Summary
Molecular imaging is a rapidly growing new discipline in gastrointestinal endoscopy that involves the development of novel imaging probes and instruments to visualize the molecular expression pattern of mucosa in the digestive tract. Several platforms for imaging agents, including antibody and peptide, are being developed to target over expressed biomolecules in cancer. In addition, novel imaging instruments, including fluorescence endoscopy and confocal microscopy, are being developed to provide wide area surveillance and microscopic examination. These methods are being applied to detect the presence of flat and depressed colonic neoplasms and to identify the tumor margins.
Keywords: molecular imaging, colonic neoplasms, dysplasia, flat and depressed, targets
Introduction
The presence of nonpolypoid colorectal neoplasms is drawing significantly greater attention in the effort to improve methods for the early detection of colorectal cancer [1]. These lesions are much more difficult to identify on conventional white light endoscopy because their architectural changes are subtle and can be difficult to distinguish from that of normal colonic mucosa. By comparison, nonpolypoid lesions can appear slightly elevated, completely flat, or slightly depressed [2]. In particular, depressed lesions are the most difficult to detect, and have the highest malignant potential. Recent studies have reported a significant miss rate on colonoscopy, and subsequent endoscopic therapy requires an accurate definition of tumor margins. Molecular imaging is a novel, emerging methodology that identifies functional properties of tissue based on the specific molecular signature of the mucosa. This field has been driven in part by recent advances in our understanding of tumor genetics that allow for future personalized oncological therapy. New molecular probes and imaging instruments are being developed to visualize the unique patterns of molecular expression in the mucosa in the digestive tract. Progress is being made in a number of imaging agent platforms that target biomolecules that are over expressed in cancer. Moreover, novel imaging instruments, including fluorescence endoscopy and confocal microscopy, are being developed to provide wide area surveillance and microscopic examination.
Molecular Probes
Molecular probes are used to reveal subtle molecular changes in the cells and tissues that are present even in the absence of structural abnormalities, and can identify the over expression of targets to guide therapy in addition to performing diagnostics. Even with the highest resolution optical imaging instruments, exogenous probes are needed to observed important biological processes, including up regulation of growth factors, presence of proteolytic enzymes, and expression of cell adhesion molecules, that drive the progression of disease [3]. These probes are fluorescent-labeled for enhancing image contrast on endoscopic detection and for overcoming background tissue autofluorescence. Specific applications include performing in vivo lesion characterization, providing risk stratification, and assessing response to specific therapies. Furthermore, because disease develops from genetic changes that are unique to each individual patient, targeting of specific molecular mechanisms can be used to tailor therapy that will maximize efficacy.
The antibody is one of the first targeting agents used for optical imaging, and is best suited for detection of extra-cellular targets and cell-surface receptors. These Y-shaped gamma globulins (IgG) express light chains on the distal end of either arm that binds selectively to over expressed targets with high affinity and specificity. The light chains can accommodate a large number of different amino acid sequences, resulting in a high diversity. Antibodies have been developed for several molecular targets that have great therapeutic relevance, including human epidermal growth factor receptor (ERBB2) and epidermal growth factor receptor (EGFR). However, there are several disadvantages for use of whole antibodies in molecular imaging. Their long circulatory half-life results in greater non-specific binding, and increased tumor vascular permeability often produces a high background level. Moreover, they may elicit an immune reaction with repeated use, and are costly to produce in large quantities.
Peptides have tremendous advantages for performing targeted detection and therapy in the colon because of their high diversity, rapid binding kinetics, and potential for deep penetration into diseased mucosa. In addition, peptides can be labeled easily, are generally non-toxic, and not immunogenic. These molecular probes have been developed using techniques of phage display, a powerful combinatorial method that uses recombinant DNA technology to generate a library of peptides that bind preferentially to cell surface targets. The protein coat of bacteriophage, such as the filamentous M13 or T7, is genetically engineered to express a very large number (>109) of different peptides with unique sequences. Selection of sequences with high affinity binding is then performed by biopanning the library against cultured cells that over-express desired targets. The DNA sequences are then recovered and used to synthesize the candidate peptides. Techniques of phage display have been successfully used to identify peptides that bind preferentially to dysplastic colonic mucosa and not to normal mucosa by employing a biopanning strategy that uses cultured cells and freshly excised normal and dysplastic tissue.
Imaging Instruments
Novel endoscopic instruments that are sensitive to fluorescence are needed to perform wide area surveillance as well as microscopic examination. The endoscope, shown in Fig. 1, has two detectors for collecting white light (WL) images and fluorescence separately [4]. The white light image is collected by the center detector, and the fluorescence image is collected by a second detector located near the periphery. Illumination for both modes is delivered through the two fiber light guides. In the WL mode, the full visible spectrum (400 to 700 nm) is provided, while in the fluorescence mode, a second filter wheel enters the illumination path, and provides fluorescence excitation in the 395 to 475 nm spectral band. In addition, illumination from 525 to 575 nm provides reflected light in the green spectral regime centered at 550 nm. The fluorescence image is collected by the peripherally located CCD detector that has a 490–625 nm band pass filter for blocking the excitation light. Because the increased vasculature in neoplastic mucosa absorbs autofluorescence, it appears with decreased intensity.
Fig. 1. Novel imaging instruments.
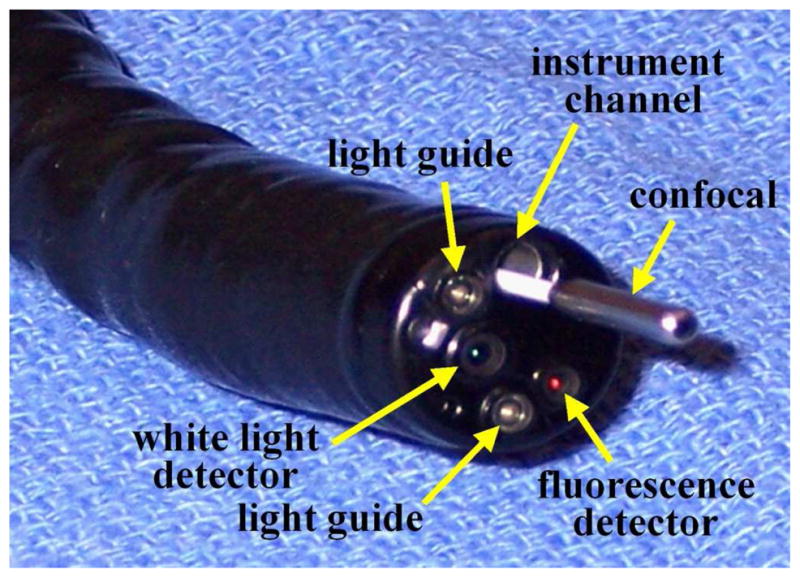
Wide area endoscopic images are collected by fluorescence detector, and microscopic images are acquired by confocal miniprobe. These instruments can be used together to guide ‘optical biopsy.”
The confocal miniprobe (Cellvizio®-GI), shown in Fig. 1, consists of a flexible fiber imaging bundle that uses a 488 nm laser for excitation light [5]. The beam is scanned by a set of rotating mirrors located in the instrument unit. A microlens located at the distal end of the miniprobe focuses the beam into the tissue as well as collects the returning fluorescence. Fluorescence is collected by the same fibers and transmitted back to the detector. The cores of the fiber act as collection pinholes for rejecting out of focus light to perform optical sectioning. A long pass filter rejects the excitation light, and fluorescence is detected with an avalanche photodiode. The small outer diameter (<3 mm) allows for easy passage of the miniprobe through the instrument channel of the endoscope, allowing for the wide area view to accurate guide placement onto the mucosal surface. Separate miniprobes provide distinct working distances up to 100 μm, and have a resolution between 1 and 3.5 μm. Images are collected in a horizontal plane (en face) at 12 frames per second with a field of view of either 600 μm. The frame rate is adequate to achieve consistent images with little interference from motion artifact.
Imaging Results
Clinical imaging has been performed with fluorescence-labeled peptides to evaluate the spatial extent of non-polypoid lesions in the colon. In Fig. 2A, a standard white light endoscopic image shows a sessile mass approximately 10 mm in size found to be carcinoma-in-situ (CIS) on histology. The fluorescence image, shown in Fig. 2B, collected after topical administration of the fluorescent-labeled peptide ‘VRPMPLQ’ reveals increased intensity at the site of the lesion compared to that of the adjacent normal mucosa [6]. Moreover, the presence of neoplastic crypts can be validated on the cellular level with confocal microscopy. A standard white light image of a dysplastic polyp is shown in Fig. 3A. The confocal image in Fig. 3B, collected after topical administration of the fluorescent-labeled peptide ‘VRPMPLQ’ reveals increased fluorescence intensity from the colonocytes in dysplastic but not in the normal crypts [7]. These images demonstrate the importance of targeted imaging and its future role in the management of non-polypoid colonic neoplasms. Because of their morphology and indistinct margins, these lesions are difficult to remove in entirety under white light guidance alone. The addition of the peptide stained images provides functional information about neoplastic target over expression that can help improve the completeness of resection.
Fig. 2. Targeted endoscopic imaging.
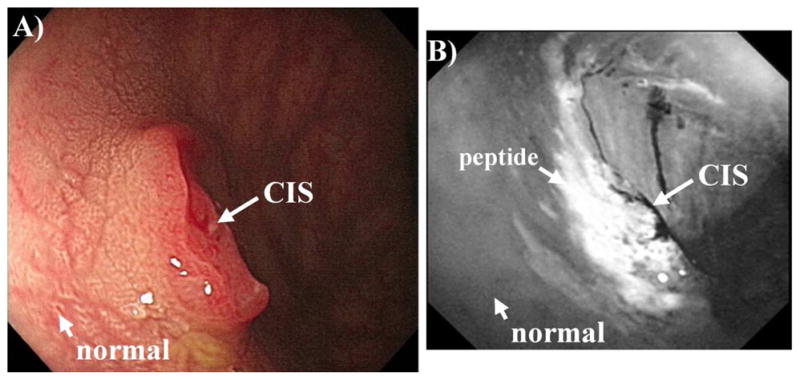
A) A 10 mm lesion (carcinoma-in-situ) is seen on white light endoscopy. B) Targeted image using topical administration of fluorescent-labeled peptides reveals tumor margins.
Fig. 3. Targeted confocal imaging.
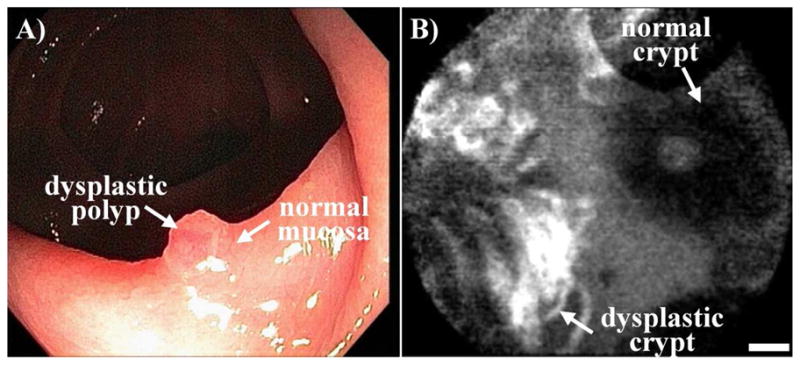
A) A dysplastic polyp sits on a colonic fold consisting of normal mucosa. B) Confocal image following administration of fluorescent-labeled peptides demonstrates preferential binding to dysplastic crypt (left) comparison to adjacent normal crypt (right), scale bar 20 μm.
Future Challenges
There exists an important clinical need for more accurate endoscopic detection of flat and depressed colonic neoplasms to improve our effectiveness in performing cancer screening and surveillance. These lesions often contain severe dysplasia and are more aggressive in nature. In addition, high specificity is needed to distinguish neoplastic from non-neoplastic lesions that may include normal mucosa, hyperplasic polyps, colitis foci, and scar tissue. These lesions can be small (<10 mm in dimension) and patchy, thus techniques of wide area surveillance and high magnification are needed. Molecular imaging represents an exciting new methodology that targets over expressed biomolecules in cancer that can identify and outline flat and depressed lesions on routine colonoscopy. A variety of probe platforms and imaging instruments are being developed to use fluorescence to enhance image contrast. Future advancements include the detection of important cell surface targets, such as EGFR and c-MET, to perform image guided therapy in addition to diagnosis.
Acknowledgments
This work was supported by Grant No. U54 CA136429 and P50 CA93990 from the National Institutes of Health.
Footnotes
The author has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaramillo E, Watanabe M, Slezak P, Rubio C. Flat neoplastic lesions of the colon and rectum detected by high-resolution video endoscopy and chromoscopy. Gastrointest Endosc. 1995;42:114–22. doi: 10.1016/s0016-5107(95)70066-8. [DOI] [PubMed] [Google Scholar]
- 2.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 3.Goetz M, Wang TD. Molecular Imaging in Gastrointestinal Endoscopy. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uedo N, Higashino K, Ishihara R, Takeuchi Y, Iishi H. Diagnosis of Colonic Adenomas by New Autofluorescence Imaging System: a Pilot Study. Digestive Endoscopy. 2007;19:S134–8. [Google Scholar]
- 5.Wang TD, Friedland S, Sahbaie P, Soetikno R, Hsiung P, Liu JTC, Crawford JM, Contag C. Functional Imaging of Colonic Mucosa with a Fibered Confocal Microscope for Real Time In Vivo Pathology. Clinical Gastroenterology & Hepatology. 2007;5:1300–05. doi: 10.1016/j.cgh.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsiung PL, Wang TD. In Vivo Biomarkers for Targeting Colorectal Neoplasms. Cancer Biomarkers. 2008;4:329–40. doi: 10.3233/cbm-2008-4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiung P, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH, Wang TD. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nature Medicine. 2008;14:454–58. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]


