Abstract
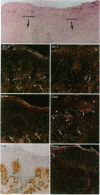
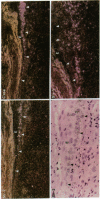
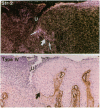
Wound repair involves cell migration and tissue remodeling, and these ordered and regulated processes are facilitated by matrix-degrading proteases. We reported that interstitial collagenase is invariantly expressed by basal keratinocytes at the migrating front of healing epidermis (Saarialho-Kere, U. K., E. S. Chang, H. G. Welgus, and W. C. Parks, 1992. J. Clin. Invest. 90:1952-1957). Because of the limited substrate specificity of collagenase, principally for interstitial fibrillar collagens, other enzymes must also be produced in the wound environment to effectively restructure tissues with a complex matrix composition. Stromelysins-1 and -2 are closely related, yet distinct metalloproteinases, and both can degrade many noncollagenous connective tissue macromolecules. Using in situ hybridization and immunohistochemistry, we found that both stromelysins are produced by distinct populations of keratinocytes in a variety of chronic ulcers. Stromelysin-1 mRNA and protein were detected in basal keratinocytes adjacent to but distal from the wound edge in what probably represents the sites of proliferating epidermis. In contrast, stromelysin-2 mRNA was seen only in basal keratinocytes at the migrating front, in the same epidermal cell population that expresses collagenase. Stromelysin-1-producing keratinocytes resided on the basement membrane, whereas stromelysin-2-producing keratinocytes were in contact with the dermal matrix. Furthermore, stromelysin-1 expression was prominent in dermal fibroblasts, whereas no signal for stromelysin-2 was seen in any dermal cell. These findings demonstrate that stromelysins-1 and -2 are produced by different populations of basal keratinocytes in response to wounding and suggest that these two matrix metalloproteinases serve distinct roles in tissue repair.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinckerhoff C. E., Sirum-Connolly K. L., Karmilowicz M. J., Auble D. T. Expression of stromelysin and stromelysin-2 in rabbit and human fibroblasts. Matrix Suppl. 1992;1:165–175. [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Cooper T. W., Bauer E. A., Eisen A. Z. Enzyme-linked immunosorbent assay for human skin collagenase. Coll Relat Res. 1983 May;3(3):205–215. doi: 10.1016/s0174-173x(83)80004-1. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch S. M., Ruley H. E. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987 Dec 5;262(34):16300–16304. [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K. A., Pourmotabbed T. F., Goldberg G. I., Thompson J. P., Spinella D. G., Stevens R. M., Mainardi C. L. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990 Jul 15;265(20):11421–11424. [PubMed] [Google Scholar]
- Herron G. S., Werb Z., Dwyer K., Banda M. J. Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J Biol Chem. 1986 Feb 25;261(6):2810–2813. [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. C., Secrist H., Wu L. C., Mecham R. P. Developmental regulation of tropoelastin isoforms. J Biol Chem. 1988 Mar 25;263(9):4416–4423. [PubMed] [Google Scholar]
- Pellegrini G., De Luca M., Orecchia G., Balzac F., Cremona O., Savoia P., Cancedda R., Marchisio P. C. Expression, topography, and function of integrin receptors are severely altered in keratinocytes from involved and uninvolved psoriatic skin. J Clin Invest. 1992 Jun;89(6):1783–1795. doi: 10.1172/JCI115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Faure M. P., DiBattista J. A., Wilhelm S., Visco D., Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993 Jan;142(1):95–105. [PMC free article] [PubMed] [Google Scholar]
- Pentland A. P., Mahoney M., Jacobs S. C., Holtzman M. J. Enhanced prostaglandin synthesis after ultraviolet injury is mediated by endogenous histamine stimulation. A mechanism for irradiation erythema. J Clin Invest. 1990 Aug;86(2):566–574. doi: 10.1172/JCI114746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser I. W., Stenmark K. R., Suthar M., Crouch E. C., Mecham R. P., Parks W. C. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Pulford K. A., Rigney E. M., Micklem K. J., Jones M., Stross W. P., Gatter K. C., Mason D. Y. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest. 1992 Nov;90(5):1952–1957. doi: 10.1172/JCI116073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. Expression of interstitial collagenase, 92-kDa gelatinase, and tissue inhibitor of metalloproteinases-1 in granuloma annulare and necrobiosis lipoidica diabeticorum. J Invest Dermatol. 1993 Mar;100(3):335–342. doi: 10.1111/1523-1747.ep12470032. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Welgus H. G., Parks W. C. Distinct mechanisms regulate interstitial collagenase and 92-kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J Biol Chem. 1993 Aug 15;268(23):17354–17361. [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Shapiro S. D., Campbell E. J., Kobayashi D. K., Welgus H. G. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Invest. 1990 Oct;86(4):1204–1210. doi: 10.1172/JCI114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirum K. L., Brinckerhoff C. E. Cloning of the genes for human stromelysin and stromelysin 2: differential expression in rheumatoid synovial fibroblasts. Biochemistry. 1989 Oct 31;28(22):8691–8698. doi: 10.1021/bi00448a004. [DOI] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Parks W. C. 92-kd gelatinase is actively expressed by eosinophils and stored by neutrophils in squamous cell carcinoma. Am J Pathol. 1993 Apr;142(4):995–1000. [PMC free article] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sudbeck B. D., Eisen A. Z., Welgus H. G., Parks W. C. Expression of 92-kDa type IV collagenase mRNA by eosinophils associated with basal cell carcinoma. J Invest Dermatol. 1992 Oct;99(4):497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- Symington B. E., Takada Y., Carter W. G. Interaction of integrins alpha 3 beta 1 and alpha 2 beta 1: potential role in keratinocyte intercellular adhesion. J Cell Biol. 1993 Jan;120(2):523–535. doi: 10.1083/jcb.120.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori E. N., Bair M. J., Bauer E. A., Amento E. P. Stromelysin expression regulates collagenase activation in human fibroblasts. Dissociable control of two metalloproteinases by interferon-gamma. J Biol Chem. 1991 Dec 5;266(34):23477–23482. [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor L. J., Grenett H., Birkedal-Hansen B., Bodden M. K., Engler J. A., Birkedal-Hansen H. Cell type-specific regulation of SL-1 and SL-2 genes. Induction of the SL-2 gene but not the SL-1 gene by human keratinocytes in response to cytokines and phorbolesters. J Biol Chem. 1993 Aug 15;268(23):17341–17347. [PubMed] [Google Scholar]