Abstract
Proinflammatory cytokines are infamous for their catabolic effects on tissues and joints in both inflammatory diseases and following the implantation of biomedical devices. However, recent studies indicate that many of these same molecules are critical for triggering tissue regeneration following injury. This review will discuss the role of inflammatory signals in regulating bone regeneration and the impact of both immunomodulatory and antiinflammatory pharmacologic agents on fracture healing, to demonstrate the importance of incorporating rational control of inflammation into the design of tissue engineering strategies.
Introduction
The limitations of current bone reconstruction techniques have led to increased interest in bone tissue engineering. Bone has the remarkable capacity to heal without scar formation, but this regenerative process fails in patients with large bone lesions or impaired wound healing, requiring clinical intervention. Autologous bone grafts are the current gold-standard solution to repair critical-size bone defects, but their use is limited by the availability of suitable donor tissue. Another problem is the necessity for a second surgery to harvest graft tissue, with potentially severe complications such as hemorrhage, nerve damage, infection, and deformity. Bone tissue engineering has emerged as a new interdisciplinary strategy to promote healing of large bone defects using bioactive implantable materials. Cells and bioactive factors are incorporated into these scaffolds to provide temporal and spatial cues guiding bone regeneration.1,2 The rational design of these scaffolds is informed by cell and developmental biology, which provides increasingly detailed knowledge of the cellular and molecular signals directing bone healing.
Fracture healing involves complex molecular signaling and induces significant changes in the expression of several thousand genes.3,4 To develop an effective tissue engineering strategy for bone regeneration, the “master switches” controlling this process must be identified and incorporated.5 The importance of osteogenic factors, including bone morphogenetic proteins, in bone regeneration is well known, and they are consequently widely used in bone tissue engineering systems, though with limited success. However, the critical role of proinflammatory cytokines in initiating regeneration remains poorly recognized in both biology and tissue engineering.6,7 As such, to our knowledge, limited work has been done in tissue engineering to harness inflammatory signaling. This review will discuss the role of inflammatory signals in regulating bone regeneration and the impact of antiinflammatory and immunomodulatory pharmacologic agents on fracture healing, to demonstrate the importance of incorporating rational control of inflammation into the design of tissue engineering strategies.
Bone Regeneration Following Injury: An Overview of Molecular Signaling
Bone fractures heal by either a direct (primary) or an indirect (secondary) mechanism. Primary healing refers to direct tunneling of remodeling units from the cortex across the fracture line and into the cortex on the other side. This type of healing is rare, as it requires that fracture fragments be restored to their original anatomic location and rigidly stabilized at the site. Since typical treatments, for example, plaster cast placement, do not completely immobilize the injured bone, fractures typically heal by secondary healing.8,9 This process involves overlapping phases of inflammation, renewal, and remodeling.
Inflammatory phase
Bone fracture is an injury, and thus incites an inflammatory response, which peaks 24 h following the injury and is complete by the first week.10 During this time, a complex cascade of proinflammatory signals and growth factors are released in a temporally and spatially controlled manner.11 Levels of several inflammatory mediators, including interleukin-1 (IL-1), IL-6, IL-11, IL-18, and tumor necrosis factor-α (TNF-α), are significantly elevated within the first few days.3,11 These signals recruit inflammatory cells and promote angiogenesis.11,12 Platelets are activated by injury to blood vessels at the fracture site, and release transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor. Osteoprogenitor cells at the fracture site express bone morphogenetic proteins.10,13 These factors, along with inflammatory mediators, recruit mesenchymal stem cells and then guide their differentiation and proliferation.8,14,15
Renewal phase
At the periphery of the fracture site, stem cells differentiate into osteoblasts. As a result, bone forms via intramembranous ossification 7–10 days after injury.8 Chondrogenesis occurs in the bulk of the injured tissue, which is mechanically less stable.10,15 Inflammatory mediators are absent during this phase. TGF-β2 and -β3, bone morphogenetic proteins, and other molecular signals induce endochondral bone formation in the cartilaginous callus.10,11 The cartilage calcifies, and then is replaced with woven bone.12,14
Remodeling
Osteoprogenitor cells differentiate into osteoblasts, which express IL-1, IL-6, and IL-11, and other factors that promote osteoclast formation.14 The renewing and resorptive actions of these two cell types replace the initial woven bone with lamellar bone. This remodeling phase is regulated by several proinflammatory signals. In addition to IL-1, IL-6, and IL-11, elevated levels of TNF-α, IL-12, and interferon-γ (IFN-γ) are also detectable at the fracture site.3,11 Rodent and porcine models indicate that growth hormone and parathyroid hormone also play key roles in this phase, speeding healing and strengthening the fracture callus.16–19 Although the original structure and mechanical properties of the skeleton are restored within several weeks of fracture, molecular and cellular signaling can take up to several years to return to its normal state. In human patients, hormones regulating bone metabolism remain at elevated levels for up to 1 year after hip fracture.20 Bone turnover is significantly accelerated in humans for at least 6 months following bone fracture, and does not return to baseline for several years.20–24
Comparison to embryonic bone development
Although fracture healing is very similar to skeletogenesis in the developing embryo, there are key differences in the regulation of each process. Mechanical stimulation has a unique effect in fracture healing. An unstable environment promotes chondrogenesis, while a stable environment promotes osteogenesis.25 The inflammatory response is also unique to fracture healing. Since bone fracture is an injury, it incites an inflammatory response. Inflammatory signals result in a local increase in macrophages, which in turn release factors and cytokines that promote healing. In contrast, bone formation occurs throughout the skeleton in a developing embryo, so systemic osteogenic signals prevail.25 Although tissue engineering strategies typically involve materials that mimic the structure and function of healed bone, recent progress in developmental biology suggests that rapid regeneration may be achieved via scaffolds that simply trigger the “master switch” for regeneration.5 A growing body of evidence suggests that inflammatory signals are critical for the initiation of the fracture healing response.
Role of Proinflammatory Molecules in Fracture Healing
Proinflammatory cytokines are best known for their destructive effects on bone in a number of clinical contexts, and as such have not been extensively explored as potential initiators of regeneration in bone tissue engineering strategies. In biomaterials research, TNF-α, IL-1, and other proinflammatory cytokines are best known as mediators of the foreign body reaction, an inflammatory response that can cause both severe tissue damage and premature failure of implanted materials.26 Proinflammatory molecules are also infamous for their catabolic effects on bone in patients with rheumatic diseases. High circulating levels of TNF-α and IL-1 in arthritis patients are directly linked to joint and bone destruction.27 In a mouse model of arthritis, absence of IL-1 due to a genetic mutation prevents bone and joint disease.28 Although it seems paradoxical, these same proinflammatory factors promote bone fracture healing.
Unlike the unregulated, prolonged inflammation seen in severe foreign body reactions and bone pathologies like rheumatoid arthritis, inflammatory signaling in fracture healing is highly regulated and brief. Although inflammatory cells, mainly neutrophils and macrophages, are found at the site of an injury, their absence does not adversely affect tissue repair in mice.29 Recent studies in cell and developmental biology suggest that the proregenerative function of inflammatory signals is a consequence of changes in the tissue microenvironment (e.g., expression of cell-surface receptors) triggered by injury. TNF-α, IL-1, and other inflammatory molecules are resilient molecules that readily diffuse through the extracellular matrix and are thus ideally suited for transmission of signals guiding regeneration. As discussed in this section, altered levels of TNF-α, IL-1, and other proinflammatory molecules have a major effect on the healing of bone fractures.30
Tumor necrosis factor-α
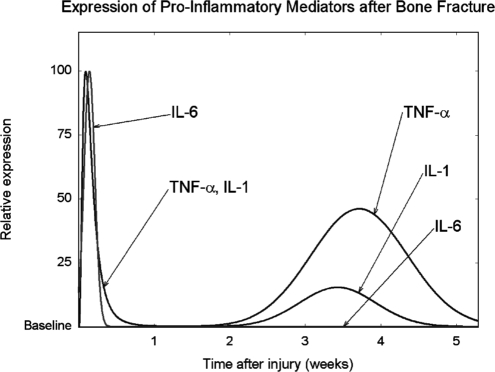
TNF-α and related molecules can either trigger cell death or promote cell survival depending on the specific cell-surface receptor they bind, the cell type, and the intracellular signaling cascade that is subsequently activated.31 In fracture healing, the expression of TNF-α and its receptors, TNFR1 and TNFR2, follows a biphasic pattern. As shown in Figure 1, TNF-α concentration peaks 24 h after bone injury in mouse models, returning to baseline levels within 72 h. During this period, TNF-α is mainly expressed by macrophages and other inflammatory cells.11,32 This brief TNF-α signaling is believed to induce the release of secondary signaling molecules and to exert a chemotactic effect, recruiting cells necessary for bone regeneration. TNF-α concentration rises again approximately 2 weeks later, during endochondral bone formation (see Fig. 1). During this period, TNF-α is expressed by osteoblasts and other cells of mesenchymal origin, including hypertrophic chondrocytes undergoing endochondral bone formation.3,32 Absence of TNF-α impairs fracture healing in mice, delaying endochondral bone formation by several weeks. However, TNF-α-deficient mice have normal skeletons, suggesting that TNF-α signaling is unique to postnatal fracture repair.33
FIG. 1.
Schematic depicting the temporal pattern expression of three proinflammatory signals, tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), and IL-6, after bone injury. The levels of each molecule are expressed as a percentage of the maximal level observed over the time period indicated. This graphic representation does not indicate concentrations of one molecule relative to another. Schematic is based on quantitative analysis of in vivo mRNA expression following bone injury.10,32
Although the effects of TNF-α on bone have been studied for decades, it has only recently been recognized that this molecule can exert opposite effects depending on the context in which it is released.31 Stimulation of the molecular signaling pathway responsible for TNF-α production induces osteogenic differentiation of mesenchymal stem cells in vitro, while signals that suppress TNF-α release decrease osteogenic differentiation.34 TNF-α regulates the differentiation and function of both osteoblasts and osteoclasts via TNFR1 and TNFR2, the two cell-surface receptors for TNF-α.35 TNFR1 is always present in bone tissue. In contrast, TNFR2 is only expressed following bone injury.32 In an in vitro culture system, TNF-α signaling promoted bone formation in cells from both normal and TNFR1-deficient mice. However, the opposite effect was observed with TNFR2-deficient cells, where TNF-α signaling stimulated osteoclast differentiation and bone resorption.36 Secondary signals arising from the activation of TNFR2 likely mediate the proregenerative effects of TNF-α in fracture healing.32,36
Interleukin-1
Although it acts through a distinct molecular signaling pathway, the effect of IL-1 on bone largely overlaps with that of TNF-α.35 Like TNF-α, IL-1 expression follows a biphasic pattern. In a mouse model of fracture healing, its concentration rises immediately after bone fracture, peaking after 24 h and returning to undetectable levels by 72 h, as shown in Figure 1. The main source of IL-1 during this inflammatory phase is macrophages.10,32 IL-1 triggers release of IL-6, prostaglandins, and other proinflammatory secondary signals.37 It also stimulates angiogenesis and promotes formation of the cartilaginous callus that stabilizes the fracture site.12 A second peak in IL-1 expression occurs approximately 3 weeks following the injury (see Fig. 1). During this period, IL-1 is mainly expressed by osteoblasts and facilitates bone remodeling by stimulating proteases to degrade callus tissue.12,32
The diverse functions of IL-1 during fracture healing can be attributed to the differential expression of the two IL-1 receptors, IL-1RI and IL-1RII. In a murine model of fracture healing, the expression of IL-1RII follows the same biphasic pattern as its ligand, IL-1. In contrast, IL-1RI is only detectable during the inflammatory phase.32 IL-1RI-deficient mice have decreased bone mass and twofold increased levels of osteoclasts,38 which underscores the importance of this molecular signal in bone homeostasis.
Interleukin-6
IL-6 is produced by osteoblasts, in response to stimulation by IL-1.12 In mouse models of bone regeneration, levels of IL-6 rise immediately after injury and return to baseline by the end of the first week (see Fig. 1).11 IL-6 regulates the differentiation of both osteoblasts and osteoclasts, and also promotes angiogenesis by stimulating release of vascular endothelial growth factor.39 Unlike TNF-α and IL-1, IL-6 expression is limited to the inflammatory phase. Levels of IL-6 remain at baseline in the remodeling phase of fracture healing.11 Absence of IL-6 significantly delays the early stages of fracture healing, including mineralization and remodeling of the fracture callus. Two weeks after bone fracture, IL-6–deficient mice have reduced mineralization and increased cartilage content at the fracture site; 4 weeks after the injury, fracture healing is comparable to that of IL-6-replete mice.39 In human patients, IL-6 remains elevated for up to several months following fracture. Higher levels correlate with decreased load-bearing capability at the injury site.40,41
Additional cytokines
The increasing recognition of the central role and unique function of proinflammatory signals in bone regeneration has stimulated research on the effects of other interleukins. There is a growing amount of evidence that a large number of cytokines play an important role in bone regeneration.
Interleukin-4
IL-4 is a so-called “inhibitory cytokine,” a term used by immunologists to describe molecules that tend to counteract the proinflammatory effects of TNF-α and IL-1.37 IL-4 targets both osteoclasts and osteoblasts, inhibiting in vivo bone remodeling. Genetically modified mice that overproduce IL-4 develop severe osteoporosis; their bones have reduced stiffness and a propensity for failure when subjected to mechanical loads.42
Interleukin-5
IL-5 has only recently been recognized as having a role in osteogenesis. A mouse model genetically engineered to express high levels of IL-5 developed ectopic ossification in the spleen. Splenic bone nodules were indistinguishable from normal bone by histology, exhibited osteoblast-specific gene expression, and had mineral content consistent with that of bone matrix.43 Additionally, bone formation was significantly increased in the long bones of these mice. When normal mice were transplanted with bone marrow from the transgenic mice, they developed identical pathology, including formation of splenic bone nodules and increased cancellous bone formation in the long bones.43
Interleukin-12 and interleukin-18
IL-12 inhibits in vitro osteoclast differentiation. This antiresorptive effect is magnified when IL-18, which is produced by osteoblasts, is simultaneously administered to murine osteoclast progenitors in vitro.44 The mechanism of IL-12 and IL-18 remains unclear. Their effect on osteoclast progenitors is indirect and involves increased production of IFN-γ.45 Overproduction of IL-18 stimulates IFN-γ production and suppresses IL-4 in vivo, resulting in cortical thinning and decreased bone volume in a mouse model.46
Interferon-γ. IFN-γ levels rise in response to bone injury and remain elevated throughout most of bone healing, returning to baseline late in the remodeling phase.11 Research on the mechanism of this cytokine has been complicated by its opposite effects on bone resorption in vitro and in vivo.37 IFN-γ stimulates alkaline phosphatase activity in human osteoblasts47 while suppressing the in vitro differentiation of osteoclasts.48 IFN-γ-receptor-deficient mice have increased osteoclast formation in the presence of bone inflammation, indicating that IFN-γ has a protective, antiresorptive effect.48 However, systemic administration of IFN-γ to rats for 8 days triggers severe osteopenia.49 IFN-γ stimulates bone resorption in human patients with osteopetrosis, a disease associated with osteoclast dysfunction.37
The effect of inflammatory cytokines on bone depends on the timing and context of their expression. A single cytokine can have both proregenerative and proresorptive effects on bone. Further research on the mechanism of these molecules will enable their incorporation into tissue engineering strategies to rationally control inflammation and induce regeneration.
Effect of Drugs that Modulate the Inflammatory Response
The number of studies documenting the effects of drugs that modulate the inflammatory response on fracture healing has grown rapidly within the past few years. This likely reflects increasing awareness amongst clinicians and pharmacologists of the key role of inflammatory signaling. However, these insights are not yet widely recognized in the field of tissue engineering, and thus few studies have attempted to integrate this knowledge into a bone tissue engineering strategy. Recent advances in molecular and cell biology have enabled researchers to specifically target bone tissue, avoiding potentially severe side effects, including systemic inflammation or, conversely, immunosuppression.50 This section summarizes the effects of pharmacologic modulation of the inflammatory response on bone regeneration in vivo, and the limited cases in which this knowledge has already been incorporated into tissue engineering strategies.
Cytokine-specific agents
Four inhibitory agents that specifically target proinflammatory molecules are currently used in human patients: infliximab (Remicade®), adalimumab (Humira®), and etanercept (Enbrel®) are antibodies that block the function of TNF-α, while anakinra (Kineret®) blocks binding of IL-1 to its receptor. These selective anticytokine therapies do not impair bone fracture healing in human patients, which likely reflects the low dosages used clinically, since higher dosages increase the risk of severe infections, particularly tuberculosis.51,52 However, in an in vitro model of bone healing, addition of anti-TNF-α antibodies obliterated the dose-dependent increase in bone formation triggered by TNF-α.36
Stimulation of the signaling pathways activated by TNF-α, IL-1, and other proinflammatory mediators also enhances fracture healing. A recent study indicates that the synthetic peptide TP508, which enhances in vivo bone regeneration in a variety of animal models, activates the same signaling pathways stimulated by TNF-α, IL-1, and other proinflammatory cytokines during fracture healing.53 A single injection of TP508 into a femoral fracture increased the strength of the healed bone by over 30% in a rat model.54 Molecular analysis of this same model indicated that TP508 altered protein expression for 7 days, even though the half-life of TP508 at the fracture site was less than 12 h. The differentially expressed proteins belonged to molecular signaling pathways that regulate osteoclasts and osteoblasts.53 Similar effects on bone healing in rabbits have been reported with controlled release of TP508 from poly (DL-lactic-co-glycolic acid) (PLGA) microspheres and poly(propylene fumarate) scaffolds.55,56 A recent placebo-controlled phase I/II study indicates that TP508 significantly accelerates the healing rate of human diabetic foot ulcers.57
Corticosteroids
Numerous studies have indicated that corticosteroids suppress in vivo fracture healing in rodents and rabbits.58–60 However, corticosteroids are known to promote in vitro osteogenic differentiation of mesenchymal stem cells.61 A recent study reported that intramuscular injections of methylprednisolone improved in vivo osteogenesis in tissue engineering scaffolds subcutaneously implanted in New Zealand white rabbits. The scaffolds were seeded with autologous chondrocytes, and the authors suggest that corticosteroids stimulated the chondrocytes to undergo endochondral bone formation.62 In human patients, prolonged corticosteroid treatment is known to cause bone necrosis.63 In fact, corticosteroids are clinically used to reduce osteogenesis in patients with fibrodysplasia ossificans progressiva, a disease associated with excessive bone formation.64,65 Further studies of the effect of these agents are necessary to establish their effects on the various phases of bone healing.
Prostaglandins and nonsteroidal antiinflammatory drugs
Prostaglandins are proinflammatory molecules that enhance bone regeneration by promoting angiogenesis and stimulating both osteoclasts and osteoblasts.66 The rate-limiting step in their synthesis is catalyzed by the enzyme cyclooxygenase (COX), which exists in two forms, COX-1 and COX-2. COX-1 is present in normal bone and triggers the production of low levels of prostaglandins. COX-2 expression is triggered by bone injury and results in high levels of prostaglandin synthesis.50,67 Absence of COX-2, due to genetic mutation, impairs fracture healing in mice.68,69
Nonsteroidal antiinflammatory drugs (NSAIDs), the most common class of antiinflammatory medication, reduce inflammation by inhibiting prostaglandin synthesis. Numerous publications over the past three decades have reported that both nonselective NSAIDs, which target both COX-1 and COX-2, and selective COX-2 inhibitors (e.g., celecoxib) delay or inhibit in vivo fracture healing (recently reviewed by Aspenberg50 and Vuolteenaho et al.66). A recent study indicates that indomethacin, a nonselective NSAID, delays in vivo femoral fracture healing in rats, while celecoxib (Celebrex®) and rofecoxib (Vioxx®), both COX-2 selective inhibitors, cause osseous nonunion.68 A significant increase in the proportion of nonunions, along with a significant decrease in fracture callus strength, is evident after just 5 days of celecoxib therapy.70 The effect of these drugs is limited to the initial period of fracture healing, corresponding to the inflammatory phase; giving celecoxib either prior to fracture or 14 days after fracture has no effect on fracture healing.70 The inhibitory effect of these drugs on fracture healing is reversible. Rats given valdecoxib (Bextra®), another COX-2 selective inhibitor, for 21 days following femoral fracture had significantly increased incidence of osseous nonunion; 2 weeks after treatment was stopped, there were no differences between experimental and control groups.71
Clinical studies of human patients support the experimental findings in animal models. Use of NSAIDs after long-bone fracture significantly increases the risk of either delayed union or nonunion.72,73 NSAIDs are consequently used to prevent heterotopic bone formation after hip surgery. Just 5–7 days of postoperative treatment with NSAIDs effectively prevents ectopic bone formation.74 As expected from these findings, in hip surgery patients with simultaneous long-bone fractures, NSAIDs significantly increase the risk of nonunion at long-bone fracture sites. Incidence of nonunion increases by as much as fourfold, suggesting that these patients should be treated with alternative methods to suppress ectopic bone formation.75
Selective prostaglandin agonists
Increased bone formation is a known side effect of prostaglandins, which are commonly used to maintain a patent ductus arteriosus in infants with congenital heart disease.76 However, they have not been pursued as a therapeutic agent to promote fracture healing because of the risk of severe side effects, including systemic inflammation.50 Recent studies indicate that there are several prostaglandin receptors, each with a distinct tissue distribution and secondary signaling cascade. The main effects on bone are exerted via the Prostaglandin E2 type 2 (EP2) and EP4 receptors.66 Selective EP2 and EP4 receptor ligands may be used for bone repair while avoiding systemic side effects. A recent study indicates that selective EP2 receptor agonists stimulate in vivo fracture healing in rodents and dogs.77 For the dog studies, EP2 agonists encapsulated in a PLGA carrier enhanced in vivo healing of critical-size radial defects and tibial bone defects.77 A similar effect has been reported for EP4 agonists in rat femoral defects.78
Conclusions
The impact of TNF-α, IL-1, and other proinflammatory signals on bone regeneration underscores the importance of incorporating rational control of inflammation into the design of tissue engineering strategies. Modulation of the inflammatory response is a new direction in the field of tissue engineering. To our knowledge, few studies have attempted to integrate inflammatory modulation into tissue engineering strategies to enhance bone regeneration. Novel strategies that exploit inflammatory signals have the potential to induce greater regeneration than current systems, which only deliver growth factors. In bone tissue engineering, a better combination of renewal signals will decrease the required dose of factors like bone morphogenetic protein-2 (BMP-2), limiting their release to the injury site and thus minimizing ectopic effects likely in current systems. Rational control of the inflammatory response will provide a powerful strategy to induce healing of engineered tissue.
Acknowledgment
The work on bone regeneration has been supported by the National Institutes of Health (R01 DE15164 and R01 DE17441).
References
- 1.Freed L.E. Guilak F. Guo X.E. Gray M.L. Tranquillo R. Holmes J.W. Radisic M. Sefton M.V. Kaplan D. Vunjak-Novakovic G. Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng. 2006;12:3285. doi: 10.1089/ten.2006.12.3285. [DOI] [PubMed] [Google Scholar]
- 2.Langer R. Tirrell D.A. Designing materials for biology and medicine. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Rundle C.H. Wang H. Yu H. Chadwick R.B. Davis E.I. Wegedal J.E. Lau K.-H.W. Mohan S. Ryaby J.T. Baylink D.J. Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone. 2006;38:521. doi: 10.1016/j.bone.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Hecht J. Kuhl H. Haas S.A. Bauer S. Poustka A.J. Lienau J. Schell H. Stiege A.C. Seitz V. Reinhardt R. Duda G.N. Mundolos S. Robinson P.N. Gene identification and analysis of transcripts differentially regulated in fracture healing by EST sequencing in the domestic sheep. BMC Genomics. 2006;7:172. doi: 10.1186/1471-2164-7-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingber D.E. Mow V.C. Butler D. Niklason L. Huard J. Mao J. Yannas I. Kaplan D. Vunjak-Novakovic G. Tissue engineering and developmental biology: going bio-mimetic. Tissue Eng. 2006;12:3265. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 6.Filbin M.T. How inflammation promotes regeneration. Nat Neurosci. 2006;9:715. doi: 10.1038/nn0606-715. [DOI] [PubMed] [Google Scholar]
- 7.Lumelsky N.L. Commentary: engineering of tissue healing and regeneration. Tissue Eng. 2007;13:1393. doi: 10.1089/ten.2007.0100. [DOI] [PubMed] [Google Scholar]
- 8.Dimitriou R. Tsiridis E. Giannoudis P.V. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn T.A. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;355(Suppl):S7. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 10.Cho T.-J. Gerstenfeld L.C. Einhorn T.A. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 11.Gerstenfeld L.C. Cullinane D.M. Barnes G.L. Graves D.T. Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 12.Sfeir C. Ho L. Doll B.A. Azari K. Hollinger J.O. Fracture repair. In: Lieberman J.R., editor; Friedlaender G.E., editor. Bone Regeneration and Repair. Totowa, NJ: Humana Press; 2005. pp. 21–44. [Google Scholar]
- 13.Lieberman J.R. Daluiski A. Einhorn T.A. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Doll B.A. Sfeir C. Azari K. Holland S. Hollinger J.O. Craniofacial repair. In: Lieberman J.R., editor; Friedlaender G.E., editor. Bone Regeneration and Repair. Totowa, NJ: Humana Press; 2005. pp. 337–358. [Google Scholar]
- 15.Barnes G.L. Kostenuik P.J. Gerstenfeld L.C. Einhorn T.A. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 16.Andreassen T.T. Oxlund H. Local anabolic effects of growth hormone on intact bone and healing fractures in rats. Calcif Tissue Int. 2003;73:258. doi: 10.1007/s00223-002-2074-6. [DOI] [PubMed] [Google Scholar]
- 17.Bail H.J. Kolbeck S. Krummrey G. Schmidmaier G. Haas N.P. Raschke M.J. Systemic application of growth hormone for enhancement of secondary and intramembranous fracture healing. Horm Res. 2002;58(Suppl 3):39. doi: 10.1159/000066481. [DOI] [PubMed] [Google Scholar]
- 18.Andreassen T.T. Ejersted C. Oxlund H. Intermittent parathyroid hormone (1–34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999;14:960. doi: 10.1359/jbmr.1999.14.6.960. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima A. Shimoji N. Shiomi K. Shimizu S. Moriya H. Einhorn T.A. Yamazaki M. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1–34) J Bone Miner Res. 2002;17:2038. doi: 10.1359/jbmr.2002.17.11.2038. [DOI] [PubMed] [Google Scholar]
- 20.Yu-Yahiro J.A. Michael R.H. Dubin N.H. Fox K.M. Sachs M. Hawkes W.G. Hebel J.R. Zimmerman S.I. Shapiro J. Magaziner J. Serum and urine markers of bone metabolism during the year after hip fracture. J Am Geriatr Soc. 2001;49:877. doi: 10.1046/j.1532-5415.2001.49177.x. [DOI] [PubMed] [Google Scholar]
- 21.Veitch S.W. Findlay S.C. Hamer A.J. Blumsohn A. Eastell R. Ingle B.M. Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos Int. 2006;17:364. doi: 10.1007/s00198-005-2025-y. [DOI] [PubMed] [Google Scholar]
- 22.Ingle B.M. Hay S.M. Bottjer H.M. Eastell R. Changes in bone mass and bone turnover following ankle fracture. Osteoporos Int. 1999;10:408. doi: 10.1007/s001980050247. [DOI] [PubMed] [Google Scholar]
- 23.Ivaska K.K. Gerdhem P. Kesson K. Garnero P. Obrant K.J. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res. 2007;22:1155. doi: 10.1359/jbmr.070505. [DOI] [PubMed] [Google Scholar]
- 24.Obrant K.J. Ivaska K.K. Gerdhem P. Alatalo S.L. Pettersson K. Vaananen H.K. Biochemical markers of bone turnover are influenced by recently sustained fracture. Bone. 2005;36:786. doi: 10.1016/j.bone.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Miclau T. Schneider R.A. Eames B.F. Helms J.A. Common molecular mechanisms regulating fetal bone formation and adult fracture repair. In: Lieberman J.R., editor; Friedlaender G.E., editor. Bone Regeneration and Repair. Totowa, NJ: Humana Press; 2005. pp. 45–55. [Google Scholar]
- 26.Luttikhuizen D.T. Harmsen M.C. van Luyn M.J.A. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12:1955. doi: 10.1089/ten.2006.12.1955. [DOI] [PubMed] [Google Scholar]
- 27.Romas E. Gillespie M.T. Martin T.J. Involvement of receptor activator of NFkB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone. 2002;30:340. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 28.Glasson S. Identification of targets through histologic evaluation of osteoarthritis in knock out mice. Osteoarthritis Cartilage. 2005;13(Suppl A):S3. [Google Scholar]
- 29.Martin P. D'Souza D. Martin J. Grose R. Cooper L. Maki R. McKercher S.R. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13 doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 30.Tsiridis E. Upadhyay N. Giannoudis P.V. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Locksley R.M. Killeen N. Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 32.Kon T. Cho T.-J. Aizawa T. Yamazaki M. Nooh N. Graves D.T. Gerstenfeld L.C. Einhorn T.A. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegrin ligand) and related pro-inflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 33.Gerstenfeld L.C. Cho T.-J. Kon T. Aizawa T. Tsay A. Fitch J. Barnes G.L. Graves D.T. Einhorn T.A. Impaired fracture healing in the absence of TNF-α signaling: the role of TNF-α in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 34.Cho H.H. Bae Y.C. Jung J.S. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24:2744. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- 35.Nanes M.S. Pacifici R. Inflammatory cytokines. In: Bronner F., editor; Farach-Carson M.C., editor; Rubin J., editor. Bone Resorption. New York, NY: Springer; 2005. pp. 67–90. [Google Scholar]
- 36.Balga R. Wetterwald A. Portenier J. Dolder S. Mueller C. Hofstetter W. Tumor necrosis factor-alpha: alternative role as an inhibitor of osteoclast formation in vitro. Bone. 2006;39 doi: 10.1016/j.bone.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Lee S.-K. Lorenzo J.A. Cytokines regulating osteoclast formation and function. Curr Opin Rheumatol. 2006;18:411. doi: 10.1097/01.bor.0000231911.42666.78. [DOI] [PubMed] [Google Scholar]
- 38.Bajayo A. Goshen I. Feldman S. Csernus V. Iverfeldt K. Shohami E. Yirmiya R. Bab I. Central IL-1 receptor signaling regulates bone growth and mass. Proc Natl Acad Sci USA. 2005;102:12956. doi: 10.1073/pnas.0502562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X. Ricciardi B.F. Hernandez-Soria A. Shi Y. Camacho N.P. Bostrom M. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller R.R. Cappola A.R. Shardell M.D. Hawkes W.G. Yu-Yahiro J.A. Hebel J.R. Magaziner J. Persistent changes in interleukin-6 and lower extremity function following hip fracture. J Gerontol A Biol Sci Med Sci. 2006;61:1053. doi: 10.1093/gerona/61.10.1053. [DOI] [PubMed] [Google Scholar]
- 41.Pape H.-C. Schmidt R.E. Rice J. van Griensven M. das Gupta R. Krettek C. Tscherne H. Biochemical changes after trauma and skeletal surgery of the lower extremity: quantification of the operative burden. Crit Care Med. 2000;28:3441. doi: 10.1097/00003246-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Lewis D.B. Liggitt H.D. Effmann E.L. Motley S.T. Teitelbaum S.L. Jepsen K.J. Goldstein S.A. Bonadio J. Carpenter J. Perlmutter R.M. Osteoporosis induced in mice by overproduction of interleukin 4. Proc Natl Acad Sci USA. 1993;90:11618. doi: 10.1073/pnas.90.24.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macias M.P. Fitzpatrick L.A. Brenneise I. McGarry M.P. Lee J.J. Lee N.A. Expression of IL-5 alters bone metabolism and induces ossification of the spleen in transgenic mice. J Clin Invest. 2001;107:949. doi: 10.1172/JCI11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horwood N.J. Elliott J. Martin T.J. Gillespie M.T. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. 2001;166 doi: 10.4049/jimmunol.166.8.4915. [DOI] [PubMed] [Google Scholar]
- 45.Nagata N. Kiataura H. Yoshida N. Nakayama K. Inhibition of RANKL-induced osteoclast formation in mouse bone marrow cells by IL-12: involvement of IFN-gamma possibly induced from non-T cell population. Bone. 2003;33:721. doi: 10.1016/s8756-3282(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 46.Kawase Y. Hoshino T. Yokota K. Kuzuhara A. Nakamura M. Maeda Y. Nishiwaki E. Zenmyo M. Hiraoka K. Aizawa H. Yoshino K. Bone malformations in interleukin-18 transgenic mice. J Bone Miner Res. 2003;18:975. doi: 10.1359/jbmr.2003.18.6.975. [DOI] [PubMed] [Google Scholar]
- 47.Gowen M. MacDonald B.R. Russell R.G. Actions of recombinant human gamma-interferon and tumor necrosis factor alpha on the proliferation and osteoblastic characteristics of human trabecular bone cells in vitro. Arthritis Rheum. 1988;31 doi: 10.1002/art.1780311206. [DOI] [PubMed] [Google Scholar]
- 48.Takayanagi H. Ogasawara K. Hida S. Chiba T. Murata S. Sato K. Takaoka A. Yokochi T. Oda H. Tanaka K. Nakamura K. Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 49.Mann G.N. Jacobs T.W. Buchinsky F.J. Armstrong E.C. Li M. Ke H.Z. Ma Y.F. Jee W.S.S. Epstein S. Interferon-gamma causes loss of bone volume in vivo and fails to ameliorate cyclosporin A-induced osteopenia. Endocrinology. 1994;135:1077. doi: 10.1210/endo.135.3.8070349. [DOI] [PubMed] [Google Scholar]
- 50.Aspenberg P. Drugs and fracture repair. Acta Orthop. 2005;76:741. doi: 10.1080/17453670510045318. [DOI] [PubMed] [Google Scholar]
- 51.Goodman S.B. Jiranek W. Petrow E. Yasko A.W. The effects of medications on bone. J Am Acad Orthop Surg. 2007;15:450. doi: 10.5435/00124635-200708000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Pieringer H. Stuby U. Biesenbach G. Patients with rheumatoid arthritis undergoing surgery: how should we deal with anti-rheumatic treatment? Semin Arthritis Rheum. 2007;36:278. doi: 10.1016/j.semarthrit.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Li X. Wang H. Touma E. Qi Y. Rousseau E. Quigg R.J. Ryaby J.T. TP508 accelerates fracture repair by promoting cell growth over cell death. Biochem Biophys Res Commun. 2007;364:187. doi: 10.1016/j.bbrc.2007.07.202. [DOI] [PubMed] [Google Scholar]
- 54.Wang H. Li X. Tomin E. Doty S.B. Lane J.M. Carney D.H. Ryaby J.T. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J Orthop Res. 2005;23:671. doi: 10.1016/j.orthres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Sheller M.R. Crowther R.S. Kinney J.H. Yang J. Di Jorio S. Breunig T. Carney D.H. Ryaby J.T. Repair of rabbit segmental defects with the thrombin peptide, TP508. J Orthop Res. 2004;22:1094. doi: 10.1016/j.orthres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Hedberg E.L. Kroese-Deutman H.C. Shih C.K. Crowther R.S. Carney D.H. Mikos A.G. Jansen J.A. Effect of varied release kinetics of the osteogenic thrombin peptide TP508 from biodegradable, polymeric scaffolds on bone formation in vivo. J Biomed Mater Res A. 2005;72 doi: 10.1002/jbm.a.30265. [DOI] [PubMed] [Google Scholar]
- 57.Fife C. Mader J.T. Stone J. Brill L. Satterfield K. Norfleet A. Zwernemann A. Ryaby J.T. Carney D.H. Thrombin peptide Chrysalin(R) stimulates healing of diabetic foot ulcers in a placebo-controlled phase I/II study. Wound Repair Regen. 2007;15:23. doi: 10.1111/j.1524-475X.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 58.Ho M.L. Chang J.K. Wang G.J. Antiinflammatory drug effects on bone repair and remodeling in rabbits. Clin Orthop Relat Res. 1995;313:270. [PubMed] [Google Scholar]
- 59.Lyritis G. Papadopoulou Z. Nikiforidis P. Batrinos M. Varonos D. Effects of cortisone and anabolic steroid upon plasma hydroxyproline during fracture healing in rabbits. Acta Orthop Scand. 1975;1975:25. doi: 10.3109/17453677508989188. [DOI] [PubMed] [Google Scholar]
- 60.Waters R.V. Gamradt S.C. Asnis P. Vickery B.H. Avnur Z. Hill E. Bostrom M. Systemic corticosteroids inhibit bone healing in a rabbit ulnar osteotomy model. Acta Orthop Scand. 2000;71:316. doi: 10.1080/000164700317411951. [DOI] [PubMed] [Google Scholar]
- 61.Jaiswal N. Haynesworth S.E. Caplan A.I. Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64 [PubMed] [Google Scholar]
- 62.Haisch A. Wanjura F. Radke C. Leder-Johrens K. Groger A. Endres M. Klaering S. Loch A. Sittinger M. Immunomodulation of tissue-engineered transplants: in vivo bone generation from methylprednisolone-stimulated chondrocytes. Eur Arch Otorhinolaryngol. 2004;261:216. doi: 10.1007/s00405-003-0646-3. [DOI] [PubMed] [Google Scholar]
- 63.Orwin J.F. Use of the uncemented bipolar endoprosthesis for the treatment of steroid-induced osteonecrosis of the hip in renal transplantation patients. J Arthroplasty. 1991;6:1. doi: 10.1016/s0883-5403(06)80151-8. [DOI] [PubMed] [Google Scholar]
- 64.Brantus J.F. Meunier P.J. Effects of intravenous eti-dronate and oral corticosteroids in fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998;346:117. [PubMed] [Google Scholar]
- 65.Kaplan F.S. Shore E.M. Glaser D.L. Emerson S. The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Clin Proc Intl Clin Consort FOP. 2005;1:1. [Google Scholar]
- 66.Vuolteenaho K. Moilanen T. Moilanen E. Non-steroi-dal anti-inflammatory drugs, cyclooxygenase-2 and the bone healing process. Basic Clin Pharmacol Toxicol. 2007;102:10. doi: 10.1111/j.1742-7843.2007.00149.x. [DOI] [PubMed] [Google Scholar]
- 67.Gerstenfeld L.C. Thiede M.A. Seibert K. Mielke C. Phippard D. Svagr B. Cullinane D.M. Einhorn T.A. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 68.Simon A.M. Manigrasso M.B. O'Connor J.P. Cyclooxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X. Schwarz E.M. Young D.A. Puzas J.E. Rosier R.N. O'Keefe R.J. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simon A.M. O'Connor J.P. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture healing. J Bone Joint Surg Am. 2007;89:500. doi: 10.2106/JBJS.F.00127. [DOI] [PubMed] [Google Scholar]
- 71.Gerstenfeld L.C. Al-Ghawas M. Alkhiary Y.M. Cullinane D.M. Krall E.A. Fitch J.L. Webb E.G. Thiede M.A. Einhorn T.A. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. J Bone Joint Surg Am. 2007;89:114. doi: 10.2106/JBJS.F.00495. [DOI] [PubMed] [Google Scholar]
- 72.Giannoudis P.V. MacDonald D.A. Matthews S.J. Smith R.M. Furlong A.J. de Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal antiinflammatory drugs. J Bone Joint Surg Br. 2000;82:655. doi: 10.1302/0301-620x.82b5.9899. [DOI] [PubMed] [Google Scholar]
- 73.Bhattacharyya T. Levin R. Vrahas M.S. Solomon D.H. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53:364. doi: 10.1002/art.21170. [DOI] [PubMed] [Google Scholar]
- 74.Neal B.C. Rodgers A. Clark T. Gray H. Reid I.R. Dunn L. MacMahon S.W. A systematic survey of 13 randomized trials of non-steroidal anti-inflammatory drugs for the prevention of heterotopic bone formation after major hip surgery. Acta Orthop Scand. 2000;71:122. doi: 10.1080/000164700317413076. [DOI] [PubMed] [Google Scholar]
- 75.Burd T.A. Hughes M.S. Anglen J.O. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br. 2003;85:700. [PubMed] [Google Scholar]
- 76.Clyman R.I. Ibuprofen and patent ductus arteriosus. N Engl J Med. 2000;343:728. doi: 10.1056/NEJM200009073431009. [DOI] [PubMed] [Google Scholar]
- 77.Paralkar V.M. Borovecki F. Ke H. Cameron K.O. Lefker B. Grasser W.A. Owen T.A. Li M. DaSilva-Jardine P. Zhou M. Dunn R.L. Dumont F. Korsmeyer R. Krasney P. Brown T.A. Plowchalk D. Vukicevic S. Thompson D.D. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci USA. 2003;100:6736. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka M. Sakai A. Uchida S. Tanaka S. Nagashima M. Katayama T. Yamaguchi K. Nakamura T. Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819.CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone. 2004;34 doi: 10.1016/j.bone.2004.01.002. [DOI] [PubMed] [Google Scholar]