Abstract
The postauricular reflex is a relatively new psychophysiological measure of appetitive emotional processing during picture viewing. However, the degree to which other auricular (i.e., superior and anterior auricular) muscles might exhibit reflexive activity congruent with that found in the postauricular muscle has not been investigated, nor has the robustness of postauricular reflex modulation across stimulus modality. In this study, postauricular reflexes were the only reflexes that showed consistent emotional modulation across ears and genders. Additionally, postauricular reflexes were significantly modulated for both emotional pictures and sounds; in both cases, postauricular reflexes were greatest during pleasant stimuli.
Keywords: postauricular reflex, auricular, startle, emotion, pictures, sounds
The postauricular reflex is a vestigial muscle response in humans that acts to pull the ear upward and backward (Berzin & Fortinguerra, 1993; Gray, 1901/1995) and is recorded from electrodes positioned over the postauricular muscle behind the ear (O’Beirne & Patuzzi, 1999). Kiang, Crist, French, and Edwards (1963) first isolated the postauricular reflex as an electrical potential evoked by noise clicks in awake humans. This potential was also shown to be myogenic in origin, suggesting that the moniker “postauricular reflex” is an appropriate one: Its magnitude increased with forward pressure on the back of the scalp to tense the postauricular muscle, decreased substantially with backward pressure on the forehead to relax the muscle, and was abolished when the postauricular muscle was injected with the muscular paralytic curare (Bickford et al., 1964). Further supporting the myogenic origin of the postauricular reflex is the finding that the magnitude of the postauricular reflex recorded over the skin is proportional to the number of postauricular muscle motor units activated, as measured by transdermal recordings (De Grandis & Santoni, 1980). Nevertheless, it is important to note that this reflex is vestigial and does not generate movement of the pinna.
Based on studies of individuals with severe sensorineural hearing loss, the postauricular reflex appears to originate from the cochlea, not the vestibular system (Yoshie & Okudaira, 1969; but see Cody, Jacobson, Walker, & Bickford, 1964, for data regarding the possible vestibular origin of the similar inion response to clicks). The facial nerve also appears to be the efferent pathway to the postauricular muscle in generating the postauricular reflex, as postauricular reflexes are abolished on the side ipsilateral to intracranial facial nerve palsies of various etiologies but not in patients with extracranial facial nerve palsies (Bochenek & Bochenek, 1976). Indeed, postauricular muscle activity can be used as an indicator of facial nerve conduction velocities (De Meirsman, Claes, & Geerdens, 1980), and postauricular reflexes are often the first EMG responses to recover from facial Bell’s palsy (Serra, Tugnoli, Cristofori, Eleopra, & De Grandis, 1986). However, the circuitry of the postauricular reflex between the cochlea and the facial nerve remains unstudied.
Benning et al. (2004) investigated the effects of emotional picture presentation on the postauricular reflex in undergraduate males. The magnitude of the postauricular reflex was generally smaller during pictures than during intertrial intervals (ITIs), indicating that perceptual engagement with a foreground stimulus generally inhibited the reflex (cf. Hackley et al., 1987). In opposition to this effect, the magnitude of the postauricular reflex was potentiated during pleasant pictures and inhibited during aversive pictures, yielding a linear pattern of emotional modulation opposite that observed for the startle blink reflex. These results were not attributable to differences in baseline postauricular EMG activity, as baseline postauricular EMG activity did not differ during pleasant, neutral, and aversive pictures. Additionally, these modulatory effects were stronger for high-intensity affective pictures than for low-intensity pictures (cf. Cuthbert et al., 1996).
These effects have been replicated in undergraduates (Gable & Harmon-Jones, 2009; Sandt, Sloan, & Johnson, 2009) and late pubertal (but not early pubertal) adolescents (Quevedo, Benning, Gunnar, & Dahl, 2009). They have also been extended in a study demonstrating that only happy facial expressions posed by women significantly potentiated postauricular reflex magnitudes, congruent with a functional equivalence hypothesis of facial expressivity (Hess, Sabourin, & Kleck, 2007). Additionally, postauricular reflexes are greater during anticipation of gustatory reward than punishment (Hackley, Muñoz, Hebert, Valle-Inclán, & Vila, 2009), indicating that postauricular reflexes may be measures of appetitive processing in the absence of a foreground emotional stimulus. Nevertheless, the validity of the postauricular reflex as a measure of appetitive processing during emotional sounds has yet to be studied.
This pattern of valence modulation is also counterintuitive, given that the postauricular muscle may have acted defensively to pull the ear back to protect hearing when the muscle’s function was more than vestigial (Berzin & Fortinguerra, 1993; Cassella & Davis, 1986), and decreased tonic postauricular muscle activity is involved in the orienting response (Stekelenburg & van Boxtel, 2001). Thus, postauricular reflex activity may be an artifact of cross-talk from another auricular muscle, the function of which is more obviously appetitive than the postauricular muscle. The two additional extrinsic auricular muscles may be promising targets. The superior auricular muscle acts to perk up the ear rather than flatten it against the head, and the anterior auricular muscle also pulls the ear forward (Gray, 1901/1995), it would be sensible to expect that these auricular muscles might also show appetitive reflexive modulation. It is notable that of the three extrinsic auricular muscles, the superior auricular muscle is largest, followed by the postauricular muscle, with the anterior auricular muscle being smallest. Thus, there may be mean level differences in reflex magnitude based on the sheer area of the muscles.
Assessing the reflexive activity of these muscles may also narrow which branch of the facial nerve is responsible for generating the appetitive modulation of the postauricular reflex. Whereas the postauricular muscle is innervated by the postauricular branch of the facial nerve, the superior and anterior auricular muscles are instead innervated by the temporal branch of the facial nerve (Sataloff & Selber, 2003). Thus, if only postauricular reflexes are potentiated during pleasant vs. neutral pictures, then a relatively restricted branch of the facial nerve (namely, its postauricular branch) would be implicated in its emotional modulation. Conversely, if other auricular reflexes are also potentiated during pleasant vs. neutral pictures, then either multiple branches of the facial nerve conduct activity related to appetitive processing, or the neural activity responsible for potentiating the auricular reflexes arises at a point upstream from the postauricular branch of the facial nerve.
There have been no reports in the literature about the reflexive activity of the superior auricular or anterior auricular muscles. Thus, the current study explored whether the superior auricular and anterior auricular muscles also show reflexive activity to noise probes, and whether reflexes observed in these muscles would show a pattern of modulation by emotional stimuli similar to that for the postauricular reflex. This study also explored whether these auricular muscles had comparable patterns of modulation during emotional pictures and sounds.
Method
Participants
Participants in this study were 38 undergraduates (21 women) from an introductory psychology course. Four participants were excluded because they failed to show significant reactivity to the startle probes (n = 2) or due to equipment failure (n = 2), leaving a final sample of 34 participants (19 women).
Stimuli
The startle probe was a bilateral 50 ms, 105 dB white noise probe with nearly instantaneous rise time; startle probes were presented 3000, 4000, or 5000 ms after picture onset. A total of 55 pictures from the International Affective Picture System (IAPS; CSEA-NIMH, 1999) were used in the experiment.1 Maximally intense exemplars of pleasant and aversive picture contents depicting stimuli that are directly (pleasant: erotic, food; aversive: mutilation, threat) or indirectly (pleasant: adventure, nurturant; aversive: disgust, victim) related to an organism’s survival were included, and all picture contents were gender balanced on dimensions of normatively rated valence (median t(6) between men and women = 0.35, p = .735) and arousal (median t(6) between men and women = 0.13, p = .897).
The first three pictures (IAPS numbers 4650, 7080, and 9252) were startled at the beginning of the experiment to habituate abnormally large initial startle magnitudes (Graham, 1979); data from these pictures were not analyzed. Startle probes were not presented during four pictures during the experiment (IAPS numbers 2220, 5460, 7233, and 8485); instead, probes were presented during the inter-trial interval (ITI) after these pictures to examine the magnitude of postauricular reflexes in the absence of a visual foreground and to reduce the predictability of the startle probes.
Furthermore, 16 sounds from the IADS were presented throughout the experiment. Because there are many fewer sounds in the IADS than there are pictures in the IAPS, examining content categories of sounds was not possible. Therefore, four sounds of each valence (pleasant, neutral, and aversive) were used. Additionally, four sounds (IADS numbers 115, 311, 352, and 425) were presented during the ITI. All sounds were gender-balanced on normative ratings of valence (median t(6) = 0.17, p = .874) and arousal (median t(6) = 0.35, p = .735). Pleasant and aversive sounds were chosen to be maximally extreme in valence and high in arousal, and neutral sounds were chosen to be near the midpoint of the valence scale and low in arousal.
A total of eight run orders were used in this study: Four different serial positions of the stimuli were used, with appropriate stimulus substitutions made for women and men in the study. In each run order, no more than two stimuli of the same valence occurred contiguously, and pictures of the same content did not follow each other.
Psychophysiology
All physiological channels were sampled at 2000 Hz with a Neuroscan NuAmps bioamplifier at DC with a 500 Hz lowpass filter to avoid aliasing of the physiological signals. Recordings of the EMG activity in the postauricular, superior auricular, and anterior auricular muscles were taken from each ear. Postauricular electrodes were placed according to locations detailed in O’Beirne and Patuzzi (1999). Superior auricular and anterior auricular electrode placements were guided by Berzin and Fortinguerra (1993) and Gray (1901/1995). Superior auricular muscle activity was recorded with one electrode on the scalp by the tendon of insertion of the muscle and the other electrode placed immediately posterior to it. Anterior auricular muscle activity was recorded with one electrode placed anterior to the tragus (the cartilaginous protrusion over the ear canal), and the other electrode was placed immediately anterior to the first electrode.
Because the postauricular reflex is a microreflex, reflexive auricular muscle activity was assessed using aggregate rectified waveforms. Auricular EMG activity to noise probes was averaged across all pictures of a given content category, yielding average waveforms composed of 4 trials. In each aggregation, auricular reflex magnitudes were assessed as the peak EMG activity occurring 8–30 ms after the onset of the startle probe minus the mean 50 ms pre-probe EMG baseline activity (Benning et al., 2004). Visual inspection confirmed the appropriateness of this window for scoring these reflexes (cf. Sandt et al., 2009). Because of the large inter-individual variation in responses to startle probes, auricular reflex magnitudes were then z-scored within each participant for valence and content analyses.
Procedure
Participants completed a consent form and a biographical questionnaire to screen for hearing and visual impairments before being escorted into the laboratory. They then completed the study questionnaires in the order noted above as the electrodes were attached. Once hookups were completed, participants were told to follow the directions on the screen, keeping as still as possible at all times. Participants were instructed to watch each picture the entire time it appeared on the screen and to keep their gaze directed toward the fixation cross whenever no picture was on the screen. They were also told that they would hear brief noises through the headphones which they could simply ignore. They were then presented with the three habituation pictures and given a demonstration of the ratings procedure they would use to rate each picture. After the demonstration, participants attended to each stimulus in the sequence determined by the run order.
Pictures and sounds were preceded by a 3 s baseline consisting of a blank screen with a fixation point; each was presented for 6 s, followed by a 3 s recovery period, during which the ITI startle probes were presented halfway through. After each trial, participants completed valence and arousal ratings of their current emotional state using the Self-Assessment Manikin (SAM; Bradley & Lang, 1994) via computer keyboard, and the ratings were followed by a blank screen lasting 3 s to allow participants to prepare for the next picture or sound. After participants viewed and rated all pictures and sounds, they received experimental debriefing and compensation.
Data Analysis
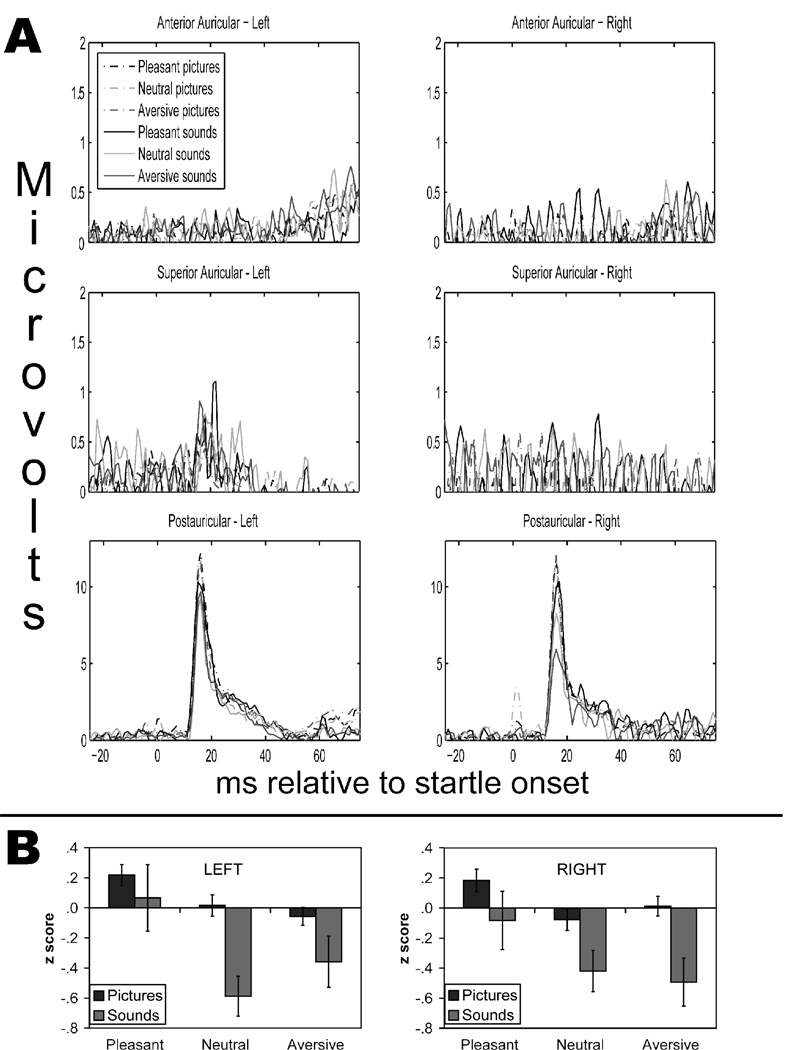
To investigate the relative strength of each auricular response to the noise probes, the mean raw peak magnitude for the anterior, superior, and posterior auricular reflexes of each ear during pictures were entered into a mixed ANOVA, with gender as the between-subjects factor and ear and auricular muscle as the within-subjects factors. Only the auricular Muscle main effect was significant, F(2,29) = 7.01, p = .002, partial η2 = .350 (all other Fs < 2.5, ps > .1, partial η2s < .15). Helmert contrasts revealed that postauricular reflexes (M = 13.9 µV, SE = 3.16) were larger than superior auricular reflexes (M = 2.74 µV, SE = 0.41) and anterior auricular reflexes (M = 1.68 µV, SE = 0.08), F(1,30) = 14.3, p = .001, partial η2 = .322; superior auricular reflexes were also greater than anterior auricular reflexes, F(1,30) = 6.78, p = .014, partial η2 = .184. However, as evidenced in Figure 1A, visual inspection of the grand average waveforms for each auricular reflex by ear revealed that only the postauricular and superior auricular waveforms had reflexive activity that could be discerned from that of the background EMG.2 Thus, only these reflex magnitudes were analyzed further.
Figure 1.
Panel A: Grand average auricular reflex magnitude by picture and sound valence, separated by ear of recording. Panel B: Mean within-subject z scored postauricular reflex magnitude by picture and sound valence, separated by ear of recording. Error bars represent the standard error of the mean.
Postauricular and superior reflex magnitudes were entered into mixed ANOVAs, with gender as the between-subjects factor and ear and valence as the within-subjects factors with planned follow-up polynomial contrasts. A critical α level of .05 was used for all analyses, and within-subjects degrees of freedom were adjusted using the Huyhn-Feldt correction for non-sphericity.
Results
Postauricular Reflexes
As shown in Figure 1B, there was a significant effect of picture valence on postauricular reflex magnitude, F(1.92,61.3) = 4.67, p = .014, partial η2 = .127. In particular, postauricular reflexes were greater during pleasant pictures than during aversive pictures, linear F(1,32) = 9.81, p = .004, partial η2 = .235 (quadratic F(1,32) = 1.97, p = .170, partial η2 = .058). None of the other main effects or interactions were significant, Fs < 1.1, ps > .35. Compared to those during neutral pictures (M = −0.03, SE = 0.06), postauricular reflexes were larger during erotic pictures (M = 0.39, SE = 0.14), t(33) = 2.52, p = .017, and during nurturant pictures (M = 0.28, SE = 0.13), t(33) = 2.09, p = .044. There were no significant differences between postauricular reflexes during any aversive picture contents and those during neutral pictures, ts < 1, ps > .3.
Likewise, postauricular reflexes during sounds were modulated by sound valence, F(1.99,63.8) = 4.03, p = .023, partial η2 = .112. Postauricular reflexes were greater during pleasant sounds than during aversive sounds, linear F(1,32) = 6.05, p = .020, partial η2 = .159; postauricular reflex magnitudes during pleasant and aversive sounds also tended to be greater than those during neutral sounds, quadratic F(1,32) = 3.52, p = .070, partial η2 = .099. Across both stimulus modalities, all other main effects and interactions were not significant, Fs < 1.5, ps > .2. Thus, in the following analyses, postauricular reflexes were averaged across ears and genders.
Analyses of the consistency of the observed valence modulation pattern for pictures (cf. Benning et al., 2004) revealed that 82% of participants (95% confidence interval 66%–92%) had numerically larger postauricular reflexes during pleasant than during aversive pictures. However, only 62% of participants (95% confidence interval 45%–76%) had greater postauricular reflexes during pleasant than during neutral pictures. During emotional sounds, 65% of participants (95% confidence interval 48%–79%) showed numerically greater postauricular reflexes during pleasant sounds than either neutral or aversive sounds.
An ANOVA in which mean postauricular reflexes during stimuli and during ITIs were compared showed that postauricular reflexes during ITIs (M = 0.14, SE = 0.09) were greater than those during stimuli (M = −0.13, SE = 0.04), F(1,33) = 5.23, p = .029, partial η2 = .137. Postauricular reflexes during or after pictures (M = 0.20, SE = 0.08) were also greater than those during or after sounds (M = −0.19, SE = 0.09), F(1,33) = 7.40, p = .010, partial η2 = .183. However, there was no interaction between probe time (during stimulus vs. ITI) and stimulus modality (picture vs. sound), F(1,33) = 0.08, p = .785, partial η2 = .002.
Superior Auricular Reflexes
For superior auricular reflexes during pictures, the strongest effect was the three-way Valence × Ear × Gender interaction, F(2.00,62.0) = 6.68, p = .002, partial η2 = .177. For men, the left ear’s superior auricular reflexes were greater during pleasant (M = 0.16, SE = 0.09) than during aversive pictures (M = −0.19, SE = 0.09), linear Valence F(1,13) = 7.48, p = .017, partial η2 = .365, with those during neutral pictures falling in between (M = −0.10, SE = 0.11), quadratic Valence F(1,13) = 0.29, p = .601, partial η2 = .022. However, in the right ear, the opposite pattern was found, with superior auricular reflexes greater during aversive pictures (M = 0.35, SE = 0.13) than during pleasant pictures (M = −0.16, SE = 0.12), linear Valence F(1,13) = 5.14, p = .041, partial η2 = .283, with those during neutral pictures (M = −0.14, SE = 0.12) falling in between, quadratic Valence F(1,13) = 2.75, p = .121, partial η2 = .174.
For women, left superior auricular reflexes were greater during pleasant (M = 0.21, SE = .10) and aversive pictures (M = 0.10, SE = 0.11) than during neutral pictures (M = −0.22, SE = 0.09), quadratic Valence F(1,18) = 8.44, p = .009, partial η2 = .319. However, there was no effect of Valence in the right ear, F(2.00,36.0) = 1.63 p = .211, partial η2 = .083 (pleasant M = 0.10, SE = 0.09; neutral M = 0.13, SE = 0.08; aversive M = −0.08, SE = 0.08). Thus, these effects substantially qualified the significant Valence × Ear effect, F(2.00,62.0) = 3.91, p = .031, partial η2 = .106. No other effects were significant for superior auricular reflexes during pictures, Fs < 2.2, ps > .12.
There were no significant effects for superior auricular reflexes during sounds, Fs < 2.5, ps > .13.
Discussion
Because superior auricular muscle reflexes were at best inconsistently modulated by emotional stimuli and because anterior auricular muscle reflexes seemed absent, it appears that it is the postauricular muscle, innervated by the postauricular branch of the facial nerve, whose reflex is responsible for the observed patterns of emotional modulation. Furthermore, the postauricular reflex appears to be a measure of appetitive processing during both pictures and sounds, indicating that this reflex may be used to study emotional processing across stimulus modalities. Additional attentional work should be performed to confirm that postauricular reflexes are smaller during sounds than during pictures, which may be an intriguing method of studying unimodal vs. cross-modal attention inhibition. It is possible that electrodes measuring the superior and anterior auricular muscles were not optimally placed to record a signal from them. However, this seems unlikely as even suboptimally placed postauricular electrodes yield interpretable signals (O’Beirne & Patuzzi, 1999), Furthermore, the superior auricular muscle is relatively large compared to the postauricular muscle, so its reflexive activity should be comparatively easy to record.
Nevertheless, it should be reiterated that the neural pathways underlying each of the auricular reflexes remain unclear. Thus, the interpretation of potentiation of the postauricular reflex remains ambiguous. Bickford et al. (1964) reported that “preliminary studies on unanesthetized monkeys, cats, rabbits, and dogs have failed to reveal a similar response to that recorded in the human” (p. 213), implying that finding animal models of this reflex may be difficult. There is also reason to doubt that the pinna reflex is an appropriate analog of the postauricular reflex, as pinna reflex magnitudes were greater in mice during presentation of a CS+ than at baseline in a single-cue conditioning experiment (Casella & Davis, 1986). Dissection of human cadavers or anterograde tracing may be needed to confirm the neural circuitry of the postauricular reflex (as has been done in examinations of the anatomy of the postauricular muscle; Guerra et al., 2004).
Acknowledgments
This work was completed in partial fulfillment for the doctoral degree of Stephen D. Benning from the University of Minnesota. Funding for this work was provided by the National Institutes of Mental Health Ruth L. Kirschstein National Research Service Award MH070104-01.
Footnotes
The following IAPS pictures comprised each content category: adventure: 5623, 8034, 8180, 8210; nurturant: (1811, 2071, 2160, 2340 / 1463, 1722, 2341, 2655); erotic: 4640, 4660, 4680, (4255 / 4572); food: 7200, 7230, 7260, 7460; buildings: 5731, 7180, 7490, 7491; humans: 2190, 2393, 2870, 2890; landscapes: 5120, 5390, 5740, 9210; objects: 7002, 7004, 7034, (7031 / 7038); disgust: 9342, 9520, 9560, 9830; mutilation: (3051, 3061, 9253, 9420 / 9042, 9265, 9440, 9490); threat: 6250, 6260, 9630, (6243 / 6190); victim: 6570, 9920, (6312, 6540 / 6530, 6561). Pictures not in parentheses were presented to participants of either gender; pictures within parentheses to the left of the slash were presented only to men, and those within parentheses to the right of the slash were presented only to women. Likewise, the following IADS stimuli comprised each sound valence: pleasant: 215, 815, (205, 226 / 201, 220); neutral: 358, 704, 708, 726; aversive: 286, 290, (277, 600 / 261, 501).
References
- Benning SD, Patrick CJ, Lang AR. Emotional modulation of the post-auricular reflex. Psychophysiology. 2004;41:426–432. doi: 10.1111/j.1469-8986.00160.x. [DOI] [PubMed] [Google Scholar]
- Berzin F, Fortinguerra CR. EMG study of the anterior, superior, and posterior auricular muscles in man. Anatomischer Anzeiger. 1993;175:195–197. doi: 10.1016/s0940-9602(11)80182-2. [DOI] [PubMed] [Google Scholar]
- Bickford RG, Jacobson JL, Cody TR. Nature of average evoked potentials to sound and other stimuli in man. Annals of the New York Academy of Sciences. 1964;112:204–223. doi: 10.1111/j.1749-6632.1964.tb26749.x. [DOI] [PubMed] [Google Scholar]
- Bochenek W, Bochenek Z. Postauricular (12 msec latency) responses to acoustic stimuli in patients with peripheral, facial nerve palsy. Acta Otolaryngologica. 1976;81:264–269. doi: 10.3109/00016487609119961. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Cassella JV, Davis M. Habituation, prepulse inhibition, fear conditioning, and drug modulation of the acoustically elicited pinna reflex in rats. Behavioral Neuroscience. 1986;100:39–44. doi: 10.1037//0735-7044.100.1.39. [DOI] [PubMed] [Google Scholar]
- Cody DTR, Jacobson JL, Walker JC, Bickford RG. Averaged evoked myogenic and cortical potentials to sound in man. Annals of Ootology, Rhinology, and Laryngology. 1964;73:763–777. doi: 10.1177/000348946407300315. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33:103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- De Grandis D, Santoni P. The post-auricular response: A single motor unit study. Electroencephalography and Clinical Neurophysiology. 1980;50:437–440. doi: 10.1016/0013-4694(80)90009-7. [DOI] [PubMed] [Google Scholar]
- De Meirsman J, Claes G, Geerdens L. Normal latency value of the facial nerve with detection in the posterior auricular muscle and normal amplitude value of the evoked action potential. Electromyography and Clinical Neurophysiology. 1980;20:481–485. [PubMed] [Google Scholar]
- Gable PA, Harmon-Jones E. Postauricular reflex responses to pictures varying in valence and arousal. Psychophysiology. 2009;46:487–490. doi: 10.1111/j.1469-8986.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Graham FK. Distinguishing among orienting, defense, and startle reflexes. In: Kimmel HD, Van Olst EH, Orlebeke JF, editors. The orienting reflex in humans. Hillsdale, NJ: Erlbaum; 1979. pp. 137–167. [Google Scholar]
- Gray H. Anatomy: Descriptive and surgical. 15th ed. New York, NY: Barnes and Noble Books, Inc; 1901/1995. [Google Scholar]
- Guerra AB, Metzinger SE, Metzinger RC, Xie C, Xie Y, Rigby PL. Variability of the postauricular muscle complex: Analysis of 40 hemicadaver dissections. Archives of Facial and Plastic Surgery. 2004;6:342–347. doi: 10.1001/archfaci.6.5.342. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Muñoz MA, Hebert K, Valle-Inclán F, Vila J. Reciprocal modulation of eye-blink and pinna-flexion components of startle during reward anticipation. Psychophysiology. 2009;46:1154–1159. doi: 10.1111/j.1469-8986.2009.00867.x. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Woldorff M, Hillyard SA. Combined use of microreflexes and event-related brain potentials as measures of auditory selective attention. Psychophysiology. 1987;24:632–647. doi: 10.1111/j.1469-8986.1987.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44:431–435. doi: 10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Kiang NY-S, Crist AH, French MA, Edwards AG. Quarterly Progress Report, Research Laboratory of Electronics. Vol. 44. Cambridge, MA: Massachusetts Institute of Technology; Postauricular electric response to acoustic stimuli in humans; pp. 218–225. (91963) [Google Scholar]
- O’Beirne GA, Patuzzi RB. Basic properties of the sound-evoked post-auricular muscle response (PAMR) Hearing Research. 1999;138:115–132. doi: 10.1016/s0378-5955(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: Effects on the psychophysiology of defensive and appetitive motivation. Development and Psychopathology. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandt AR, Sloan DM, Johnson KJ. Measuring appetitive processing with the postauricular reflex. Psychophysiology. 2009;46:491–497. doi: 10.1111/j.1469-8986.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Sataloff RT, Selber JC. Phylogeny and embryology of the facial nerve and related structures: Part II – embryology. Ear, Nose, and Throat Journal. 2003;82:764–766. 769–772, 774, 779. [PubMed] [Google Scholar]
- Serra G, Tugnoli V, Cristofori MC, Eleopra R, De Grandis D. The electromyographic examination of the posterior auricular muscle. Electromyography and Clinical Neurophysiology. 1986;26:661–665. [PubMed] [Google Scholar]
- Stekelenburg JJ, van Boxtel A. Inhibition of pericranial muscle activity, respiration, and heart rate enhances auditory sensitivity. Psychophysiology. 2001;38:629–641. [PubMed] [Google Scholar]
- Yoshie N, Okudairi T. Myogenic evoked potential responses to clicks in man. Acta Oto-Laryngologica Supplementum. 1969;252:89–103. doi: 10.3109/00016486909120515. [DOI] [PubMed] [Google Scholar]