Abstract
Studies on metastasic lesions from human carcinomas are scarce. Therefore there is a need for such studies to identify the expression of the biological factors that will help in the assessment of the natural history of breast cancer. Here an immunohistochemical study was performed using tissue arrays and specific antibodies against matrix metalloproteinases (MMPs)-1, 2, 7, 9, 11, 13, 14 and tissue inhibitors of metalloproteases (TIMPs)-1, 2 and 3 in 39 patients with breast cancer. Specimens from 39 patients with node-positive carcinomas were examined and the analysis was performed at the central core of the tumour, at the invasive front, and in the metastasic axillary lymph nodes (MALNs). Global expression of MMP-1, 7 and 14, TIMP-1, and 3, were significantly higher at the centre of the tumour compared with the invasive front or the MALNs. Significantly higher expression of MMP-7 and 14, and TIMP-3, by fibroblast-like cells and mononuclear inflammatory cells (MICs) was seen in MALNs. In addition, in the tumour centre, the expression of MMP-11 and TIMP-1 and 2 by MICs, as well as TIMP-2 expression by fibroblast-like cells, were associated significantly with the occurrence of distant metastasis. In contrast, TIMP-3 expression by tumour cells or by fibroblast-like cells in this same tumour locations, as well as TIMP-1 expression by fibroblast-like cells at the invasive front, were associated significantly with poor prognosis. However, the expression of all of these biological factors in MALNs was not associated with the development of distant metastasis. Our data suggest that there is prognostic relevance to the expression of MMPs and TIMPs in the stromal cells of primary tumours, rather than to the expression of these enzymes in MALNs.
Keywords: axillary lymph node, invasive front, leucocytes, matrix metalloproteinase, tissue inhibitors of metalloproteases, tumour heterogeneity
Few studies in the literature have focused on the metastasic lesions associated with human carcinomas. With regard to breast cancer, studies on paired primary tumours and metastasic axillary lymph nodes (MALNs) from the same patients have demonstrated that both lesions show similar phenotype in histopathology, proliferation activity (Feichter et al. 1989; Daidone et al. 1990; Tommasi et al. 1992; Chang et al. 1993; Goodson et al. 1993) oestrogen and progesterone receptors (Andersen & Poulsen 1988; van Agthoven et al. 1995; Umekita et al. 1998), and oncogene expression (Moffett et al. 1992; Tommasi et al. 1992). However, acquired abnormalities in MALNs of breast carcinomas have been described, such as increased levels of epidermal growth factor receptor (Mori et al. 1991) or heat shock protein 27 over-expression (Storm et al. 1996), and these genetic alterations are present with higher frequency in the metastatic areas than in primary breast carcinomas (Nishizaki et al. 1997). This propensity to high number of genetic changes in MALNs suggests the acquisition, along with tumour progression, of a more aggressive phenotype. Therefore, in order to assess the natural history of breast cancer, it is necessary to investigate the expression of many other biological factors within the metastasic lesions from breast cancer patients. In particular, molecules implicated in invasion and metastasis are among the candidate biological factors.
The human MMP family currently consists of 28 members of homologous zinc-dependent endopeptidases. These can either be divided into eight structural classes or grouped depending on their substrate specificity and primary structure. MMPs can also be clustered into subgroups of collagenases (MMP-1, -8 and -13), gelatinases (MMP-2 and -9), stromelysins (MMP-3, -10, -11), membrane -associated MMPs (MMP-14, -15, -16, -17, -23, -24, -25) and other novel MMPs (Overall & Lopez-Otin 2002; Demers et al. 2004).
MMPs are synthesized as inactive zymogens, which are then activated by other MMPs or by serine proteases in a pericellular manner. MMPs’ activity is specifically inhibited by tissue inhibitors of metalloproteases (TIMPs) or by nonspecific protease inhibitors (e.g. α2-macroglogulin). TIMPs 1, 2, 3 and 4 are the four different TIMPs currently known to exist. The balance between MMPs and their inhibitors is critically altered in those physiologic conditions where rapid remodelling of extracellular matrix happens, such as in cancer. Interestingly, MMPs are expressed by different tissues at various stages of their development, while they are conspicuously absent in normal cells of adult organisms (Stamenkovic 2000). Growth factors and cytokines secreted by either tumour or stromal cells (Meller et al. 2000), regulate, in a paracrine manner, the expression of MMPs in neoplastic tissues.
Old theories state that MMPs promote metastases exclusively by modulating the remodelling of extracellular matrix. But this dogma can now be easily challenged by robust available data. Indeed, researchers have been able to identify MMPs’ ability to impact tumour cell behaviour in vivo through their ability to cleave growth factors, cell surface receptors, cell adhesion molecules, and chemokins/cytokins (Rifkin et al. 1999; Sternlicht & Werb 2001; Egeblad & Werb 2002). Furthermore, MMPs may produce a more aggressive tumour phenotype, via the generation of apoptotic resistant cells due to cleaving of proapoptotic factors (Fingleton et al. 2001). MMPs may also regulate angiogenesis in cancer in two opposite directions: positively, through their ability to mobilize proangiogenic factors (Stetler-Stevenson 1999); negatively, generating angiogenesis inhibitors, such as angiostatin and endostatin, which are then cleaved from large protein precursors (Rifkin et al. 1999). Consequently, several MMPs, in particular the gelatinases MMP-2 (Cornelius et al. 1998; Jones et al. 1999; Stetler-Stevenson 1999; Duffy et al. 2000; Ferreras et al. 2000; Fingleton et al. 2001; Talvensaari-Mattila et al. 2003) and MMP-9 (Jones et al. 1999; Duffy et al. 2000; Talvensaari-Mattila & Turpeenniemi-Hujanen 2005), have been studied recently as prognostic factors in breast cancer, and associated with a poor outcome in various subsets of breast cancer patients. Likewise, it has been reported that several other MMPs, such as MMP-7 (Vizoso et al. 2007), MMP-11 (Duffy et al. 2000; Vizoso et al. 2007), MT1-MMP (MMP-14) (Talvensaari-Mattila et al. 2003), and MMP-13 (Nielsen et al. 2001), may be over-expressed and/or related to the clinical outcome of breast cancers. On the other hand, it is now assumed that TIMPs are multifactorial proteins also involved in the induction of proliferation as well as in the inhibition of apoptosis (Chantrain et al. 2004; Wurtz et al. 2005). Thus, it was also reported that TIMPs, such as TIMP-1 (Baker et al. 1999; Jiang et al. 2002) or TIMP-2 (Ree et al. 1997; Talvensaari-Mattila et al. 2003), may be over-expressed and/or related to clinical outcome in breast cancer.
The aim of the present study was to investigate differences in the expression of MMPs and TIMPs between primary tumours and MALNs in breast cancer, as well as their possible impact on the development of distant metastasis.
Materials and methods
Patient selection, characteristics and tissue specimen handling
The study included 39 women with a histologically confirmed diagnosis of early breast cancer and treated between 1990 and 2003. We selected women with the following inclusion criteria: invasive ductal carcinoma, lymph node involvement, at least six histopathologically assessed axillary lymph nodes, and a minimum of five years of follow-up for those women without tumour recurrence. The exclusion criteria were the following: metastatic disease at presentation, prior history of any kind of malignant tumour, bilateral breast cancer at presentation, having received any type of neoadjuvant therapy, development of loco-regional recurrence during the follow-up period, development of a second primary cancer, and absence of sufficient tissue in the paraffin blocks used for manufacturing the TAs. From a total of 692 patients fulfilling these criteria, we selected a random sample size of 39 patients into two groups with similar size tumours and stratified them according to the tumour recurrence. Table 1 shows the patients/pathological characteristics of the population.
Table 1.
Basal characteristics of 39 patients with invasive ductal carcinoma of the breast
| Patient groups |
|||
|---|---|---|---|
| No tumour recurrence |
Tumour recurrence |
||
| Characteristics | N (%) | N (%) | P |
| Total cases | 25 | 14 | |
| Age (years) | |||
| <62 | 17 (68) | 7 (50) | n.s. |
| >62 | 8 (32) | 7 (50) | |
| Menopausal status | |||
| Premenopausal | 5 (20) | 2 (14.3) | n.s. |
| Postmenopausal | 20 (80) | 12 (85.7) | |
| Tumoral size | |||
| T1 | 10 (40) | 4 (28.6) | n.s. |
| T2 | 15 (60) | 10 (71.4) | |
| Tumoral status | |||
| Ii | 19 (76) | 3 (21.4) | 0.001 |
| Iii | 6 (24) | 11 (78.6) | |
| Histological grade | |||
| Well differentiated | 8 (33.3) | 9 (7.1) | n.s. |
| Mod differentiated | 11 (45.8) | 22 (78.6) | |
| Poorly differentiated | 5 (20.8) | 7 (14.3) | |
| Nottingham pronostic index | |||
| <3.4 | 8 (32) | 3 (21.4) | n.s. |
| 3.4–5.4 | 11 (44) | 10 (71.4) | |
| >5.4 | 6 (24) | 1 (7.1) | |
| Estrogen receptors | |||
| Negative | 6 (28.6) | 7 (63.6) | n.s. |
| Positive | 15 (71.4) | 4 (36.4) | |
| Progesterone receptors | |||
| Negative | 7 (33.3) | 5 (45.5) | n.s. |
| Positive | 14 (66.7) | 6 (54.5) | |
| Adjuvant radiotherapy | |||
| No | 18 (72) | 6 (42.9) | n.s. |
| Yes | 7 (28) | 8 (57.1) | |
| Adjuvant systemic therapy | |||
| Chemotherapy | 7 (28) | 2 (14.3) | n.s. |
| Adjuvant chemotherapy tamoxifen* | 6 (24) | 7 (50) | |
| Tamoxifen | 11 (44) | 2 (14.3) | |
| No treatment | 1 (4) | 3 (21.4) | |
Chemotherapy and sequential tamoxifen
Ethical approval
Women were treated according to the guidelines used in our institution. Patients signed consent for the use of the tissue samples for this assay. The study adhered to national regulations and was approved by our institution Ethics and Investigation Committee. The end-point was distant metastatic relapse. The median follow-up period in patients without metastases was 85 months, and 46 months in patients with metastases.
Tissue arrays and immunohistochemistry
Breast carcinoma tissue samples were obtained at the time of surgery. Routinely fixed (overnight in 10% buffered formalin), paraffin-embedded tumour samples stored in our pathology laboratories were used. Histopathologically representative tumour areas were defined on haematoxylin and eosin-stained sections and marked on the slide. Tumour tissue array (TA) blocks were obtained by punching a tissue cylinder (core) with a diameter of 1.5 mm through a histologically representative area of each ‘donor’ tumour block, which was then inserted into an empty ‘recipient’ tissue array paraffin block using a manual tissue arrayer (Beecker Instruments, Sun Praerie, WI, USA) as described elsewhere (Del Casar et al. 2009). Collection of tissue cores was carried out under highly controlled conditions. A total of four cores of the primary tumours were used for each case. Two of these cores in each case corresponded to the tumour central area, other two cores corresponded to the invasive front. This method, evaluating two cores (double redundancy) of each tumour area has been shown to correlate well with conventional immunohistochemical staining (Del Casar et al. 2009). In addition, we performed one core per each MALN, at least per three lymph nodes in patients with more than three MALNs. The invasive front was defined as the tumoral advancing edge. This corresponds to a 2 mm margin surrounding the tumour and containing cancerous cells.
Serial 5 μm sections of the high-density TA blocks were consecutively cut with a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and transferred to adhesive-coated slides. One section from each TA block was stained with H&E, and these slides were then reviewed to confirm that the sample was representative of the original tumour. Immunohistochemistry was done on these sections of TA fixed in 10% buffered formalin and embedded in paraffin using a TechMate TM50 autostainer (Dako, Glostrup, Denmark). Antibodies for MMPs and TIMPs were obtained from Neomarker (Lab Vision Corporation, Fremont, CA, USA). The dilution for each antibody was established based on negative and positive controls (1/50 for MMP-2, -7, -14 and TIMP-2; 1/100 for MMP-9, -13, TIMP-1 and -3; and 1/200 for MMP-1, -11). The negative control was DakoCytomation mouse serum diluted to the same mouse IgG concentration as the primary antibody. All the dilutions were made in Antibody Diluent, (Dako) and incubated for 30 min at room temperature. As positive controls were used cases in which we confirmed the presence of the evaluated proteins by Western blot analysis of breast tumour cytosol samples as in previous reports (Gonzalez et al. 2007; Del Casar et al. 2009). A single band of the expected molecular mass was observed for each protein (Gonzalez et al. 2007).
Tissue sections were deparaffinized in xylene and then rehydrated in graded concentrations of ethyl alcohol (100%, 96%, 80%, 70%, then water). To enhance antigen retrieval, only for some antibodies, TA sections were microwave treated in a H2800 Microwave Processor (EBSciences, East Granby, CT, USA) in citrate buffer (Target Retrieval Solution; Dako) at 99 °C for 16 min. Endogenous peroxidase activity was blocked by incubating the slides in peroxidase-blocking solution (Dako) for 5 min. The EnVision Detection Kit (Dako) was used as the reactivity detection system. Sections were counterstained with haematoxylin, dehydrated with ethanol and permanently coverslipped.
For each antibody preparation studied, the location of immunoreactivity and intensity were determined. To evaluate immunostaining intensity we used a numeric score ranging from 0 to 3, reflecting the intensity as follows: 0, no reactivity; 1, weak reactivity; 2, moderate reactivity; and 3, intense reactivity. Nevertheless, in the present work we also evaluate the immunohistochemical staining by each main cellular type: tumoral cells, fibroblastic-like cells and monuclear inflammatory cells (MICs). In cases with cellular types positives for each MMPs or TIMPs, at least as 70% of these corresponding cell types showed a positive immunostaining at each evaluated field. We distinguished stromal cells from cancer cells because these latter cells are larger in size. In addition, fibroblasts are spindle cells whereas MICs are round cells. On the other hand, while cancer cells are arranged forming either acinar or trabecullar pattern, stromal cells are spreaded. Moreover, we used two markers to distinguish fibroblast-like cells from tumoral cells: cytokeratins and vimentin, and several markers to distinguish mononuclear inflammatory cells (T lymphocites (CD 3, CD 45Ro, CD 4,CD 8), B lymphocites (CD 20,CD 79a) and macrophages (CD68).
Statistical analysis
Differences in percentages were calculated using the chi-square test. Immunostaining score values for each protein were expressed as a mean ± standard deviation (SD. Correlation between score values was calculated by using the Spearman correlation test. Comparison of immunostaining values between groups was made with the Mann-Whitney or Kruskall–Wallis tests. For metastasis-free survival analysis we used the Cox’s univariate method. Cox’s regression model was used to examine interactions of different prognostic factors in a multivariate analysis. Expression profiles were analysed by an unsupervised hierarchical clustering method that organizes proteins in tree structures, based on their similarity. Data was reformatted as follows: ‘-3’ designated negative staining, ‘3’ positive staining, and missing data was left blank. We used the Cluster 3.0 program (average linkage, uncentred correlation). Results were displayed with the Treeview program (Eisen et al. 1998). The spss 17.0 program (SPSS Inc., Chicago, IL, USA) was used for all calculations. P< 0.05 was considered significant.
Results
Figure 1 shows representative examples of MMPs and TIMPs expressions by MICs in the centre of the tumour, at the invasive front and at MALNs of breast carcinomas. Immunostaining for these proteins has a cytoplasmic location in all positive cases. With regard to MMP-14 expression, the immunostaining showed both cytoplasmic and membrane location. As Table 2 shows, the global expressions (intensity of staining) for MMPs and TIMPs varied among tumours in the different tumoral localizations. In addition, we found that the intensity of staining values of MMP-1, 7 and 14, TIMP-1, and 3, were significantly higher at the centre of the tumour than at the invasive front or at MALNs.
Figure 1.
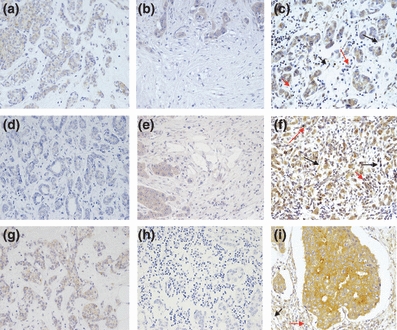
Representative examples of MMPs and TIMPs expressions in the centre of the tumour, at the invasive front and at MALNs of breast carcinomas. (a) 200 ×. MMP-7 expression in the centre of the tumour. (b) 200 ×. MMP-7 expression at the invasive front. (c) 200x MMP-7 expression at MALN. (d) 200 ×. MMP-14 expression in the centre of the tumour. (e) 200 ×. MMP-14 expression at the invasive front. (f) 200 ×. MMP-14 expression at MALN. (g) 200 ×. TIMP-3 expression in the centre of the tumour. (h) 200 ×. Weak expression of TIMP-3 at the invasive front. (i) 200 ×. TIMP-3 expression at MALN. Black arrows: expression in fibroblast-like cells. Red arrows: expression in MICs.
Table 2.
Score values and intensity of immunostaining for MMPs and TIMPs in tumoral centre, invasive front and in metastasic axillary lymph nodes (MALN)
| Intensity of immunostaining |
||||
|---|---|---|---|---|
| Factor | Tumoral Centre | Invasive front | MALN | P* |
| MMP-1 | 1.89 ± 0.82 | 1.63 ± 0.54 | 1.03 ± 0.67 | 0.0001 |
| MMP-2 | 0.49 ± 0.79 | 0.32 ± 0.63 | 0.42 ± 0.5 | n.s. |
| MMP-7 | 2.11 ± 0.78 | 0.84 ± 0.5 | 0.79 ± 0.62 | 0.0001 |
| MMP-9 | 0.85 ± 0.71 | 1.11 ± 0.51 | 0.89 ± 0.46 | n.s. |
| MMP-11 | 1.46 ± 1.02 | 1.67 ± 0.67 | 1.43 ± 0.81 | n.s. |
| MMP-13 | 0.85 ± 0.63 | 0.83 ± 0.56 | 0.94 ± 0.64 | n.s. |
| MMP-14 | 1.21 ± 0.76 | 0.89 ± 0.45 | 0.7 ± 0.52 | 0.003 |
| TIMP-1 | 1.67 ± 0.81 | 1.08 ± 0.36 | 1.16 ± 0.5 | 0.0001 |
| TIMP-2 | 1.44 ± 0.94 | 1.11 ± 0.52 | 1.22 ± 0.63 | n.s. |
| TIMP-3 | 1.54 ± 0.88 | 0.27 ± 0.45 | 0.95 ± 0.74 | 0.0001 |
Data are represented as median and standard deviation (SD). MALN, metastasis axillary lymph nodes.
Kruskal–Wallis test.
Table 3 shows the correlation coefficients between the immunostining values for MMPs and TIMPs belonging to the different tumoral localizations. There were several significant but low correlations between these different localizations. Nevertheless, it was of note that we found higher number of significant correlations between MALNs or between invasive front and MALNs, than between tumoral centre and MALNs.
Table 3.
Correlations between immunostaining values for MMPs and TIMPs from the different tumoral localizations
| Spearman correlation coefficients (rS) |
||||
|---|---|---|---|---|
| Factors | Between TC and IF | Between TC and LN | Between IF and LN | Between MALNs |
| MMP-1 | 0.29 | 0.01 | 0.41* | 0.69*** |
| MMP-2 | 0.38* | 0.37* | 0.43* | 0.65** |
| MMP-7 | −0.11 | −0.14 | 0.35*** | 0.67*** |
| MMP-9 | 0.32* | 0.19 | 0.31 | 0.68*** |
| MMP-11 | 0.33* | 0.36* | 0.46* | 0.81*** |
| MMP-13 | 0.29 | 0.15 | 0.32 | 0.69**** |
| MMP-14 | 0.11 | 0.23 | 0.33* | 0.69*** |
| TIMP-1 | 0.19 | 0.56*** | 0.35** | 0.58** |
| TIMP-2 | 0.30 | 0.01 | −0.05 | 0.67*** |
| TIMP-3 | −0.07 | −0.10 | 0.03 | 0.75*** |
P< 0.05;
P < 0.01;
P < 0.001.
TC, tumoral centre; IF, invasive front; MALNs, metastasic axillary lymph nodes.
For correlations between MALNs, these ones with higher score valur and these ones with the lower score value were chosen in each case for each factor.
We also compared the expression of MMPs and TIMPs by the different cellular types at various tumour locations. The results (Table 4) demonstrated significant differences in the expression of MMPs and TIMPs between tumour cells in different locations. However, these location-related expression differences where even more significant in stromal cells. Indeed, fibroblast-like cells and MICs in MALNs displayed significantly higher percentages of expression of MMP-7 and 14, and TIMP-3.
Table 4.
Expressions of MMPs and TIMPs by the different cellular types in tumoral centre, invasive front and in metastasic axillary lymph nodes
| Tumoral cell |
Fibroblast-like cells |
MICs |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | TC | IF | MALN | P | TC | IF | MALN | P | TC | IF | MALN | P |
| MMP-1 | 31 (79.5%) | 37 (94.9%) | 36 (92.3%) | 0.037 | 20 (51.3%) | 37 (94.9%) | 31 (79.5%) | 0.0001 | 21 (53.8%) | 24 (61.5%) | 29 (74.4%) | 0.106 |
| MMP-2 | 19 (48.7%) | 9 (23.1%) | 13 (33.3%) | 0.048 | 6 (15.4%) | 4 (10.3%) | 4 (10.3%) | 0.688 | 4 (10.3%) | 0 (0%) | 0 (0%) | 0.014 |
| MMP-7 | 30 (76.9%) | 29 (74.4%) | 36 (92.3%) | 0.014 | 10 (25.6%) | 3 (7.7%) | 33 (84.6%) | 0.0001 | 10 (25.6%) | 1 (2.6%) | 19 (48.7%) | 0.0001 |
| MMP-9 | 31 (79.5%) | 35 (89.7%) | 26 (66.7%) | 0.005 | 31 (79.5%) | 13 (33.3%) | 3 (7.7%) | 0.015 | 9 (23.1%) | 8 (20.5%) | 1 (2.6%) | 0.034 |
| MMP-11 | 33 (84.6%) | 35 (89.7%) | 32 (82.1%) | 0.083 | 19 (48.7%) | 27 (69.2%) | 26 (66.7%) | 0.136 | 18 (46.2%) | 18 (46.2%) | 5 (12.8%) | 0.001 |
| MMP-13 | 31 (79.5%) | 27 (69.2%) | 28 (71.8%) | 0.186 | 15 (38.5%) | 13 (33.3%) | 3 (7.7%) | 0.834 | 18 (46.2%) | 4 (10.3%) | 16 (41%) | 0.001 |
| MMP-14 | 30 (76.9%) | 31 (79.5%) | 32 (94.9%) | 0.953 | 11 (28.2%) | 12 (30.8%) | 27 (69.2%) | 0.001 | 9 (23.1%) | 7 (17.9%) | 23 (59%) | 0.0001 |
| TIMP-1 | 35 (89.7%) | 36 (92.3%) | 37 (94.9%) | 0.823 | 15 (38.5%) | 11 (28.2%) | 3 (7.7%) | 0.358 | 15 (38.5%) | 6 (15.4%) | 10 (25.6%) | 0.038 |
| TIMP-2 | 35 (89.7%) | 34 (87.2%) | 29 (74.4%) | 0.018 | 11 (28.2%) | 10 (25.6%) | 13 (33.3%) | 0.834 | 11 (28.2%) | 12 (30.8%) | 8 (20.5%) | 0.472 |
| TIMP-3 | 29 (74.4%) | 10 (25.6%) | 34 (87.2%) | 0.001 | 11 (28.2%) | 3 (7.7%) | 24 (61.5%) | 0.0001 | 9 (23.1%) | 0 (0%) | 26 (66.7%) | 0.0001 |
Data are represented as numbers of cases (%).
TC, tumoral centre; IF, invasive front; MALN, metastasic axillary lymph nodes.
To identify specific groups of tumours with distinct MMP/TIMP immunohistochemical expression profiles in MALNs, protein expression data were evaluated by unsupervised hierarchical cluster analysis for each cellular type. This algorithm placed proteins on the horizontal axis and samples on the vertical axis based on similarity of their expression profiles. This study did not produce a dendrogram with well-defined cluster of cases when tumour cells were analysed (Figure 2a). However, for fibroblast-like cells, the dendogram shows a first-order division of all tumours into two distinct MMP/TIMP molecular profiles, designated group 1 (with high molecular profile MMPs/TIMPs expression; n=10) and group 2 (with low molecular profile; n=29) (Figure 2b). For MICs the dendogram also shows a first-order division of the tumours into two distinct MMP/TIMP molecular profiles, designated group 1 (n=15) and group 2 (n=24) (Figure 2c). It is of mention that these different patterns correlate with each other (P= 0.0001). Thus, whereas 24 tumours (61.5%) showed low molecular profile MMPs/TIMPs expression of either fibroblasts-like cells and MICs, 10 tumours (25.6%) high molecular profile MMPs/TIMPs expression of either fibroblasts-like cells and MICs. The remainder five tumours (12.6%) showed and high molecular profile MMPs/TIMPs expression of MICs and low molecular profile MMPs/TIMPs expression of fibroblasts-like cells.
Figure 2.
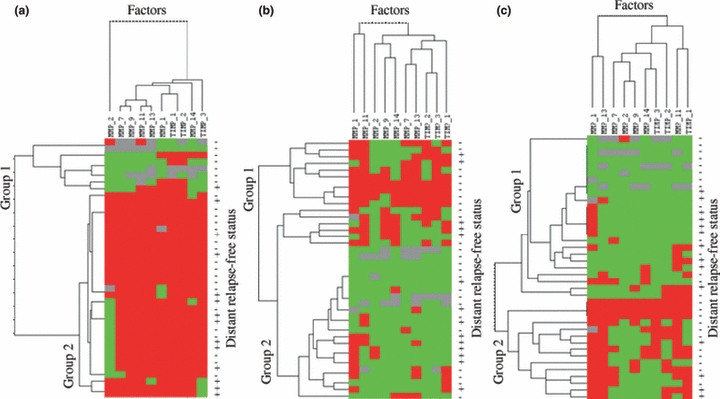
Hierarchical clustering analysis of global MMPs/TIMPs expression in the different cells types of breast cancer as measured by immunohistochemistry on TA. Graphical representation of hierarchical clustering results in tumoral cells (a), fibroblasts (b) and mononuclear inflammatory cells (c). Rows, tumour samples; columns, MMPs/TIMPs. Protein expressions are depicted according to a colour scale: red, positive staining; green, negative staining; grey, missing data. The status columns: + = with recurrence; − = without recurrence, at the census point. Two major cluster of tumours are shown for fibroblast-like cells and mononuclear inflammatory cells.
In the present study we also investigated the possible influence of MMPs and TIMPs expression levels and locations on distant relapse-free survival. As shown in Table 5, univariate and multivariate analysis showed that only the expression of some of these factors at the primary tumours was significantly associated with relapse-free survival, whereas expression of none of the proteins studied at MANLs was associated with the occurrence of distant metastasis (data not shown). We found, however, that the expression of MM-11 and TIMP-1 and 2 by MICs, as well as the expression of TIMP-2 by fibroblast-like cells, at the tumour centre were significantly associated with the occurrence of distant metastasis. Notably, and contrary to those results, TIMP-3 expression by tumoral cells or by fibroblast-like cells in those same tumour locations, were significantly associated with lower occurrence of distant metastasis. In addition, our results also showed that TIMP-1 expression by fibroblast-like cells at the invasive front was significantly associated with poor prognosis (Table 5). Multivariate analysis according to a Cox model demonstrated that tumour stage was significantly and independently associated with relapse-free survival. However, this same analysis also demonstrated that TIMP-1 and TIMP-3 expressions were variables significantly and independently associated with prognosis (Table 5).
Table 5.
Cox’s univariate (HR) and multivariate (RR) analysis of the relationships between MMPs and TIMPs expressions and relapse-free survival
| Factor | Number of patients | Event frequency | HR (95%CI) | RR (95%CI) |
|---|---|---|---|---|
| Stage II vs. Stage III | 22/17 | 3/11 | 9.75 (2.6–35.9)**** | 6.3 (1.6–24.3)**** |
| Tumoral centre | ||||
| MMP-11 | ||||
| MICs(-)vs.(+) | 34/5 | 9/5 | 5.24 (1.7–16.1)*** | |
| TIMP-1 | ||||
| MICs(-)vs.(+) | 31/8 | 8/6 | 2.75 (1.2–7.9) | 3.46 (1.02–11.7)* |
| TIMP-2 | ||||
| Fibroblast-like c. (-)vs(+) | 26/13 | 6/8 | 4.13 (1.41–12.04)** | |
| MICs(-)vs.(+) | 31/8 | 7/7 | 6.60 (2.20–19.79)**** | |
| TIMP-3 | ||||
| Tumoral cells (-)vs(+) | 5/34 | 4/10 | 0.21 (0.06–0.72)* | 1.61 (0.03–0.71)* |
| MICs(-)vs.(+) | 13/26 | 8/6 | 0.15 (0.04–0.53)*** | 0.23 (0.06–0.9)* |
| Invasive front | ||||
| TIMP-1 | ||||
| Fibroblast-like c. (-)vs.(+) | 26/11 | 6/8 | 5.01 (1.6–15.5)*** | 3.59 (0.99–13.06)* |
In the multivariate analysis were included, for each MMPs or TIMPs, the classical clinico-pathological parameters (tumour stage, tumours grade, oestrogen and progesterone receptors status) and adjunvant therapy (log rank test).
With regard to the validity of the Cox regression analysis for proportionality, we analysed the normal distribution of value survival function of events by using the Shapiro–Wilk test (P= 0.13).
P< 0.05;
P< 0.01;
P< 0.05;
P< 0.001.
Discussion
There have been no studies on MMPs and TIMPs expression profiles in draining lymph nodes of breast cancer patients. Draining lymph nodes are of great interest because of their exposure to all soluble factors coming from the tumour; also, they may be colonized by aggressive clones deriving from primary tumour cells. The present study shows new aspects of the expression of MMPs and TIMPs in different tumour locations in node-positive breast cancer: tumour centre, invasive front and MALNs. Our data demonstrate that there is no single pattern of MMP and TIMPs expression in the different tumour compartments. However, we found changes in the expression of these proteins that have been implicated consistently in invasion and metastasis, which seem to correspond to evolutionary changes in tumoral progression.
When we compared the immunostaining values of proteins between the different tumour locations, the higher positive correlations were found between MALNs. This finding suggests that clones of primary tumour cells which colonize regional lymph nodes show a similar phenotype of MMPs/TIMPs expressions. We also found major similarities in MMPs/TIMPs expression between the invasive front and the MALNs in breast cancer. These similarities could not be found when we compared the tumour centre with the MALNs. This could be explained by the reorganization of the lymphatic network that is found in breast cancer. Indeed, whereas it is well known the putative absence of intratumoral lymphatics in invasive breast carcinomas (Vleugel et al. 2004), there are data indicating an increased density of peritumour lymphatic vessels in breast cancer as compared with normal breast (Agarwal et al. 2005), which may facilitate the lymphatic migration of tumoral cells to the axillary lymph nodes.
We also found the existence of higher global expression levels of several factors, such as MMP-1, 7, and 14, TIMP-1, 2 and 3, in the tumour centre compared with the invasive front or with the MALNs. These different patterns of expression of MMPs and TIMPs may correspond to differences in cellular density, which is higher in the centre of the tumours, and/or to different biological mechanisms of interaction between tumour cells and the fibroblast-like cell population of those two different tumour areas. This suggestion is based upon the notion that cell-cell contact between cancer cells and fibroblasts enhances the production and activation of MMPs by cancer cells, which in turn promotes pericellular proteolysis, angiogenesis, and tumour cell invasion (Behrens et al. 2001; Sato et al. 2004).
Despite the fact that there were significant differences in the expression MMPs by tumour cells from the different tumour locations, however, the more conspicuous differences were found between stromal cells when we compared their different locations (i.e. the higher levels of expression of MMP-7 and -14, and TIMP-3 were found in fibroblast-like cells and MICs at MALNs). This seems to indicate that metastasic cancer cells have the ability to induce the production of these proteins in host cells within the lymph nodes, which emphasizes the importance of the stromal-epithelial interactions in tumour progression. This is especially relevant with regard to the expressions of MMP-7 and 14 by stromal cells of MALNs, due to their role in tumour progression. Hence, MMP-7 (matrilysin 1) is a stromelysin which degrades type IV collagen, fibronectin and laminin. MMP-7 is also able to cleave integrins on the surface of cancer cells, including breast cancer cells (Abdel-Ghany et al. 2001). MMP-7 forms a complex with CD44 at the cell surface of cancer cells, possibly to coordinate the matrix degradation process by cancer cells (Yu et al. 2002). MMP-7 can also cleave E-cadherin to induce the invasive potential of cancer cells (Davies et al. 2001; Noe et al. 2001). Likewise, it has been recently reported that MMP-7 over-expression in breast cancer (MCF-7) cells enhances cellular invasiveness and activation of proMMP-2 and MMP-9 (Wang et al. 2006). MMP-14 (membrane type 1 MMP, or MT1-MMP) is a key metalloprotease involved in the degradation of extracellular matrix, activates pro-MMP-13 (Knauper et al. 1996)and pro-MMP-2 (Strongin et al. 1995) on the cell surface, and plays crucial roles in molecular carcinogenesis, tumour cell growth, invasion and angiogenesis.
In this context, however, it was remarkable the high expression of TIMP-3 in stromal cells of MALNs. In fact, previous experimental studies have shown that TIMP-3 may inhibit angiogenesis and also induce apoptosis which would not be coherent with a pro-tumour role of this protein (Ahonen et al. 1998; Spurbeck et al. 2002). However, in a prior study we also found that TIMP-3 expression by fibroblast-like cells, but not by cancer cells, correlates positively with the occurrence of distant metastases, reflecting the existence of additional molecular mechanisms in the molecular biology of the breast tumours in which TIMP-3 might have a functional role (Vizoso et al. 2007).
Remarkably, we found a subset of breast tumours having a phenotype of MICs or fibroblast-like cells expressing high MMPs and TIMPs in their MALNs. This finding seems to indicate that, after contacting with metastasis tumour cells, stromal cells of the nodal lymphs diverge into two different behaviours with regard to MMPs/TIMPs expression. However, we could only find a significant prognostic impact of the expression of all the different proteins in different tumour locations, particularly in node-positive tumours, with the occurrence of distant metastasis. These findings point to the importance of the expression patterns in primary tumours, rather than in MALNs, in the occurrence of distant metastasis in breast cancer. This also support the concept that lymph node metastasis, while providing a staging or prognostic value, probably have no governing or controlling role in developing later distant vital organ metastasis (Cady 2007). A reasonable hypothesis to explain this is that metastatic cells having the ability to lodge and grow in lymph nodes may have no capacity to lodge in, extravasate, and grow progressively in other organs, although retaining the ability to involve lymph nodes sequentially. Accordingly, it has been demonstrated that human breast cancer lymph node metastatic cells when injected systemically in animal models specifically lodged in lymph node stromal sites (Irjala et al. 2003). This is in agreement with recent studies showing that metastasis from human breast cancer metastasic cell lines injected in animal models had selective propensity to lodge in those same organs with a similar genetic profile, indicating highly complex selective metastasic site choice behaviour (Kang et al. 2003; Minn et al. 2005).
It was also remarkable our data showing the prognostic value of the expressions of TIMPs in node-positive breast cancer. Our results showing a significant association between TIMP-1 and TIMP-2 and a high occurrence of distant metastases, are in accordance to similar findings reported in other studies (Ree et al. 1997; Remacle et al. 2000; Schrohl et al. 2004; Gonzalez et al. 2007; Vizoso et al. 2007). If TIMPs inhibit MMPs in vivo, it should be expected that high levels of these inhibitors would prevent tumour progression and thus be related with good outcome in patients with cancer. However, TIMPs are multifunctional proteins that in addition to its MMP-inhibitory effect also demonstrate distinct tumour-stimulatory functions (Jiang et al. 2002). By contrary, our data suggest the possible importance of the expression of TIMP-3 in tumoral centre of primary tumours, by cancer cells or MICs, to predict a more favourable prognosis in patients with node-positive breast cancer. In addition to the biological aspects on TIMP-3 referred more above, it has been published that high TIMP-3 mRNA levels are associated with a good prognosis in breast cancer (Kotzsch et al. 2005). Likewise, Span et al have reported that high levels of TIMP-3 predicted a longer relapse-free survival in patients treated with tamoxifen (Span et al. 2004). All of these findings suggest that TIMP-3 is involved in specific pathways of tamoxifen-induced apoptosis. In accordance with this, previous results showed a significantly higher TIMP-3 expression in ER-positive tumours (Span et al. 2004; Vizoso et al. 2007). Thus, we consider that TIMP-3 expression by primary tumours may be a possible prognostic and/or predictive factor in node-positive patients with breast cancer.
Conclusions
Our data point the prognostic importance of the expression of MMPs and TIMPs by stromal cells in primary tumours, rather than their expression in MALNs. We found the association of a high expression of those proteins in tumour stromal cells with the occurrence of distant metastasis in patients with node-positive breast cancer. Nevertheless, we also found significant changes in the expression of these factors between primary tumours and MALNs, which may underline the evolutionary behaviour of breast cancer across to their different localizations. We propose that some of these findings may applied to design further studies in breast cancer, such as for instance, to predict the invasion of non-sentinel lymph nodes in breast cancer.
Author contribution
Conception and design: García M. Fernanda, Medina María and Vizoso Francisco. Provision of study materials or patients: Junquera Sara, del Casar José M and Berdidze Nana. Collection and analysis of samples: González and González-Reyes S. Data analysis and interpretation: García M. Fernanda, González-Reyes S, Gonzalez Luis O, and Vizoso Francisco. Final approval of manuscript: García M. Fernanda, González-Reyes Salomé, González Luis.O., Junquera Sara, Berdidze Nana, del Casar José M., Medina María and Vizoso Francisco.
Competing interests
The authors indicated no potential conflicts of interest.
Grant support
This work was supported by grants from Fondo de Investigación Sanitaria del Instituto Carlos III (FIS-PI070306 and EXPTE: PI08/90043).
References
- Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. The breast cancer beta 4 integrin and endothelial human CLCA2 mediate lung metastasis. J. Biol. Chem. 2001;276:25438–25446. doi: 10.1074/jbc.M100478200. [DOI] [PubMed] [Google Scholar]
- Agarwal B, Saxena R, Morimiya A, Mehrotra S, Badve S. Lymphangiogenesis does not occur in breast cancer. Am. J. Surg. Pathol. 2005;29:1449–1455. doi: 10.1097/01.pas.0000174269.99459.9d. [DOI] [PubMed] [Google Scholar]
- Ahonen M, Baker AH, Kahari VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998;58:2310–2315. [PubMed] [Google Scholar]
- Andersen J, Poulsen HS. Relationship between estrogen receptor status in the primary tumour and its regional and distant metastases. An immunohistochemical study in human breast cancer. Acta Oncol. 1988;27:761–765. doi: 10.3109/02841868809091782. [DOI] [PubMed] [Google Scholar]
- Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br. J. Cancer. 1999;79:1347–1355. doi: 10.1038/sj.bjc.6690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens P, Rothe M, Wellmann A, Krischler J, Wernert N. The Ets-1 transcription factor is up-regulated together with MMP 1 and MMP 9 in the stroma of pre-invasive breast cancer. J. Pathol. 2001;194:43–50. doi: 10.1002/path.844. [DOI] [PubMed] [Google Scholar]
- Cady B. Regional lymph node metastases; a singular manifestation of the process of clinical metastases in cancer: contemporary animal research and clinical reports suggest unifying concepts. Ann. Surg. Oncol. 2007;14:1790–1800. doi: 10.1245/s10434-006-9234-2. [DOI] [PubMed] [Google Scholar]
- Chang DB, Yang PC, Chang KJ, Luh KT, Kuo SH. Comparison of DNA stemline and cell kinetics between primary breast cancer and its lymph node metastasis. Anal. Quant. Cytol. Histol. 1993;15:32–38. [PubMed] [Google Scholar]
- Chantrain CF, Shimada H, Jodele S, et al. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer. Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- Cornelius LA, Nehring LC, Harding E, et al. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J. Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- Daidone MG, Silvestrini R, Valentinis B, et al. Proliferative activity of primary breast cancer and of synchronous lymph node metastases evaluated by [3H]-thymidine labelling index. Cell. Tissue. Kinet. 1990;23:401–408. doi: 10.1111/j.1365-2184.1990.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Davies G, Jiang WG, Mason MD. Matrilysin mediates extracellular cleavage of E-cadherin from prostate cancer cells: a key mechanism in hepatocyte growth factor/scatter factor-induced cell-cell dissociation and in vitro invasion. Clin. Cancer Res. 2001;7:3289–3297. [PubMed] [Google Scholar]
- Del Casar JM, Gonzalez LO, Alvarez E, et al. Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumor stromal fibroblasts and those at the invasive front of breast carcinomas. Breast. Cancer Res. Treat. 2009;116 doi: 10.1007/s10549-009-0351-z. [DOI] [PubMed] [Google Scholar]
- Demers M, Couillard J, Belanger S, St-Pierre Y. New roles for matrix metalloproteinases in metastasis. Crit. Rev. Immunol. 2005;25:493–523. doi: 10.1615/critrevimmunol.v25.i6.30. [DOI] [PubMed] [Google Scholar]
- Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast. Cancer Res. 2000;2:252–257. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichter GE, Kaufmann M, Muller A, et al. DNA index and cell cycle analysis of primary breast cancer and synchronous axillary lymph node metastases. Breast Cancer Res. Treat. 1989;13:17–22. doi: 10.1007/BF01806546. [DOI] [PubMed] [Google Scholar]
- Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia. 2001;3:459–468. doi: 10.1038/sj.neo.7900190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LO, Pidal I, Junquera S, et al. Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br. J. Cancer. 2007;97:957–963. doi: 10.1038/sj.bjc.6603963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson WH, III, Ljung BM, Moore DH, II, et al. Tumor labeling indices of primary breast cancers and their regional lymph node metastases. Cancer. 1993;71:3914–3919. doi: 10.1002/1097-0142(19930615)71:12<3914::aid-cncr2820711219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Irjala H, Alanen K, Grenman R, et al. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)-1 direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res. 2003;63:4671–4676. [PubMed] [Google Scholar]
- Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- Jones JL, Glynn P, Walker RA. Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. J. Pathol. 1999;189:161–168. doi: 10.1002/(SICI)1096-9896(199910)189:2<161::AID-PATH406>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Kotzsch M, Farthmann J, Meye A, et al. Prognostic relevance of uPAR-del4/5 and TIMP-3 mRNA expression levels in breast cancer. Eur. J. Cancer. 2005;41:2760–2768. doi: 10.1016/j.ejca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Meller D, Li DQ, Tseng SC. Regulation of collagenase, stromelysin, and gelatinase B in human conjunctival and conjunctivochalasis fibroblasts by interleukin-1beta and tumor necrosis factor-alpha. Invest. Ophthalmol. Vis. Sci. 2000;41:2922–2929. [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett BF, Baban D, Bao L, Tarin D. Fate of clonal lineages during neoplasia and metastasis studied with an incorporated genetic marker. Cancer Res. 1992;52:1737–1743. [PubMed] [Google Scholar]
- Mori T, Morimoto T, Komaki K, Monden Y. Comparison of estrogen receptor and epidermal growth factor receptor content of primary and involved nodes in human breast cancer. Cancer. 1991;68:532–537. doi: 10.1002/1097-0142(19910801)68:3<532::aid-cncr2820680314>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Rank F, Lopez JM, et al. Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res. 2001;61:7091–7100. [PubMed] [Google Scholar]
- Nishizaki T, DeVries S, Chew K, et al. Genetic alterations in primary breast cancers and their metastases: direct comparison using modified comparative genomic hybridization. Genes Chromosomes Cancer. 1997;19:267–272. doi: 10.1002/(sici)1098-2264(199708)19:4<267::aid-gcc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J. Cell. Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat. Rev. Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- Ree AH, Florenes VA, Berg JP, et al. High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin. Cancer Res. 1997;3:1623–1628. [PubMed] [Google Scholar]
- Remacle A, McCarthy K, Noel A, et al. High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int. J. Cancer. 2000;89:118–121. doi: 10.1002/(sici)1097-0215(20000320)89:2<118::aid-ijc3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rifkin DB, Mazzieri R, Munger JS, Noguera I, Sung J. Proteolytic control of growth factor availability. Apmis. 1999;107:80–85. doi: 10.1111/j.1699-0463.1999.tb01529.x. [DOI] [PubMed] [Google Scholar]
- Sato T, Sakai T, Noguchi Y, et al. Tumor-stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol. Oncol. 2004;92:47–56. doi: 10.1016/j.ygyno.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Schrohl AS, Holten-Andersen MN, Peters HA, et al. Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin. Cancer Res. 2004;10:2289–2298. doi: 10.1158/1078-0432.ccr-03-0360. [DOI] [PubMed] [Google Scholar]
- Span PN, Lindberg RL, Manders P, et al. Tissue inhibitors of metalloproteinase expression in human breast cancer: TIMP-3 is associated with adjuvant endocrine therapy success. J. Pathol. 2004;202:395–402. doi: 10.1002/path.1528. [DOI] [PubMed] [Google Scholar]
- Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002;100:3361–3368. doi: 10.1182/blood.V100.9.3361. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell. Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J. Clin. Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm FK, Mahvi DM, Gilchrist KW. Heat shock protein 27 overexpression in breast cancer lymph node metastasis. Ann. Surg. Oncol. 1996;3:570–573. doi: 10.1007/BF02306091. [DOI] [PubMed] [Google Scholar]
- Strongin AY, Collier I, Bannikov G, et al. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Talvensaari-Mattila A, Turpeenniemi-Hujanen T. Preoperative serum MMP-9 immunoreactive protein is a prognostic indicator for relapse-free survival in breast carcinoma. Cancer Lett. 2005;217:237–242. doi: 10.1016/j.canlet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer. 2003;89:1270–1275. doi: 10.1038/sj.bjc.6601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi S, Giannella C, Paradiso A, et al. HER-2/neu gene in primary and local metastatic axillary lymph nodes in human breast tumors. Int. J. Biol. Markers. 1992;7:107–113. doi: 10.1177/172460089200700207. [DOI] [PubMed] [Google Scholar]
- Umekita Y, Sagara Y, Yoshida H. Estrogen receptor mutations and changes in estrogen receptor and progesterone receptor protein expression in metastatic or recurrent breast cancer. Jpn. J. Cancer Res. 1998;89:27–32. doi: 10.1111/j.1349-7006.1998.tb00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Agthoven T, Timmermans M, Dorssers LC, Henzen-Logmans SC. Expression of estrogen, progesterone and epidermal growth factor receptors in primary and metastatic breast cancer. Int. J. Cancer. 1995;63:790–793. doi: 10.1002/ijc.2910630607. [DOI] [PubMed] [Google Scholar]
- Vizoso FJ, Gonzalez LO, Corte MD, et al. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br. J. Cancer. 2007;96:903–911. doi: 10.1038/sj.bjc.6603666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugel MM, Bos R, van der Groep P, et al. Lack of lymphangiogenesis during breast carcinogenesis. J. Clin. Pathol. 2004;57:746–751. doi: 10.1136/jcp.2003.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Reierstad S, Fishman DA. Matrilysin over-expression in MCF-7 cells enhances cellular invasiveness and pro-gelatinase activation. Cancer Lett. 2006;236:292–301. doi: 10.1016/j.canlet.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Wurtz SO, Schrohl AS, Sorensen NM, et al. Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr. Relat. Cancer. 2005;12:215–227. doi: 10.1677/erc.1.00719. [DOI] [PubMed] [Google Scholar]
- Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes. Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]


