Abstract
Background
For processes that follow first order kinetics, exponential decay nonlinear regression analysis (EDNRA) may delineate curve characteristics and suggest processes affecting curve shape. We conducted a preliminary feasibility assessment of EDNRA of patient survival curves.
Methods
EDNRA was performed on Kaplan-Meier overall survival (OS) and time-to-relapse (TTR) curves for 323 patients with resected NSCLC and on OS and progression-free survival (PFS) curves from selected publications.
Results and Conclusions
In our resected patients, TTR curves were triphasic with a “cured” fraction of 60.7% (half-life [t1/2] >100,000 months), a rapidly-relapsing group (7.4%, t1/2=5.9 months) and a slowly-relapsing group (31.9%, t1/2=23.6 months). OS was uniphasic (t1/2=74.3 months), suggesting an impact of co-morbidities; hence, tumor molecular characteristics would more likely predict TTR than OS. Of 172 published curves analyzed, 72 (42%) were uniphasic, 92 (53%) were biphasic, 8 (5%) were triphasic. With first-line chemotherapy in advanced NSCLC, 87.5% of curves from 2-3 drug regimens were uniphasic vs only 20% of those with best supportive care or 1 drug (p<0.001). 54% of curves from 2-3 drug regimens had convex rapid-decay phases vs 0% with fewer agents (p<0.001). Curve convexities suggest that discontinuing chemotherapy after 3-6 cycles “synchronizes” patient progression and death. With postoperative adjuvant chemotherapy, the PFS rapid-decay phase accounted for a smaller proportion of the population than in controls (p=0.02) with no significant difference in rapid-decay t1/2, suggesting adjuvant chemotherapy may move a subpopulation of patients with sensitive tumors from the relapsing group to the cured group, with minimal impact on time to relapse for a larger group of patients with resistant tumors. In untreated patients, the proportion of patients in the rapid-decay phase increased (p=0.04) while rapid-decay t1/2 decreased (p=0.0004) with increasing stage, suggesting that higher stage may be associated with tumor cells that both grow more rapidly and have a higher probability of surviving metastatic processes than in early stage tumors.
This preliminary assessment of EDNRA suggests that it may be worth exploring this approach further using more sophisticated, statistically rigorous nonlinear modelling approaches. Using such approaches to supplement standard survival analyses could suggest or support specific testable hypotheses.
Keywords: exponential decay, nonlinear regression analysis, non-small cell lung cancer Running Title: Nonlinear regression of NSCLC patient survival time
Introduction
Biological processes such as drug disappearance1 and enzymatic reactions2 may follow first order kinetics, with disappearance of a given proportion (rather than amount) of remaining drug, substance, etc, in a given time. For processes such as drug disappearance that follow first order kinetics, plotting linear drug concentration, etc vs time will give a curved line, while plotting log concentration vs time will give a straight line, and the slope of the line can be used to calculate the half-life1. In pharmacokinetic (PK) analyses, nonlinear regression analysis of the drug disappearance curve may reveal inflection points in the curve, and presence of an inflection point in the log-linear curve may indicate a distinct process driving the rate of drug disappearance. For example, the rate of drug disappearance during the initial portion of the curve (the “distribution phase”) is driven by drug uptake into tissues, the second portion (following the first inflection point) may be driven by metabolism/excretion, and the third portion (following a second inflection point) may be driven by saturation of metabolism/excretion processes, by redistribution of drug from tissues to blood, etc1. Additional inflection points and curve segments may also occur with some biological processes.
Here we explored the use of exponential decay nonlinear regression analysis (EDNRA) in assessing patient overall survival (OS), progression-free survival (PFS) or relapse-free survival (RFS) curves, and examined the impact of treatment methods and stage on curve characteristics and inflection points. This was done as a preliminary feasibility assessment of whether adding similar approaches to standard clinical trial survival analyses could be used to generate specific testable hypotheses that might not necessarily be suggested by assessment of standard Kaplan-Meier plots.
Methods
We used data both from a set of 323 NSCLC patients undergoing tumor resection with curative intent at our institution as well as using OS and PFS curves from arbitrarily selected NSCLC trials published from 1986 to 2006 involving front-line chemotherapy in advanced disease3-20, adjuvant chemotherapy in resected stage I-III disease6, 21-31 and survival as a function of stage4, 6, 14, 20-24, 26-38, and also used curves from best supportive care arms from 2 second line therapy trials39, 40. For this preliminary feasibility assessment, the only criteria used to select studies were that they dealt with NSCLC and that they had published OS or PFS curves related to therapy or stage that were sufficiently legible to permit us to perform the planned manual measurements. This was not intended as a comprehensive assessment of all or most published trials, nor was it intended to assess one particular type of chemotherapy. All published chemotherapy and staging studies that we assessed and for which we were able to perform EDNRA were included in this report. As noted above, this was a hypothesis-generating exercise, with the only pre-stated hypothesis being that modelling the curves in this way would generate half-lives and estimates of sizes of subpopulations accounting for curve inflection points. The results presented are intended to illustrate a few potential uses of this approach, rather than being offered as proof of any specific therapy question.
For our 323 patients, we did Kaplan-Meier plots of OS and time to relapse (TTR). For OS, surviving patients were censored at last follow-up. For TTR, relapse-free patients were censored at last follow-up. They were also censored at time of death or at development of a second primary if apparently relapse-free from their first NSCLC at that time. From the Kaplan-Meier analyses, % of patients alive or relapse-free was estimated for different time points, and these estimates were used for 1-phase, 2-phase and 3-phase EDNRA by the GraphPad Prism Version 5.0 program. The value of Y at time=0 was set as a constant at 100%. Analyses were repeated for a subset of 222 stage I and II patients who had not received any adjuvant chemotherapy.
For published OS and PFS curves, height of curve above baseline was measured manually in mm for different time points from therapy initiation, then converted to a percent of the curve height at time 0. EDNRA was then performed as above. For the purpose of these preliminary assessments, curves were arbitrarily considered to conform to a one-phase model rather than a two-phase model (or to a two-phase model rather than a three-phase model) if one of the phases accounted for <1% of the patient population, or if half-lives for two phases differed by <10%.
Across published studies, the median percent of patients in each decay phase and the median half-life of the rapid-decay phase were calculated for different groups. Groups were compared using Wilcoxon signed rank tests for matched groups (patients treated with adjuvant therapy vs control groups from the same study), and Kruskal-Wallis testing was used for comparisons of non-matched groups. We stress that for this preliminary feasibility assessment only adjuvant therapies were compared to randomized control groups. All other comparisons were done across multiple studies. Fisher's Exact Test was used to compare groups with respect to proportion of curves fit by 2-3 phase decay models vs proportion fit by only one phase decay models, and with respect to proportion of curves with major convexities.
Results
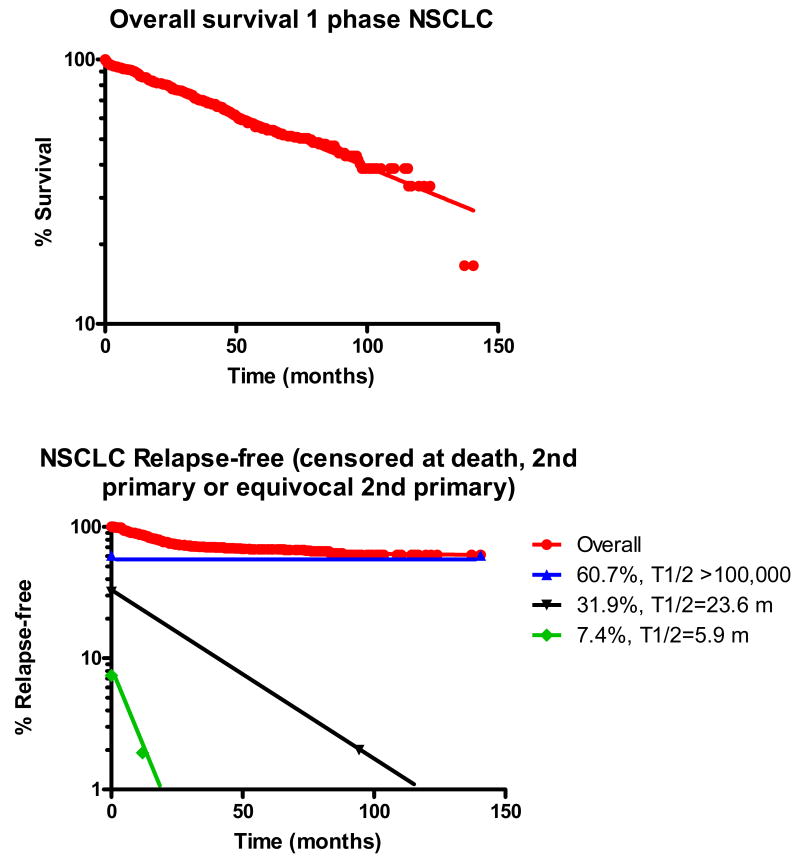
Included in our group of 323 patients with resected NSCLC were 202 stage I patients, 63 stage II patients, 48 stage III patients and 10 stage IV patients, of whom 107 had relapsed at last follow-up and 157 had died. In this group of resected NSCLC patients, the OS curve was uniphasic (half-life [t1/2] = 74.3 mo) (Figure 1a). The TTR curve was triphasic (Figure 1b), and suggested a “cured” fraction of 60.7% (t1/2 >100,000 months), a rapidly-relapsing group (7.4%, t1/2=5.9 months) and a slowly-relapsing group (31.9%, t1/2=23.6 months). When this analysis was repeated on a subset of 222 patients with stage I-II NSCLC patients who had not received any adjuvant chemotherapy, the OS curve remained uniphasic, with t1/2 = 89 months, while TTR was now biphasic, with a “cured” fraction (TTR t1/2 > 1,000 months) consisting of 70% of the patients and a relapsing fraction consisting of 30% of the patients, with a TTR t1/2 of 20 months.
Figure 1.
Semilog plots of overall survival and time-to-relapse (TTR) for a population of 323 patients with resected NSCLC, with exponential decay nonlinear regression analysis. For TTR, patients who had developed no evidence of relapse were censored at time of last follow-up, at death from other causes or at development of a non-localized second primary. Overall survival was uniphasic, with a half-life of 74.3 months, while relapse-free survival was triphasic, with a rapidly-relapsing population (7.4% of the original population, half-line=5.9 months, a slowly-relapsing population (31.9% of the original population, half-life=23.6 months), and an apparently cured population (60.7% of the original population).
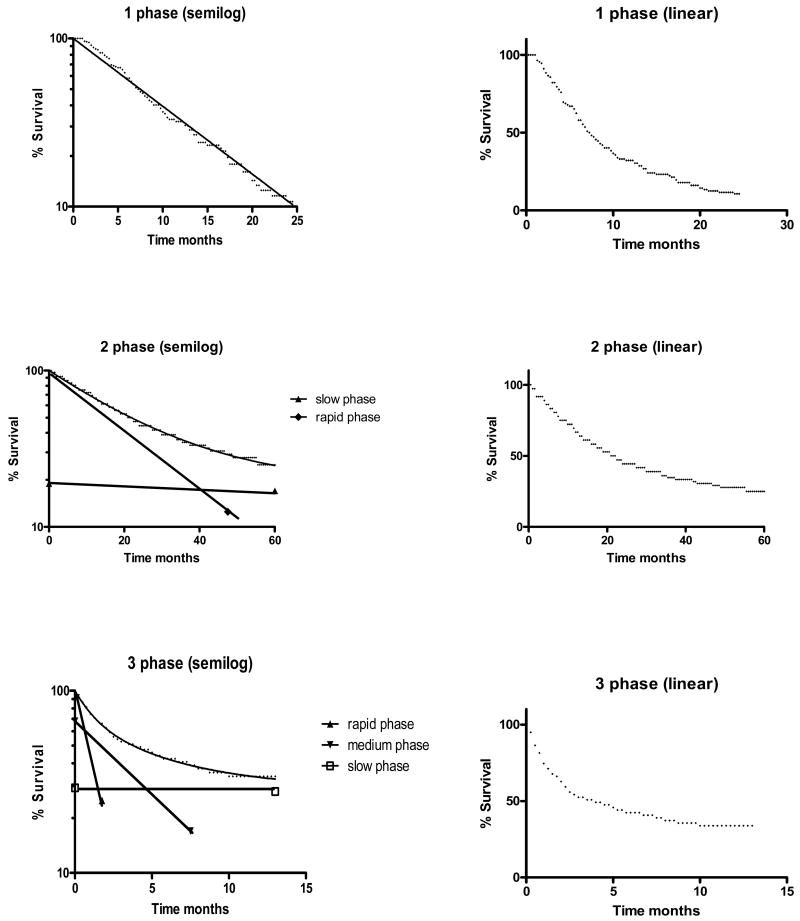
Of 172 published OS or PFS curves analyzed, 72 (42%) were fit by one-phase exponential decay models, 92 (53%) were fit by two-phase exponential decay models (single inflection point) and 8 (5%) were fit by three-phase exponential decay models (two inflection points). Examples of each are presented in Figure 2.
Figure 2.
Typical one, two and three phase decay survival curves showing both semilog plots and the corresponding linear plots
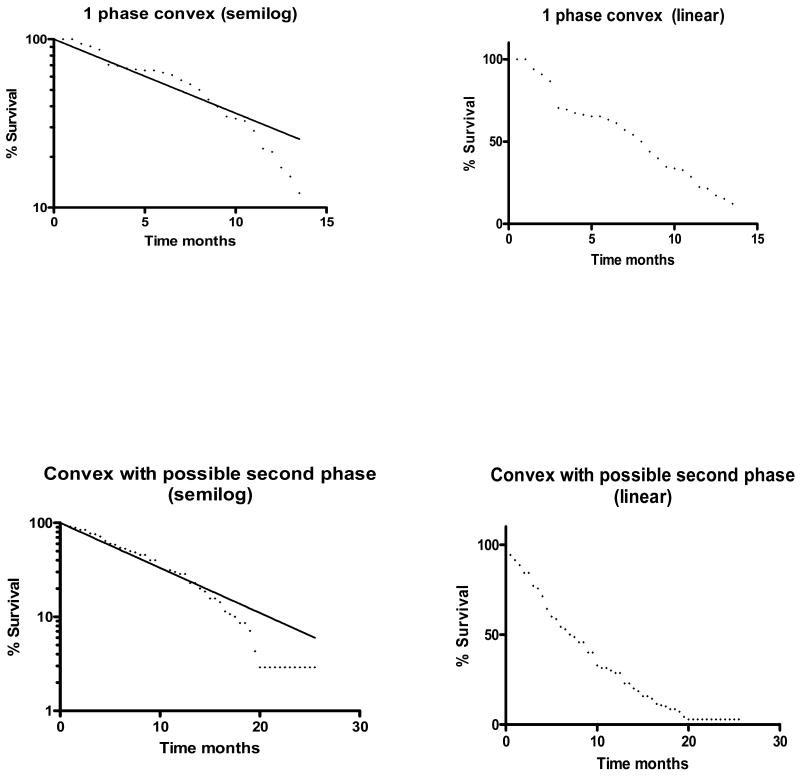
Overall, 41 of the curves (24%) from published studies were convex over much of the rapid-decay phase (Figure 3). Curve characteristics varied with therapy (Table 1). In patients on front-line chemotherapy for advanced disease, 12 of 15 (80%) OS or PFS curves from patients receiving best supportive care or single agent chemotherapy could be fit by 2-3 phase decay models, compared to only 6 of 48 (12.5%) curves from patients treated with regimens involving 2 or more agents (p<0.001). The proportion of curves fit by only a single phase decay model increased with the number of agents used in therapy. In addition, 54% of the 48 curves from patients treated with regimens involving ≥ 2 agents appeared to have convex rapid-decay phases, compared to none of 15 curves for patients treated with best supportive care or single agent therapy (p<0.001).
Figure 3.
Examples of semilog plots with convex rapid phases (with and without a possible second phase), and corresponding linear plots
Table 1.
Effect of therapy details on number of curve phases and on presence of rapid phase convexity in chemotherapy of advanced NSCLC
| No. drugs | No. curves | No. (%) with 2-3 phase decay* | No. (%) with convexity* |
|---|---|---|---|
| 0 | 8 | 7 (88) | 0 (0) |
| 1 | 7 | 5 (71) | 0 (0) |
| 2 | 39 | 6 (15) | 19 (49) |
| ≥ 3 | 9 | 0 (0) | 7 (78) |
p <0.001 for 0-1 drugs vs ≥ 2 drugs (Fisher's Exact Test)
Proportion of patients in the rapid-decay phase and rapid-decay phase half-lives as a function of number of chemotherapy agents used for advanced NSCLC is presented in Table 2. For 2- and 3-phase decay curves in these and other analyses, the slow-decay phase half-lives were generally very long with very wide 95% confidence intervals, and hence are not presented in this preliminary analysis. For curves that could be fit by either a 2- or 3-phase-decay model, data from the 2-phase-decay models were used for comparisons. In advanced NSCLC, as the number of chemotherapy agents in the treatment regimen increased, the proportion of the entire patient population that was in the rapid-decay phase increased (since there was an increase in curves fit only by one phase), but the rapid-decay half-life also increased.
Table 2.
For overall survival in advanced NSCLC, effect of number of chemotherapy agents in frontline treatment regimen on % of patients in rapid-decay phase and on rapid-decay phase half-life
| Therapy | No. studies | % rapid-decay phase | P* | Rapid phase t1/2** | P* | ||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | ||||
| Best supportive care | 6 | 96.7 | 82.6-100 | 0.006 | 4.4 | 3.8-4.7 | 0.0008 |
| Single Agent | 5 | 94.8 | 66.4-100 | 4.9 | 1.5-7.1 | ||
| Two Drugs | 28 | 100 | 12.9-100 | 7.5 | 2.5-11.5 | ||
| ≥ Three drugs | 7 | 100 | 100-100 | 6.8 | 4.6-12.2 | ||
Kruskal-Wallis comparison of groups
months
With adjuvant chemotherapy of resected NSCLC, for PFS the rapid-decay phase accounted for a smaller proportion of the entire population than in matched untreated randomized control groups and there was also a trend towards a reduction in the rapid-decay phase in OS, suggesting that adjuvant therapy was shifting some patients from the relapsing (rapid-decay) to the potentially cured (slow-decay) groups. There was no significant prolongation of rapid-decay phase half-life with adjuvant therapy (Table 3). If single phase models were compared, adjuvant chemotherapy was associated with a significant increase in median half-lives for both PFS (p=0.03) and OS (p=0.0002).
Table 3.
For overall and progression-free survival in resected NSCLC, effect of adjuvant chemotherapy on % of patients in rapid-decay phase and on rapid-decay phase half-life
| Therapy | No. studies | % rapid-decay | P* | Rapid-decay t1/2 | P* | ||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | ||||
| Overall survival | |||||||
| Adjuvant chemo | 24 | 89.3 | 1.3-100 | 0.12 | 63.7 | 4.4-245.0 | 0.47 |
| Controls | 24 | 94.4 | 43.9-100 | 45.1 | 7.0-339.0 | ||
| Progression-free survival | |||||||
| Adjuvant chemo | 13 | 68.2 | 29.9-100 | 0.02 | 12.6 | 5.0-58.2 | 0.54 |
| Controls | 13 | 85.0 | 44.0-100 | 10.9 | 5.1-98.6 | ||
Wilcoxon signed rank test, comparison to matched control group
With respect to stage, 6 of 11 (55%) OS curves from stage I untreated patients were best fit by one-phase decay models (with very long half-lives), compared to 4 of 24 (17%) OS curves from stage II-IV untreated patients (Table 4). For stages II-IV, the proportion of patients in the rapid-decay phase increased and the half-life of the rapid-decay phase decreased with increasing stage (Table 4).
Table 4.
For overall survival in NSCLC, effect of stage on % of patients in the rapid-decay phase and on rapid-decay phase half-life
| Stage | No. studies | % rapid phase | P* | Rapid phase t1/2 (months) | P* | ||
|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | ||||
| I | 11 | 100**** | 40.6-100 | 0.23** 0.04*** |
105.6 | 15.3-339 | 0.0001** 0.0004*** |
| II | 8 | 79.1 | 49.4-100 | 19.1 | 12.7-58.5 | ||
| III | 10 | 92.5 | 62.8-100 | 11.5 | 6.6-33.6 | ||
| IV | 6 | 96.9 | 82.9-100 | 4.3 | 3.1-5.9 | ||
Kruskal-Wallis comparison of stages
comparing stages I, II, III and IV
comparing stages II, III, IV (excluding stage I)
since most studies had uniphasic curves for stage I, this could be regarded as a “slow-decay phase” (with a median of 0% of patients in the rapid-decay phase) or alternatively as a “rapid-decay phase” (with a median of 0% patients in the slow-decay phase)
Discussion
In this preliminary assessment, we took a simplistic approach to analyzing survival data and published survival curves as a first look at the feasibility and potential utility of such an exercise. Given the limitations of our methods, this assessment did suggest that similar analyses could be useful in suggesting specific testable hypotheses when added to more standard survival analyses. Our assessments here suggest that EDNRA might potentially facilitate utilization of previously published survival curves (and new data) to generate or support testable hypotheses. The analysis of our set of 323 patients with resected NSCLC suggests the possibility that there is “true cure” of approximately 61% of this particular population, and that there may be 2 distinct relapsing populations: a rapidly-relapsing group (7.4% of the original population) and a more slowly-relapsing group (32% of the original population). Assessment of group half-lives suggests that by 2 years after resection, 87% of the rapidly-relapsing group would have relapsed (4 half-lives) as opposed to only 50% of the slowly-relapsing group (one half-life). Relative population sizes and relapse-free half-lives of the different groups suggest that eventual relapses will occur in approximately 12%, 6%, 3% and 1.5% of those alive and relapse-free at 4, 6, 8 & 10 years, respectively.
We plan to do molecular assessments in the resected tumors to explore the hypothesis that the rapidly-relapsing group differs from the slowly-relapsing group with respect to factors driving tumor cell proliferation rates. We will also explore the hypothesis that, after correcting for primary tumor size and other relevant clinical variables, probability of relapse for both groups will vary with expression of factors that may facilitate the metastatic process (eg, anti-apoptotic factors), and that will lead to increased probability of survival of one metastatic cell. If so, tumor molecular factors that correlated with number of metastatic deposits in patients who present initially with advanced disease could also correlate with probability of eventually developing recurrence in patients who present initially with localized disease.
The fact that OS was uniphasic in our patient population suggests that multiple other factors (and not just recurrence of the original NSCLC) contributed to mortality. If co-morbidities are playing a major role in patient survival, then trying to correlate tumor molecular characteristics with OS may not be very informative, since the impact of co-morbidities would dilute out the impact of tumor molecular characteristics. Hence, it may be preferable to assess impact of tumor molecular characteristics on TTR rather than assessing OS.
The observation of convexities on the rapid decay phase of OS and PFS curves could be due to artefact, but it also raises a question: Is the routine practice in advanced NSCLC of discontinuing chemotherapy after 4-6 cycles partially synchronizing patient deaths? Synchronization of patient deaths might also happen as a result of stopping therapy at time of progression in patients in whom the therapy was slowing tumor growth despite not being able to completely stop it. The increasing trend to curve convexities and to uniphasic curves with increasing number of agents used would be in keeping with a greater biological impact on tumor of more intense therapy, with greater synchronization of patient death. Randomized trials have generally failed to identify a benefit of continuing chemotherapy beyond 3-6 cycles in advanced NSCLC41-44, but some older studies45 as well as some more recent trials46 have suggested a possible benefit of maintenance therapy in selected patients. When newer maintenance studies it will be of interest to see whether or not maintenance to the time of progression is associated with reduced curve convexity. If so, then in patients not receiving maintenance therapy, assessing clinical and tumor molecular characteristics of patients relapsing or dying along the leading edge of the convexity might possibly identify a distinct subpopulation most likely to benefit from maintenance therapy.
In at least some stages of surgically-resected NSCLC, randomized trials21, 31 and meta-analyses47 indicate a benefit of adjuvant chemotherapy. In our EDNRA assessment of selected randomized trials, we found that the adjuvant arms had a smaller rapid-decay phase than did the control arm (significant for PFS and with a trend for OS), but had only a slight increase in PFS and OS rapid-phase half-life in the treated arms. This would suggest that the major benefit from adjuvant chemotherapy is that it shifts patients from the relapsing population to the potentially cured population, with only a minor part of its effect being due to prolongation of survival or time to relapse of those who are destined to relapse despite the adjuvant therapy. This in turn would suggest the possibility that the therapy is being highly effective in a small, sensitive subset of patients but is having only a very modest effect in a larger resistant population, rather than it having a “graded” effect across the entire population. In so, then seeing how adjuvant chemotherapy impacted on overall probability of relapse in different clinical and molecular subgroups might be a more effective way of assessing who will benefit from adjuvant chemotherapy than would analysis of impact of adjuvant therapy on time to relapse in different subgroups.
In order to have sufficient studies to permit this preliminary analysis, some of the staging studies we assessed were based on preoperative stage and some were based on postoperative stage, and it is stressed that this could potentially have impacted comparisons. Published survival curves for Stage I patients were more likely to be uniphasic than were survival curves for patients with other stages. This was probably due at least in part to follow-up times being too short to properly define a second survival phase, and the long half-life for this group is probably due in part to its being a mixture of rapid-decay and slow-decay groups. For stages II-IV, increasing stage was characterized by a combination of a higher proportion of patients in the rapid-decay group and a shorter half-life for the rapid-decay group. Hence, not only were patients with higher stages more likely to relapse, but those who relapsed did so on average more rapidly than did patients with lower stages. This would lead us to hypothesize that tumors of patients who present with higher stage disease would be more likely to have molecular characteristics that would promote rapid tumor cell growth in addition to having characteristics that promote metastases. Hence, one might hypothesize that patients with recurrent stage I NSCLC would on average have more indolent disease than would patients with equal bulk disease that was stage IV at presentation, and that the two would tend to have different molecular characteristics and possibly different treatment susceptibilities. In addition, this would suggest that molecular characteristics drive both prognosis and stage- ie, a patient with relatively indolent disease might tend to have it discovered when it was still in an early stage since it would stay at an early stage longer, while patients with more rapidly growing disease would be less likely to have the disease discovered by chance while it was still early stage. This is in keeping with the concept that stage at presentation is a surrogate for tumor cell growth rate in addition to being a surrogate for presence of viable micrometastatic disease.
Hence, use of EDNRA or related methods to supplement standard survival analyses could potentially prove helpful by suggesting specific testable biological hypotheses. Prior to initiating this assessment, we were uncertain whether the approach would prove feasible or whether it would yield useful information. Furthermore, we did not start out with pre-specified statistical rules, and the studies we assessed were very heterogeneous with respect to patient populations, therapies, duration of follow-up, patient numbers, etc, and studies were not weighted based on patient numbers or other factors. Now that we have found that potentially interesting observations can be generated by such an approach, we would suggest exploring it further using appropriate adaptations of more sophisticated and statistically rigorous and informative nonlinear modelling methods that have been described elsewhere48-50. While a variety of more commonly used approaches are quite adequate for routine analyses, nonlinear approaches may help provide insight into the existence of specific subpopulations and into variability between groups in different parts of the survival curve.
Acknowledgments
This work was conducted under the auspices of the Lung Cancer Program of MD Anderson Cancer Center, and was supported in part by Cancer Center Support Grant number 5-P30 CA16672-32 and by Department of Defense grant number W81XWH-07-1-0306
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benet L, Mitchell J, Sheiner L. Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, and Elimination. In: Goodman Gilman A, Rall T, Nies A, Taylor P, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics Eighth. New York, NY: Pergamon Press; 1990. pp. 3–32. [Google Scholar]
- 2.White A, Handler P, Smith E. Enzymes II Kinetics, Inhibition, Metabolic Inhibitors, Control of Enzymatic Activity. In: White A, Handler P, Smith E, editors. Principles of Biochemistry. New York, NY: McGraw-Hill; 1968. pp. 223–46. [Google Scholar]
- 3.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–31. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 4.Buccheri G, Ferrigno D, Rosso A, Vola F. Further evidence in favour of chemotherapy for inoperable non-small cell lung cancer. Lung Cancer. 1990;6:87–98. [Google Scholar]
- 5.Lilenbaum RC, Herndon JE, 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730) J Clin Oncol. 2005;23:190–6. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 6.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. Bmj. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 7.Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21:3016–24. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 8.Fukuoka M, Masuda N, Furuse K, et al. A randomized trial in inoperable non-small-cell lung cancer: vindesine and cisplatin versus mitomycin, vindesine, and cisplatin versus etoposide and cisplatin alternating with vindesine and mitomycin. J Clin Oncol. 1991;9:606–13. doi: 10.1200/JCO.1991.9.4.606. [DOI] [PubMed] [Google Scholar]
- 9.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–8. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 10.Klastersky J, Sculier JP, Ravez P, et al. A randomized study comparing a high and a standard dose of cisplatin in combination with etoposide in the treatment of advanced non-small-cell lung carcinoma. J Clin Oncol. 1986;4:1780–6. doi: 10.1200/JCO.1986.4.12.1780. [DOI] [PubMed] [Google Scholar]
- 11.Klastersky J, Sculier JP, Bureau G, et al. Cisplatin versus cisplatin plus etoposide in the treatment of advanced non-small-cell lung cancer. Lung Cancer Working Party, Belgium. J Clin Oncol. 1989;7:1087–92. doi: 10.1200/JCO.1989.7.8.1087. [DOI] [PubMed] [Google Scholar]
- 12.Le Chevalier T, Brisgand D, Douillard JY, et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: results of a European multicenter trial including 612 patients. J Clin Oncol. 1994;12:360–7. doi: 10.1200/JCO.1994.12.2.360. [DOI] [PubMed] [Google Scholar]
- 13.Mylonakis N, Tsavaris N, Bacoyiannis C, et al. A randomized prospective study of cisplatin and vinblastine versus cisplatin, vinblastine and mitomycin in advanced non-small cell lung cancer. Ann Oncol. 1992;3:127–30. doi: 10.1093/oxfordjournals.annonc.a058127. [DOI] [PubMed] [Google Scholar]
- 14.Rapp E, Pater JL, Willan A, et al. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer--report of a Canadian multicenter randomized trial. J Clin Oncol. 1988;6:633–41. doi: 10.1200/JCO.1988.6.4.633. [DOI] [PubMed] [Google Scholar]
- 15.Rosso R, Ardizzoni A, Salvati F, et al. Etoposide v etoposide and cisplatin in the treatment of advanced non-small cell lung cancer: a FONICAP randomized study. Semin Oncol. 1988;15:49–51. [PubMed] [Google Scholar]
- 16.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 17.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 18.Shinkai T, Saijo N, Eguchi K, et al. Cisplatin and vindesine combination chemotherapy for non-small cell lung cancer: a randomized trial comparing two dosages of cisplatin. Jpn J Cancer Res. 1986;77:782–9. [PubMed] [Google Scholar]
- 19.Veeder MH, Jett JR, Su JQ, et al. A phase III trial of mitomycin C alone versus mitomycin C, vinblastine, and cisplatin for metastatic squamous cell lung carcinoma. Cancer. 1992;70:2281–7. doi: 10.1002/1097-0142(19921101)70:9<2281::aid-cncr2820700912>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Woods RL, Williams CJ, Levi J, et al. A randomised trial of cisplatin and vindesine versus supportive care only in advanced non-small cell lung cancer. Br J Cancer. 1990;61:608–11. doi: 10.1038/bjc.1990.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 22.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 23.Imaizumi M. Postoperative adjuvant cisplatin, vindesine, plus uracil-tegafur chemotherapy increased survival of patients with completely resected p-stage I non-small cell lung cancer. Lung Cancer. 2005;49:85–94. doi: 10.1016/j.lungcan.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–21. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 25.The benefit of adjuvant treatment for resected locally advanced non-small-cell lung cancer. The Lung Cancer Study Group. J Clin Oncol. 1988;6:9–17. doi: 10.1200/JCO.1988.6.1.9. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa M, Tanaka F, Tsubota N, Ohta M, Takao M, Wada H. A randomized phase III trial of adjuvant chemotherapy with UFT for completely resected pathological stage I non-small-cell lung cancer: the West Japan Study Group for Lung Cancer Surgery (WJSG)--the 4th study. Ann Oncol. 2005;16:75–80. doi: 10.1093/annonc/mdi008. [DOI] [PubMed] [Google Scholar]
- 27.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 28.Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–8. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 29.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86:673–80. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 30.Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst. 2003;95:1453–61. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 31.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 32.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94:1099–106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonomi P, Faber L. Neoadjuvant chemoradiation therapy in non-small cell lung cancer: the Rush University experience. Lung Cancer. 1993;9:383–90. [Google Scholar]
- 34.Gralla RJ. Preoperative and adjuvant chemotherapy in non-small cell lung cancer. Semin Oncol. 1988;15:8–12. [PubMed] [Google Scholar]
- 35.Lin E, Karp D. Color-matrix cancer staging and chemotherapy handbook. 2. Houston, TX: The University of Texas M.D. Anderson Cancer Center; 2003. [Google Scholar]
- 36.Mountain CF. A new international staging system for lung cancer. Chest. 1986;89:225S–33S. doi: 10.1378/chest.89.4_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 37.Wisnivesky JP, Yankelevitz D, Henschke CI. The effect of tumor size on curability of stage I non-small cell lung cancers. Chest. 2004;126:761–5. doi: 10.1378/chest.126.3.761. [DOI] [PubMed] [Google Scholar]
- 38.Wisnivesky JP, Henschke C, McGinn T, Iannuzzi MC. Prognosis of Stage II non-small cell lung cancer according to tumor and nodal status at diagnosis. Lung Cancer. 2005;49:181–6. doi: 10.1016/j.lungcan.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 40.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 41.von Plessen C, Bergman B, Andresen O, et al. Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer. 2006;95:966–73. doi: 10.1038/sj.bjc.6603383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westeel V, Quoix E, Moro-Sibilot D, et al. Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:499–506. doi: 10.1093/jnci/dji096. [DOI] [PubMed] [Google Scholar]
- 43.Smith IE, O'Brien ME, Talbot DC, et al. Duration of chemotherapy in advanced non-small-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol. 2001;19:1336–43. doi: 10.1200/JCO.2001.19.5.1336. [DOI] [PubMed] [Google Scholar]
- 44.Buccheri GF, Ferrigno D, Curcio A, Vola F, Rosso A. Continuation of chemotherapy versus supportive care alone in patients with inoperable non-small cell lung cancer and stable disease after two or three cycles of MACC. Results of a randomized prospective study Cancer. 1989;63:428–32. doi: 10.1002/1097-0142(19890201)63:3<428::aid-cncr2820630305>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 45.Rinaldi M, Belvedere O, Cauchi C, Defferrari C, Viola G, Grossi F. Maintenance chemotherapy in non-small cell lung cancer. Ann Oncol. 2006;17 2:ii67–70. doi: 10.1093/annonc/mdj928. [DOI] [PubMed] [Google Scholar]
- 46.Belani CP, Brodowicz T, Ciuleanu T, et al. Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: A randomized phase III study in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2009;27:A#CRA8000. [Google Scholar]
- 47.Berghmans T, Paesmans M, Meert AP, et al. Survival improvement in resectable non-small cell lung cancer with (neo)adjuvant chemotherapy: results of a meta-analysis of the literature. Lung Cancer. 2005;49:13–23. doi: 10.1016/j.lungcan.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Davidian M, Giltinan DM. Nonlinear models for repeated measurement data. New York: Chapman & Hall; 1995. [Google Scholar]
- 49.Pinheiro JC, Bates DM. Approximations to the Log-likelihood Function in the Nonlinear Mixed-effects Model. Journal of Computational and Graphical Statistics. 1995;4:12–25. [Google Scholar]
- 50.Vonesh EF, Chinchilli VM. Linear and Nonlinear Models for the Analysis of Repeated Measurements. New York: Marcel Dekker; 1996. [Google Scholar]