosphorylated ERK-1/2 (33.1 ± 6.4% increase, n= 4, P < 0.05, Figure 4A). The SK1 inhibitor, SKi, 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole (French et al., 2003) also had a marked effect on responses to anandamide, almost completely inhibiting relaxation (maximal response 2.3 ± 6.2%, n= 5, P < 0.05, Figure 4B; Table 2), suggesting that anandamide-mediated vasorelaxation requires SK1/S1P. In contrast to this, incubation with SKi had no effect on anandamide-mediated relaxation in endothelium-denuded vessels (n= 4, Figure 4C; Table 2), highlighting a role for SK1/S1P specifically within the vascular endothelium.
Figure 4.
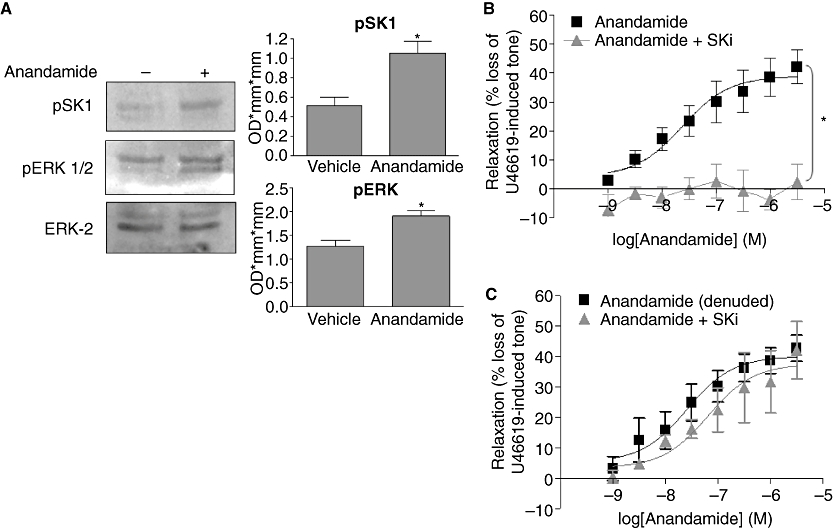
Sphingosine kinase is required for anandamide-induced relaxation in the rat CA. (A) Representative immunoblot showing the effect of anandamide (10 µM) on pSK1, pERK-1/2 and ERK2 levels in rat CA rings. Graph shows the densitometry results quantified and expressed as mean ± SEM for four separate samples. *P < 0.05 versus vehicle as determined by Student's unpaired two-tailed t-test. (B) Concentration–response curves for the relaxation to anandamide alone (1 nM–30 µM, n= 13) and in the presence of SKi, a selective SK1 inhibitor 10 µM (n= 5), in U46619-pre-contracted endothelium-intact CA and (C) endothelium-denuded CA (n= 4). Data are expressed as a % of U46619-induced tone and shown as mean ± SEM for n arteries from different animals. *P < 0.05 versus anandamide alone as determined by two-way anova.
Anandamide-induced relaxation is mediated by S1P3 within the vascular endothelium
Selective S1P antagonists were utilized to determine if the S1P that would be potentially formed by the anandamide-mediated phosphorylation and activation of SK1 can mediate its effects by acting via S1P receptors. The selective S1P1/3 antagonist, VPC23019, significantly attenuated anandamide-mediated relaxation in the rat CA (maximal response 14.85 ± 1.36%, n= 8, P < 0.05, Figure 5A; Table 2), but had no effect on the anandamide response in denuded arteries, suggesting any S1P produced in response to anandamide exerts its effect by acting on S1P1/3 receptors on the vascular endothelium (maximal relaxation 40.19 ± 2.86%, n= 8, Figure 5B; Table 2). To determine the individual effects of S1P1 and S1P3 in mediating the anandamide, response-selective S1P1 and S1P3 were employed. W146, a selective S1P1 antagonist, had no effect on anandamide-induced relaxation (Figure 5C; Table 2). Similar to its effects on exogenously added S1P, the S1P3 antagonist CAY10444 was found to significantly reduce the relaxation to anandamide in endothelium-intact vessels (maximal relaxation 16.15 ± 2.9%, n= 5, Figure 5D; Table 2). However, in endothelium-denuded vessels, the inhibitory effect of CAY10444 was no longer observed (maximal relaxation 47.33 ± 3.32 %, n= 6, Figure 5E; Table 2), indicating that anandamide-induced relaxation involves the activation of S1P3 on the vascular endothelium. Furthermore, combined pretreatment with both AM630 (100 nM) and CAY10444 (10 µM), while inhibiting the anandamide response, did not have an additive effect in attenuating vascular relaxation, suggesting that anandamide does not act directly on S1P3 (Figure 5F; Table 2).
Figure 5.
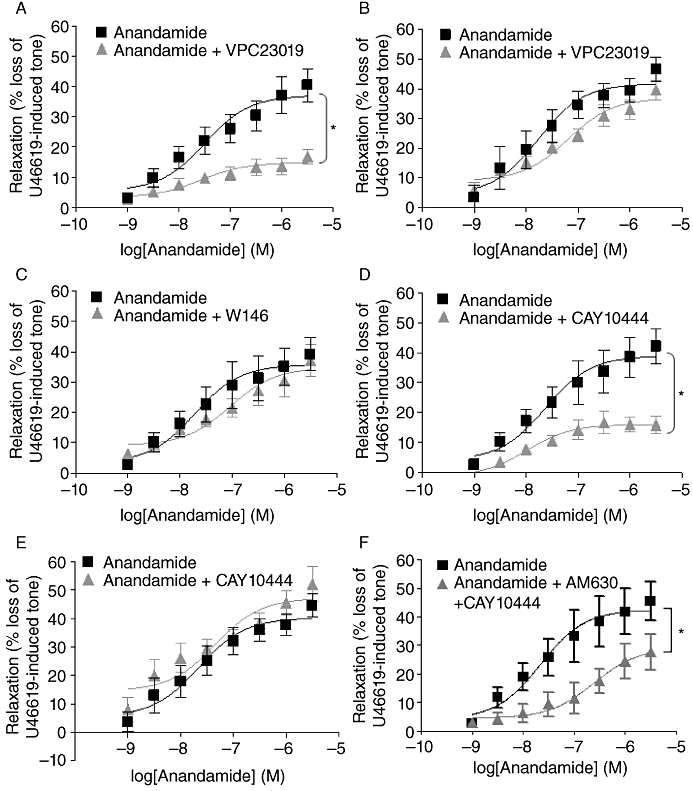
Anandamide-induced relaxation is mediated by S1P3 within the vascular endothelium. Concentration–response curves for the relaxation to anandamide alone (1 nM–30 µM, n= 13) and in the presence of VPC23019, an S1P1/3 antagonist (10 µM) in rat (A) endothelium-intact (n= 8) and (B) endothelium-denuded (n= 8) U46619-pre-contracted CA. Concentration–response curves for the relaxation to anandamide alone (1 nM–30 µM, n= 13) and in the presence of (C) W146, a selective S1P1 receptor antagonist (10 µM) (n= 5) and (D) CAY10444, a selective S1P3 antagonist (10 µM) (n= 5) in U46619-pre-contracted endothelium-intact CA and (E) in the presence of CAY10444 10 µM in endothelium-denuded U46619-pre-contracted arteries (n= 6). (F) Concentration–response curves for anandamide alone (n= 6) and in the presence of a combination of AM630 (100 nM) and CAY10444 (10 µM) (n= 4) in endothelium-intact CA. Data are expressed as a % of U46619-induced tone and shown as mean ± SEM for n arteries from different animals. *P < 0.05 versus anandamide alone as determined by two-way anova.
S1P-induced relaxation involves CB2 receptors and requires SK to mediate its effect
To further investigate the relationship between S1P and anandamide-mediated responses, we examined whether S1P responses involved CB receptors. Pretreatment of the rat CA with the CB2 antagonist AM630 significantly attenuated S1P-mediated relaxation, reducing the maximal relaxation to 23.48 ± 3.78%, n= 6, P < 0.05 (Figure 6A; Table 3). The CB2 inverse agonist JTE907 also markedly reduced relaxation in response to S1P (maximum relaxation 18.51 ± 2.44%, n= 5, P < 0.05, Figure 6B; Table 3). Furthermore, in MCF-7 cells where ERK-1/2 activation responses to S1P are mediated by S1P3 (Sukocheva et al., 2006) and can be completely abolished by pretreatment with CAY10444, neither AM630 nor JTE907 had any effect on the ability of S1P to activate ERK-1/2 (n= 4, Figure 6C), demonstrating that neither AM630 nor JTE907 is an S1P3 antagonist. Therefore, we can rule out the possibility that these antagonists block S1P3 receptors in the vessels. In addition, similar to anandamide, relaxant responses to S1P were significantly decreased in the rat CA in the presence of the SK1 inhibitor, SKi (23.14 ± 4.52%, n= 5, P < 0.05, Figure 6D; Table 3), suggesting that S1P-induced vasorelaxation requires SK1.
Figure 6.
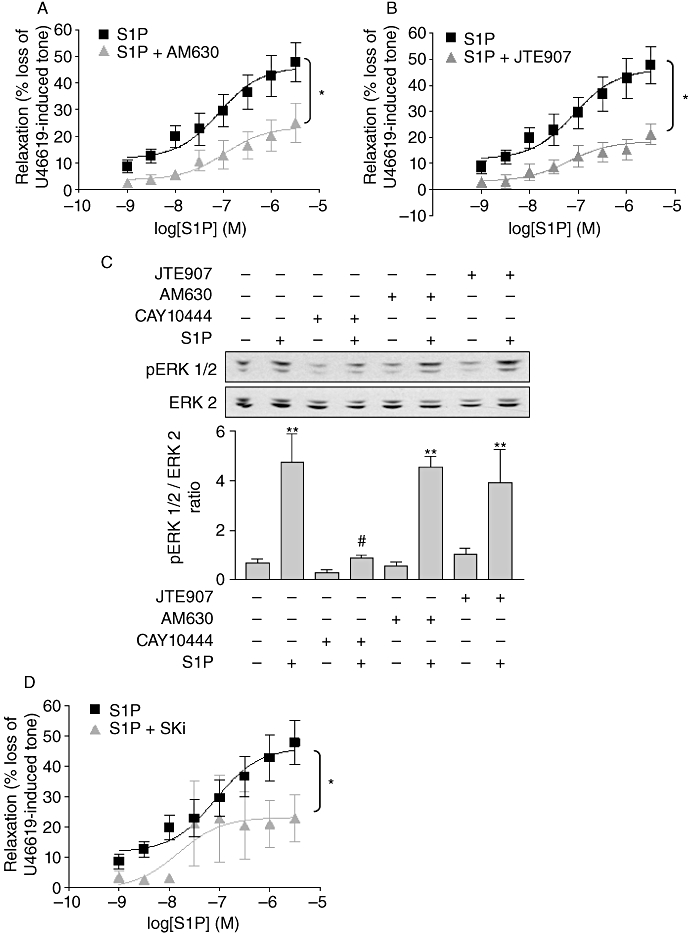
S1P induced relaxation via the CB2 receptor and required SK to mediate its effect. Concentration–response curves showing the vasorelaxant effect of S1P alone (1 nM–30 µM, n= 10) and in the presence of (A) AM630, a selective CB2 receptor antagonist (10 µM) (n= 6) and (B) JTE907, a selective CB2 receptor antagonist (10 µM) (n= 5) in endothelium-intact U46619-pre-contracted rat CA. (C) Representative immunoblots showing the effect of CAY10444 10 µM (a selective S1P3 antagonist), AM630 10 µM and JTE907 10 µM (both selective CB2 receptor antagonists) on S1P-mediated (1 µM) ERK-1/2 phosphorylation in MCF-7 cells. Graph shows the densitometry results quantified and expressed as mean ± SEM for four separate experiments. (D) Concentration–response curves showing the effect of S1P alone (1 nM–30 µM, n= 10) and in the presence of Ski, a selective SK1 inhibitor (10 µM) (n= 5) in endothelium-intact U46619-pre-contracted CA. Myography data are expressed as a percentage of U46619-induced tone and shown as mean ± SEM for n arteries from different animals. *P < 0.05 versus S1P alone as determined by two-way anova. **P < 0.01 versus unstimulated cells and #P < 0.05 versus S1P-stimulated cells as determined by one-way anova with Newman–Keuls post-test.
S1P functionally interacts with CB2 receptor activation
The findings presented in Figure 5 suggest that S1P may activate CB2 receptors in the rat CA. In order to further establish whether S1P can functionally interact with CB2 receptors, cell-based assays using HEK293 cells, which stably over-express CB2 receptors (CB2HEK293s), were carried out. Experiments were also undertaken using parental HEK293s for comparison; these cells also endogenously express S1P receptors (see later for effect of S1P and CB2 receptor antagonists). Firstly, [35S]-GTPγS binding assays were undertaken in these cells to establish if S1P can activate CB2 receptors. HU210, a known CB1/2 receptor agonist, was found to stimulate [35S]-GTPγS binding in CB2HEK293 cells, but not parental HEK293s (n= 3, P < 0.05, Figure 7A). Similarly, treatment with S1P also resulted in an increase in [35S]-GTPγS binding in CB2HEK293s, but not parental cells (n= 3, P < 0.05, Figure 7B). Furthermore, the elevation in [35S]-GTPγS binding to CB2HEK293 membranes stimulated by both HU210 and S1P was significantly attenuated by the CB2 antagonist AM630 (n= 4, P < 0.05, Figure 7C), suggesting both HU210 and S1P are agonists of the CB2 receptor.
Figure 7.
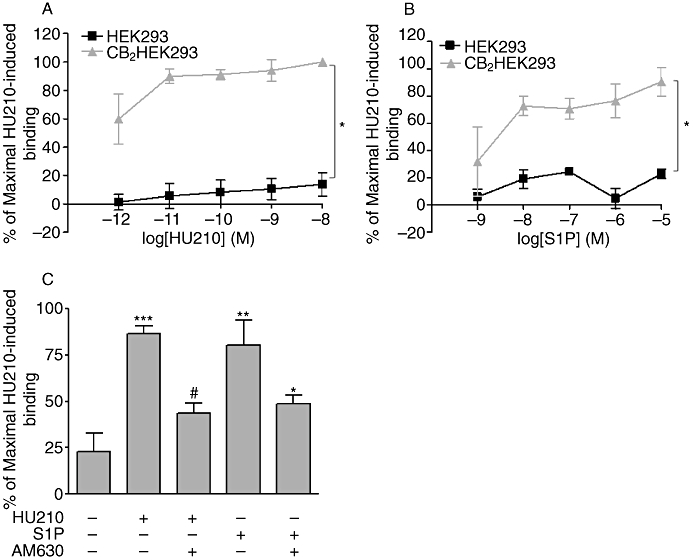
S1P stimulated CB2 receptor activation. The effects of (A) HU210, a CB1/2 agonist (1 pM–10 nM) (n= 3) and (B) S1P (1 nM–10 µM) (n= 3) on [35S]-GTPγS binding to HEK293 and CB2HEK293 cell membranes. Data expressed as a % of maximal HU210-induced [35S]-GTPγS binding. Results shown as mean ± SEM for three separate experiments. *P < 0.05 versus HEK293 membranes as determined by two-way anova. (C) The effects of AM630, a selective CB2 receptor antagonist (10 µM) on HU210 (10 µM) and S1P (10 µM) mediated [35S]-GTPγS binding to CB2HEK293 cell membranes. Data expressed as a % of maximal HU210-induced [35S]-GTPγS binding. Results shown as mean ± SEM for four separate experiments. ***P < 0.001 versus vehicle, **P < 0.01 versus vehicle, #P < 0.05 versus HU210 and *P < 0.05 versus S1P as determined by one-way anova with Newman–Keuls post-test.
The effects of S1P and antagonists on the activation of the ERK-1/2 pathway in parental and CB2HEK293 cells were also assessed as a reporter of CB2 receptor function. Stimulation of both parental HEK293 and CB2HEK293s cells with S1P resulted in a marked elevation in the levels of phoshorylated ERK-1/2 (Figure 8A, P < 0.01, n= 3). Pretreatment with either of the CB2 antagonists, AM630 or JTE907, alone had no effect on the ability of S1P to induce ERK-1/2 phosphorylation in either cell type (Figure 8A). However, the S1P response was completely abolished following pretreatment of HEK293 cells with a combination of CAY10444 (20 µM) and W146 (20 µM), suggesting that in the parental HEK293 cells, S1P induces activation of ERK-1/2 via S1P1/3 (Figure 8B). In contrast to this, pretreatment of CB2HEK293 cells with combined CAY10444 (20 µM) and W146 (20 µM) had no effect on the ability of S1P to induce ERK-1/2 activation (Figure 8B), suggesting that the major effect of S1P in these cells is mediated via a distinct receptor. Evidence that this distinct receptor might be CB2 was obtained by results showing that the stimulant effect of S1P on ERK-1/2 activation was no longer observed in CB2HEK293 cells that were treated with AM630 in addition to CAY10444 and W146 (Figure 8B). Similar results were also obtained using JTE907 in the presence of S1P antagonists (data not shown). Pretreatment with the combination of W146 and CAY10444 and AM630 or JTE907 did not significantly alter basal levels of phosphorylated ERK in either cell type.
Figure 8.
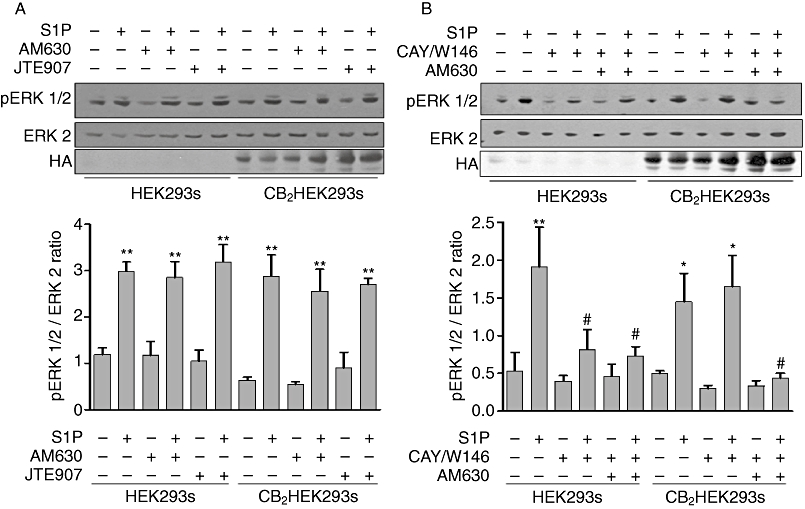
S1P stimulated ERK-1/2 activation involves CB2 receptor. (A) Representative immunoblots showing the effect of AM630 (10 µM) and JTE907 (10 µM) (both selective CB2 receptor antagonists) and (B) representative immunoblots showing the effect of combined pretreatment with W146, a selective S1P1 antagonist (20 µM), CAY10444 (CAY), a selective S1P3 antagonist (20 µM) and AM630 (10 µM) on S1P-mediated (5 µM) ERK1/2 activation in lysates prepared from parental HEK293 cells and CB2HEK293s. Graph shows the densitometry data quantified and expressed as mean ± SEM for three separate experiments. *P < 0.05 and **P < 0.01 versus unstimulated cells. #P < 0.05 versus S1P stimulated cells as determined by one-way anova with Newman–Keuls post-test.
Discussion
Anandamide and S1P both play an important role in regulating vascular tone. Here, we showed that both these lipid mediators stimulate relaxation in rat isolated CAs, and that a functional interaction between these receptor systems exists within the vascular endothelium to regulate vasorelaxation.
Various studies have reported the role of the CB1 receptor in mediating relaxation to anandamide (O'Sullivan et al., 2004; Romano and Lograno, 2006). However, the present study found that AM251, a selective CB1 antagonist, had no effect on anandamide-induced vasorelaxation. Blocking CB2 receptors, on the other hand, with either AM630 or JTE907, significantly attenuated the effects of anandamide. Similar effects were also observed on relaxation to the synthetic CB agonist HU210. These findings suggest the CB2 receptor is important in mediating the effects of CB agonists on rat CA reactivity. Moreover, the inhibitory effect of AM630 on anandamide-mediated relaxation was abolished in endothelium-denuded vessels, specifically implicating the vascular endothelium in CB2 receptor-mediated vascular relaxation. The CB2 receptor has mainly been suggested to play a role in cardioprotection. For instance, remote ischaemic preconditioning is thought to involve the release of endogenous CBs, which exert their cardioprotective effect by acting on the CB2 receptor (Hajrasouliha et al., 2008).
Similar to anandamide, we found that S1P also induced relaxation of the rat CA and that this effect was significantly attenuated by removal of the vascular endothelium. Furthermore, this vasodilator effect of S1P appears to be mediated by activation of S1P3, as CAY10444 was able to significantly attenuate S1P-induced vascular relaxation. A large body of evidence exists, implicating S1P3 in the vasodilator effects of S1P, mainly via activation of eNOS (Nofer et al., 2004). However, in this instance, pretreatment of the coronary vessels with l-NAME had no effect on S1P-stimulated relaxation, suggesting NO is not involved in the vasoactive effects of S1P in this vessel (data not shown).
Both anandamide and S1P induce relaxation of a similar magnitude in the rat CA, which is partially sensitive to the COX inhibitor indomethacin, suggesting a common downstream pathway involving a vasodilator prostanoid. Only the endothelium-dependent component of anandamide-induced relaxation is inhibited by indomethacin. Furthermore, the effects of S1P are also dependent on the endothelial layer, suggesting any common pathway shared by anandamide and S1P may occur specifically within the vascular endothelium.
Recently, CBs have been implicated in the regulation of sphingolipid metabolism (Giuliano et al., 2006). In this study, we investigated the potential role of SK1, the enzyme responsible for synthesizing S1P, in mediating the effects of anandamide. There are two mammalian SK isoforms, SK1 and SK2, which are ubiquitously expressed. SK1 can be phosphorylated by ERK-1/2 (Pitson et al., 2003), which results in the phosphorylation of the enzyme on Ser225. This phosphorylation is required for translocation of SK1 to the plasma membrane, where it produces S1P for ‘inside-out’ signalling at S1P receptors. This involves the release of S1P into the extracellular milieu and/or partition into lipid micro-environments in close proximity to the S1P receptor, therefore allowing privileged and efficient binding of S1P to the S1P receptor. Indeed, various stimuli shown to activate SK1 induce its translocation to the plasma membrane (Melendez and Khaw, 2002; Sarkar et al., 2005). Here, we demonstrate that stimulation of the rat CA with anandamide stimulates Ser225 phosphorylation of SK1, suggesting a potential role for SK1 in ‘inside-out’ signalling. The pretreatment of rat CAs with the SK inhibitor SKi also completely abolished the ability of anandamide to induce relaxation, thereby suggesting a role for SK1 in mediating the effects of anandamide. SKi also had similar effects on HU210-induced relaxation (data not shown). Additionally, the inhibitory effect of SKi on anandamide-mediated relaxation was dependent on the vascular endothelium. Moreover, CAY10444, an S1P3 antagonist, significantly attenuated anandamide-induced relaxation. This effect was also abolished by the removal of the vascular endothelium. These findings suggest that SK1 is required downstream of CBs and the S1P that is likely to be formed then acts at S1P3 to induce vasorelaxation, a process that appears to occur specifically within the endothelial layer. To support this model, others have demonstrated that the generation of intracellular S1P by the activation of SK1 is involved in mediating the effects of various agonists and phorbol esters (Taha et al., 2006; Alemany et al., 2007). Furthermore, S1P can be released from cells to exert its effects extracellularly via S1P receptors (Kobayashi et al., 2006). Endothelial cells have been reported to release both S1P and active SK to form S1P outside the cell (Ancellin et al., 2002; Venkataraman et al., 2006). Finally, in cardiac myocytes, a similar mechanism of action has been proposed for the adipose-derived plasma protein adiponectin (APN), whereby APN was found to activate SK to generate S1P, which then exerted its effects via activation of the S1P1/3 (Ikeda et al., 2008).
The role of the CB2, S1P/SK1 and S1P3 in anandamide-induced relaxation appears to be confined to the endothelial layer. However, in denuded vessels, anandamide still induced relaxation to a magnitude similar to that observed in endothelium-intact vessels. This suggests that a compensatory mechanism exists within the vascular smooth muscle that allows anandamide to mediate vasorelaxation in the event of endothelial damage. Others have also reported the existence of distinct mechanisms that regulate the effects of anandamide in vascular endothelium and smooth muscle (Mukhopadhyay et al., 2002; O'Sullivan et al., 2004; Romano and Lograno, 2006). Although not investigated in the current study in rat CA, one potential compensatory mechanism that may mediate anandamide-induced relaxation in the absence of the vascular endothelium is the activation of large conductance Ca2+-activated K+ channels (BKca). BKca have previously been reported to be activated by anandamide within vascular smooth muscle, inducing relaxation (White et al., 2001).
We conclude from the current findings that two distinct mechanisms of anandamide-induced relaxation exist, one dependent on the vascular endothelium involving the CB2 receptor, S1P/S1P3 and COX, in addition to another compensatory mechanism that acts independently of the endothelial layer.
In addition to this, S1P may elicit its effects via a process that requires the CB2 receptor, as both AM630 and JTE907 significantly reduced the vasorelaxant response to S1P in the rat CA. This effect is not due to the blockade of S1P3 as neither AM630 nor JTE907 attenuated the ability of S1P to induce ERK-1/2 activation in MCF-7 cells, a breast cancer cell line where the effects of S1P are via S1P3 activation (Sukocheva et al., 2006). Moreover, AM630 and JTE907 did not modify the activation of ERK-1/2 by S1P in parental HEK293s, which express functional S1P1 and S1P3. The finding that S1P-induced relaxation was blocked by CB2 receptor antagonists suggests that S1P might use the CB2 receptor in order to induce vascular relaxation. This is supported by our studies using CB2HEK293 cells where S1P was shown to stimulate GTPγS binding, and this was blocked by AM630. Furthermore, ERK-1/2 activation in response to S1P in these cells was still observed following pretreatment with a combination of S1P1 and S1P3 antagonists sufficient to significantly reduce S1P-induced ERK-1/2 activation in the parental HEK293 cells. In addition, the ability of S1P to stimulate ERK activation in CB2HEK293s when S1P1/3 are blocked was completely ablated when these cells were concurrently treated with CB2 antagonists. Given that CB2 antagonists on their own have no inhibitory effect on S1P-mediated ERK activation unless S1P1/3 are blocked, these findings suggest that S1P may activate CB2 receptors in HEK293 cells to initiate downstream signalling cascades when the functionality of S1P receptors is experimentally impaired. Others have also highlighted a link between lysolipids and CB receptors. For instance, sphingosine has been shown to interact with CB receptors; this study reported that sphingosine and its analogue FTY720 were able to bind to CB1 receptors where they acted as antagonists (Paugh et al., 2006). Moreover, both CBs and lysophosphatidylinositol (LPI), an acidic lysophospholipid similar to S1P, have been proposed to act as agonists at the orphan GPCR, GRP55 (Oka et al., 2007).
We also observed that relaxation to S1P in the rat CA was significantly reduced by SKi, suggesting a role for SK1 downstream of CB2 receptors. Thus, in rat CA, stimulation of CB2 receptors in response to S1P may provide an alternative mechanism by which S1P mediates vascular relaxation through the generation of intracellular S1P, which is then released locally to access S1P3. This mechanism may deliver S1P more efficiently to S1P3 compared with exogenous S1P, suggesting privileged access to S1P receptors, possibly via efficient disposition of S1P in a lipid microdomain in close proximity to S1P3.
In summary, both anandamide and S1P induced vascular relaxation in the rat CA, with the involvement of both CB2 and S1P3. Within the vascular endothelium, both lipid mediators required COX to exert their vasodilator effects, potentially by prostaglandin production. Phosphorylation by ERK-1/2 is known to activate cytosolic phospholipase A2, resulting in an increase in arachidonate production and subsequent prostanoid production via COX (Pyne et al., 1997). Therefore, ERK activation within the CA in response to anandamide may result in the production of vasodilator prostaglandins. Furthermore, for the first time, we demonstrate that SK1 has an important role in anandamide-induced relaxation and suggest that activation of SK1 by anandamide may result in the generation of S1P, which can then exert its effects via activation of S1P3 on the vascular endothelium. We also provide evidence that S1P might use the CB2 receptor to stimulate localized production of S1P in the vicinity of S1P3, the activation of which mediates coronary vascular relaxation.
Acknowledgments
Parental HEK293 cells and CB2HEK293 cells were kindly gifted by Prof Ken Mackie, Indiana University. This work was supported by the British Heart Foundation (grant numbers FS/04/046, 22305).
Glossary
Abbreviations
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AM630
6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone
- anandamide
arachidonylethanolamide
- CA
coronary artery
- CAY10444
2-undecyl-thiazolidine-4-carboxylic acid
- CB
cannabinoid
- eNOS
endothelial NOS
- ERK-1/2
extracellular signal-regulated kinase-1/2
- GTPγS
guanosine 5′- [γ-thio]triphosphate
- GPCR
G-protein-coupled receptor
- HA
haemagglutinin; HU210 (6aR)-trans-3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol
- JTE-013
1-[1,3-dimethyl-4-(2-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl]-4-(3,5-dichloro-4-pyridinyl)-semicarbazide
- JTE907
N-(1,3-benzodioxol-5-ylmethyl)-1,2-dihydro-7-methoxy-2-oxo-8-(pentyloxy)-3-quinolinecarboxamide
- pERK-1/2
phospho-extracellular signal-regulated kinase-1/2
- pSK
phospho-sphingosine kinase
- SK
sphingosine kinase
- SKi
2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole
- S1P
sphingosine-1-phosphate
- U46619
9,11-dideoxy-9α,11-methanoepoxy prostaglandin F2α
- VPC23019
(R)-phosphoric acid mono-[2-amino-2-(3-octyl-phenylcarbamoyl)-ethyl] ester
- W146
(R)-3-amino-(3-hexylphenylamino)-4-oxobutylphosphonic acid
Conflict of interest
None declared.
References
- Alemany R, Van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:413–428. doi: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- Alewijnse AE, Peters SL, Michel MC. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br J Pharmacol. 2004;143:666–684. doi: 10.1038/sj.bjp.0705934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn.) 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Sanchez C, Daza A, Galve-Roperh I, Guzman M. The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. J Neurochem. 1999;72:1759–1768. doi: 10.1046/j.1471-4159.1999.721759.x. [DOI] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Coussin F, Scott RH, Wise A, Nixon GF. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JLM, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- Galve-Roperh I, Sanchez C, Cortes ML, Gomez Del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Giuliano M, Calvaruso G, Pellerito O, Portanova P, Carlisi D, Vento R, et al. Anandamide-induced apoptosis in Chang liver cells involves ceramide and JNK/AP-1 pathway. Int J Mol Med. 2006;17:811–819. [PubMed] [Google Scholar]
- Grainger J, Boachie-Ansah G. Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br J Pharmacol. 2001;134:1003–1012. doi: 10.1038/sj.bjp.0704340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajrasouliha AR, Tavakoli S, Ghasemi M, Jabehdar-Maralani P, Sadeghipour H, Ebrahimi F, et al. Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur J Pharmacol. 2008;579:246–252. doi: 10.1016/j.ejphar.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Hemmings DG. Signal transduction underlying the vascular effects of sphingosine 1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:18–29. doi: 10.1007/s00210-006-0046-5. [DOI] [PubMed] [Google Scholar]
- Hemmings DG, Xu Y, Davidge ST. Sphingosine 1-phosphate-induced vasoconstriction is elevated in mesenteric resistance arteries from aged female rats. Br J Pharmacol. 2004;143:276–284. doi: 10.1038/sj.bjp.0705752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herradon E, Martin MI, Lopez-Miranda V. Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br J Pharmacol. 2007;152:699–708. doi: 10.1038/sj.bjp.0707404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br J Pharmacol. 2003;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29:307–313. [PubMed] [Google Scholar]
- Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J Biol Chem. 2000;275:32363–32370. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. Differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Ohashi K, Shibata R, Pimentel DR, Kihara S, Ouchi N, et al. Cyclooxygenase-2 induction by adiponectin is regulated by a sphingosine kinase-1 dependent mechanism in cardiac myocytes. FEBS Lett. 2008;582:1147–1150. doi: 10.1016/j.febslet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, et al. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- Long JS, Natarajan V, Tigyi G, Pyne S, Pyne NJ. The functional PDGFbeta receptor–S1P1 receptor signaling complex is involved in regulating migration of mouse embryonic fibroblasts in response to platelet derived growth factor. Prostaglandins Other Lipid Mediat. 2006;80:74–80. doi: 10.1016/j.prostaglandins.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Van Der Giet M, Tolle M, Wolinska I, Von Wnuck Lipinski K, Baba HA, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br J Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Cassidy MP, He H, Milstien S, Sim-Selley LJ, Spiegel S, et al. Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor. Mol Pharmacol. 2006;70:41–50. doi: 10.1124/mol.105.020552. [DOI] [PubMed] [Google Scholar]
- Peters SL, Alewijnse AE. Sphingosine-1-phosphate signaling in the cardiovascular system. Curr Opin Pharmacol. 2007;7:186–192. doi: 10.1016/j.coph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Tolan D, Pyne S. Bradykinnin stimulates cyclic AMP synthesis via a mitogen-activated protein kinase-dependent regulation of cytosolic phospholipase A2 and prostaglandin E2 release in airway smooth muscle. Biochem J. 1997;328:689–694. doi: 10.1042/bj3280689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne S, Lee SC, Long J, Pyne NJ. Role of sphingosine kinases and lipid phosphate phosphatases in regulating spatial sphingosine 1-phosphate signalling in health and disease. Cell Signal. 2009;21:14–21. doi: 10.1016/j.cellsig.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Romano MR, Lograno MD. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: evidences for CB1 receptors, nitric oxide and potassium channels. Br J Pharmacol. 2006;147:917–925. doi: 10.1038/sj.bjp.0706687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roviezzo F, Bucci M, Delisle C, Brancaleone V, Di Lorenzo A, Mayo IP, et al. Essential requirement for sphingosine kinase activity in eNOS-dependent NO release and vasorelaxation. Faseb J. 2006;20:340–342. doi: 10.1096/fj.05-4647fje. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, et al. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, et al. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


